Targeting Cancer Translational Plasticity: IRES-Driven Metabolism and Survival Within the Tumor Microenvironment
Simple Summary
Abstract
1. Introduction
2. IRES-Mediated Translation: Mechanisms and Regulation
2.1. Canonical vs. Non-Canonical Translation
2.2. IRES Mechanisms and Regulation
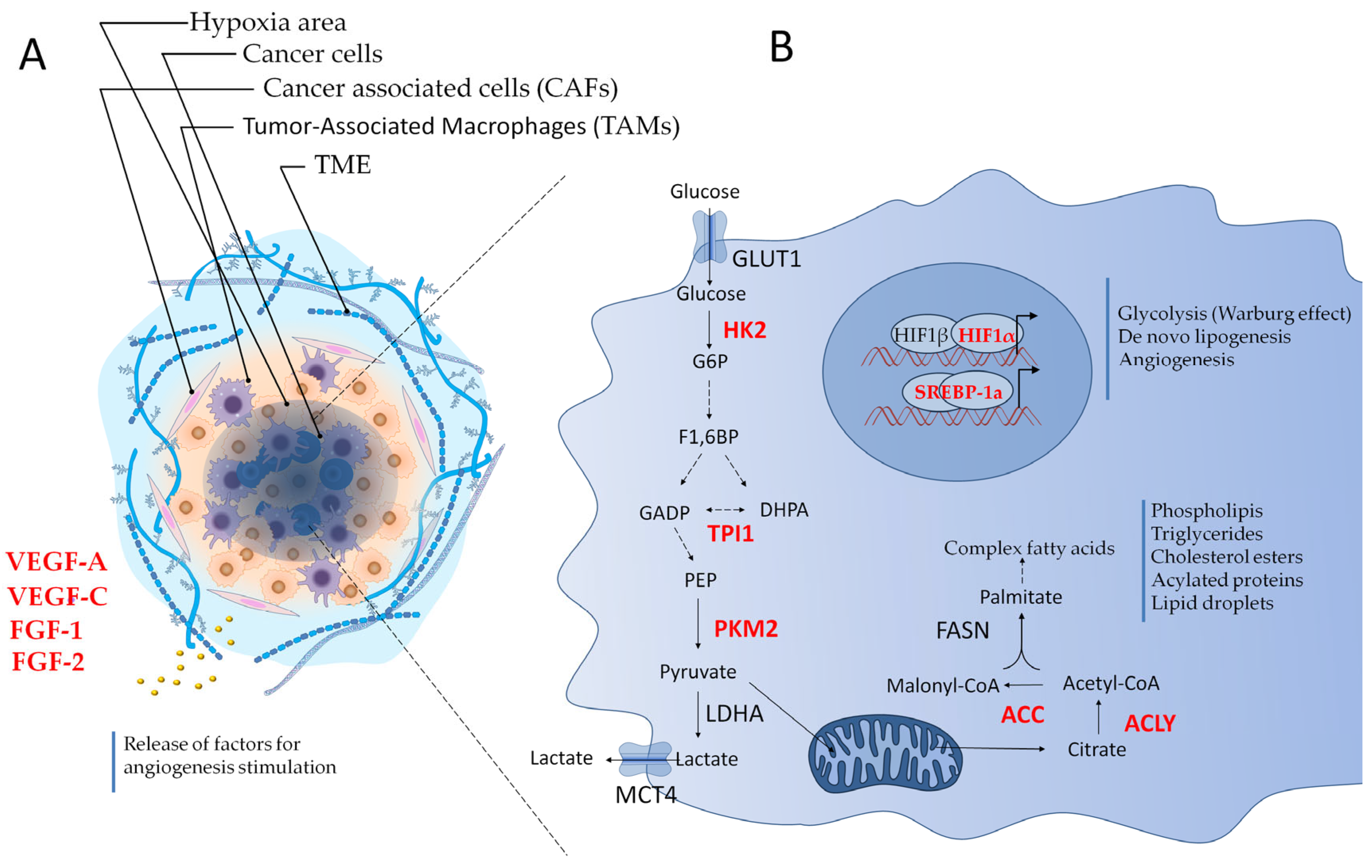
3. The Tumor Microenvironment (TME): Stress Conditions and Adaptive Translation
4. IMT of Growth Factors and Their Receptors in the TME
5. IMT of Oncogenes Driving Cell Proliferation and Cycle Progression
6. IMT Within the Integrated Stress Response (ISR) and Proteostasis Regulation in the TME
7. IMT in Hypoxia and Tumor Angiogenesis
8. Metabolic Adaptations for Tumor Survival: The Role of IMT
8.1. Glycolysis and the Warburg Effect
8.2. De Novo Lipogenesis
8.3. IMT Control of One-Carbon and Polyamine Metabolism in Cancer
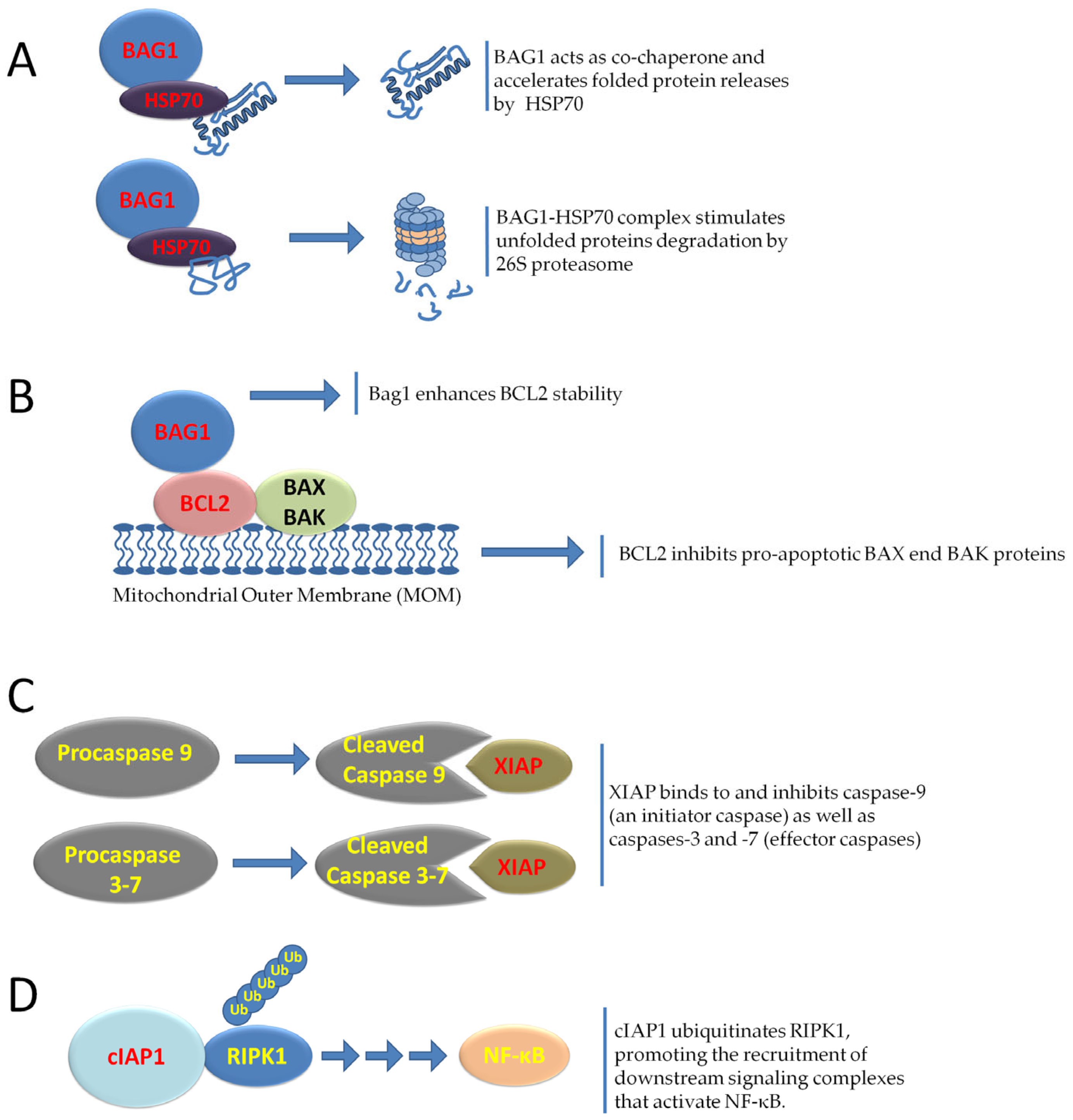
9. IRES-Driven Translation in Tumor Cell Survival, Senescence, and Therapy Resistance
10. The Role of IMT in NRF2 and NOS2 Expression in Response to Oxidative and Nitrosative Stress in Cancer
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merrick, W.C.; Pavitt, G.D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a033092. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Y.; Piao, W.; Jin, H. The Translational Regulation in mTOR Pathway. Biomolecules 2022, 12, 802. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Roiuk, M.; Neff, M.; Teleman, A.A. eIF4E-independent translation is largely eIF3d-dependent. Nat. Commun. 2024, 15, 6692. [Google Scholar] [CrossRef]
- Komar, A.A.; Hatzoglou, M. Cellular IRES-mediated translation: The war of ITAFs in pathophysiological states. Cell Cycle 2011, 10, 229–240. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- James, C.C.; Smyth, J.W. Alternative mechanisms of translation initiation: An emerging dynamic regulator of the proteome in health and disease. Life Sci. 2018, 212, 138–144. [Google Scholar] [CrossRef]
- Tidu, A.; Martin, F. The interplay between cis- and trans-acting factors drives selective mRNA translation initiation in eukaryotes. Biochimie 2024, 217, 20–30. [Google Scholar] [CrossRef]
- Mahé, M.; Rios-Fuller, T.; Katsara, O.; Schneider, R.J. Non-canonical mRNA translation initiation in cell stress and cancer. NAR Cancer 2024, 6, zcae026. [Google Scholar] [CrossRef]
- Chen, Q.M. The Odds of Protein Translation Control Under Stress. Antioxid. Redox Signal. 2024, 40, 943–947. [Google Scholar] [CrossRef]
- Romanelli, M.G.; Diani, E.; Lievens, P.M. New insights into functional roles of the polypyrimidine tract-binding protein. Int. J. Mol. Sci. 2013, 14, 22906–22932. [Google Scholar] [CrossRef]
- Godet, A.C.; David, F.; Hantelys, F.; Tatin, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.C. IRES Trans-Acting Factors, Key Actors of the Stress Response. Int. J. Mol. Sci. 2019, 20, 924. [Google Scholar] [CrossRef]
- Siculella, L.; Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Calcagnile, M.; Quarta, S.; Massaro, M.; Damiano, F. A comprehensive understanding of hnRNP A1 role in cancer: New perspectives on binding with noncoding RNA. Cancer Gene Ther. 2023, 30, 394–403. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Liu, Y.; Xie, L.; Hu, F.; Liu, H. Hypoxia-driven angiogenesis and metabolic reprogramming in vascular tumors. Front. Cell Dev. Biol. 2025, 15, 1572909. [Google Scholar] [CrossRef]
- Ribatti, D. Aberrant tumor vasculature. Facts and pitfalls. Front. Pharmacol. 2024, 15, 1384721. [Google Scholar] [CrossRef]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef]
- Yuan, Y. Spatial Heterogeneity in the Tumor Microenvironment. Cold Spring Harb. Perspect. Med. 2016, 6, a026583. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Sorensen, P.H. Translational control of aberrant stress responses as a hallmark of cancer. J. Pathol. 2018, 244, 650–666. [Google Scholar] [CrossRef]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef]
- Connolly, E.; Braunstein, S.; Formenti, S.; Schneider, R.J. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol. Cell. Biol. 2006, 26, 3955–3965. [Google Scholar] [CrossRef]
- Shin, S.; Han, M.J.; Jedrychowski, M.P.; Zhang, Z.; Shokat, K.M.; Plas, D.R.; Dephoure, N.; Yoon, S.O. mTOR inhibition reprograms cellular proteostasis by regulating eIF3D-mediated selective mRNA translation and promotes cell phenotype switching. Cell Rep. 2023, 42, 112868. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Bastide, A.; David, A. The ribosome, (slow) beating heart of cancer (stem) cell. Oncogenesis 2018, 7, 34. [Google Scholar] [CrossRef]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef]
- Mukherjee, S.; Warden, E.A.; Zhang, J. YAP/TAZ: An epitome of tumorigenesis. Cancer Lett. 2025, 625, 217806. [Google Scholar] [CrossRef]
- John, A.S.; Hu, X.; Rothman, V.L.; Tuszynski, G.P. Thrombospondin-1 (TSP-1) up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) production in human tumor cells: Exploring the functional significance in tumor cell invasion. Exp. Mol. Pathol. 2009, 87, 184–188. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov. 2020, 10, 1797–1807. [Google Scholar] [CrossRef]
- Werner, H. The IGF1 Signaling Pathway: From Basic Concepts to Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 14882. [Google Scholar] [CrossRef]
- Clark, N.K.; Harris, M.T.; Dahl, W.B.; Knotts, Z.; Marr, M.T. The Insulin Receptor and Insulin like Growth Factor Receptor 5′ UTRs Support Translation Initiation Independently of EIF4G1. Mol. Cell. Biol. 2023, 43, 485–499. [Google Scholar] [CrossRef]
- Meng, Z.; Jackson, N.L.; Choi, H.; King, P.H.; Emanuel, P.D.; Blume, S.W. Alterations in RNA-binding activities of IRES-regulatory proteins as a mechanism for physiological variability and pathological dysregulation of IGF-IR translational control in human breast tumor cells. J. Cell. Physiol. 2008, 217, 172–183. [Google Scholar] [CrossRef]
- Belfiore, A.; Rapicavoli, R.V.; Le Moli, R.; Lappano, R.; Morrione, A.; De Francesco, E.M.; Vella, V. IGF2: A Role in Metastasis and Tumor Evasion from Immune Surveillance? Biomedicines 2023, 11, 229. [Google Scholar] [CrossRef]
- Pedersen, S.K.; Christiansen, J.; Hansen, T.V.; Larsen, M.R.; Nielsen, F.C. Human insulin-like growth factor II leader 2 mediates internal initiation of translation. Biochem. J. 2002, 363, 37–44. [Google Scholar] [CrossRef]
- Dai, N.; Rapley, J.; Angel, M.; Yanik, M.F.; Blower, M.D.; Avruch, J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011, 25, 1159–1172. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-Like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef]
- Duan, S.; Rico, K.; Merchant, J.L. Gastrin: From Physiology to Gastrointestinal Malignancies. Function 2021, 3, zqab062. [Google Scholar] [CrossRef]
- Smith, J.P.; Fonkoua, L.K.; Moody, T.W. The Role of Gastrin and CCK Receptors in Pancreatic Cancer and other Malignancies. Int. J. Biol. Sci. 2016, 12, 283–291. [Google Scholar] [CrossRef]
- Grabowska, A.M.; Berry, C.A.; Hughes, J.; Bushell, M.; Willis, A.E.; Watson, S.A. A gastrin transcript expressed in gastrointestinal cancer cells contains an internal ribosome entry site. Br. J. Cancer 2008, 98, 1696–1703. [Google Scholar] [CrossRef]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating metabolism in cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef]
- Raji, L.; Tetteh, A.; Amin, A.R.M.R. Role of c-Src in Carcinogenesis and Drug Resistance. Cancers 2023, 16, 32. [Google Scholar] [CrossRef]
- Allam, H.; Ali, N. Initiation factor eIF2-independent mode of c-Src mRNA translation occurs via an internal ribosome entry site. J. Biol. Chem. 2010, 285, 5713–5725. [Google Scholar] [CrossRef]
- Stoneley, M.; Subkhankulova, T.; Le Quesne, J.P.C.; Coldwell, M.J.; Jopling, C.L.; Belsham, G.J.; Willis, A.E. Analysis of the c-myc IRES: A potential role for cell-type specific trans-acting factors and the nuclear compartment. Nucleic Acids Res. 2000, 28, 687–694. [Google Scholar] [CrossRef]
- Le Quesne, J.P.; Stoneley, M.; Fraser, G.A.; Willis, A.E. Derivation of a structural model for the c-myc IRES. J. Mol. Biol. 2001, 310, 111–126. [Google Scholar] [CrossRef]
- Jopling, C.L.; Willis, A.E. N-myc translation is initiated via an internal ribosome entry segment that displays enhanced activity in neuronal cells. Oncogene 2001, 20, 2664–2670. [Google Scholar] [CrossRef]
- Jopling, C.L.; Spriggs, K.A.; Mitchell, S.A.; Stoneley, M.; Willis, A.E. L-Myc protein synthesis is initiated by internal ribosome entry. RNA 2004, 10, 287–298. [Google Scholar] [CrossRef]
- O’Leary, C.A.; Andrews, R.J.; Tompkins, V.S.; Chen, J.L.; Childs-Disney, J.L.; Disney, M.D.; Moss, W.N. RNA structural analysis of the MYC mRNA reveals conserved motifs that affect gene expression. PLoS ONE 2019, 14, e0213758. [Google Scholar] [CrossRef]
- Jimenez, J.; Jang, G.M.; Semler, B.L.; Waterman, M.L. An internal ribosome entry site mediates translation of lymphoid enhancer factor-1. RNA 2005, 11, 1385–1399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, B.P.; Jimenez, J.; Lim, S.; Fitzgerald, K.D.; Zhang, M.; Chuah, C.T.; Axelrod, H.; Wilson, L.; Ong, S.T.; Semler, B.L.; et al. A novel Bcr-Abl-mTOR-eIF4A axis regulates IRES-mediated translation of LEF-1. Open Biol. 2014, 4, 140180. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Tian, S.; Fujii, K.; Kladwang, W.; Das, R.; Barna, M. RNA regulons in Hox 5′ UTRs confer ribosome specificity to gene regulation. Nature 2015, 517, 33–38. [Google Scholar] [CrossRef]
- Alghoul, F.; Laure, S.; Eriani, G.; Martin, F. Translation inhibitory elements from Hoxa3 and Hoxa11 mRNAs use uORFs for translation inhibition. Elife 2021, 10, e66369. [Google Scholar] [CrossRef]
- Kondrashov, N.; Pusic, A.; Stumpf, C.R.; Shimizu, K.; Hsieh, A.C.; Ishijima, J.; Shiroishi, T.; Barna, M. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 2011, 145, 383–397. [Google Scholar] [CrossRef]
- Shi, Y.; Sharma, A.; Wu, H.; Lichtenstein, A.; Gera, J. Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J. Biol. Chem. 2005, 280, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Beà, S.; Jares, P.; Campo, E. Molecular Pathogenesis of Mantle Cell Lymphoma. Hematol. Oncol. Clin. N. Am. 2020, 34, 795–807. [Google Scholar] [CrossRef]
- Valla, M.; Klæstad, E.; Ytterhus, B.; Bofin, A.M. CCND1 Amplification in Breast Cancer -associations With Proliferation, Histopathological Grade, Molecular Subtype and Prognosis. J. Mammary Gland. Biol. Neoplasia 2022, 27, 67–77. [Google Scholar] [CrossRef]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. 2016, 94, 1313–1326. [Google Scholar] [CrossRef]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Lines, C.L.; McGrath, M.J.; Dorwart, T.; Conn, C.S. The integrated stress response in cancer progression: A force for plasticity and resistance. Front. Oncol. 2023, 13, 1206561. [Google Scholar] [CrossRef] [PubMed]
- Wek, R.C. Role of eIF2alpha Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Lu, H.J.; Koju, N.; Sheng, R. Mammalian integrated stress responses in stressed organelles and their functions. Acta Pharmacol. Sin. 2024, 45, 1095–1114. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Reddy, R.K.; Mao, C.; Baumeister, P.; Austin, R.C.; Kaufman, R.J.; Lee, A.S. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: Role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 2003, 278, 20915–20924. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Lee, A.S. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007, 67, 3734–3740. [Google Scholar] [CrossRef]
- Cook, K.L.; Shajahan, A.N.; Wärri, A.; Jin, L.; Hilakivi-Clarke, L.A.; Clarke, R. Glucose-regulated protein 78 controls cross-talk between apoptosis and autophagy to determine antiestrogen responsiveness. Cancer Res. 2012, 72, 3337–3349. [Google Scholar] [CrossRef] [PubMed]
- Shani, G.; Fischer, W.H.; Justice, N.J.; Kelber, J.A.; Vale, W.; Gray, P.C. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol. Cell. Biol. 2008, 28, 666–677. [Google Scholar] [CrossRef]
- Direito, I.; Gomes, D.; Monteiro, F.L.; Carneiro, I.; Lobo, J.; Henrique, R.; Jerónimo, C.; Helguero, L.A. The Clinicopathological Significance of BiP/GRP-78 in Breast Cancer: A Meta-Analysis of Public Datasets and Immunohistochemical Detection. Curr. Oncol. 2022, 29, 9066–9087. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.P.; Sizova, D.V.; Dmitriev, S.E.; Ivanov, D.S.; Prassolov, V.S.; Shatsky, I.N. Distinctive properties of the 5′-untranslated region of human hsp70 mRNA. J. Biol. Chem. 2003, 278, 22350–22356. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Lang, K.J.; Kappel, A.; Goodall, G.J. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell 2002, 13, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Tinton, S.A.; Bruynooghe, Y.; Beyaert, R.; Cornelis, S. The polypyrimidine tract-binding protein stimulates HIF-1alpha IRES-mediated translation during hypoxia. Nucleic Acids Res. 2005, 33, 6884–6894. [Google Scholar] [CrossRef]
- Braunstein, S.; Karpisheva, K.; Pola, C.; Goldberg, J.; Hochman, T.; Yee, H.; Cangiarella, J.; Arju, R.; Formenti, S.C.; Schneider, R.J. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 2007, 28, 501–512. [Google Scholar] [CrossRef]
- El-Naggar, A.M.; Veinotte, C.J.; Cheng, H.; Grunewald, T.G.; Negri, G.L.; Somasekharan, S.P.; Corkery, D.P.; Tirode, F.; Mathers, J.; Khan, D.; et al. Translational Activation of HIF1α by YB-1 Promotes Sarcoma Metastasis. Cancer Cell 2015, 27, 682–697. [Google Scholar] [CrossRef]
- Ivanova, I.G.; Park, C.V.; Yemm, A.I.; Kenneth, N.S. PERK/eIF2α signaling inhibits HIF-induced gene expression during the unfolded protein response via YB1-dependent regulation of HIF1α translation. Nucleic Acids Res. 2018, 46, 3878–3890. [Google Scholar] [CrossRef]
- Giri, V.K.; Zaemes, J. The selection of targeted therapies for relapsed or refractory advanced renal cell carcinoma. Expert Rev. Anticancer Ther. 2025, 25, 337–349. [Google Scholar] [CrossRef]
- Huez, I.; Créancier, L.; Audigier, S.; Gensac, M.C.; Prats, A.C.; Prats, H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol. Cell. Biol. 1998, 18, 6178–6190. [Google Scholar] [CrossRef]
- Bornes, S.; Prado-Lourenco, L.; Bastide, A.; Zanibellato, C.; Iacovoni, J.S.; Lacazette, E.; Prats, A.C.; Touriol, C.; Prats, H. Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ. Res. 2007, 100, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Bastide, A.; Karaa, Z.; Bornes, S.; Hieblot, C.; Lacazette, E.; Prats, H.; Touriol, C. An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res. 2008, 36, 2434–2445. [Google Scholar] [CrossRef]
- Morfoisse, F.; Kuchnio, A.; Frainay, C.; Gomez-Brouchet, A.; Delisle, M.B.; Marzi, S.; Helfer, A.C.; Hantelys, F.; Pujol, F.; Guillermet-Guibert, J.; et al. Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1α-independent translation-mediated mechanism. Cell Rep. 2014, 6, 155–167. [Google Scholar] [CrossRef]
- Morfoisse, F.; Tatin, F.; Hantelys, F.; Adoue, A.; Helfer, A.C.; Cassant-Sourdy, S.; Pujol, F.; Gomez-Brouchet, A.; Ligat, L.; Lopez, F.; et al. Nucleolin Promotes Heat Shock-Associated Translation of VEGF-D to Promote Tumor Lymphangiogenesis. Cancer Res. 2016, 76, 4394–4405. [Google Scholar] [CrossRef]
- Hellström, M.; Phng, L.K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Jaud, M.; Philippe, C.; Van Den Berghe, L.; Ségura, C.; Mazzolini, L.; Pyronnet, S.; Laurell, H.; Touriol, C. The PERK Branch of the Unfolded Protein Response Promotes DLL4 Expression by Activating an Alternative Translation Mechanism. Cancers 2019, 11, 142. [Google Scholar] [CrossRef]
- Conte, C.; Riant, E.; Toutain, C.; Pujol, F.; Arnal, J.F.; Lenfant, F.; Prats, A.C. FGF2 translationally induced by hypoxia is involved in negative and positive feedback loops with HIF-1alpha. PLoS ONE 2008, 3, e3078. [Google Scholar] [CrossRef] [PubMed]
- Martineau, Y.; Le Bec, C.; Monbrun, L.; Allo, V.; Chiu, I.M.; Danos, O.; Moine, H.; Prats, H.; Prats, A.C. Internal ribosome entry site structural motifs conserved among mammalian fibroblast growth factor 1 alternatively spliced mRNAs. Mol. Cell. Biol. 2004, 24, 7622–7635. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Liao, M.; Yao, D.; Wu, L.; Luo, C.; Wang, Z.; Zhang, J.; Liu, B. Targeting the Warburg effect: A revisited perspective from molecular mechanisms to traditional and innovative therapeutic strategies in cancer. Acta Pharm. Sin. B 2024, 14, 953–1008. [Google Scholar] [CrossRef]
- Li, T.; Copeland, C.; Le, A. Glutamine Metabolism in Cancer. Adv. Exp. Med. Biol. 2021, 1311, 17–38. [Google Scholar] [CrossRef]
- Damiano, F.; Alemanno, S.; Gnoni, G.V.; Siculella, L. Translational control of the sterol-regulatory transcription factor SREBP-1 mRNA in response to serum starvation or ER stress is mediated by an internal ribosome entry site. Biochem. J. 2010, 429, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, S.; Yao, Y.; Zhang, G. Role of Human Monocarboxylate Transporter 1 (hMCT1) and 4 (hMCT4) in Tumor Cells and the Tumor Microenvironment. Cancer Manag. Res. 2023, 15, 957–975. [Google Scholar] [CrossRef]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J.L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2010, 463, 364–368. [Google Scholar] [CrossRef]
- Ismail, R.; Najar, I.A.; Rahamathulla, M.; Hussain, M.U.; Banday, M.S.; Devi, S.; Arora, P.; Kumar, M.; Shivanandappa, T.B.; Ahmed, M.M.; et al. IRES activation: HK2 and TPI1 glycolytic enzymes play a pivotal role in non-neuronal cell survival under hypoxia. Artif. Cells Nanomed. Biotechnol. 2025, 53, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Rousseau, A. Translation regulation in response to stress. FEBS J. 2024, 291, 5102–5122. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Q.; Cao, T.; Li, F.; Li, X.; Zhang, M.; Zhou, Y. Lactate dehydrogenase A: A potential new target for tumor drug resistance intervention. J. Transl. Med. 2025, 23, 713. [Google Scholar] [CrossRef]
- Cappello, P.; Principe, M.; Bulfamante, S.; Novelli, F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front. Biosci. 2017, 22, 944–959. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, B. Role of ENO1 and its targeted therapy in tumors. J. Transl. Med. 2024, 22, 1025. [Google Scholar] [CrossRef]
- Bartrons, R.; Simon-Molas, H.; Rodríguez-García, A.; Castaño, E.; Navarro-Sabaté, À.; Manzano, A.; Martinez-Outschoorn, U.E. Fructose 2,6-Bisphosphate in Cancer Cell Metabolism. Front. Oncol. 2018, 8, 331. [Google Scholar] [CrossRef]
- Koizume, S.; Miyagi, Y. Adaptation mechanisms in cancer: Lipid metabolism under hypoxia and nutrient deprivation as a target for novel therapeutic strategies (Review). Mol. Med. Rep. 2025, 31, 83. [Google Scholar] [CrossRef]
- Siculella, L.; Tocci, R.; Rochira, A.; Testini, M.; Gnoni, A.; Damiano, F. Lipid accumulation stimulates the cap-independent translation of SREBP-1a mRNA by promoting hnRNP A1 binding to its 5′-UTR in a cellular model of hepatic steatosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Rochira, A.; Damiano, F.; Marsigliante, S.; Gnoni, G.V.; Siculella, L. 3,5-Diiodo-l-thyronine induces SREBP-1 proteolytic cleavage block and apoptosis in human hepatoma (Hepg2) cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1679–1689. [Google Scholar] [CrossRef]
- Gao, S.; Shi, Z.; Li, X.; Li, W.; Wang, Y.; Liu, Z.; Jiang, J. Fatostatin suppresses growth and enhances apoptosis by blocking SREBP-regulated metabolic pathways in endometrial carcinoma. Oncol. Rep. 2018, 39, 1919–1929. [Google Scholar] [CrossRef]
- Icard, P.; Wu, Z.; Fournel, L.; Coquerel, A.; Lincet, H.; Alifano, M. ATP citrate lyase: A central metabolic enzyme in cancer. Cancer Lett. 2020, 471, 125–134. [Google Scholar] [CrossRef]
- Han, Q.; Chen, C.A.; Yang, W.; Liang, D.; Lv, H.W.; Lv, G.S.; Zong, Q.N.; Wang, H.Y. ATP-citrate lyase regulates stemness and metastasis in hepatocellular carcinoma via the Wnt/β-catenin signaling pathway. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, K.; Gong, D.; Zhang, J.; Li, Q.; Zhao, G.; Lin, P. ACLY: A biomarker of recurrence in breast cancer. Pathol. Res. Pract. 2020, 216, 153076. [Google Scholar] [CrossRef]
- Siculella, L.; Giannotti, L.; Testini, M.; Gnoni, G.V.; Damiano, F. In Steatotic Cells, ATP-Citrate Lyase mRNA Is Efficiently Translated through a Cap-Independent Mechanism, Contributing to the Stimulation of De Novo Lipogenesis. Int. J. Mol. Sci. 2020, 21, 1206. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, Q.; Wang, Z.; Di, Y.; Chen, X.; Ren, K. Targeting acetyl-CoA carboxylase 1 for cancer therapy. Front. Pharmacol. 2023, 14, 1129010. [Google Scholar] [CrossRef]
- Giannotti, L.; Stanca, E.; Di Chiara Stanca, B.; Spedicato, F.; Massaro, M.; Quarta, S.; Del Rio, D.; Mena, P.; Siculella, L.; Damiano, F. Coffee Bioactive N-Methylpyridinium: Unveiling Its Antilipogenic Effects by Targeting De Novo Lipogenesis in Human Hepatocytes. Mol. Nutr. Food Res. 2024, 68, e2400338. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.G.; Hurst, K.E.; Riesenberg, B.P.; Kennedy, A.S.; Gandy, E.J.; Andrews, A.M.; Pauneto, C.d.M.A.; Ball, L.E.; Wallace, E.D.; Gao, P.; et al. Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment. Cell Metab. 2024, 36, 969–983.e10. [Google Scholar] [CrossRef] [PubMed]
- Damiano, F.; Testini, M.; Tocci, R.; Gnoni, G.V.; Siculella, L. Translational control of human acetyl-CoA carboxylase 1 mRNA is mediated by an internal ribosome entry site in response to ER stress, serum deprivation or hypoxia mimetic CoCl2. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Damiano, F.; Tocci, R.; Gnoni, G.V.; Siculella, L. Expression of citrate carrier gene is activated by ER stress effectors XBP1 and ATF6α, binding to an UPRE in its promoter. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2015, 1849, 23–31. [Google Scholar] [CrossRef]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Holbert, C.E.; Casero, R.A., Jr.; Stewart, T.M. Polyamines: The pivotal amines in influencing the tumor microenvironment. Discov. Oncol. 2024, 15, 173. [Google Scholar] [CrossRef]
- Huang, D.; Cai, H.; Huang, H. Serine metabolism in tumor progression and immunotherapy. Discov. Oncol. 2025, 16, 628. [Google Scholar] [CrossRef]
- Quan, S.; Huang, H. Epigenetic contribution to cancer. Int. Rev. Cell Mol. Biol. 2024, 387, 1–25. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Darnell, A.M.; Reilly, M.F.; Kunchok, T.; Joesch-Cohen, L.; Rosenberg, D.; Ali, A.; Rees, M.G.; Roth, J.A.; Lewis, C.A.; et al. Methionine synthase is essential for cancer cell proliferation in physiological folate environments. Nat. Metab. 2021, 3, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Stover, P.J. Mechanism of the internal ribosome entry site-mediated translation of serine hydroxymethyltransferase 1. J. Biol. Chem. 2009, 284, 31085–31096. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.T.; Shin, W.K.; Caudill, M.A.; Stover, P.J. A UV-responsive internal ribosome entry site enhances serine hydroxymethyltransferase 1 expression for DNA damage repair. J. Biol. Chem. 2009, 284, 31097–31108. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Oltean, S.; Banerjee, R. A B12-responsive internal ribosome entry site (IRES) element in human methionine synthase. J. Biol. Chem. 2005, 280, 32662–32668. [Google Scholar] [CrossRef]
- Du, T.; Han, J. Arginine Metabolism and Its Potential in Treatment of Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 658861. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- You, S.; Han, X.; Xu, Y.; Sui, L.; Song, K.; Yao, Q. High expression of SLC7A1 in high-grade serous ovarian cancer promotes tumor progression and is involved in MAPK/ERK pathway and EMT. Cancer Med. 2024, 13, e7217. [Google Scholar] [CrossRef]
- Schramm, J.; Sholler, C.; Menachery, L.; Vazquez, L.; Saulnier Sholler, G. Polyamine Inhibition with DFMO: Shifting the Paradigm in Neuroblastoma Therapy. J. Clin. Med. 2025, 14, 1068. [Google Scholar] [CrossRef]
- Pyronnet, S.; Pradayrol, L.; Sonenberg, N. A cell cycle-dependent internal ribosome entry site. Mol. Cell 2000, 5, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Bode, B.; Koromilas, A.; Diehl, J.A.; Krukovets, I.; Snider, M.D.; Hatzoglou, M. Translation mediated by the internal ribosome entry site of the cat-1 mRNA is regulated by glucose availability in a PERK kinase-dependent manner. J. Biol. Chem. 2002, 277, 11780–11787. [Google Scholar] [CrossRef]
- Sammons, M.A.; Antons, A.K.; Bendjennat, M.; Udd, B.; Krahe, R.; Link, A.J. ZNF9 activation of IRES-mediated translation of the human ODC mRNA is decreased in myotonic dystrophy type 2. PLoS ONE 2010, 5, e9301. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Dobbyn, H.C.; Hill, K.; Hamilton, T.L.; Spriggs, K.A.; Pickering, B.M.; Coldwell, M.J.; de Moor, C.H.; Bushell, M.; Willis, A.E. Regulation of BAG-1 IRES-mediated translation following chemotoxic stress. Oncogene 2008, 27, 1167–1174. [Google Scholar] [CrossRef]
- Mariotto, E.; Viola, G.; Zanon, C.; Aveic, S. A BAG’s life: Every connection matters in cancer. Pharmacol. Ther. 2020, 209, 107498. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Ye, Q.; Zhuang, X.Z.; Li, J.; Zhou, X. Targeting the inhibitors of apoptosis proteins (IAPs) to combat drug resistance in cancers. Front. Pharmacol. 2025, 16, 1562167. [Google Scholar] [CrossRef]
- Krzykawski, K.; Kubina, R.; Wendlocha, D.; Sarna, R.; Mielczarek-Palacz, A. Multifaceted Evaluation of Inhibitors of Anti-Apoptotic Proteins in Head and Neck Cancer: Insights from In Vitro, In Vivo, and Clinical Studies (Review). Pharmaceuticals 2024, 17, 1308. [Google Scholar] [CrossRef]
- Bertrand, M.J.; Milutinovic, S.; Dickson, K.M.; Ho, W.C.; Boudreault, A.; Durkin, J.; Gillard, J.W.; Jaquith, J.B.; Morris, S.J.; Barker, P.A. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 2008, 30, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Gyrd-Hansen, M.; Meier, P. IAPs: From caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 2010, 10, 561–574. [Google Scholar] [CrossRef]
- Graber, T.E.; Holcik, M. Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2. Cell Death Dis. 2011, 2, e135. [Google Scholar] [CrossRef]
- Lewis, S.M.; Veyrier, A.; Hosszu Ungureanu, N.; Bonnal, S.; Vagner, S.; Holcik, M. Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol. Biol. Cell. 2007, 18, 1302–1311. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, K.W.; Byrd, M.P.; Van Eden, M.E.; Lloyd, R.E. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 2004, 279, 29066–29074. [Google Scholar] [CrossRef]
- Durie, D.; Hatzoglou, M.; Chakraborty, P.; Holcik, M. HuR controls mitochondrial morphology through the regulation of BclxL translation. Translation 2013, 1, e23980. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Du, Y.; Hao, S.; Ni, T. The Regulation of Cellular Senescence in Cancer. Biomolecules 2025, 15, 448. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Papaspyropoulos, A.; Hazapis, O.; Altulea, A.; Polyzou, A.; Verginis, P.; Evangelou, K.; Fousteri, M.; Papantonis, A.; Demaria, M.; Gorgoulis, V. Decoding of translation-regulating entities reveals heterogeneous translation deficiency patterns in cellular senescence. Aging Cell 2023, 22, e13893. [Google Scholar] [CrossRef]
- Ding, F.; Yu, Y.; Zhao, J.; Wei, S.; Zhang, Y.; Han, J.H.; Li, Z.; Jiang, H.B.; Ryu, D.; Cho, M.; et al. The interplay of cellular senescence and reprogramming shapes the biological landscape of aging and cancer revealing novel therapeutic avenues. Front. Cell Dev. Biol. 2025, 13, 1593096. [Google Scholar] [CrossRef]
- Jia, Y.; Han, L.; Ramage, C.L.; Wang, Z.; Weng, C.C.; Yang, L.; Colla, S.; Ma, H.; Zhang, W.; Andreeff, M.; et al. Co-targeting BCL-XL and BCL-2 by PROTAC 753B eliminates leukemia cells and enhances efficacy of chemotherapy by targeting senescent cells. Haematologica 2023, 108, 2626–2638. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Li, W.; Thakor, N.; Xu, E.Y.; Huang, Y.; Chen, C.; Yu, R.; Holcik, M.; Kong, A.N. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res. 2010, 38, 778–788. [Google Scholar] [CrossRef]
- Zhang, J.; Dinh, T.N.; Kappeler, K.; Tsaprailis, G.; Chen, Q.M. La autoantigen mediates oxidant induced de novo Nrf2 protein translation. Mol. Cell. Proteom. 2012, 11, M111.015032. [Google Scholar] [CrossRef]
- Tian, Y.; Tang, L.; Wang, X.; Ji, Y.; Tu, Y. Nrf2 in human cancers: Biological significance and therapeutic potential. Am. J. Cancer Res. 2024, 14, 3935–3961. [Google Scholar] [CrossRef]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, T. Unveiling the significance of inducible nitric oxide synthase: Its impact on cancer progression and clinical implications. Cancer Lett. 2024, 592, 216931. [Google Scholar] [CrossRef] [PubMed]
- Vannini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Basudhar, D.; Somasundaram, V.; de Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric Oxide Synthase-2-Derived Nitric Oxide Drives Multiple Pathways of Breast Cancer Progression. Antioxid. Redox Signal. 2017, 26, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Internal ribosomal entry site-mediated translational activity of nitric oxide synthase 2. Anim. Cells Syst. 2023, 27, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, M.E.; Mori, A.; Oberer, M.; Lindqvist, L.; Chard, L.S.; Higa, T.; Belsham, G.J.; Wagner, G.; Tanaka, J.; Pelletier, J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol. 2006, 2, 213–220. [Google Scholar] [CrossRef]
- Benavides-Serrato, A.; Saunders, J.T.; Holmes, B.; Nishimura, R.N.; Lichtenstein, A.; Gera, J. Repurposing Potential of Riluzole as an ITAF Inhibitor in mTOR Therapy Resistant Glioblastoma. Int. J. Mol. Sci. 2020, 21, 344. [Google Scholar] [CrossRef]
- Marques, R.; Lacerda, R.; Romão, L. Internal Ribosome Entry Site (IRES)-Mediated Translation and Its Potential for Novel mRNA-Based Therapy Development. Biomedicines 2022, 10, 1865. [Google Scholar] [CrossRef] [PubMed]
- Renaud-Gabardos, E.; Hantelys, F.; Morfoisse, F.; Chaufour, X.; Garmy-Susini, B.; Prats, A.C. Internal ribosome entry site-based vectors for combined gene therapy. World J. Exp. Med. 2015, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiano, F.; Di Chiara Stanca, B.; Giannotti, L.; Stanca, E.; Dinoi, A.F.; Siculella, L. Targeting Cancer Translational Plasticity: IRES-Driven Metabolism and Survival Within the Tumor Microenvironment. Cancers 2025, 17, 2731. https://doi.org/10.3390/cancers17172731
Damiano F, Di Chiara Stanca B, Giannotti L, Stanca E, Dinoi AF, Siculella L. Targeting Cancer Translational Plasticity: IRES-Driven Metabolism and Survival Within the Tumor Microenvironment. Cancers. 2025; 17(17):2731. https://doi.org/10.3390/cancers17172731
Chicago/Turabian StyleDamiano, Fabrizio, Benedetta Di Chiara Stanca, Laura Giannotti, Eleonora Stanca, Angela Francesca Dinoi, and Luisa Siculella. 2025. "Targeting Cancer Translational Plasticity: IRES-Driven Metabolism and Survival Within the Tumor Microenvironment" Cancers 17, no. 17: 2731. https://doi.org/10.3390/cancers17172731
APA StyleDamiano, F., Di Chiara Stanca, B., Giannotti, L., Stanca, E., Dinoi, A. F., & Siculella, L. (2025). Targeting Cancer Translational Plasticity: IRES-Driven Metabolism and Survival Within the Tumor Microenvironment. Cancers, 17(17), 2731. https://doi.org/10.3390/cancers17172731







