Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Inclusion Criteria
2.2. Study Quality Assessment and Data Extraction
2.3. Data Analysis and Synthesis
3. Results
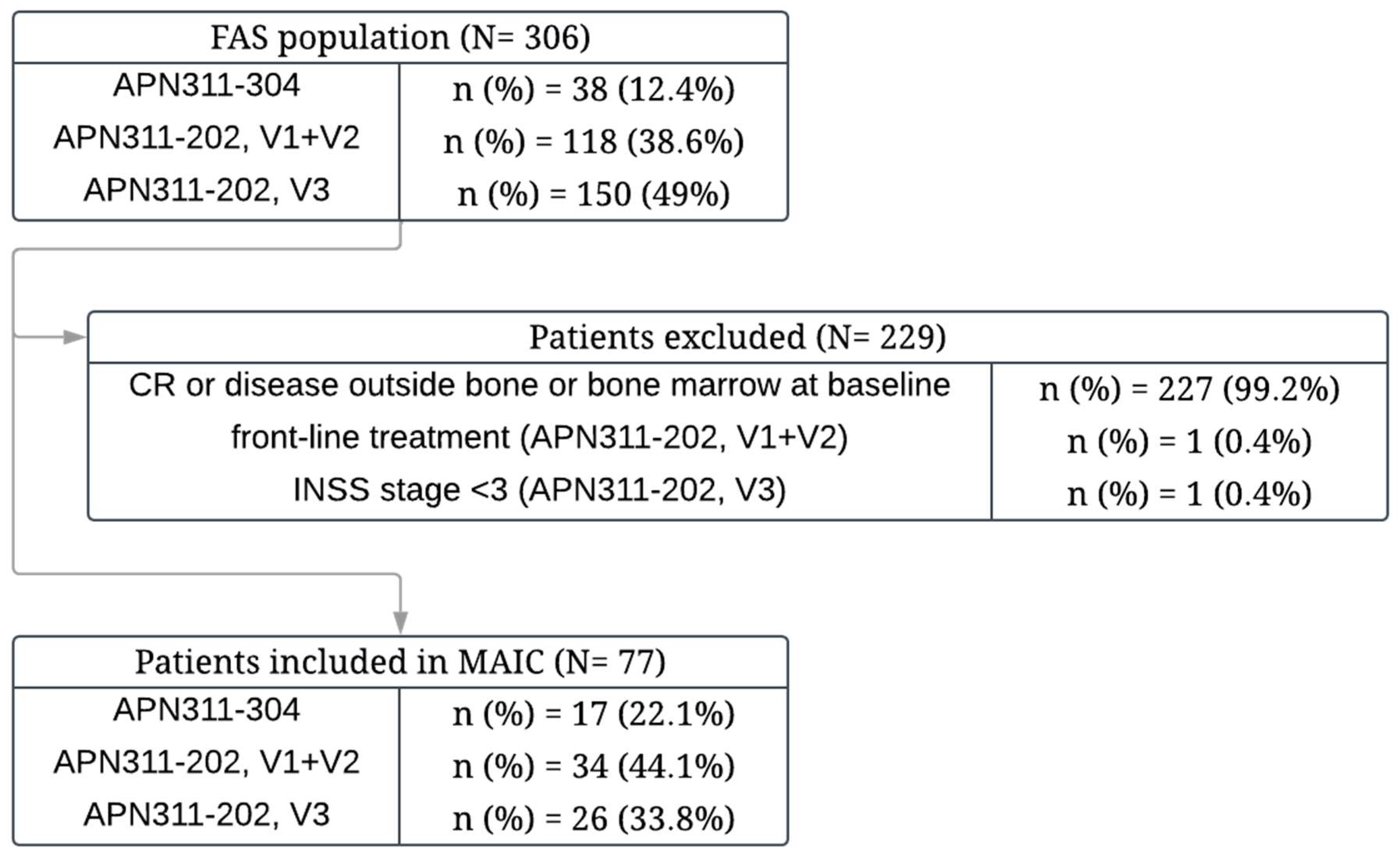
3.1. Search Results and Included Studies
3.2. Study Selection for Indirect Comparison
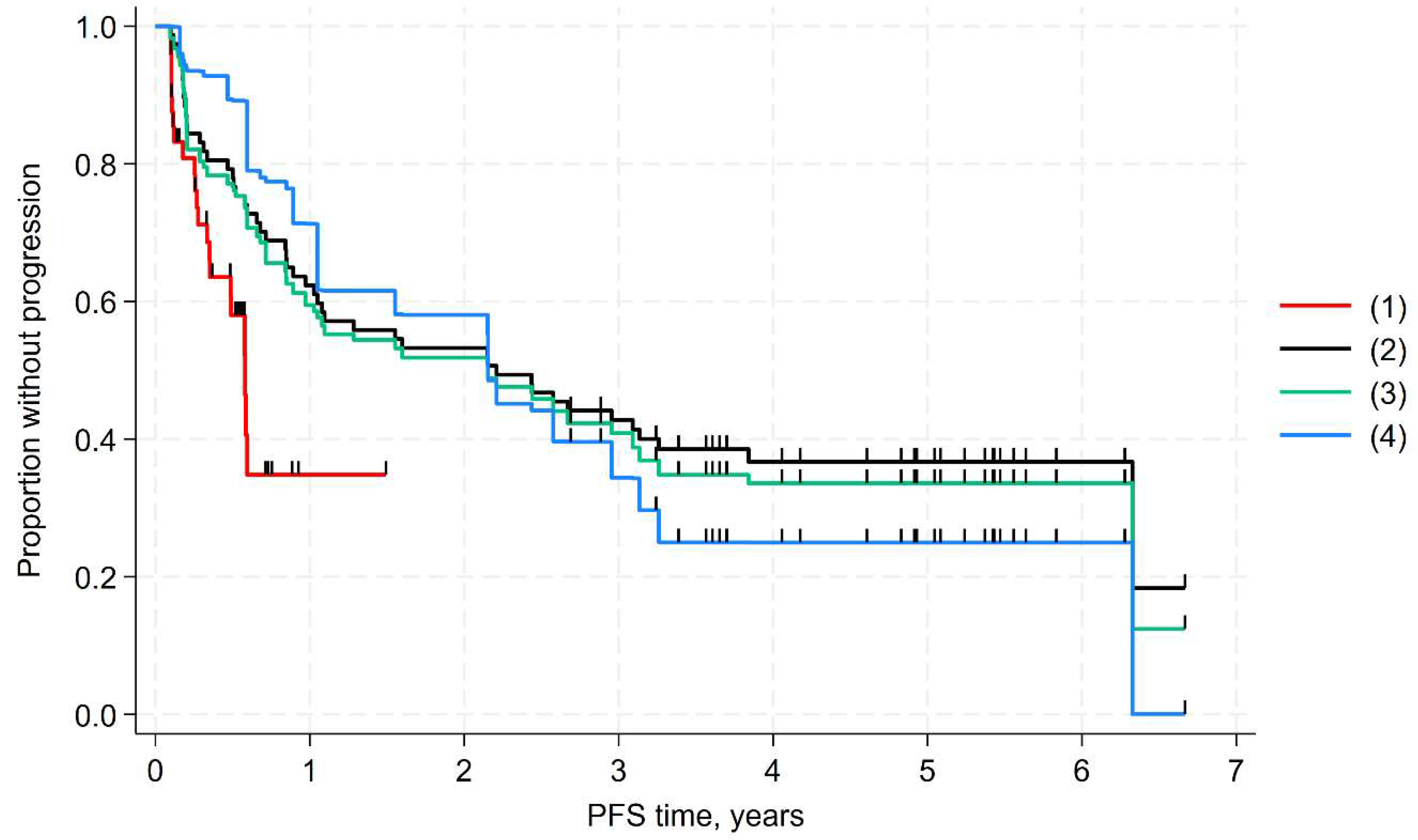
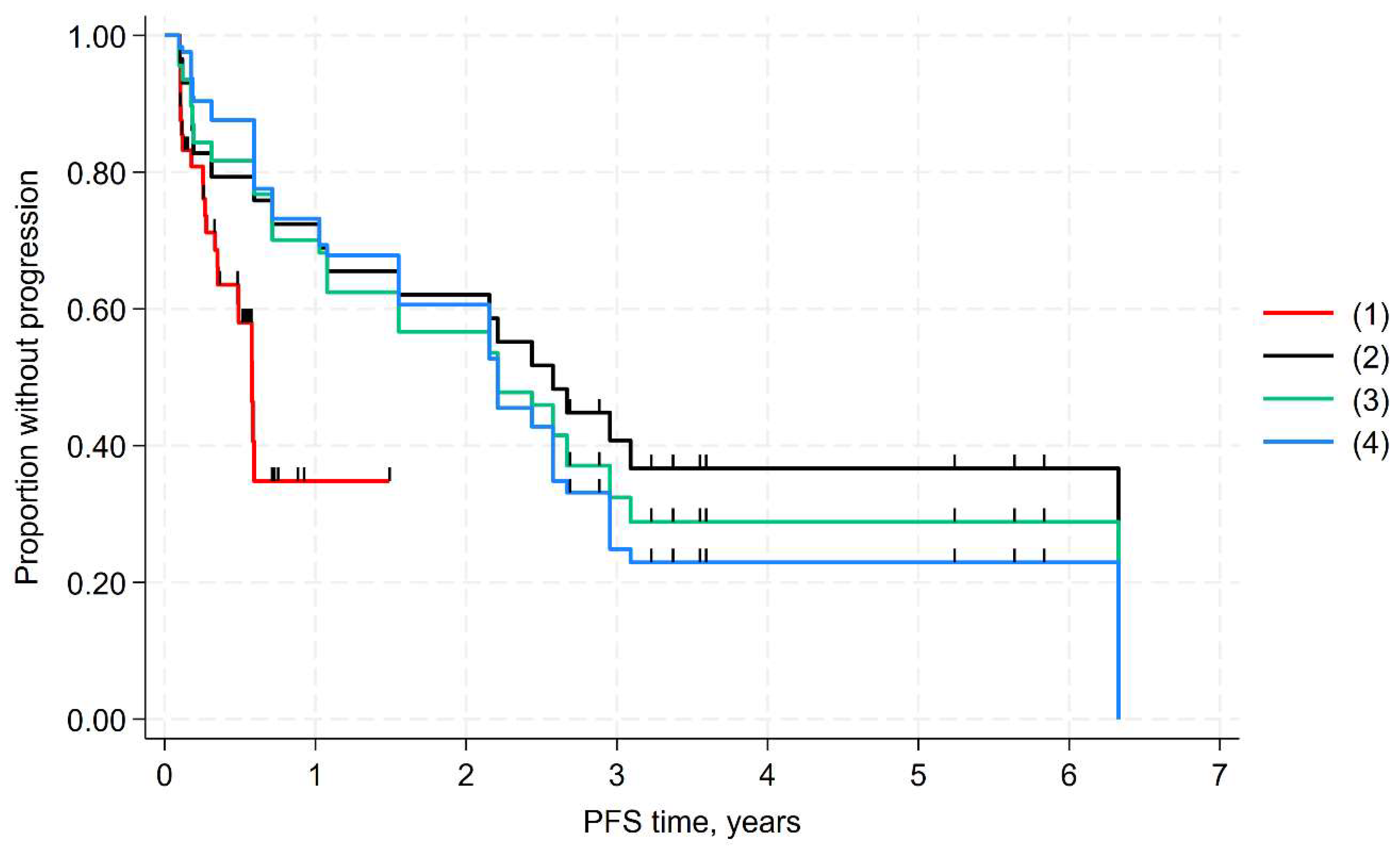
3.3. MAIC of PFS
3.4. MAIC of ORR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Matthay, K.K.; Villablanca, J.G.; Seeger, R.C.; Stram, D.O.; Harris, R.E.; Ramsay, N.K.; Swift, P.; Shimada, H.; Black, C.T.; Brodeur, G.M.; et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 1999, 341, 1165–1173. [Google Scholar] [CrossRef]
- Pritchard, J.; Cotterill, S.J.; Germond, S.M.; Imeson, J.; de Kraker, J.; Jones, D.R. High dose melphalan in the treatment of advanced neuroblastoma: Results of a randomised trial (ENSG-1) by the European Neuroblastoma Study Group. Pediatr. Blood Cancer 2005, 44, 348–357. [Google Scholar] [CrossRef]
- Abbas, A.A.; Samkari, A.M.N. High-Risk Neuroblastoma: Poor Outcomes Despite Aggressive Multimodal Therapy. Curr. Cancer Ther. Rev. 2022, 18, 14–40. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Pieniążek, B.; Cencelewicz, K.; Bździuch, P.; Młynarczyk, Ł.; Lejman, M.; Zawitkowska, J.; Derwich, K. Neuroblastoma—A Review of Combination Immunotherapy. Int. J. Mol. Sci. 2024, 25, 7730. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Bexelius, T.S.; Santopolo, G.; Yuan, Y.; Liaño-Pons, J.; Arsenian-Henriksson, M. Defining neuroblastoma: From origin to precision medicine. Neuro-Oncology 2024, 26, 2174–2192. [Google Scholar] [CrossRef]
- Balaguer, J.; Hidalgo, L.G.; Hladun, R.; Vega, C.M.; Alonso, V.P. Recent Evidence-Based Clinical Guide for the Use of Dinutuximab Beta in Pediatric Patients with Neuroblastoma. Target. Oncol. 2023, 18, 77–93. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Summary of Product Characteristic for Qarziba. Available online: https://www.ema.europa.eu/en/documents/product-information/qarziba-epar-product-information_en.pdf (accessed on 15 April 2025).
- Danyelza Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761171s008lbl.pdf (accessed on 15 April 2025).
- Furman, W.L. Monoclonal Antibody Therapies for High Risk Neuroblastoma. Biol. Targets Ther. 2021, 15, 205–219. [Google Scholar] [CrossRef]
- Cheung, N.K.V.; Guo, H.; Hu, J.; Tassev, D.V.; Cheung, I.Y. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. OncoImmunology 2012, 1, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Troschke-Meurer, S.; Zumpe, M.; Ahrenberg, P.M.; Ebeling, T.; Siebert, N.; Grabarczyk, P.; Lode, H.N. Affinity Affects the Functional Potency of Anti-GD2 Antibodies by Target-Mediated Drug Disposition. Cancers 2025, 17, 2510. [Google Scholar] [CrossRef]
- Lisby, S.; Liebenberg, N.; Bukrinski, J.; Sønderby, P.; Lund-Hansen, T. Naxitamab, An Antibody with Distinct Complementary Determining Regions and High Binding Affinity to Disialoganglioside GD2. Abstract #945. Available online: https://ir.ymabs.com/static-files/52dfb61a-bb9a-472e-a6ff-473284a00cf5 (accessed on 15 April 2025).
- Lodge, J.; Kajtar, L.; Duxbury, R.; Hall, D.; Burley, G.A.; Cordy, J.; Jates, J.W.T.; Rattray, Z. Quantifying antibody binding: Techniques and therapeutic implications. MABs 2025, 17, 2459795. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Human Use (CHMP) Dinutuximab Beta Apeiron. Assessment Report Procedure No. EMEA/H/C/003918/0000. Available online: https://www.ema.europa.eu/en/documents/assessment-report/dinutuximab-beta-apeiron-epar-public-assessment-report_en.pdf (accessed on 15 April 2025).
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.L.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan–temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957. [Google Scholar] [CrossRef]
- Moreno, L.; Dubois, S.G.; Bird, N.; Knox, L.; Ludwinski, D.; Pearson, A.D.J.; Beck-Popovic, M.; Bagatell, R. A 2035 Clinical Research Vision and Roadmap for High-Risk Neuroblastoma. Pediatr. Blood Cancer 2025, 72, e31660. [Google Scholar] [CrossRef]
- Wieczorek, A.; Śladowska, K.; Lode, N.H. Efficacy and Safety of Anti-GD2 Immunotherapy with Dinutuximab Beta in the Treatment of Relapsed/Refractory High-Risk Neuroblastoma. Target. Oncol. 2025, 20, 1–18. [Google Scholar] [CrossRef]
- National Cancer Institute. Neuroblastoma Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/neuroblastoma/hp/neuroblastoma-treatment-pdq (accessed on 2 June 2025).
- Yu, X.; Kang, S.; Ge, J.; Wang, J. A clinical observational study of dinutuximab beta as first-line maintenance treatment for patients with high-risk neuroblastoma in China. BMC Pediatr. 2025, 25, 203. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons: Chichester, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Elridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Signorovitch, J.E.; Sikirica, V.; Erder, M.H.; Hie, J.; Lu, M.; Hodgkins, P.S.; Betts, K.A.; Wu, E.Q. Matching-adjusted indirect comparisons: A new tool for timely comparative effectiveness research. Value Health. 2012, 15, 940–947. [Google Scholar] [CrossRef]
- Signorovitch, J.E.; Wu, E.Q.; Yu, A.P.; Gerrits, C.M.; Kantor, E.; Bao, Y.; Gupta, S.R.; Mulani, P.M. Comparative effectiveness without head-to-head trials: A method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics 2010, 28, 935–945. [Google Scholar] [CrossRef]
- Phillippo, D.M.; Ades, A.E.; Dias, S.; Palmer, S.; Abrams, K.R.; Welton, N.J. NICE DSU Technical Support Document 18: Methods for Population-Adjusted Indirect Comparisons in Submission to NICE. 2016. Available online: http://www.nicedsu.org.uk (accessed on 26 April 2025).
- Mora, J.; Chan, G.C.; Morgenstern, D.A.; Amoroso, L.; Nysom, K.; Faber, J.; Wingerter, A.; Bear, M.; San Simon, R.A.; Tornoe, K.; et al. 62MO Naxitamab pivotal clinical trial planned interim analysis of PFS and OS in patients with relapsed or refractory high-risk neuroblastoma. Immuno-Oncol. Technol. 2022, 16 (Suppl. S1), 100167. [Google Scholar] [CrossRef]
- Mora, J.; Chan, G.C.F.; Morgenstern, D.A.; Amoroso, L.; Nysom, K.; Faber, J.; Wingerter, A.; Bear, M.K.; Rubio-San-Simon, A.; Heras, B.M.d.L.; et al. The anti-GD2 monoclonal antibody naxitamab plus GM-CSF for relapsed or refractory high-risk neuroblastoma: A phase 2 clinical trial. Nat. Commun. 2025, 16, 1636. [Google Scholar] [CrossRef]
- Kushner, B.; Mora, J.; Chan, G.; Morgenstern, D.A.; Amoroso, L.; Nysom, K.; Faber, J.; Wingerter, A.; Bear, M.; Simón, A.R.S.; et al. Naxitamab efficacy in patients with refractory/relapsed high-risk neuroblastoma and bone metastases as assessed by Curie score. Immuno-Oncol. Technol. 2023, 20, 100601. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Application Number 761171Orig1s000. DANYELZA, naxitamab-gqgk. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761171Orig1s000TOC.cfm (accessed on 15 April 2025).
- Wieczorek, A.; Żebrowska, U.; Ussowicz, M.; Sokół, A.; Stypińska, M.; Dembowska-Bagińska, B.; Pawińska-Wąsikowska, K.; Balwierz, W. Dinutuximab Beta Maintenance Therapy in Patients with High-Risk Neuroblastoma in First-Line and Refractory/Relapsed Settings—Real-World Data. J. Clin. Med. 2023, 12, 5252. [Google Scholar] [CrossRef] [PubMed]
- Lode, H.N.; Ehlert, K.; Huber, S.; Troschke-Meurer, S.; Siebert, N.; Zumpe, M.; Loibner, H.; Ladenstein, R. Long-term, continuous infusion of single-agent dinutuximab beta for relapsed/refractory neuroblastoma: An open-label, single-arm, Phase 2 study. Br. J. Cancer 2023, 129, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Clinical Study Report. Phase I study of monoclonal antibody CH14.18/CHO Continuous Infusion in Patients with Primary Refractory or Relapsed Neuroblastoma APN311-304. Final Version 24 July 2023 [Unpublished Data on File]. Available online: https://clinicaltrials.gov/study/NCT02743429 (accessed on 15 April 2025).
- Flaadt, T.; Ladenstein, R.L.; Ebinger, M.; Lode, H.N.; Arnardóttir, H.B.; Poetschger, U.; Schwinger, W.; Meisel, R.; Schuster, F.R.; Döring, M.; et al. Anti-GD2 Antibody Dinutuximab Beta and Low-Dose Interleukin 2 after Haploidentical Stem-Cell Transplantation in Patients with Relapsed Neuroblastoma: A Multicenter, Phase I/II Trial. J. Clin. Oncol. 2023, 41, 3135–3148. [Google Scholar] [CrossRef]
- Mueller, I.; Ehlert, K.; Endres, S.; Pill, L.; Siebert, N.; Kietz, S.; Brock, P.; Garaventa, A.; Valteau-Couanet, D.; Janzek, E.; et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD2 antibody ch14.18/CHO. mAbs 2018, 10, 55–61. [Google Scholar] [CrossRef]
- Clinical Study Report. A Phase I/II Dose Schedule Finding Study of Ch14.18/CHO Continuous Infusion Combined with Subcutaneous Aldesleukin (IL-2) in patients with Primary Refractory or Relapsed Neuroblastoma. Version 3.0. February 2014 (Phase: II (randomised) (V3 cohort) and Analysis From Data Collected In The SIOPEN Long-Term CH14.18/CHO Infusion (LTI) Study A Phase I/II Dose Schedule Finding Study Of CH14.18/CHO Continuous Infusion Combined With Subcutaneous Aldesleukin (IL-2) in Patients with Primary Refractory or Relapsed Neuroblastoma. APN311-202. Version 1.0 10 July 2024 (V1+V2) [Unpublished Data on File]). Available online: https://www.orpha.net/en/research-trials/clinical-trial/530430?name=&mode=&country=&recruiting=0&terminated=0 (accessed on 15 April 2025).
- Ladenstein, R.; Weixler, S.; Baykan, B.; Bleeke, M.; Kunert, R.; Katinger, D.; Pribill, I.; Glander, P.; Bauer, S.; Pistoia, V.; et al. Ch14.18 antibody produced in CHO cells in relapsed or refractory Stage 4 neuroblastoma patients: A SIOPEN Phase 1 study. MABs 2013, 5, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.P.; Fleurence, R.; Devine, B.; Itzler, R.; Barrett, A.; Hawkins, N.; Lee, K.; Boersma, C.; Annemans, L.; Cappelleri, J.C. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health 2011, 14, 417–428. [Google Scholar] [CrossRef]
- HTA CG. Member State Coordination Group on Health Technology Assessment. Methodological Guideline for Quantitative Evidence Synthesis: Direct and Indirect Comparisons. Adopted on 8 March 2024 by the HTA CG pursuant to Article 3(7), point (d), of Regulation (EU) 2021/2282 on Health Technology Assessment. Available online: https://health.ec.europa.eu/document/download/4ec8288e-6d15-49c5-a490-d8ad7748578f_en?filename=hta_methodological-guideline_direct-indirect-comparisons_en.pdf (accessed on 4 April 2025).
- Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-anticancer-medicinal-products-man-revision-5_en.pdf (accessed on 25 April 2025).
- U.S. Department of Health and Human Services Food and Drug Administration. Clinical Study Endpoints for the Approval of Cancer Drugs and Biologics. Guidance for Industry. December 2018. Available online: https://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf (accessed on 25 April 2025).
- Huibregtsea, K.E.; Vob, K.T.; DuBoisc, S.G.; Fetzkod, S.; Neuhause, J.; Batraf, V.; Marisf, J.M.; Weissg, B.; Marachelianh, A.; Yaniki, G.A.; et al. Incidence and risk factors for secondary malignancy in patients with neuroblastoma after treatment with 131I-metaiodobenzylguanidine. Eur. J. Cancer 2016, 66, 144–152. [Google Scholar] [CrossRef]
- Royle, K.-L.; Meads, D.; Visser-Rogers, J.K.; White, I.R.; Cairns, D.A. How is overall survival assessed in randomised clinical trials in cancer and are subsequent treatment lines considered? A systematic review. Trials 2023, 24, 708. [Google Scholar] [CrossRef]
- Sherry, A.D.; Msaouel, P.; Lin, T.A.; Abi Jaoude, J.; Kouzy, R.; Beck, E.J.; Miller, A.M.; Passy, A.H.; Kupferman, G.S.; Koay, E.J.; et al. Postprogression therapy and confounding for the estimated treatment effect on overall survival in phase III oncology trials. BMJ Oncol. 2024, 3, e000322. [Google Scholar] [CrossRef]
- Żebrowska, U.; Balwierz, W.; Wechowski, J.; Wieczorek, A. Survival Benefit of Myeloablative Therapy with Autologous Stem Cell Transplantation in High-Risk Neuroblastoma: A Systematic Literature Review. Target. Oncol. 2024, 19, 143–159. [Google Scholar] [CrossRef]
- Kushner, B.H.; Ostrovnaya, I.; Cheung, I.Y.; Kuk, D.; Kramer, K.; Modak, S.; Yataghene, K.; Cheung, N.-K.V. Prolonged progression-free survival after consolidating second or later remissions of neuroblastoma with Anti-GD2 immunotherapy and isotretinoin: A prospective Phase II study. OncoImmunology 2015, 4, e1016704. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; Naranjo, A.; Diccianni, M.B.; Gan, J.; Hank, J.A.; Batova, A.; London, W.A.; Tenney, S.C.; et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021, 27, 2179–2189. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Castel, V.; Monclair, T.; Ambros, P.F.; Pearson, A.D.J.; Cohn, S.L.; Berthold, F.; Nakagawara, A.; Ladenstein, R.L.; Iehara, T.; et al. Clinical and Biologic Features Predictive of Survival After Relapse of Neuroblastoma: A Report From the International Neuroblastoma Risk Group Project. J. Clin. Oncol. 2011, 29, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Matthay, K.K.; Ambros, P.F.; Monclair, T.; Pearson, A.D.; Cohn, S.L.; Castel, V. Clinical and biological features predictive of survival after relapse of neuroblastoma: A study from the International Neuroblastoma (NB) Risk Group (INRG) Database. J. Clin. Oncol. 2010, 28, 9518. [Google Scholar] [CrossRef]
- Gartrell, J.; Shulkin, B.L.; Helmig, S.; Caldwell, K.J.; Furman, W.; Federico, S.M. Induction Chemotherapy With an Anti-GD2 Monoclonal Antibody (Dinutuximab) and Cytokines in Children With Newly Diagnosed High-risk Neuroblastoma: A Case Series. J. Pediatr. Hematol. 2021, 43, E692–E696. [Google Scholar] [CrossRef]
- Cupit-Link, M.; Federico, S.M. Treatment of High-Risk Neuroblastoma with Dinutuximab and Chemotherapy Administered in all Cycles of Induction. Cancers 2023, 15, 4609. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Larrosa, C.; Chamorro, S.; Perez-Jaume, S.; Simao, M.; Sanchez-Sierra, N.; Varo, A.; Gorostegui, M.; Castañeda, A.; Garraus, M.; et al. Early Salvage Chemo-Immunotherapy with Irinotecan, Temozolomide and Naxitamab Plus GM-CSF (HITS) for Patients with Primary Refractory High-Risk Neuroblastoma Provide the Best Chance for Long-Term Outcomes. Cancers 2023, 15, 4837. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Study | Reasons |
|---|---|---|
| Naxitamab | Study 2PR01 (compassionate use) [32] |
|
| Phase I of study 12-230 (NCT01757626) [32] (phase II of this study was included in the MAIC) |
| |
| Dinutuximab beta | Wieczorek et al., 2023 [33] |
|
| APN311-201 (Flaadt et al., 2023) [36] |
| |
| Mueller et al., 2018 [37]/APN311-303 (compassionate use) [17] |
| |
| APN311-101 (Ladenstein et al., 2013) [39] |
|
| Variable | Naxitamab, Study 201 (n = 52) | DB (n = 77) Before Weighting | p Value * | DB (n = 77) After Weighting | p Value * |
|---|---|---|---|---|---|
| Age, years | Median: 6 Range: 2–18 | Median: 6.0 Mean: 6.61 Range: 2–19 | - | Median: 6.0 Mean: 6.43 Range: 2–19 | - |
| % refractory (n/N) | 50.0% (26/52) | 50.7% (39/77) | 0.942 | 50.0% | >0.999 |
| % female (n/N) | 40.4% (21/52) | 35.1% (27/77) | 0.540 | 40.4% | >0.999 |
| Prior treatment | |||||
| % prior stem cell transplant (n/N) | 26.9% (14/52) | 96.1% (74/77) | <0.001 | 95.3% | <0.001 |
| % prior radiotherapy (n/N) | 40.4% (13/52) | 68.4% (52/77) | <0.001 | 70.7% | <0.001 |
| % prior surgery (n/N) | 88.5% (46/52) | 90.8% (69/77) | 0.837 | 90.9% | 0.659 |
| Disease site | |||||
| % bone only (n/N) | 55.8% (29/52) | 64.9% (50/77) | 0.295 | 55.8% | >0.999 |
| % bone marrow only (n/N) | 3.8% (2/52) | 9.1% (7/77) | 0.251 | 3.8% | >0.999 |
| % both (n/N) | 40.4% (21/52) | 26.0% (20/77) | 0.085 | 40.4% | >0.999 |
| Race/Ethnic origin | |||||
| % White (n/N) | 34.6% (18/52) | 85.7% (66/77) | <0.001 | 85.7% | <0.001 |
| % Black (n/N) | 3.8% (2/52) | 2.6% (2/77) | 0.688 | 2.1% | 0.570 |
| % Asian (n/N) | 55.8% (29/52) | 1.3% (1/77) | <0.001 | 2.3% | <0.001 |
| MYCN | |||||
| % amplification (n/N) | 13.5% (7/52) | 9.1% (7/77) | 0.434 | 13.5% | >0.999 |
| % missing (n/N) | 13.5% (7/52) | 2.6% (2/77) | 0.018 | 1.8% | 0.011 |
| INSS, diagnosis | |||||
| % stage 3 (n/N) | 7.7% (4/52) | 1.3% (1/77) | 0.065 | 1.7% | 0.109 |
| % stage 4 (n/N) | 88.5% (46/52) | 97.4% (75/77) | 0.039 | 97.4% | 0.047 |
| % missing (n/N) | 3.8% (2/52) | 1.3% (1/77) | 0.346 | 0.9% | 0.265 |
| Unadjusted Comparison | Base-Case MAIC A | Sensitivity Analysis #1 B | Sensitivity Analysis #2 C | Sensitivity Analysis #3 D | Sensitivity Analysis #4 E | |
|---|---|---|---|---|---|---|
| Log-rank test, p value * | 0.005 | - | - | 0.013 | - | - |
| HR (95% CI), p value * | 0.44 (0.25 to 0.79), 0.006 | 0.47 (0.26 to 0.87), 0.015 | 0.28 (0.12 to 0.63), 0.002 | 0.37 (0.16 to 0.83), 0.016 | 0.36 (0.16 to 0.84), 0.017 | 0.29 (0.12 to 0.69), 0.005 |
| Variable | Naxitamab (n = 90), Study 201 and Study 230 | DB (n = 77) Before Weighting | p Value * | DB (n = 77) After Weighting | p Value * |
|---|---|---|---|---|---|
| Age, years | Median: 6 (Study 201) Median: 5 (Study 230) Range: 2–23 | Median 6.0 Mean 6.61 Range: 2–19 | - | Median 6.0 Mean 6.48 Range: 2–19 | - |
| % refractory (n/N) | 47.8% (43/90) | 50.7% (39/77) | 0.711 | 47.8% | >0.999 |
| % female (n/N) | 44.4% (40/90) | 35.1% (27/77) | 0.218 | 44.4% | >0.999 |
| Prior treatment | |||||
| % prior stem cell transplant (n/N) | 33.3% (30/90) | 96.1% (74/77) | <0.001 | 95.3% | <0.001 |
| % prior radiotherapy (n/N) | 34.4% (31/90) | 68.4% (52/77) | <0.001 | 69.9% | <0.001 |
| % prior surgery (n/N) | 93.3% (84/90) | 90.8% (69/77) | 0.387 | 91.4% | 0.643 |
| Disease site | |||||
| % bone only (n/N) | 53.3% (48/90) | 64.9% (50/77) | 0.129 | 53.3% | >0.999 |
| % bone marrow only (n/N) | 6.7% (6/90) | 9.1% (7/77) | 0.560 | 6.7% | >0.999 |
| % both (n/N) | 40.0% (36/90) | 26.0% (20/77) | 0.056 | 40.0% | >0.999 |
| Race/Ethnic origin | |||||
| % White (n/N) | 51.1% (46/90) | 85.7% (66/77) | <0.001 | 86.4% | <0.001 |
| % Black (n/N) | 4.4% (4/90) | 2.6% (2/77) | 0.523 | 2.1% | 0.410 |
| % Asian (n/N) | 35.6% (32/90) | 1.3% (1/77) | <0.001 | 2.3% | <0.001 |
| MYCN | |||||
| % amplification (n/N) | 14.4% (13/90) | 9.1% (7/77) | 0.288 | 14.4% | >0.999 |
| % missing (n/N) | 12.2% (11/90) | 2.6% (2/77) | 0.021 | 1.5% | 0.011 |
| INSS, diagnosis | |||||
| % stage 3 (n/N) | 5.6% (5/90) | 1.3% (1/77) | 0.141 | 1.7% | 0.212 |
| % stage 4 (n/N) | 91.1% (82/90) | 97.4% (75/77) | 0.088 | 97.5% | 0.092 |
| % missing (n/N) | 3.3% (3/90) | 1.3% (1/77) | 0.391 | 0.7% | 0.270 |
| Unadjusted | Base-Case MAIC A | Sensitivity Analysis #1 B | Sensitivity Analysis #2 C | Sensitivity Analysis #3 D | Sensitivity Analysis #4 E | Sensitivity Analysis #4 F | Sensitivity Analysis #5 G | |
|---|---|---|---|---|---|---|---|---|
| ORR, naxitamab (95% CI) | 43.3% (33.1% to 53.6%) | 43.3% (33.1% to 53.6%) | 43.3% (33.1% to 53.6%) | 43.3% (33.1% to 53.6%) | 43.3% (33.1% to 53.6%) | 43.3% (33.1% to 53.6%) | 50% (36% to 64%) | 50.0% (36.4% to 63.6%) |
| ORR, dinutuximab beta (95% CI) | 61.04% (47.10% to 74.98%) | 60.1% (48.5% to 71.6%) | 58.2% (41.8% to 74.5%) | 58.62% (40.04% to 77.20%) | 62.3% (43.7% to 80.9%) | 64.6% (45.0% to 84.1%) | 61.04% (47.10% to 74.98%) | 60.4% (49.0% to 71.9%) |
| OR (95% CI) | 2.05 (1.10 to 3.81) | 1.97 (1.02 to 3.80) | 1.82 (0.74 to 4.49) | 1.85 (0.79 to 4.34) | 2.16 (0.85 to 5.47) | 2.38 (0.88 to 6.46) | 1.57 (0.77 to 3.20) | 1.53 (0.73 to 3.20) |
| p value * | 0.024 | 0.044 | 0.195 | 0.156 | 0.105 | 0.088 | 0.218 | 0.263 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lode, H.N.; Holko, P.; Wieczorek, A.; Siebert, N.; Valteau-Couanet, D.; Garaventa, A.; Cañete, A.; Anderson, J.; Yaniv, I.; Ash, S.; et al. Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison. Cancers 2025, 17, 2723. https://doi.org/10.3390/cancers17172723
Lode HN, Holko P, Wieczorek A, Siebert N, Valteau-Couanet D, Garaventa A, Cañete A, Anderson J, Yaniv I, Ash S, et al. Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison. Cancers. 2025; 17(17):2723. https://doi.org/10.3390/cancers17172723
Chicago/Turabian StyleLode, Holger N., Przemysław Holko, Aleksandra Wieczorek, Nikolai Siebert, Dominique Valteau-Couanet, Alberto Garaventa, Adela Cañete, John Anderson, Isaac Yaniv, Shifra Ash, and et al. 2025. "Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison" Cancers 17, no. 17: 2723. https://doi.org/10.3390/cancers17172723
APA StyleLode, H. N., Holko, P., Wieczorek, A., Siebert, N., Valteau-Couanet, D., Garaventa, A., Cañete, A., Anderson, J., Yaniv, I., Ash, S., Gray, J., Luksch, R., Manzitti, C., Troschke-Meurer, S., Ebeling, T., Kawalec, P., Śladowska, K., & Ladenstein, R. L. (2025). Dinutuximab Beta Versus Naxitamab in the Treatment of Relapsed/Refractory Neuroblastoma in Patients with Stable Disease, Minor Response or Partial Response and Disease in Bone or Bone Marrow: Systematic Review and Matching-Adjusted Indirect Comparison. Cancers, 17(17), 2723. https://doi.org/10.3390/cancers17172723







