Targeted Radiotherapy in Primary Cutaneous Lymphomas: Precision, Efficacy, and Evolving Strategies
Simple Summary
Abstract
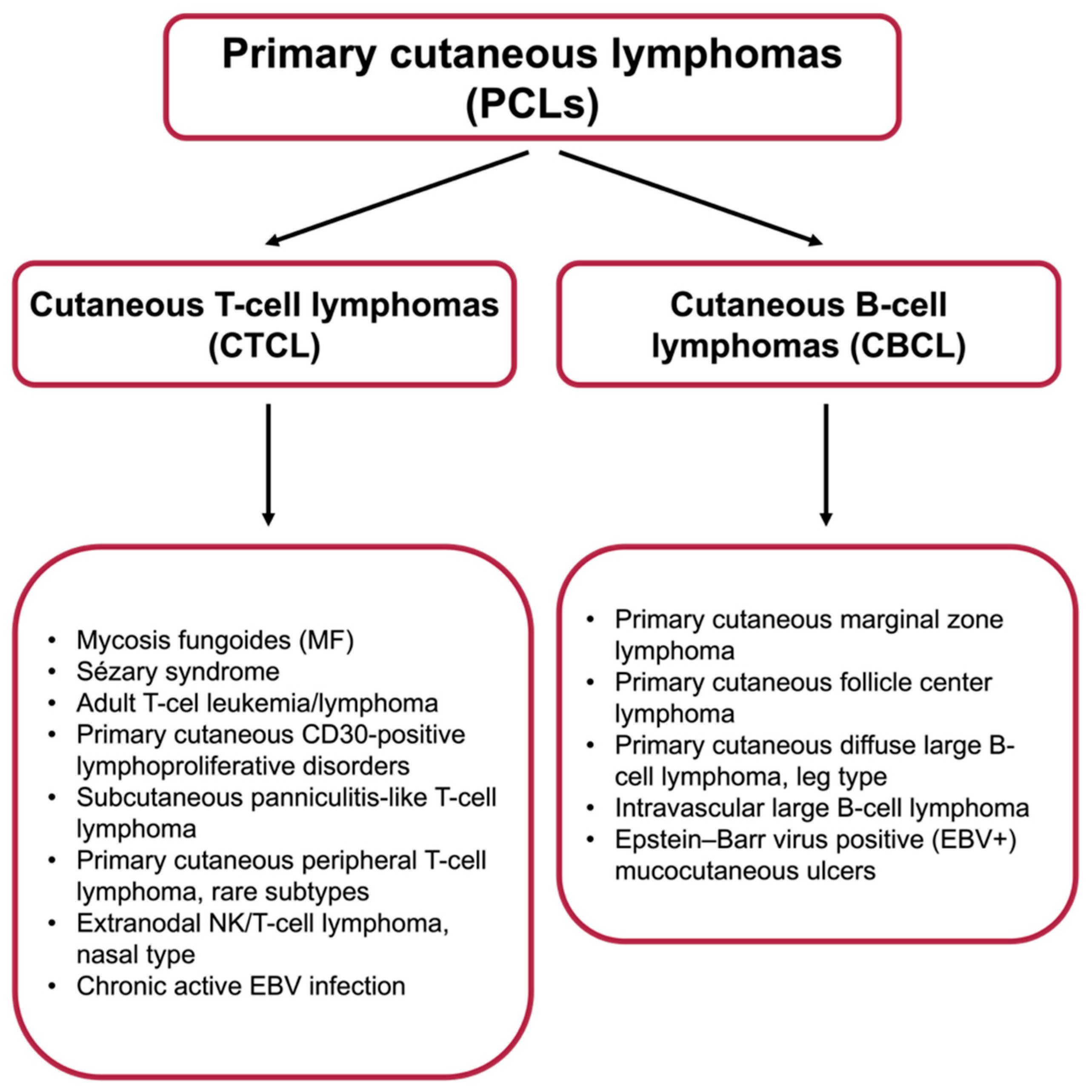
1. Introduction
2. Radiotherapy in Skin Cancers Treatment
| Modality | Indications | Pros | Cons | Outcomes | Ref. |
|---|---|---|---|---|---|
| Brachytherapy (HDR/Surface) | Superficial, small, or irregularly shaped lesions; sites requiring tissue preservation | Precise dose delivery; spares surrounding tissues; excellent cosmetic results | Limited availability of HDR units; requires specialized expertise | Excellent local control; high patient satisfaction with cosmetic outcome | [31,32,33] |
| Total Skin Electron Beam Therapy (TSEBT) | Widespread cutaneous involvement (e.g., advanced MF/SS) | Covers entire skin surface; effective palliation; rapid symptom relief | Technically complex; requires specialized equipment; relapse common | High response rates, but frequent relapses; best for palliation or induction therapy | [15,34] |
| Low-dose RT (localized or TSEBT) | Elderly/frail patients; relapsed disease; palliative settings | Low toxicity; convenient; can be repeated; immunomodulatory effects | Responses often short-lived; not curative as monotherapy | Good short-term control; useful bridge to systemic or combined therapy | [35,36] |
2.1. Radiotherapy in the Treatment of Primary Cutaneous B-Cell Lymphomas
2.2. Radiotherapy in the Treatment of Primary Cutaneous T-Cell Lymphomas
2.2.1. Localized Teleradiotherapy for MF Lesions
2.2.2. Clinical Stage IA Mycosis Fungoides: Teleradiotherapy as a Cure
2.2.3. Teleradiotherapy for Advanced-Stage MF (IIB-III)
2.2.4. Palliative Radiotherapy for Late-Stage MF
2.2.5. Brachytherapy in the Treatment of Primary Cutaneous Lymphomas
2.3. Skin Reactions During Radiotherapy
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Specht, L.; Dabaja, B.; Illidge, T.; Wilson, L.D.; Hoppe, R.T.; Group ILRO. Modern radiation therapy for primary cutaneous lymphomas: Field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood J. Am. Soc. Hematol. 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Senff, N.J.; Noordijk, E.M.; Kim, Y.H.; Bagot, M.; Berti, E.; Cerroni, L.; Dummer, R.; Duvic, M.; Hoppe, R.T.; Pimpinelli, N. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood J. Am. Soc. Hematol. 2008, 112, 1600–1609. [Google Scholar] [CrossRef]
- Kirova, Y.M.; Piedbois, Y.; Le Bourgeois, J.-P. Radiotherapy in the management of cutaneous B-cell lymphoma. Our experience in 25 cases. Radiother. Oncol. 1999, 52, 15–18. [Google Scholar] [CrossRef]
- Eich, H.T.; Eich, D.; Micke, O.; Sttzer, H.; Casper, C.; Krieg, T.; Mller, R.-P. Long-term efficacy, curative potential, and prognostic factors of radiotherapy in primary cutaneous B-cell lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 899–906. [Google Scholar] [CrossRef]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.; Sica, A.; Ronchi, A.; Caccavale, S.; Franco, R.; Argenziano, G. Primary cutaneous B-cell lymphomas: An update. Front. Oncol. 2020, 10, 651. [Google Scholar] [CrossRef]
- Geller, S.; Marghoob, A.; Scope, A.; Braun, R.; Myskowski, P. Dermoscopy and the diagnosis of primary cutaneous B-cell lymphoma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 53–56. [Google Scholar] [CrossRef]
- Pinter-Brown, L.C. Diagnosis and management of cutaneous B-cell lymphoma. Dermatol. Clin. 2015, 33, 835–840. [Google Scholar] [CrossRef][Green Version]
- Senff, N.J.; Hoefnagel, J.J.; Jansen, P.M.; Vermeer, M.H.; van Baarlen, J.; Blokx, W.A.; Canninga-van Dijk, M.R.; Geerts, M.-L.; Hebeda, K.M.; Kluin, P.M. Reclassification of 300 primary cutaneous B-cell lymphomas according to the new WHO–EORTC classification for cutaneous lymphomas: Comparison with previous classifications and identification of prognostic markers. J. Clin. Oncol. 2007, 25, 1581–1587. [Google Scholar] [CrossRef]
- Oertel, M.; Khaled, E.; Carsten, W.; Kerstin, S.; Eich, H.T. De-escalated radiotherapy for indolent primary cutaneous B-cell lymphoma. Strahlenther. Onkol. 2020, 196, 126–131. [Google Scholar] [CrossRef]
- Girardi, M.; Heald, P.W.; Wilson, L.D. The pathogenesis of mycosis fungoides. N. Engl. J. Med. 2004, 350, 1978–1988. [Google Scholar] [CrossRef]
- Agar, N.S.; Wedgeworth, E.; Crichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef]
- Zackheim, H.S. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol. Ther. 2003, 16, 283–287. [Google Scholar] [CrossRef]
- Jones, G.W.; Hoppe, R.T.; Glatstein, E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol./Oncol. Clin. 1995, 9, 1057–1076. [Google Scholar] [CrossRef]
- O’Malley, J.T.; de Masson, A.; Lowry, E.L.; Giobbie-Hurder, A.; LeBoeuf, N.R.; Larocca, C.; Gehad, A.; Seger, E.; Teague, J.E.; Fisher, D.C. Radiotherapy eradicates malignant T cells and is associated with improved survival in early-stage mycosis fungoides. Clin. Cancer Res. 2020, 26, 408–418. [Google Scholar] [CrossRef]
- Garbutcheon-Singh, K.B.; Veness, M.J. The role of radiotherapy in the management of non-melanoma skin cancer. Australas. J. Dermatol. 2019, 60, 265–272. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef]
- Elsayad, K.; Guenova, E.; Assaf, C.; Nicolay, J.P.; Trautinger, F.; Stadler, R.; Waldstein, C.; Boterberg, T.; Meijnders, P.; Kirova, Y. Radiotherapy in cutaneous lymphomas: Recommendations from the EORTC cutaneous lymphoma tumour group. Eur. J. Cancer 2024, 212, 115064. [Google Scholar] [CrossRef]
- Chen, H.H.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742. [Google Scholar] [CrossRef]
- Ferini, G.; Molino, L.; Bottalico, L.; De Lucia, P.; Garofalo, F. A small case series about safety and effectiveness of a hypofractionated electron beam radiotherapy schedule in five fractions for facial non melanoma skin cancer among frail and elderly patients. Rep. Pract. Oncol. Radiother. 2021, 26, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mukherjee, S.; Sinha, D.; Abdisalaam, S.; Krishnan, S.; Asaithamby, A. Immunomodulatory effects of radiotherapy. Int. J. Mol. Sci. 2020, 21, 8151. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Hu, S.; Jia, B.; Tuo, B.; Sun, H.; Wang, Q.; Liu, Y.; Sun, Z. Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Cell Death Dis. 2023, 14, 679. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Sezen, D.; Menon, H.; Younes, A.I.; Hu, Y.; He, K.; Puebla-Osorio, N.; Wasley, M.; Hsu, E.; Patel, R.R. High plus low dose radiation strategy in combination with TIGIT and PD1 blockade to promote systemic antitumor responses. Cancers 2022, 14, 221. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef]
- Stetson, D.B.; Ko, J.S.; Heidmann, T.; Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 2008, 134, 587–598. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Kaur, P.; Asea, A. Radiation-induced effects and the immune system in cancer. Front. Oncol. 2012, 2, 191. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kirchmair, A.; Sato, A.; Buqué, A.; Rybstein, M.; Petroni, G.; Bloy, N.; Finotello, F.; Stafford, L.; Navarro Manzano, E. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 2020, 21, 1160–1171. [Google Scholar] [CrossRef]
- Guix, B.; Finestres, F.; Tello, J.-I.; Palma, C.; Martinez, A.; Guix, J.-R.; Guix, R. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Sánchez, R.; Pons-Llanas, O.; Candela-Juan, C.; Celada-Alvarez, F.J.; de Unamuno-Bustos, B.; Llavador-Ros, M.; Ballesta-Cuñat, A.; Barker, C.A.; Tormo-Mico, A.; Botella-Estrada, R. Efficacy and safety of electronic brachytherapy for superficial and nodular basal cell carcinoma. J. Contemp. Brachyther. 2015, 7, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Chyrek, A.; Bielęda, G.; Burchardt, W.; Chicheł, A. High-dose-rate brachytherapy of primary cutaneous B-cell lymphoma: The first reported case series. J. Contemp. Brachyther. 2020, 12, 241–247. [Google Scholar] [CrossRef]
- Harrison, C.; Young, J.; Navi, D.; Riaz, N.; Lingala, B.; Kim, Y.; Hoppe, R. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e651–e657. [Google Scholar] [CrossRef]
- Kamstrup, M.R.; Lindahl, L.M.; Gniadecki, R.; Iversen, L.; Skov, L.; Petersen, P.; Loft, A.; Specht, L. Low-dose total skin electron beam therapy as a debulking agent for cutaneous T-cell lymphoma: An open-label prospective phase II study. Br. J. Dermatol. 2012, 166, 399–404. [Google Scholar] [CrossRef]
- Kamstrup, M.R.; Gniadecki, R.; Iversen, L.; Skov, L.; Petersen, P.M.; Loft, A.; Specht, L. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: An open clinical study and pooled data analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 138–143. [Google Scholar] [CrossRef]
- Yosefof, E.; Kurman, N.; Yaniv, D. The role of radiation therapy in the treatment of non-melanoma skin cancer. Cancers 2023, 15, 2408. [Google Scholar] [CrossRef]
- De Felice, F.; Grapulin, L.; Pieroni, A.; Salerno, F.; D’Elia, G.M.; Pulsoni, A.; Musio, D.; Tombolini, V. Radiotherapy in indolent primary cutaneous B-cell lymphoma. Hematol. Oncol. 2018, 36, 610. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Cutaneous B-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 1326–1332. [Google Scholar] [CrossRef]
- Kraft, R.M.; Ansell, S.M.; Villasboas, J.C.; Bennani, N.N.; Wang, Y.; Habermann, T.M.; Thanarajasingam, G.; Lester, S.C.; Macon, W.; Inwards, D.J. Outcomes in primary cutaneous diffuse large B-cell lymphoma, leg type. Hematol. Oncol. 2021, 39, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Perrone, F.; Stoico, V.; Pichiri, I.; Salvotelli, L.; Teobaldi, I.; Bruti, M.; Conti, M.; Cima, L.; Eccher, A. Primary cutaneous B-cell lymphoma and chronic leg ulcers in a patient with type 2 diabetes. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 17-0032. [Google Scholar] [CrossRef] [PubMed]
- Mount, C.; Ferraro, D.; Gru, A.; Bradbury, M.; Cornelius, L.; Gay, H. Treatment of pagetoid reticulosis with intensity modulated radiation therapy. Dermatol. Online J. 2014, 20. [Google Scholar] [CrossRef]
- Besch-Stokes, J.G.; Shahin, A.; Bhullar, P.; Hwang, A.; Kechter, J.; Puri, P.; Butterfield, R.; Costello, C.; Rule, W.G.; Rosenthal, A. Intralesional and systemic rituximab in the treatment of primary cutaneous B-cell lymphoma. JEADV Clin. Pract. 2023, 2, 872–881. [Google Scholar] [CrossRef]
- Senff, N.J.; Hoefnagel, J.J.; Neelis, K.J.; Vermeer, M.H.; Noordijk, E.M.; Willemze, R. Results of radiotherapy in 153 primary cutaneous B-Cell lymphomas classified according to the WHO-EORTC classification. Arch. Dermatol. 2007, 143, 1520–1526. [Google Scholar] [CrossRef]
- Smith, B.D.; Glusac, E.J.; McNiff, J.M.; Smith, G.L.; Heald, P.W.; Cooper, D.L.; Wilson, L.D. Primary cutaneous B-cell lymphoma treated with radiotherapy: A comparison of the European Organization for Research and Treatment of Cancer and the WHO classification systems. J. Clin. Oncol. 2004, 22, 634–639. [Google Scholar] [CrossRef]
- Neelis, K.J.; Schimmel, E.C.; Vermeer, M.H.; Senff, N.J.; Willemze, R.; Noordijk, E.M. Low-dose palliative radiotherapy for cutaneous B-and T-cell lymphomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 154–158. [Google Scholar] [CrossRef]
- Lowry, L.; Smith, P.; Qian, W.; Falk, S.; Benstead, K.; Illidge, T.; Linch, D.; Robinson, M.; Jack, A.; Hoskin, P. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother. Oncol. 2011, 100, 86–92. [Google Scholar] [CrossRef]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome–Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef]
- Nath, S.K.; James, B.Y.; Wilson, L.D. Poorer prognosis of African-American patients with mycosis fungoides: An analysis of the SEER dataset, 1988 to 2008. Clin. Lymphoma Myeloma Leuk. 2014, 14, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, J.; Kim, Y.; Whittaker, S.; Wood, G.; Vermeer, M.; Prince, H.; Quaglino, P. Prognostic factors, prognostic indices and staging in mycosis fungoides and Sézary syndrome: Where are we now? Br. J. Dermatol. 2014, 170, 1226–1236. [Google Scholar] [CrossRef]
- Lo, T.; Salzman, F.A.; Wright, K.A. Dose considerations in total skin electron irradiation for mycosis fungoides. Am. J. Roentgenol. 1979, 132, 261–263. [Google Scholar] [CrossRef]
- Hoppe, R.T. Mycosis fungoides: Radiation therapy. Dermatol. Ther. 2003, 16, 347–354. [Google Scholar] [CrossRef]
- Becker, M.; Hoppe, R.T.; Knox, S.J. Multiple courses of high-dose total skin electron beam therapy in the management of mycosis fungoides. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Quiros, P.A.; Kolenik, S.A.; Heald, P.W.; Braverman, I.M.; Edelson, R.L.; Kacinski, B.M. Additional courses of total skin electron beam therapy in the treatment of patients with recurrent cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 1996, 35, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bunn, P.; Lamberg, S.I. Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treat. Rep. 1979, 63, 725–728. [Google Scholar]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood J. Am. Soc. Hematol. 2007, 110, 1713–1722. [Google Scholar]
- Elsayad, K.; Stadler, R.; Steinbrink, K.; Eich, H.T. Combined total skin radiotherapy and immune checkpoint inhibitors: A promising potential treatment for mycosis fungoides and Sezary syndrome. JDDG J. Dtsch. Dermatol. Ges. 2020, 18, 193–197. [Google Scholar] [CrossRef]
- Wilson, L.D.; Kacinski, B.M.; Jones, G.W. Local superficial radiotherapy in the management of minimal stage IA cutaneous T-cell lymphoma (mycosis fungoides). Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Micaily, B.; Miyamoto, C.; Kantor, G.; Lessin, S.; Rook, A.; Brady, L.; Goodman, R.; Vonderheid, E.C. Radiotherapy for unilesional mycosis fungoides. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 361–364. [Google Scholar] [CrossRef]
- Piccinno, R.; Caccialanza, M.; Percivalle, S. Minimal stage IA mycosis fungoides. Results of radiotherapy in 15 patients. J. Dermatol. Treat. 2009, 20, 165–168. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Harrison, C.; Tavallaee, M.; Bashey, S.; Sundram, U.; Li, S.; Million, L.; Dabaja, B.; Gangar, P.; Duvic, M. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: Results of a pooled analysis from 3 phase-II clinical trials. J. Am. Acad. Dermatol. 2015, 72, 286–292. [Google Scholar] [CrossRef]
- Morris, S.; Scarisbrick, J.; Frew, J.; Irwin, C.; Grieve, R.; Humber, C.; Kuciejewska, A.; Bayne, S.; Weatherhead, S.; Child, F. The results of low-dose total skin electron beam radiation therapy (TSEB) in patients with mycosis fungoides from the UK Cutaneous Lymphoma Group. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Aral, İ.P.; Göçer Gürok, N.; Konuk, A.O.; Üçer, Ö. Ultra-Low-Dose Radiotherapy for Palliation of Mycosis Fungoides. Case Rep. Dermatol. Med. 2020, 2020, 4216098. [Google Scholar] [CrossRef]
- Thawani, N.; Jani, S.; Kang, B.; Yang, M.; Sorenson, S.; Srivastava, S.; Pinnaduwage, D.; Ellefson, S.; Diaz, A.; Patel, S. PO09: Treatment of Facial Folliculotropic Mycosis Fungoides with High Dose Rate Brachytherapy in Two Fractions. Brachy 2022, 21, S74–S75. [Google Scholar] [CrossRef]
- Skowronek, J. Current status of brachytherapy in cancer treatment–short overview. J. Contemp. Brachyther. 2017, 9, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Linggonegoro, D.W.; McCormack, L.; Grenier, P.O.; Vrooman, L.M.; Devlin, P.M.; Huang, J.T. Pediatric primary cutaneous anaplastic large cell lymphoma treated with brachytherapy. Pediatr. Dermatol. 2021, 38, 712–713. [Google Scholar] [CrossRef]
- Goddard, A.L.; Vleugels, R.A.; LeBoeuf, N.R.; O’Farrell, D.A.; Cormack, R.A.; Hansen, J.L.; Kupper, T.S.; Devlin, P.M. Palliative therapy for recalcitrant cutaneous T-cell lymphoma of the hands and feet with low-dose, high dose-rate brachytherapy. JAMA Dermatol. 2015, 151, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, J.A.; Guenova, E.; Carter, J.B.; Chaney, K.S.; Aldridge, J.R.; Noell, C.M.; Dorosario, A.A.; Hansen, J.L.; Kupper, T.S.; Devlin, P.M. Low-dose high-dose-rate brachytherapy in the treatment of facial lesions of cutaneous T-cell lymphoma. J. Am. Acad. Dermatol. 2013, 69, 61–65. [Google Scholar] [CrossRef]
- Devlin, P.M.; James, S.L.S.; Goddard, A.L.; O’Farrell, D.A.; Buzurovic, I.; Friesen, S.A.; Bhagwat, M.S.; Kupper, T.S.; Cormack, R.A. Dose Fidelity and Conformality for High-Dose-Rate Surface Applicator Brachytherapy for Cutaneous Lymphoma Lesions of the Hands and Feet. Brachy 2014, 13, S110. [Google Scholar] [CrossRef][Green Version]
- Shukla, G.; Lockamy, V.; Keller, J.; Sahu, J.; Pro, B.; Alpdogan, O.; Shi, W. High dose rate (HDR) brachytherapy for mycosis fungoides of the wrist. Int. J. Clin. Med. 2015, 6, 154. [Google Scholar] [CrossRef]
- Rosenthal, A.; Israilevich, R.; Moy, R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J. Am. Acad. Dermatol. 2019, 81, 558–567. [Google Scholar] [CrossRef]
- Burke, G.; Faithfull, S.; Probst, H. Radiation induced skin reactions during and following radiotherapy: A systematic review of interventions. Radiography 2022, 28, 232–239. [Google Scholar] [CrossRef]
- Vickers, E.R.; Liang, J.; Vickers, P.G.; Wen, H. Regenerative Medicine to Reduce the Side Effects from Radiotherapy Causing Skin Cancer, Fibrosis, Neuropathic Pain and Hair Loss. J. Cancer Ther. 2021, 12, 461–477. [Google Scholar] [CrossRef]
- Park, S.-Y.; Park, J.M.; Kim, J.-i.; Choi, C.H.; Chun, M.; Chang, J.H.; Kim, J.H. Quantitative radiomics approach to assess acute radiation dermatitis in breast cancer patients. PLoS ONE 2023, 18, e0293071. [Google Scholar] [CrossRef]
- Marak, S.K.; Mahawar, R.; Devi, N.D.; Devi, N.N.; Devi, Y.S.; Baidya, K. A Comparative Study of the Toxicities and Local Recurrence of Conventional External Beam Radiotherapy versus Hypofractionated Radiotherapy in Breast Cancer Patients. Int. J. Curr. Sci. Res. Rev. 2023, 6, 7918–7926. [Google Scholar] [CrossRef]
- Chugh, R.; s Bisht, Y.; Nautiyal, V.; Jindal, R. Factors influencing the severity of acute radiation-induced skin and mucosal toxicity in head and neck cancer. Cureus 2021, 13, e18147. [Google Scholar] [CrossRef]
- Trueman, E. Management of radiotherapy-induced skin reactions. Int. J. Palliat. Nurs. 2015, 21, 187–192. [Google Scholar] [CrossRef]
- Jd, C. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar]
- Malik, D.; Singh, A.; Birajdar, M.M.; Vyas, V.J. Feasibility, Tolerance, and Quality of Life for Hypofractionation Versus Conventional Fractionation for Post-mastectomy Radiotherapy in Indian Patients. Cureus 2022, 14, e23497. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, Y.; Han, D.; Zhao, Q.; Liu, C.; Sun, H.; Wang, Z.; Lin, H.; Huang, W. Hypofractionated simultaneous integrated boost radiotherapy versus conventional fractionation radiotherapy of early breast cancer after breast-conserving surgery: Clinical observation and analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211064719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Fenton, G.; White, R. Radiation dermatitis: The evaluation of a new topical therapy for the treatment and prevention of radiation-induced skin damage and moist desquamation: A multicentre UK case cohort study. J. Radiother. Pract. 2021, 20, 461–465. [Google Scholar] [CrossRef]
- Ding, J.; Guo, Y.; Li, Q.; Chen, J.; Hu, P.; Liu, Q.; Cao, Y.; Wu, J. The incidence of postoperative radiotherapy-induced acute dermatitis in breast cancer and its influencing factors for Chinese women. OncoTargets Ther. 2018, 11, 1665–1670. [Google Scholar] [CrossRef]
- Kiprian, D.; Szykut-Badaczewska, A.; Gradzińska, A.; Czuwara, J.; Rudnicka, L. How to manage radiation-induced dermatitis? Nowotwory. J. Oncol. 2022, 72, 86–95. [Google Scholar] [CrossRef]
- Berger, A.; Regueiro, C.; Hijal, T.; Pasquier, D.; De La Fuente, C.; Le Tinier, F.; Coche-Dequeant, B.; Lartigau, E.; Moyal, D.; Seité, S. Interest of supportive and barrier protective skin care products in the daily prevention and treatment of cutaneous toxicity during radiotherapy for breast cancer. Breast Cancer Basic Clin. Res. 2018, 12, 1178223417752772. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, P.; Kumar, G.; Sampadarao, B. Study of the Various Cutaneous Adverse Reactions to Radiotherapy. Int. J. Res. Dermatol. 2021, 7, 250. [Google Scholar] [CrossRef]
- Laetsch, B.; Hofer, T.; Lombriser, N.; Lautenschlager, S. Irradiation-induced morphea: X-rays as triggers of autoimmunity. Dermatology 2011, 223, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Ulff, E.; Maroti, M.; Serup, J.; Nilsson, M.; Falkmer, U. Late cutaneous effects of a local potent steroid during adjuvant radiotherapy for breast cancer. Clin. Transl. Radiat. Oncol. 2017, 7, 9–12. [Google Scholar] [CrossRef]
- Bensadoun, R.-J.; Humbert, P.; Krutman, J.; Luger, T.; Triller, R.; Rougier, A.; Seite, S.; Dreno, B. Daily baseline skin care in the prevention, treatment, and supportive care of skin toxicity in oncology patients: Recommendations from a multinational expert panel. Cancer Manag. Res. 2013, 5, 401–408. [Google Scholar] [CrossRef]
- McAlinden, L.; Mullan, A.; Shepherd, P. An evaluation of the skincare management of patients receiving radiotherapy for breast cancer. J. Radiother. Pract. 2020, 19, 365–369. [Google Scholar] [CrossRef]
- Chan, R.J.; Keller, J.; Cheuk, R.; Blades, R.; Tripcony, L.; Keogh, S. A double-blind randomised controlled trial of a natural oil-based emulsion (Moogoo Udder Cream®) containing allantoin versus aqueous cream for managing radiation-induced skin reactions in patients with cancer. Radiat. Oncol. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Haruna, F.; Lipsett, A.; Marignol, L. Topical management of acute radiation dermatitis in breast cancer patients: A systematic review and meta-analysis. Anticancer. Res. 2017, 37, 5343–5353. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Khalaf, S.A.; Khalaf, F.R.; Mohamed, S.H. Education Program to Promote Skin Integrity and Reduce Pain for Patients Receiving External Beam Radiotherapy. Assiut Sci. Nurs. J. 2022, 10, 191–199. [Google Scholar] [CrossRef]
- Haley, A.C.; Calahan, C.; Gandhi, M.; West, D.P.; Rademaker, A.; Lacouture, M.E. Skin care management in cancer patients: An evaluation of quality of life and tolerability. Support. Care Cancer 2011, 19, 545–554. [Google Scholar] [CrossRef]
- Wong, J.H.D.; Zaili, Z.; Abdul Malik, R.; Bustam, A.Z.; Saad, M.; Jamaris, S.; Mosiun, J.A.; Mohd Taib, N.A.; Ung, N.M.; See, M.H. Evaluation of skin dose and skin toxicity in patients undergoing intraoperative radiotherapy for early breast cancer. J. Appl. Clin. Med. Phys. 2021, 22, 139–147. [Google Scholar] [CrossRef]
- Levy, R.; Pope, E. Primary cutaneous lymphoma. In Harper’s Textbook of Pediatric Dermatology; Wiley: Hoboken, NJ, USA, 2019; pp. 1044–1062. [Google Scholar]
- Izu-Belloso, R.; García-Ruiz, J. Treatment of cutaneous lymphomas: An update. Actas Dermo-Sifiliográficas (Engl. Ed.) 2012, 103, 694–707. [Google Scholar] [CrossRef]
- Poltorak, M.; Banatkiewicz, P.; Poltorak, L.; Sobolewski, P.; Zimon, D.; Szwast, M.; Walecka, I. Brachytherapy and 3D printing for skin cancer: A review paper. J. Contemp. Brachyther. 2024, 16, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, M.; Banatkiewicz, P.; Poltorak, L.; Sobolewski, P.; Zimon, D.; Szwast, M.; Walecka, I. Reproducibility and air gap pockets of 3D-printed brachytherapy applicator placement in high-dose-rate skin cancer. Phys. Medica 2024, 123, 103401. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, M.; Banatkiewicz, P.; Poltorak, L.; Sobolewski, P.; Zimon, D.; Szwast, M.; Walecka, I. Individualized 3D printing for skin cancer brachytherapy: Development, implementation, clinical applications, and treatment assessment. J. Contemp. Brachyther. 2024, 16, 173–183. [Google Scholar] [CrossRef]
- Jones, G.W.; Kacinski, B.M.; Wilson, L.D.; Willemze, R.; Spittle, M.; Hohenberg, G.; Handl-Zeller, L.; Trautinger, F.; Knobler, R.; Group, E.C.L.P. Total skin electron radiation in the management of mycosis fungoides: Consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J. Am. Acad. Dermatol. 2002, 47, 364–370. [Google Scholar] [CrossRef]
- Skowronek, J. Brachytherapy in the treatment of skin cancer: An overview. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2015, 32, 362–367. [Google Scholar] [CrossRef]
- Alam, M.; Nanda, S.; Mittal, B.B.; Kim, N.A.; Yoo, S. The use of brachytherapy in the treatment of nonmelanoma skin cancer: A review. J. Am. Acad. Dermatol. 2011, 65, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Di Stefani, A.; Tagliaferri, L.; Lancellotta, V.; Fionda, B.; Fossati, B.; Balducci, M.; Federico, F.; Hohaus, S.; De Simone, C.; Gambacorta, M.A. The safety of radiotherapy in the treatment of primary cutaneous B-cell lymphoma: A multidisciplinary systematic review. Front. Oncol. 2020, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Jennings, T.; Duffy, R.; Gochoco, A.; Knoblauch, K.; Shi, W.; Alpdogan, S.O.; Porcu, P.; Werner-Wasik, M.; Sahu, J. Valchlor maintenance therapy for patients with mycosis fungoides who received low dose total skin electron beam treatment. Chin. Clin. Oncol. 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
| Grade | Observation |
|---|---|
| Grade 0 | None |
| Grade 1 | Follicular, faint, or dull erythema, epilation, dry desquamation, decreased sweating. Mild tightness of skin and itching may occur. |
| Grade 2 | Brisk erythema/dry desquamation. Skin may feel tight, sore and itchy. |
| Grade 2.5 | Patchy moist desquamation. Yellow/pale green exudate may be visible on surface. Soreness and oedema. |
| Grade 3 | Confluent, moist desquamation other than skin folds, pitting edema. Yellow/pale green exudate visible. Soreness. Bleeding may occur. |
| Grade 4 | Ulceration, necrosis, hemorrhage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewski, P.; Koper, M.; Ciechanowicz, P.; Walecka, I. Targeted Radiotherapy in Primary Cutaneous Lymphomas: Precision, Efficacy, and Evolving Strategies. Cancers 2025, 17, 2722. https://doi.org/10.3390/cancers17172722
Sobolewski P, Koper M, Ciechanowicz P, Walecka I. Targeted Radiotherapy in Primary Cutaneous Lymphomas: Precision, Efficacy, and Evolving Strategies. Cancers. 2025; 17(17):2722. https://doi.org/10.3390/cancers17172722
Chicago/Turabian StyleSobolewski, Piotr, Mateusz Koper, Piotr Ciechanowicz, and Irena Walecka. 2025. "Targeted Radiotherapy in Primary Cutaneous Lymphomas: Precision, Efficacy, and Evolving Strategies" Cancers 17, no. 17: 2722. https://doi.org/10.3390/cancers17172722
APA StyleSobolewski, P., Koper, M., Ciechanowicz, P., & Walecka, I. (2025). Targeted Radiotherapy in Primary Cutaneous Lymphomas: Precision, Efficacy, and Evolving Strategies. Cancers, 17(17), 2722. https://doi.org/10.3390/cancers17172722







