A Comparison of the Flow Cytometric Analysis Results of Benign and Malignant Serous Tumors of the Ovary
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
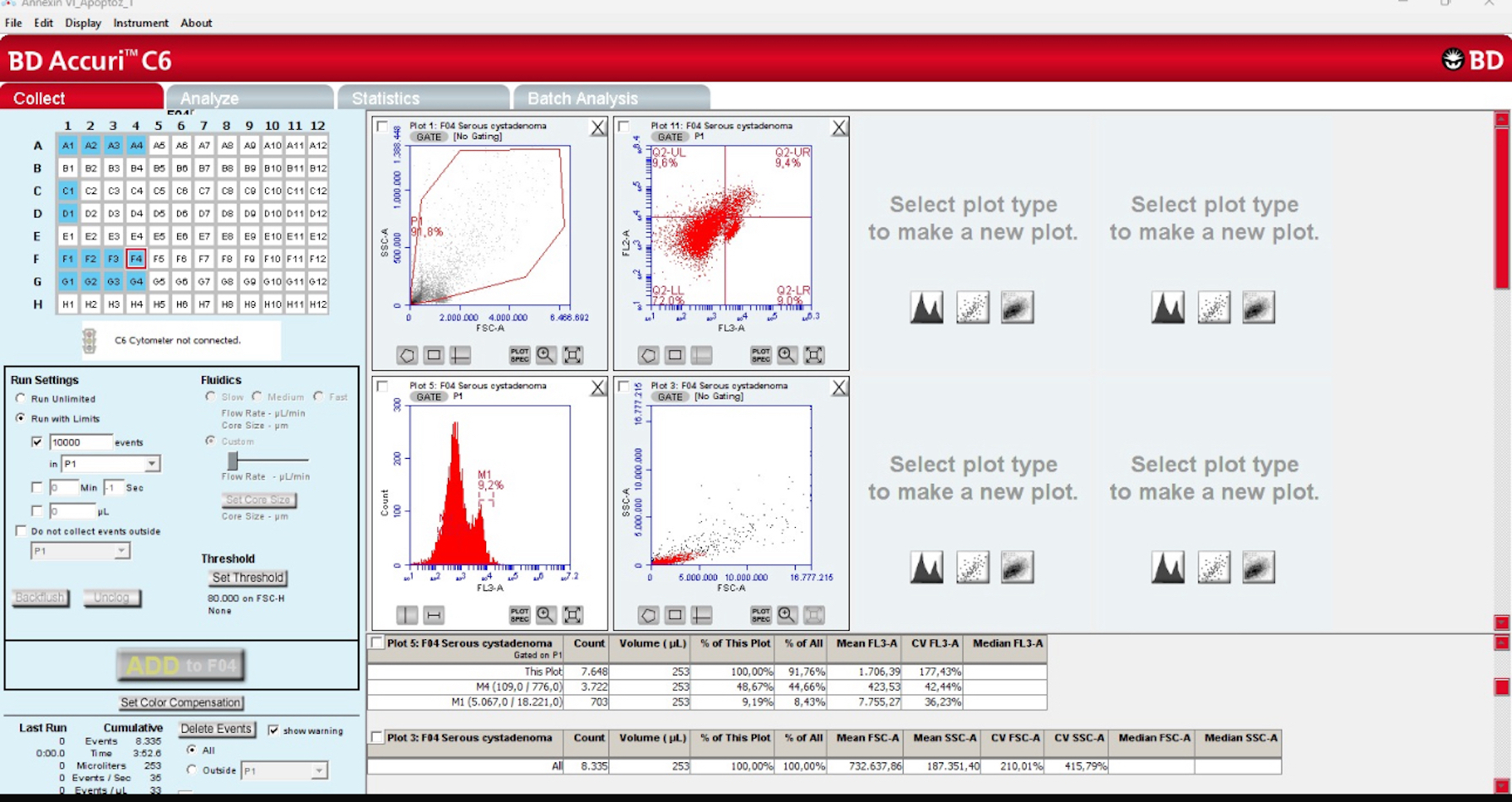
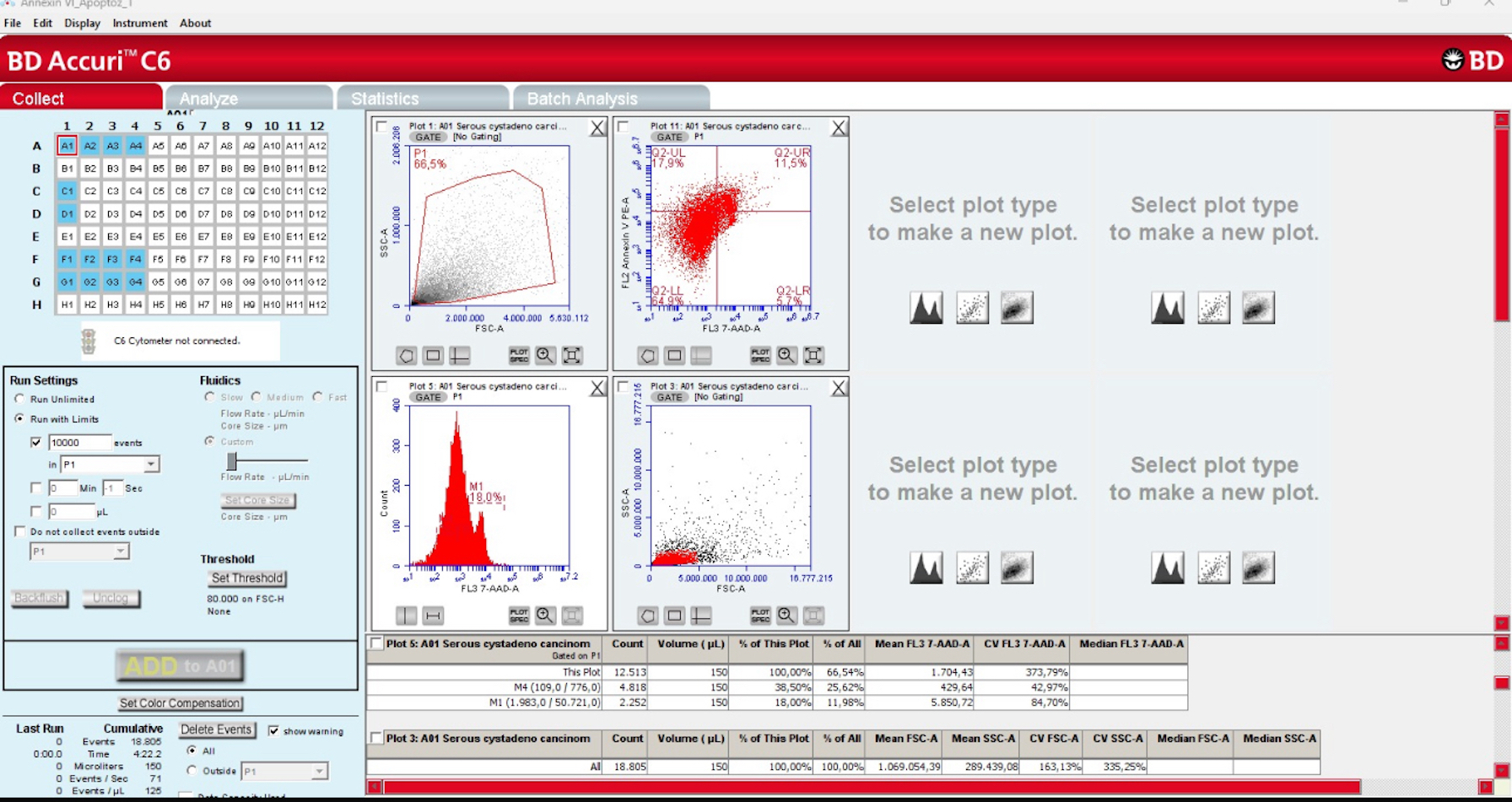
2.1. Deparaffinization and Flow Cytometry
2.2. Apoptotic Index
2.3. Statistical Analyses
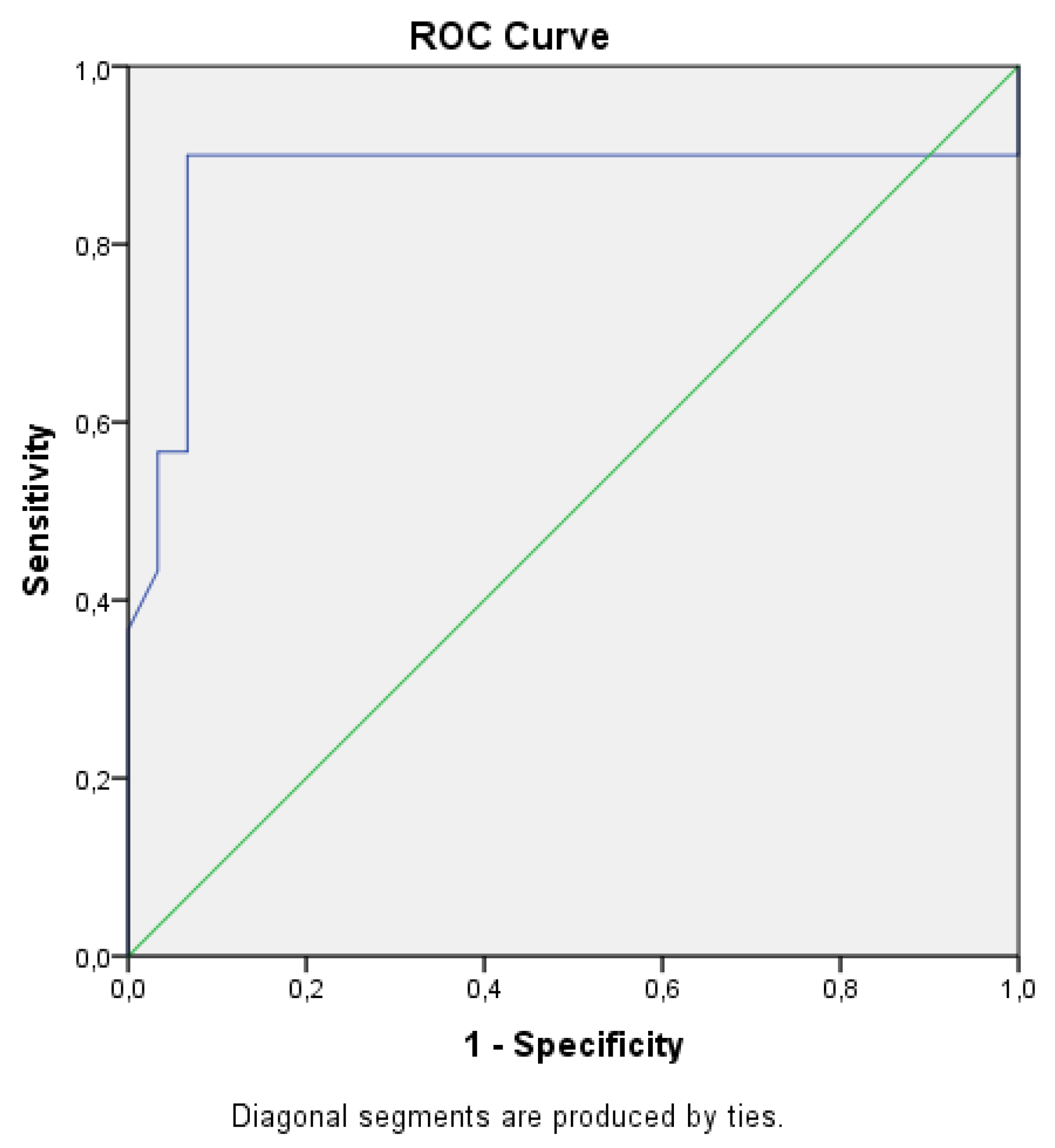
3. Results
Student’s t-Test Was Used for Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STIC | Serous tubal intraepithelial carcinoma |
| TAH + BSO/USO | Total abdominal hysterectomy + bilateral/unilateral salpingo-oophorectomy |
| SPF | S-phase fraction |
| PI | Proliferative index |
References
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 61–85. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, M.; Matsumura, N.; Konishi, I. Recent concepts of ovarian carcinogenesis: Type I and type II. Biomed. Res. Int. 2014, 2014, 934261. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Shih, I.-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer—Shifting the paradigm. Hum. Pathol. 2011, 42, 918–931. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Szpakowski, M.; Malinowski, A.; Romanowicz, H.; Wieczorek, A.; Szpakowski, A.; Wilczynski, J.R.; Maciolek-Blewniewska, G.; Kolasa, D. Ovarian tumors in the reproductive age group. Ginekol. Pol. 2002, 73, 354–358. [Google Scholar]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Telford, W.G.; King, L.E.; Fraker, P.J. Rapid quantitation of apoptosis in pure and heterogeneous cell populations using flow cytometry. J. Immunol. Methods 1994, 172, 1–16. [Google Scholar] [CrossRef]
- Arur, S.; Uche, U.E.; Rezaul, K.; Fong, M.; Scranton, V.; Cowan, A.E.; Mohler, W.; Han, D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 2003, 4, 587–598. [Google Scholar] [CrossRef]
- Novikov, F.V.; Anufriev, A.G.; Efremov, G.D. High-grade Serous Carcinoma Occurring in a Serous Cystadenoma on the Background of a Serous Tubal Intraepithelial Carcinoma (STIC)-like Lesion: A Case Report With Literature Review. Int. J. Gynecol. Pathol. 2024, 43, 626–630. [Google Scholar] [CrossRef]
- Prat, J. FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.D.; Clevenger, C.V.; Endow, R.K.; Murad, T.; Epstein, A.L.; Scarpelli, D.G. Simultaneous nuclear antigen and DNA content quantitation using paraffin-embedded colonic tissue and multiparameter flow cytometry. Cancer Res. 1986, 46, 2428–2434. [Google Scholar] [PubMed]
- Hedley, D.W. Flow cytometry using paraffin-embedded tissue: Five years on. Cytometry 1989, 10, 229–241. [Google Scholar] [CrossRef]
- Hedley, D.W.; Friedlander, M.L.; Taylor, I.W.; Rugg, C.A.; Musgrove, E.A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J. Histochem. Cytochem. 1983, 31, 1333–1335. [Google Scholar] [CrossRef] [PubMed]
- Akman, A.U.; Erisgin, Z.; Turedi, S.; Tekelioglu, Y. Methotrexate-induced hepatotoxicity in rats and the therapeutic properties of vitamin E: A histopathologic and flowcytometric research. Clin. Exp. Hepatol. 2023, 9, 359–367. [Google Scholar] [CrossRef]
- Merchant, S.H.; Gonchoroff, N.J.; Hutchison, R.E. Apoptotic index by Annexin V flow cytometry: Adjunct to morphologic and cytogenetic diagnosis of myelodysplastic syndromes. Cytometry 2001, 46, 28–32. [Google Scholar] [CrossRef]
- Nair, A.; Manohar, S.M. A flow cytometric journey into cell cycle analysis. Bioanalysis 2021, 13, 1627–1644. [Google Scholar] [CrossRef]
- Griffiths, A.P.; Cross, D.; Kingston, R.E.; Harkin, P.; Wells, M.; Quirke, P. Flow cytometry and AgNORs in benign, borderline, and malignant mucinous and serous tumours of the ovary. Int. J. Gynecol. Pathol. 1993, 12, 307–314. [Google Scholar] [CrossRef]
- Braly, P.S.; Klevecz, R.R. Flow cytometric evaluation of ovarian cancer. Cancer 1993, 71, 1621–1628. [Google Scholar] [CrossRef]
- Kim, Y.T.; Zhao, M.; Kim, S.H.; Lee, C.S.; Kim, J.H.; Kim, J.W. Prognostic significance of DNA quantification by flow cytometry in ovarian tumors. Int. J. Gynaecol. Obstet. 2005, 88, 286–291. [Google Scholar] [CrossRef]
- Friedlander, M.L.; Taylor, I.W.; Russell, P.; Musgrove, E.A.; Hedley, D.H.; Tattersall, M.H. Ploidy as a prognostic factor in ovarian cancer. Int. J. Gynecol. Pathol. 1983, 2, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.S.; Heath, T.M.; Morris, L.S.; Rushbrook, S.M.; Bird, K.; Vowler, S.L.; Arends, M.J.; Coleman, N. A novel immunohistochemical method for estimating cell cycle phase distribution in ovarian serous neoplasms: Implications for the histopathological assessment of paraffin-embedded specimens. Br. J. Cancer 2004, 90, 1583–1590. [Google Scholar] [CrossRef]
- Gomes, F.C.; Figueiredo, E.R.L.; Araujo, E.N.; Andrade, E.M.; Carneiro, C.D.L.; Almeida, G.M.; Dias, H.; Teixeira, L.I.B.; Almeida, M.T.; Farias, M.F.; et al. Social, Genetics and Histopathological Factors Related to Titin (TTN) Gene Mutation and Survival in Women with Ovarian Serous Cystadenocarcinoma: Bioinformatics Analysis. Genes 2023, 14, 1092. [Google Scholar] [CrossRef] [PubMed]
- Szafron, L.A.; Iwanicka-Nowicka, R.; Sobiczewski, P.; Koblowska, M.; Dansonka-Mieszkowska, A.; Kupryjanczyk, J.; Szafron, L.M. The Diversity of Methylation Patterns in Serous Borderline Ovarian Tumors and Serous Ovarian Carcinomas. Cancers 2024, 16, 3524. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Koma, Y.I.; Urakami, S.; Takahashi, R.; Nagamata, S.; Omori, M.; Torigoe, R.; Yokoo, H.; Nakanishi, T.; Ishihara, N.; et al. YKL40/Integrin beta4 Axis Induced by the Interaction between Cancer Cells and Tumor-Associated Macrophages Is Involved in the Progression of High-Grade Serous Ovarian Carcinoma. Int. J. Mol. Sci. 2024, 25, 10598. [Google Scholar] [CrossRef]
- Kanno, K.; Nakayama, K.; Razia, S.; Islam, S.H.; Farzana, Z.U.; Sonia, S.B.; Sasamori, H.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; et al. Molecular Analysis of High-Grade Serous Ovarian Carcinoma Exhibiting Low-Grade Serous Carcinoma and Serous Borderline Tumor. Curr. Issues Mol. Biol. 2024, 46, 9376–9385. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Chen, Y.; Dong, J. Amplification of Hippo Signaling Pathway Genes Is Governed and Implicated in the Serous Subtype-Specific Ovarian Carcino-Genesis. Cancers 2024, 16, 1781. [Google Scholar] [CrossRef]
- Hassan, H.A.; Salem, M.L.; Gouida, M.S.; El-Azab, K.M. Comparative expression of caspases and annexin V in benign and malignant ovarian tumors. J. Cancer Res. Ther. 2018, 14, 1042–1048. [Google Scholar] [CrossRef]
- Reiner, A.T.; Tan, S.; Agreiter, C.; Auer, K.; Bachmayr-Heyda, A.; Aust, S.; Pecha, N.; Mandorfer, M.; Pils, D.; Brisson, A.R.; et al. EV-Associated MMP9 in High-Grade Serous Ovarian Cancer Is Preferentially Localized to Annexin V-Binding EVs. Dis. Markers 2017, 2017, 9653194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; He, H.; Bai, Y.; Liu, Z.; Sabihi, S.S. Mechanisms of apoptosis-related non-coding RNAs in ovarian cancer: A narrative review. Apoptosis 2025, 30, 553–578. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kohn, E.C.; Brill, E.; Lee, J.M. Apoptosis is augmented in high-grade serous ovarian cancer by the combined inhibition of Bcl-2/Bcl-xL and PARP. Int. J. Oncol. 2017, 50, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Krzystyniak, J.; Ceppi, L.; Dizon, D.S.; Birrer, M.J. Epithelial ovarian cancer: The molecular genetics of epithelial ovarian cancer. Ann. Oncol. 2016, 27 (Suppl. 1), i4–i10. [Google Scholar] [CrossRef] [PubMed]
- Andreou, M.; Vartholomatos, E.; Harissis, H.; Markopoulos, G.S.; Alexiou, G.A. Past, Present and Future of Flow Cytometry in Breast Cancer—A Systematic Review. Electron. J. Int. Fed. Clin. Chem. Lab. Med. EJIFCC 2019, 30, 423–437. [Google Scholar]
- Georvasili, V.K.; Markopoulos, G.S.; Batistatou, A.; Mitsis, M.; Messinis, T.; Lianos, G.D.; Alexiou, G.; Vartholomatos, G.; Bali, C.D. Detection of cancer cells and tumor margins during colorectal cancer surgery by intraoperative flow cytometry. Int. J. Surg. 2022, 104, 106717. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Vartholomatos, G.; Alexiou, G.A.; Markopoulos, G.S. The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies. Methods Protoc. 2021, 4, 11. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Basiari, L.; Kastanioudakis, I.; Psichogios, G.; Alexiou, G.A. The Role of Intraoperative Flow Cytometry in Surgical Margins of Head and Neck Malignancies. Ear Nose Throat J. 2021, 100, 989S–990S. [Google Scholar] [CrossRef]
- Dorfman, D.M. The Flow Cytometric Evaluation of B- and T-Lymphoblastic Leukemia/Lymphoma. Cancers 2025, 17, 1111. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 2.2025. Acute Myeloid Leukemia (Age ≥ 18 Years); National Comprehensive Cancer Network: Montgomery, PA, USA, 2025.

| Clinical Data | Patients with Serous Cyst Adenoma (n = 30) | Patients with Serous Cystadenocarcinoma (n = 30) | p |
|---|---|---|---|
| Age a | 50.13 ± 15.01 | 57.80 ± 11.10 | 0.028 |
| Gravida a | 3.96 ± 3.54 | 2.60 ± 2.06 | 0.181 |
| Parity a | 3.04 ± 2.87 | 2.33 ± 1.95 | 0.404 |
| Systemic disease (%) b | 0.399 | ||
| Absent | 63.3% (19) | 76.7% (23) | |
| Present (hypertension, diabetes, thyroid disease, other) | 36.7% (11) | 23.3% (7) | |
| Surgery b | 0.001 | ||
| TAH + USO or BSO/Staging | 60.0% (18) | 100.0% (30) | |
| Salpingo-oophorectomy | 13.3% (4) | - | |
| Cystectomy | 26.7% (8) | - | |
| FIGO stage | - | ||
| Stage I | - | 33.3% (10) | |
| Stage II | - | 26.7% (8) | |
| Stage III–IV | - | 40.0% (12) |
| Patients with Serous Cyst Adenoma (n = 30) | Patients with Serous Cystadenocarcinoma (n = 30) | p | |
|---|---|---|---|
| G0/G1 phase (%) | 82.18 ± 2.25 | 73.09 ± 4.36 | <0.001 |
| G2/M phase (%) | 8.55 ± 3.25 | 9.53 ± 1.96 | 0.163 |
| S phase (%) | 11.18 ± 1.66 | 18.25 ± 3.27 | <0.001 |
| Proliferative index (%) | 18.89 ± 7.49 | 27.91 ± 4.36 | <0.001 |
| Aneuploidy cell ratio (%) | 10.47 ± 1.96 | 18.24 ± 3.95 | <0.001 |
| Annexin V apoptotic index (%) | 19.29 ± 5.40 | 34.04 ± 8.38 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozdemir, O.; Duygulu, M.E.; Tekelioglu, Y.; Ersoz, S.; Guven, S. A Comparison of the Flow Cytometric Analysis Results of Benign and Malignant Serous Tumors of the Ovary. Cancers 2025, 17, 2691. https://doi.org/10.3390/cancers17162691
Ozdemir O, Duygulu ME, Tekelioglu Y, Ersoz S, Guven S. A Comparison of the Flow Cytometric Analysis Results of Benign and Malignant Serous Tumors of the Ovary. Cancers. 2025; 17(16):2691. https://doi.org/10.3390/cancers17162691
Chicago/Turabian StyleOzdemir, Ozgur, Mustafa Emre Duygulu, Yavuz Tekelioglu, Safak Ersoz, and Suleyman Guven. 2025. "A Comparison of the Flow Cytometric Analysis Results of Benign and Malignant Serous Tumors of the Ovary" Cancers 17, no. 16: 2691. https://doi.org/10.3390/cancers17162691
APA StyleOzdemir, O., Duygulu, M. E., Tekelioglu, Y., Ersoz, S., & Guven, S. (2025). A Comparison of the Flow Cytometric Analysis Results of Benign and Malignant Serous Tumors of the Ovary. Cancers, 17(16), 2691. https://doi.org/10.3390/cancers17162691






