Unexpectedly Low Rate of Metastasis and Death Among Patients Treated for Uveal Melanoma with Brachytherapy, Vitrectomy, and Silicone Oil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Design
2.2. Risk Stratification
2.3. Treatment of Ocular Primary
2.4. Post-Treatment Surveillance
2.5. Outcome Assessment
2.6. Diagnostic Test Performance Analysis
2.7. Sensitivity
2.8. Specificity
2.9. Positive Prognostic Value (PPV)
2.10. Negative Prognostic Value (NPV)
2.11. Prognostic Accuracy
2.12. Confidence Intervals
3. Results
3.1. Demographics
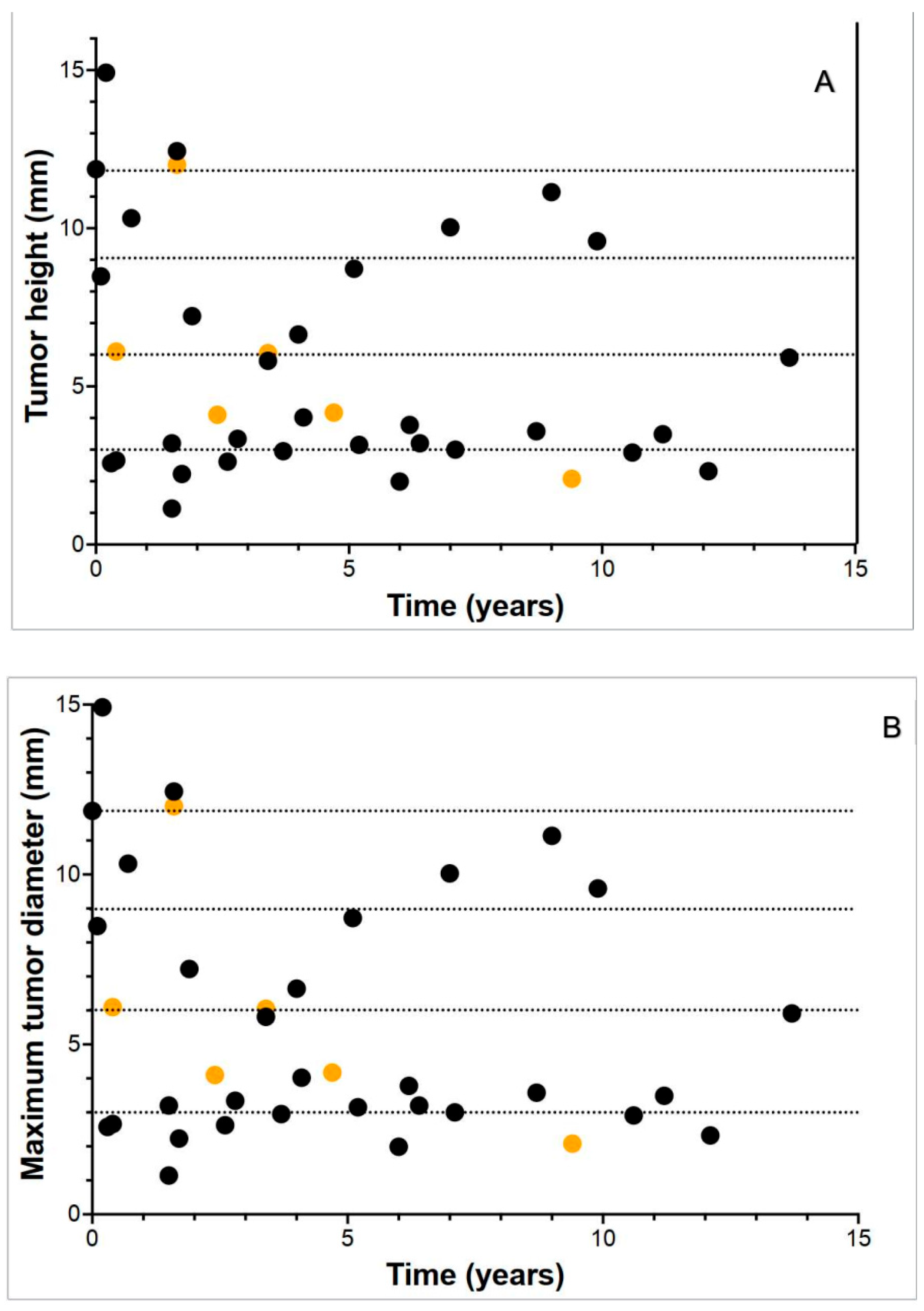
3.2. Tumor Dimensions
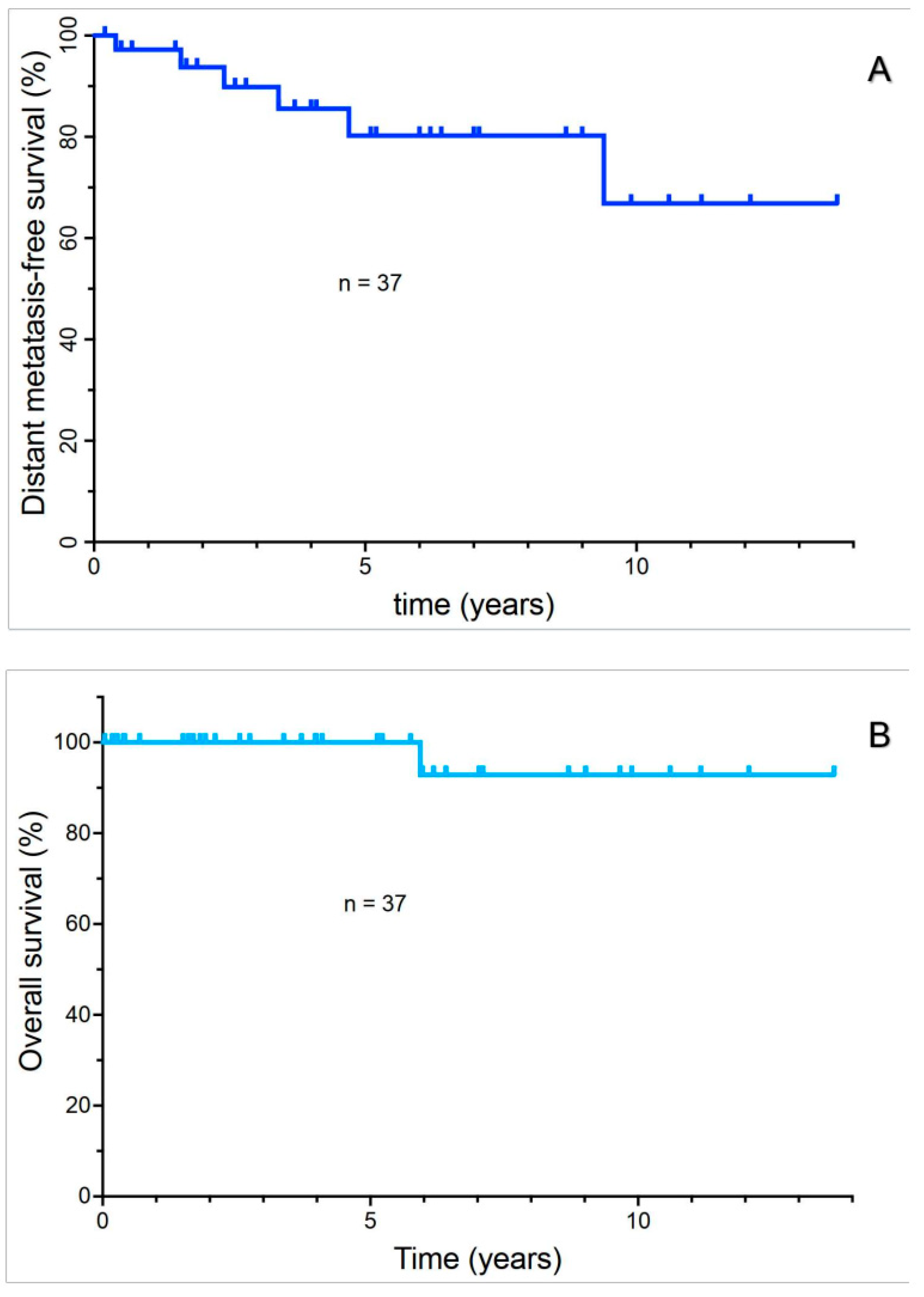
3.3. Analysis of DMFS and Overall Survival
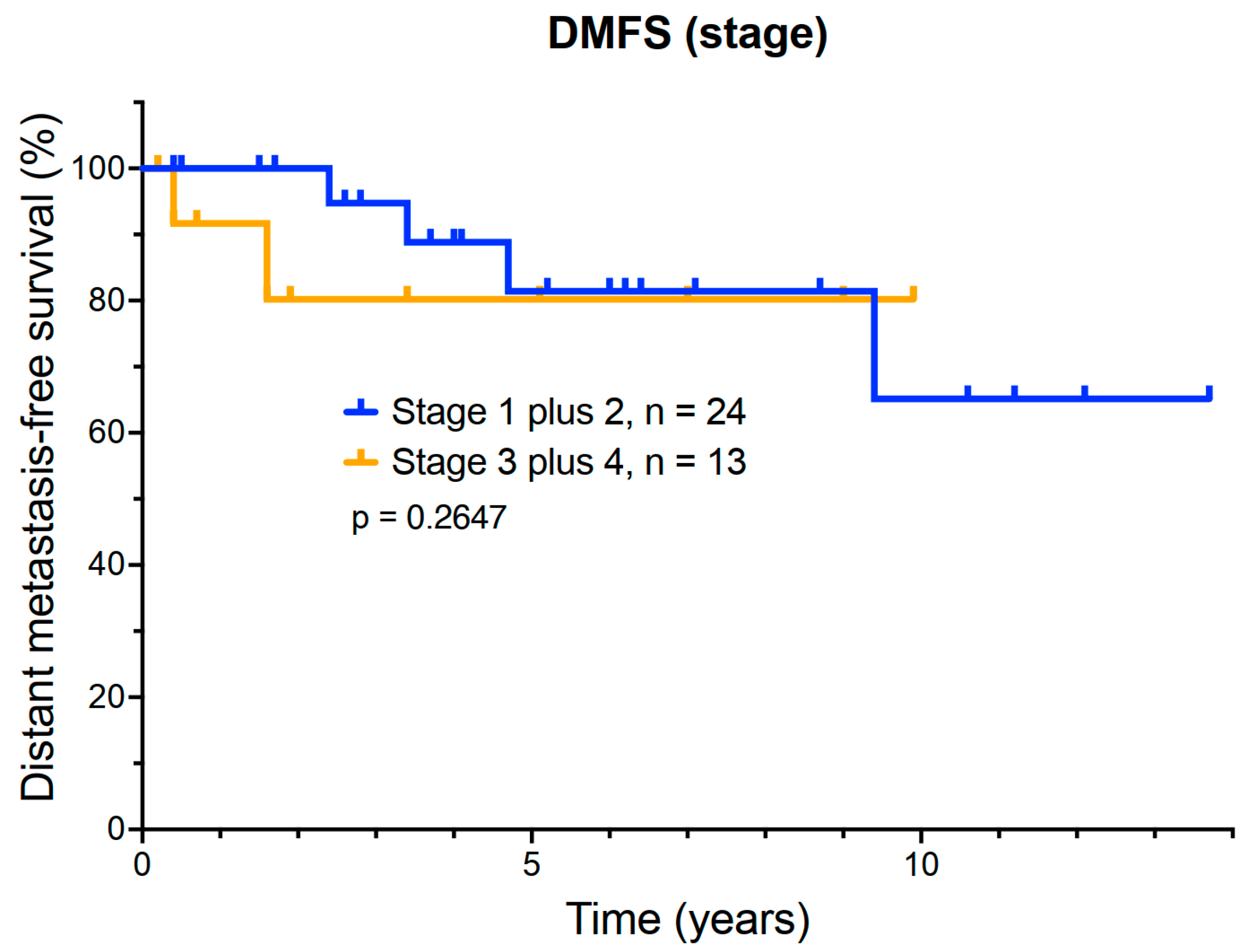
3.4. Exploratory Analysis of the Relationship Between DMFS and AJCC Stage
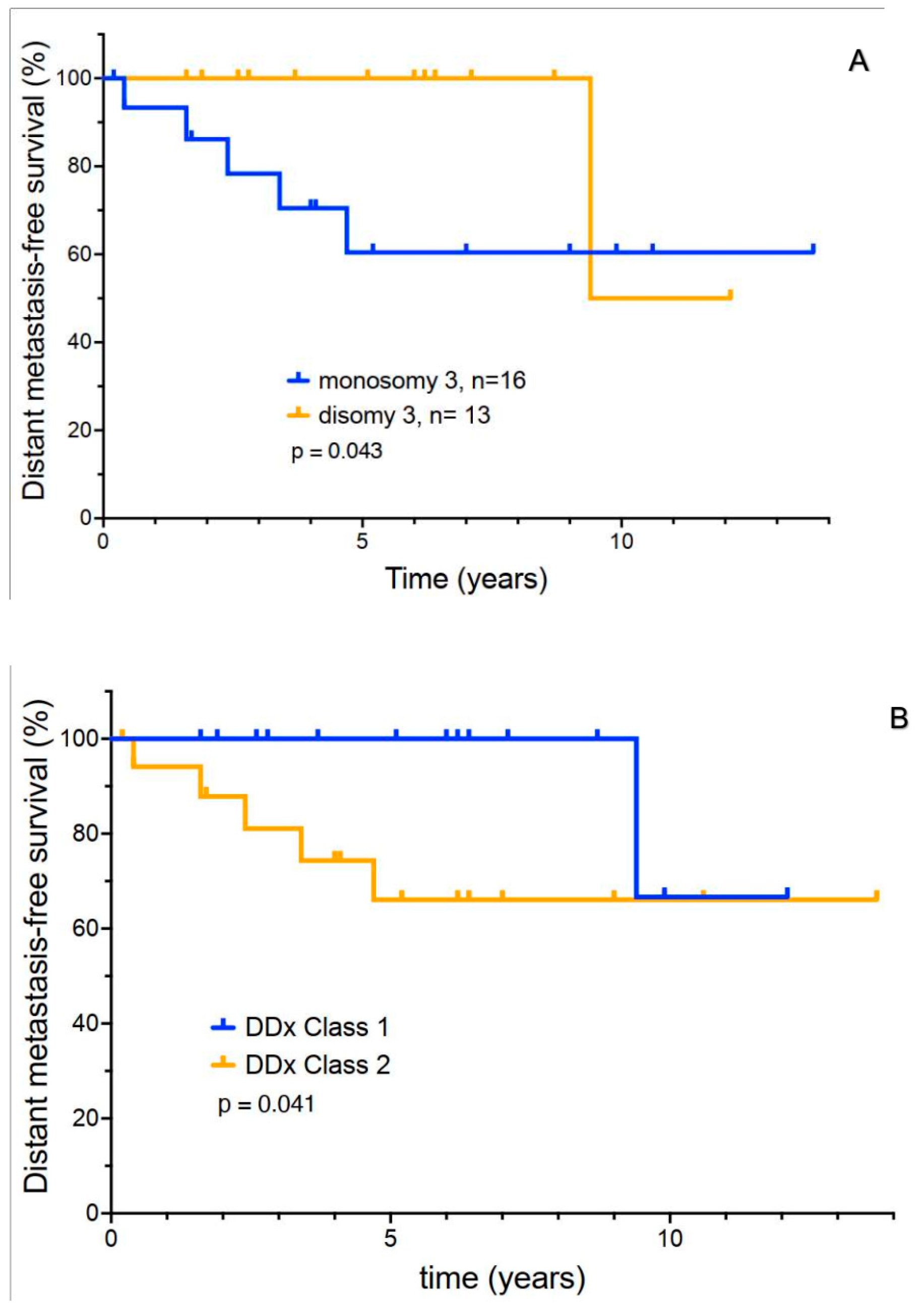
3.5. Exploratory Analysis of Molecular Prognostic Testing
3.6. Analysis of the Accuracy of Prognostic Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; Garcia-Honduvilla, N.; Coca, S.; Alvarez-Mon, M.; Bujan, J.; Teus, M.A. Update on uveal melanoma: Translational research from biology to clinical practice (Review). Int. J. Oncol. 2020, 57, 1262–1279. [Google Scholar] [CrossRef]
- Chalada, M.; Ramlogan-Steel, C.A.; Dhungel, B.P.; Layton, C.J.; Steel, J.C. The Impact of Ultraviolet Radiation on the Aetiology and Development of Uveal Melanoma. Cancers 2021, 13, 1700. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.M.; Piperno-Neumann, S.; Grossniklaus, H.E.; Kivela, T.T. Metastatic uveal melanoma: The final frontier. Prog. Retin. Eye Res. 2022, 90, 101041. [Google Scholar] [CrossRef]
- Rantala, E.S.; Hernberg, M.; Kivela, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef]
- Lamas, N.J.; Martel, A.; Nahon-Esteve, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Makitie, T.; Kivela, T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Damato, B.; Coupland, S.E.; Desjardins, L.; Bechrakis, N.E.; Grange, J.D.; Kivela, T. Staging of ciliary body and choroidal melanomas based on anatomic extent. J. Clin. Oncol. 2013, 31, 2825–2831. [Google Scholar] [CrossRef]
- Aoude, L.G.; Vajdic, C.M.; Kricker, A.; Armstrong, B.; Hayward, N.K. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment. Cell Melanoma Res. 2013, 26, 278–279. [Google Scholar] [CrossRef]
- Prescher, G.; Bornfeld, N.; Hirche, H.; Horsthemke, B.; Jockel, K.H.; Becher, R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996, 347, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Miguez, S.; Lee, R.Y.; Chan, A.X.; Demkowicz, P.C.; Jones, B.; Long, C.P.; Abramson, D.H.; Bosenberg, M.; Sznol, M.; Kluger, H.; et al. Validation of the Prognostic Usefulness of the Gene Expression Profiling Test in Patients with Uveal Melanoma. Ophthalmology 2023, 130, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Kivela, T.; Simpson, E.R.; Grossniklaus, H.E.; Jager, M.J.; Singh, A.D.; Caminal, J.M.; Pavlick, A.C.; Kujala, E.; Coupland, S.E.; Finger, P.T. Uveal Melanoma. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; Springer Publishing Company: New York, NY, USA, 2017; pp. 805–817. [Google Scholar]
- Oliver, S.C.; Leu, M.Y.; DeMarco, J.J.; Chow, P.E.; Lee, S.P.; McCannel, T.A. Attenuation of iodine 125 radiation with vitreous substitutes in the treatment of uveal melanoma. Arch. Ophthalmol. 2010, 128, 888–893. [Google Scholar] [CrossRef] [PubMed]
- McCannel, T.A.; McCannel, C.A. Iodine 125 brachytherapy with vitrectomy and silicone oil in the treatment of uveal melanoma: 1-to-1 matched case-control series. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 347–352. [Google Scholar] [CrossRef]
- McCannel, T.A.; Kamrava, M.; Demanes, J.; Lamb, J.; Bartlett, J.D.; Almanzor, R.; Chun, M.; McCannel, C.A. 23-mm iodine-125 plaque for uveal melanoma: Benefit of vitrectomy and silicone oil on visual acuity. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 2461–2467. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Simundic, A.M. Measures of Diagnostic Accuracy: Basic Definitions. Electron. J. Int. Fed. Clin. Chem. Lab. Med. 2009, 19, 203–211. [Google Scholar]
- Tong, T.M.L.; Bastiaannet, E.; Speetjens, F.M.; Blank, C.U.; Luyten, G.P.M.; Jager, M.J.; Marinkovic, M.; Vu, T.H.K.; Rasch, C.R.N.; Creutzberg, C.L.; et al. Time Trends in the Treatment and Survival of 5036 Uveal Melanoma Patients in The Netherlands over a 30-Year Period. Cancers 2023, 15, 5419. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Fulco, E.; Alarcon, C.; Shields, J.A. American Joint Committee on Cancer Classification of Uveal Melanoma (Anatomic Stage) Predicts Prognosis in 7,731 Patients: The 2013 Zimmerman Lecture. Ophthalmology 2015, 122, 1180–1186. [Google Scholar] [CrossRef]
- Djulbegovic, M.B.; Taylor, D.J.; Uversky, V.N.; Galor, A.; Shields, C.L.; Karp, C.L. Intrinsic Disorder in BAP1 and Its Association with Uveal Melanoma. Genes 2022, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Glasgow, B.J.; McCannel, T.A. Correlation of Immunocytochemistry of BRCA1-associated Protein-1 (BAP1) With Other Prognostic Markers in Uveal Melanoma. Am. J. Ophthalmol. 2018, 189, 122–126. [Google Scholar] [CrossRef]
- Onken, M.D.; Worley, L.A.; Char, D.H.; Augsburger, J.J.; Correa, Z.M.; Nudleman, E.; Aaberg, T.M., Jr.; Altaweel, M.M.; Bardenstein, D.S.; Finger, P.T.; et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012, 119, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Ehlers, J.P.; Harbour, J.W. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004, 64, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- Aaberg, T.M.; Covington, K.R.; Tsai, T.; Shildkrot, Y.; Plasseraud, K.M.; Alsina, K.M.; Oelschlager, K.M.; Monzon, F.A. Gene Expression Profiling in Uveal Melanoma: Five-Year Prospective Outcomes and Meta-Analysis. Ocul. Oncol. Pathol. 2020, 6, 360–367. [Google Scholar] [CrossRef]
- Plasseraud, K.M.; Cook, R.W.; Tsai, T.; Shildkrot, Y.; Middlebrook, B.; Maetzold, D.; Wilkinson, J.; Stone, J.; Johnson, C.; Oelschlager, K.; et al. Clinical Performance and Management Outcomes with the DecisionDx-UM Gene Expression Profile Test in a Prospective Multicenter Study. J. Oncol. 2016, 2016, 5325762. [Google Scholar] [CrossRef]
| T Stage (n = 37) | Monosomy 3 (n = 29) | GEP (n = 16) | |
|---|---|---|---|
| Sensitivity | 33.33% (CI 4.3–77.7%) | 83.33% (CI 35.9–99.6) | 100% (CI 15.8–100) |
| Specificity | 64.52% (CI 45.4–80.8) | 52.17% (CI 30.6–73.2) | 57.14% (CI 28.9–82.3) |
| Positive Predictive Value | 15.38% (CI 5.1–38.3) | 31.25% (CI 20.7–44.2) | 25.0% (CI 15.4–37.9) |
| Negative Predictive Value | 83.33% (CI 72.8–90.3) | 92.31% (CI 65.8–98.7) | 100% (CI 63.1–100) |
| Diagnostic Accuracy | 59.46% (CI 42.1–75.3) | 58.62% (CI 38.9–76.5) | 62.5% (CI 35.4–84.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, A.; Samlowski, W.; McCannel, T.A. Unexpectedly Low Rate of Metastasis and Death Among Patients Treated for Uveal Melanoma with Brachytherapy, Vitrectomy, and Silicone Oil. Cancers 2025, 17, 2683. https://doi.org/10.3390/cancers17162683
Rivas A, Samlowski W, McCannel TA. Unexpectedly Low Rate of Metastasis and Death Among Patients Treated for Uveal Melanoma with Brachytherapy, Vitrectomy, and Silicone Oil. Cancers. 2025; 17(16):2683. https://doi.org/10.3390/cancers17162683
Chicago/Turabian StyleRivas, Axel, Wolfram Samlowski, and Tara A. McCannel. 2025. "Unexpectedly Low Rate of Metastasis and Death Among Patients Treated for Uveal Melanoma with Brachytherapy, Vitrectomy, and Silicone Oil" Cancers 17, no. 16: 2683. https://doi.org/10.3390/cancers17162683
APA StyleRivas, A., Samlowski, W., & McCannel, T. A. (2025). Unexpectedly Low Rate of Metastasis and Death Among Patients Treated for Uveal Melanoma with Brachytherapy, Vitrectomy, and Silicone Oil. Cancers, 17(16), 2683. https://doi.org/10.3390/cancers17162683







