Antitumor Effects of Combination Therapy with Oncolytic Vaccinia Virus and Tepotinib on Lung Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Virus Preparation
2.4. Cell Viability Assay
2.5. Animal Models
2.6. Determination of ATP Content
2.7. Histology and Immunohistochemistry
2.8. Western Blotting
2.9. Real-Time Quantitative PCR
2.10. Statistical Analysis
3. Results
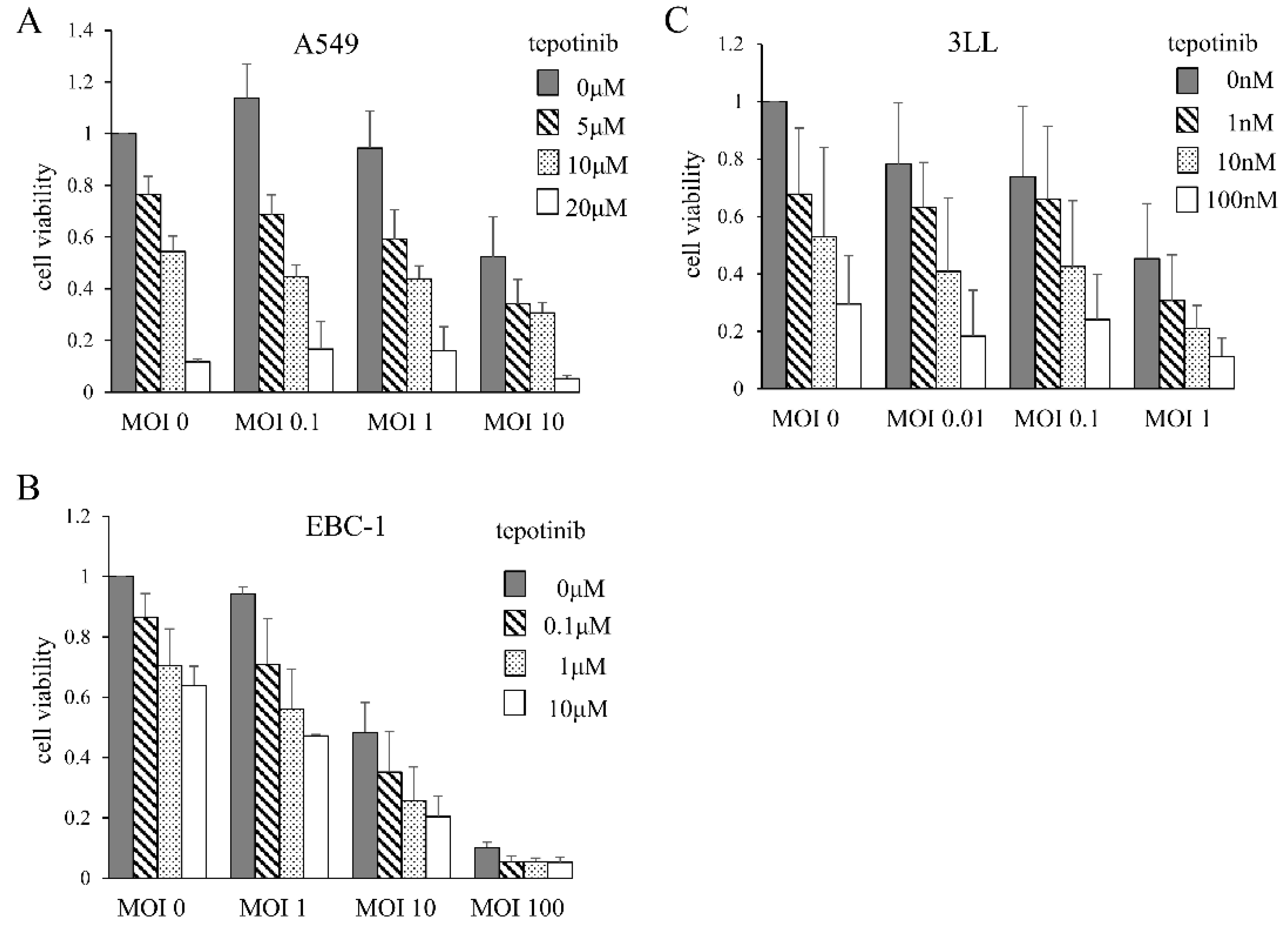
3.1. Cytotoxic Effects of MDRVV and Tepotinib on Lung Cancer Cell Lines
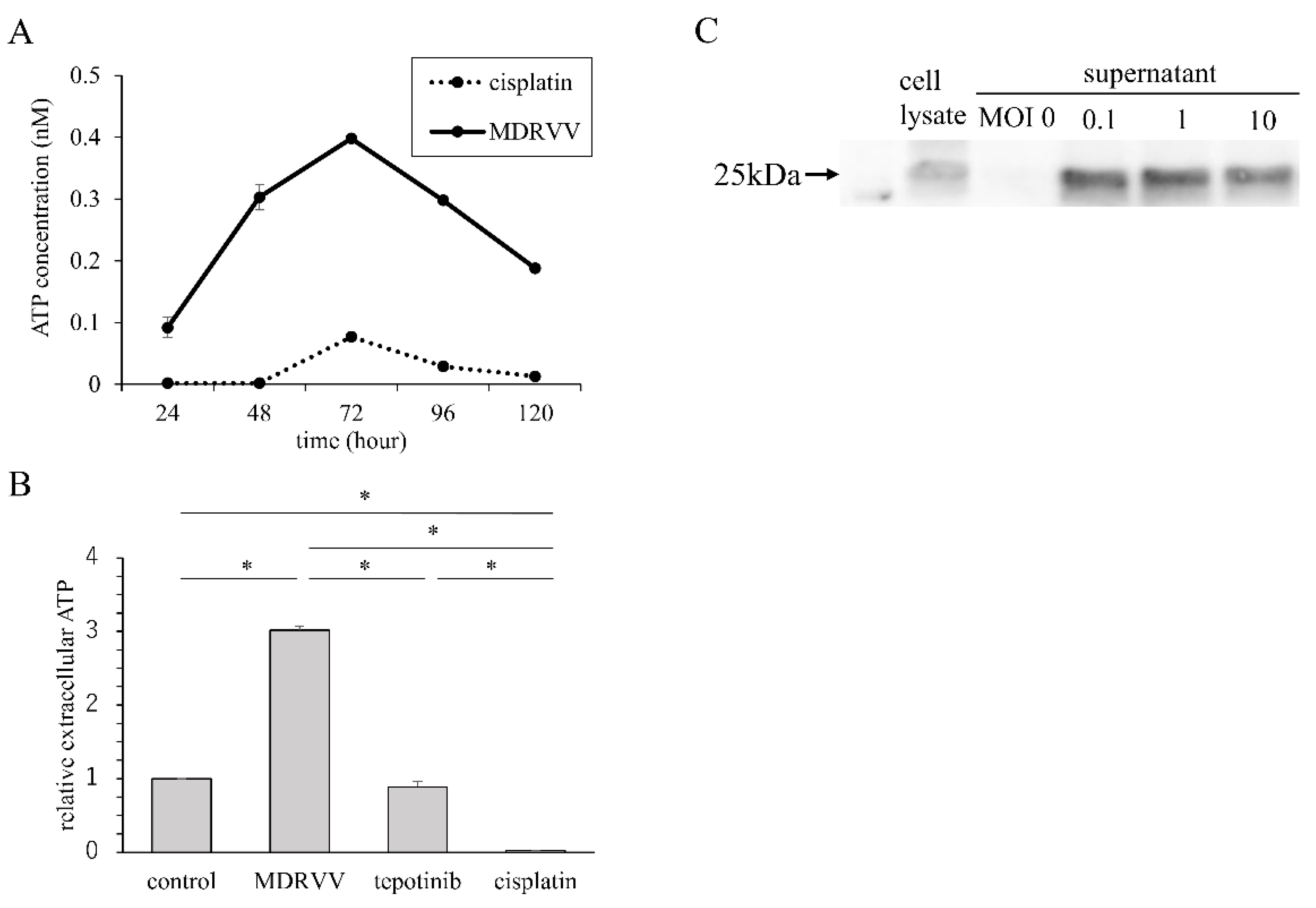
3.2. ATP Concentration in Culture Supernatant and HMGB-1 Expression
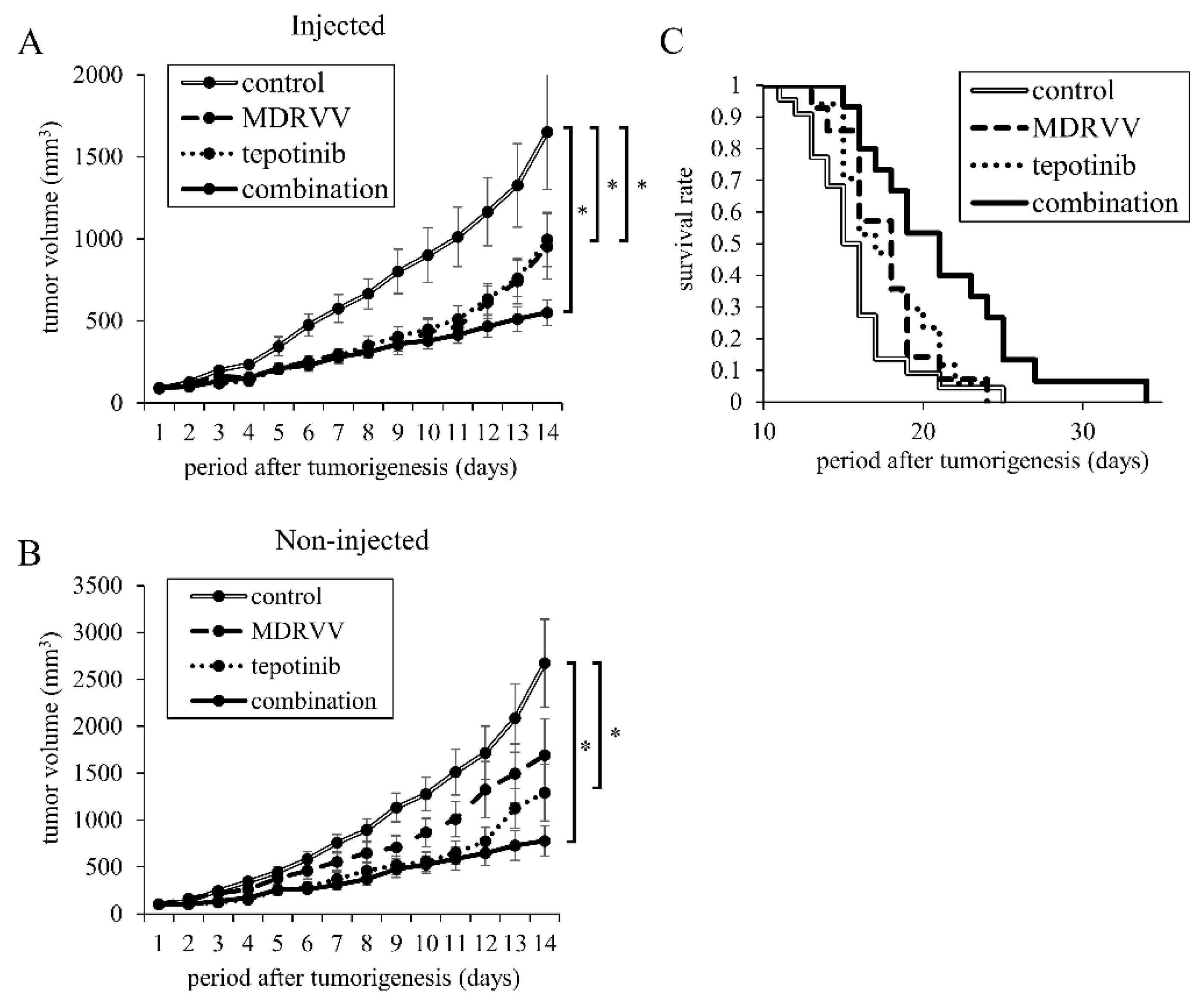
3.3. Combination Treatment Inhibits Tumor Progression in Mouse
3.4. Detection of Vaccinia Virus in MDRVV-Injected and Non-Injected Tumors
3.5. Pathological Analysis of Tumors After Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Dómine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with Oncolytic Viruses: Progress and Challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, X.; Sander, M.; Zhang, H.; Yan, G.; Lin, Y. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front. Immunol. 2021, 12, 721830. [Google Scholar] [CrossRef]
- Truong, C.S.; Yoo, S.Y. Oncolytic Vaccinia Virus in Lung Cancer Vaccines. Vaccines 2022, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized Dose-Finding Clinical Trial of Oncolytic Immunotherapeutic Vaccinia JX-594 in Liver Cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Deraffele, G.; Mitcham, J.; Moroziewicz, D.; Cohen, S.M.; Hurst-Wicker, K.S.; Cheung, K.; Lee, D.S.; Divito, J.; Voulo, M.; et al. Targeting the Local Tumor Microenvironment with Vaccinia Virus Expressing B7.1 for the Treatment of Melanoma. J. Clin. Investig. 2005, 115, 1903–1912. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Schuetz, T.J.; Blumenstein, B.A.; Glode, L.M.; Bilhartz, D.L.; Wyand, M.; Manson, K.; Panicali, D.L.; Laus, R.; Schlom, J.; et al. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2010, 28, 1099–1105. [Google Scholar] [CrossRef]
- Gulley, J.L.; Arlen, P.M.; Madan, R.A.; Tsang, K.Y.; Pazdur, M.P.; Skarupa, L.; Jones, J.L.; Poole, D.J.; Higgins, J.P.; Hodge, J.W.; et al. Immunologic and Prognostic Factors Associated with Overall Survival Employing a Poxviral-Based PSA Vaccine in Metastatic Castrate-Resistant Prostate Cancer. Cancer Immunol. Immunother. 2010, 59, 663–674. [Google Scholar] [CrossRef]
- Monge, C.; Xie, C.; Myojin, Y.; Coffman, K.; Hrones, D.M.; Wang, S.; Hernandez, J.M.; Wood, B.J.; Levy, E.B.; Juburi, I.; et al. Phase I/II Study of PexaVec in Combination with Immune Checkpoint Inhibition in Refractory Metastatic Colorectal Cancer. J. Immunother. Cancer 2023, 11, e005640. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.; Nieva, J.; Hwang, T.H.; Moon, A.; Patt, R.; et al. Intravenous Delivery of a Multi-Mechanistic Cancer-Targeted Oncolytic Poxvirus in Humans. Nature 2011, 477, 99–102. [Google Scholar] [CrossRef]
- Chintala, N.K.; Choe, J.K.; McGee, E.; Bellis, R.; Saini, J.K.; Banerjee, S.; Moreira, A.L.; Zauderer, M.G.; Adusumilli, P.S.; Rusch, V.W. Correlative Analysis from a Phase I Clinical Trial of Intrapleural Administration of Oncolytic Vaccinia Virus (Olvi-vec) in Patients with Malignant Pleural Mesothelioma. Front. Immunol. 2023, 14, 1112960. [Google Scholar] [CrossRef]
- Kurosaki, H.; Nakatake, M.; Sakamoto, T.; Kuwano, N.; Yamane, M.; Ishii, K.; Fujiwara, Y.; Nakamura, T. Anti-Tumor Effects of MAPK-Dependent Tumor-Selective Oncolytic Vaccinia Virus Armed with CD/UPRT against Pancreatic Ductal Adenocarcinoma in Mice. Cells 2021, 10, 985. [Google Scholar] [CrossRef]
- Yao, S.; Liu, X.; Feng, Y.; Li, Y.; Xiao, X.; Han, Y.; Xia, S. Unveiling the Role of HGF/c-Met Signaling in Non-Small Cell Lung Cancer Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 9101. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, N.; Yokohira, M.; Nakano-Narusawa, Y.; Watanabe, N.; Imaida, K.; Kadowaki, N.; Bandoh, S. Hepatocyte Growth Factor Produced in Lung Fibroblasts Enhances Non-Small Cell Lung Cancer Cell Survival and Tumor Progression. Respir. Res. 2017, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Mok, T.; Wu, Y.L.; Han, J.Y.; Ahn, M.J.; Ramalingam, S.S.; John, T.; Okamoto, I.; Yang, J.C.; Shepherd, F.A.; et al. Analysis of Acquired Resistance Mechanisms to Osimertinib in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer from the AURA3 Trial. Nat. Commun. 2023, 14, 1071. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14-Skipping Non-Small Cell Lung Cancer: Long-Term Follow-Up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260–1266. [Google Scholar] [CrossRef]
- Glodde, N.; Bald, T.; van den Boorn-Konijnenberg, D.; Nakamura, K.; O’Donnell, J.S.; Szczepanski, S.; Brandes, M.; Eickhoff, S.; Das, I.; Shridhar, N.; et al. Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy. Immunity 2017, 47, 789–802.e9. [Google Scholar] [CrossRef]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte Growth Factor Inhibits CNS Autoimmunity by Inducing Tolerogenic Dendritic Cells and CD25+Foxp3+ Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef]
- Machida, K.; Suemizu, H.; Kawai, K.; Ishikawa, T.; Sawada, R.; Ohnishi, Y.; Tsuchiya, T. Higher Susceptibility of NOG Mice to Xenotransplanted Tumors. J. Toxicol. Sci. 2009, 34, 123–127. [Google Scholar] [CrossRef]
- Albers, J.; Friese-Hamim, M.; Clark, A.; Schadt, O.; Walter-Bausch, G.; Stroh, C.; Johne, A.; Karachaliou, N.; Blaukat, A. The Preclinical Pharmacology of Tepotinib—A Highly Selective MET Inhibitor with Activity in Tumors Harboring MET Alterations. Mol. Cancer Ther. 2023, 22, 833–843. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef]
- Zhu, Z.; McGray, A.J.R.; Jiang, W.; Lu, B.; Kalinski, P.; Guo, Z.S. Improving Cancer Immunotherapy by Rationally Combining Oncolytic Virus with Modulators Targeting Key Signaling Pathways. Mol. Cancer 2022, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Clements, D.R.; Sterea, A.M.; Jang, H.W.; Gujar, S.A.; Lee, P.W.K. Dendritic Cells in Oncolytic Virus-Based Anti-Cancer Therapy. Viruses 2015, 7, 6506–6525. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The Cancer-Immunity Cycle: Indication, Genotype, and Immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Tang, G.; Wang, D.; Zhao, X.; Feng, Z.; Chen, Q.; Shen, Y. The Dilemma of HSV-1 Oncolytic Virus Delivery: The Method Choice and Hurdles. Int. J. Mol. Sci. 2023, 24, 3681. [Google Scholar] [CrossRef]
- Nakatake, M.; Kuwano, N.; Kaitsurumaru, E.; Kurosaki, H.; Nakamura, T. Fusogenic Oncolytic Vaccinia Virus Enhances Systemic Antitumor Immune Response by Modulating the Tumor Microenvironment. Mol. Ther. 2021, 29, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; et al. Intratumoral Expression of IL-7 and IL-12 Using an Oncolytic Virus Increases Systemic Sensitivity to Immune Checkpoint Blockade. Sci. Transl. Med. 2020, 12, eaax7992. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, L.; Waltermire, J.; Zhao, J.; Ren, J.; Guo, Z.; Bartlett, D.L.; Liu, Z. Intratumoral Delivery of Interleukin 9 via Oncolytic Vaccinia Virus Elicits Potent Antitumor Effects in Tumor Models. Cancers 2024, 16, 1021. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Kanaji, N.; Nakamura, T.; Yokohira, M.; Komori, Y.; Ohara, Y.; Mizoguchi, H.; Watanabe, N.; Kadowaki, N. Antitumor Effects of Combination Therapy with Oncolytic Vaccinia Virus and Tepotinib on Lung Cancer Cells. Cancers 2025, 17, 2681. https://doi.org/10.3390/cancers17162681
Inoue T, Kanaji N, Nakamura T, Yokohira M, Komori Y, Ohara Y, Mizoguchi H, Watanabe N, Kadowaki N. Antitumor Effects of Combination Therapy with Oncolytic Vaccinia Virus and Tepotinib on Lung Cancer Cells. Cancers. 2025; 17(16):2681. https://doi.org/10.3390/cancers17162681
Chicago/Turabian StyleInoue, Takuya, Nobuhiro Kanaji, Takafumi Nakamura, Masanao Yokohira, Yuta Komori, Yasuhiro Ohara, Hitoshi Mizoguchi, Naoki Watanabe, and Norimitsu Kadowaki. 2025. "Antitumor Effects of Combination Therapy with Oncolytic Vaccinia Virus and Tepotinib on Lung Cancer Cells" Cancers 17, no. 16: 2681. https://doi.org/10.3390/cancers17162681
APA StyleInoue, T., Kanaji, N., Nakamura, T., Yokohira, M., Komori, Y., Ohara, Y., Mizoguchi, H., Watanabe, N., & Kadowaki, N. (2025). Antitumor Effects of Combination Therapy with Oncolytic Vaccinia Virus and Tepotinib on Lung Cancer Cells. Cancers, 17(16), 2681. https://doi.org/10.3390/cancers17162681






