Exogenous Estrogens as Breast Cancer Risk Factors: A Perspective
Simple Summary
Abstract
1. Introduction
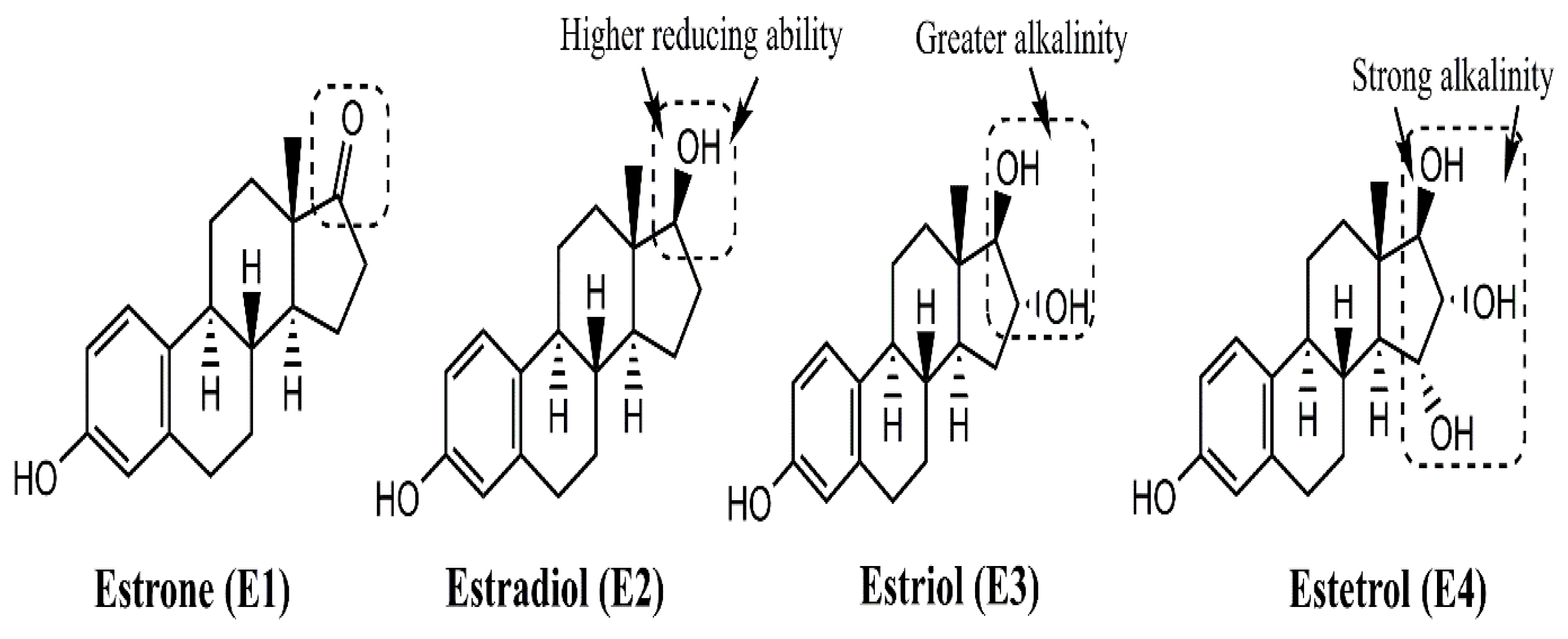
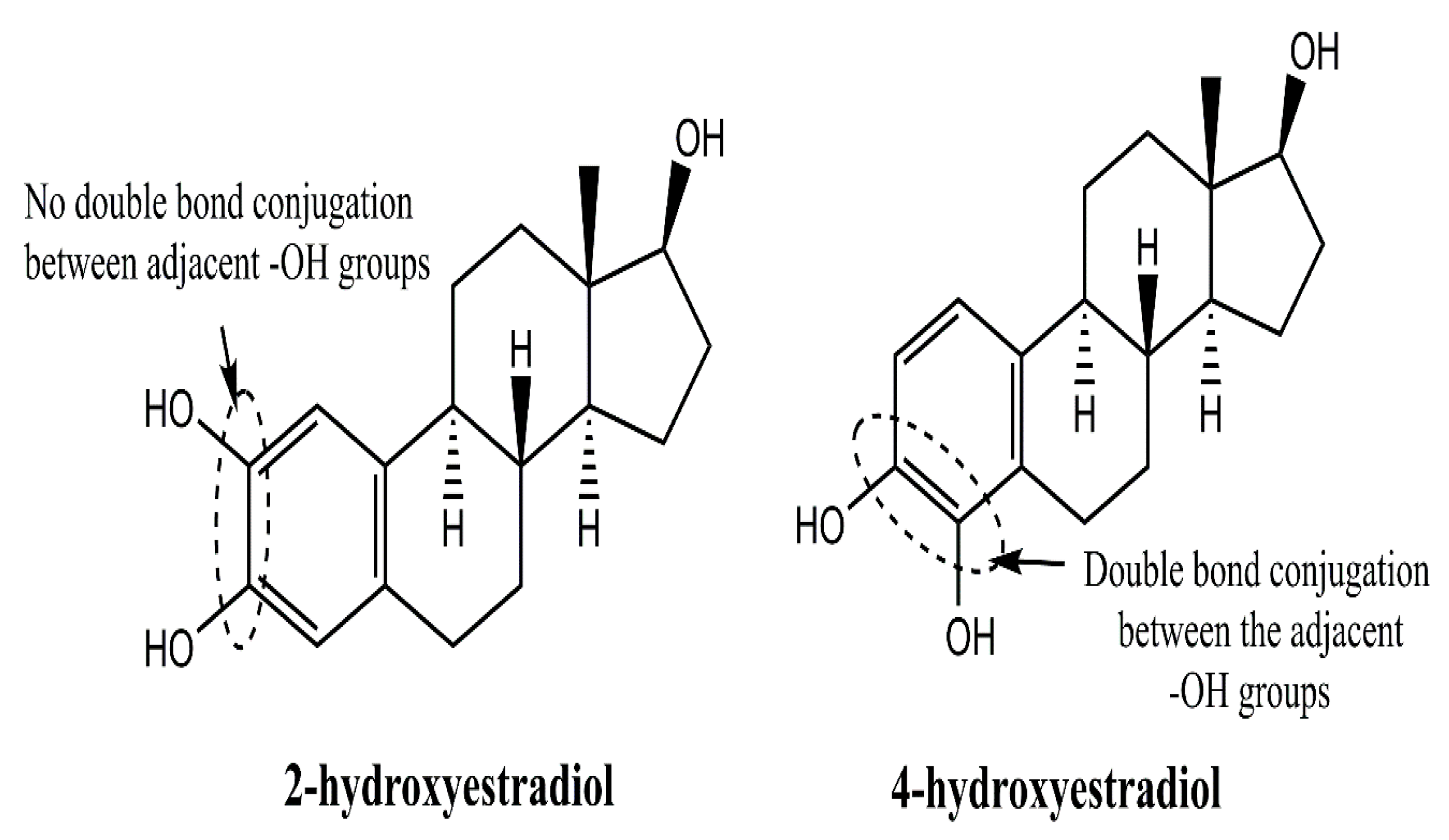
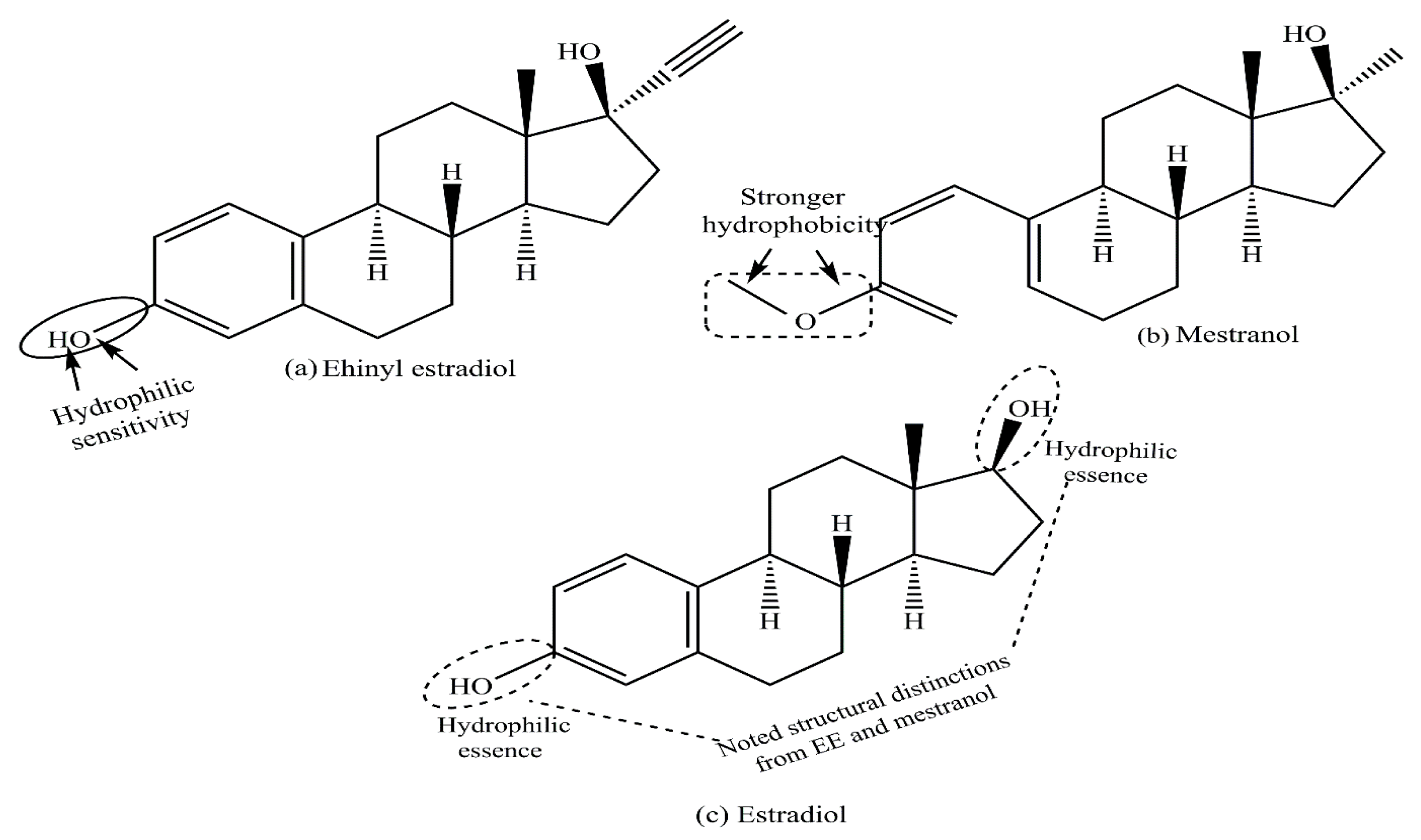
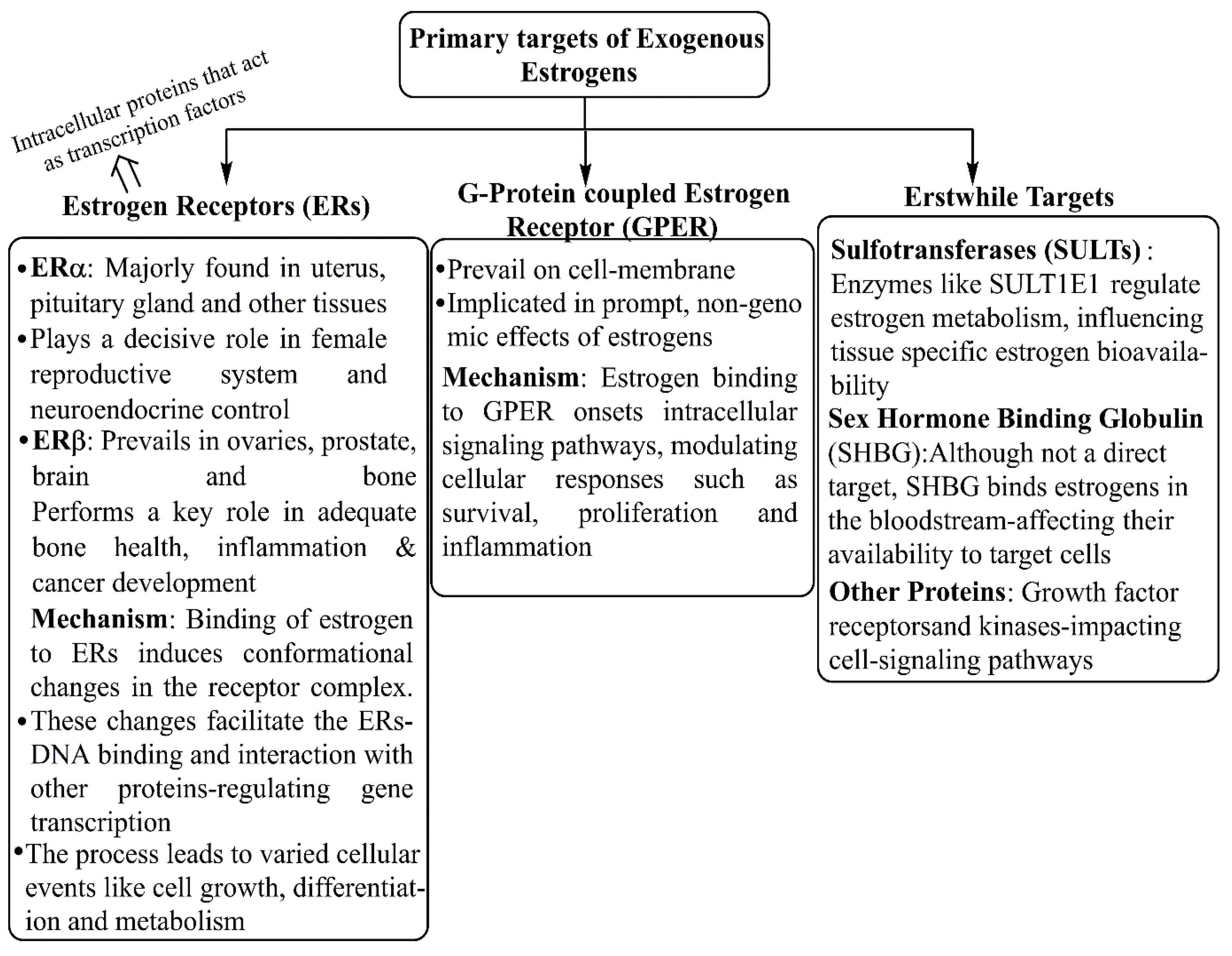
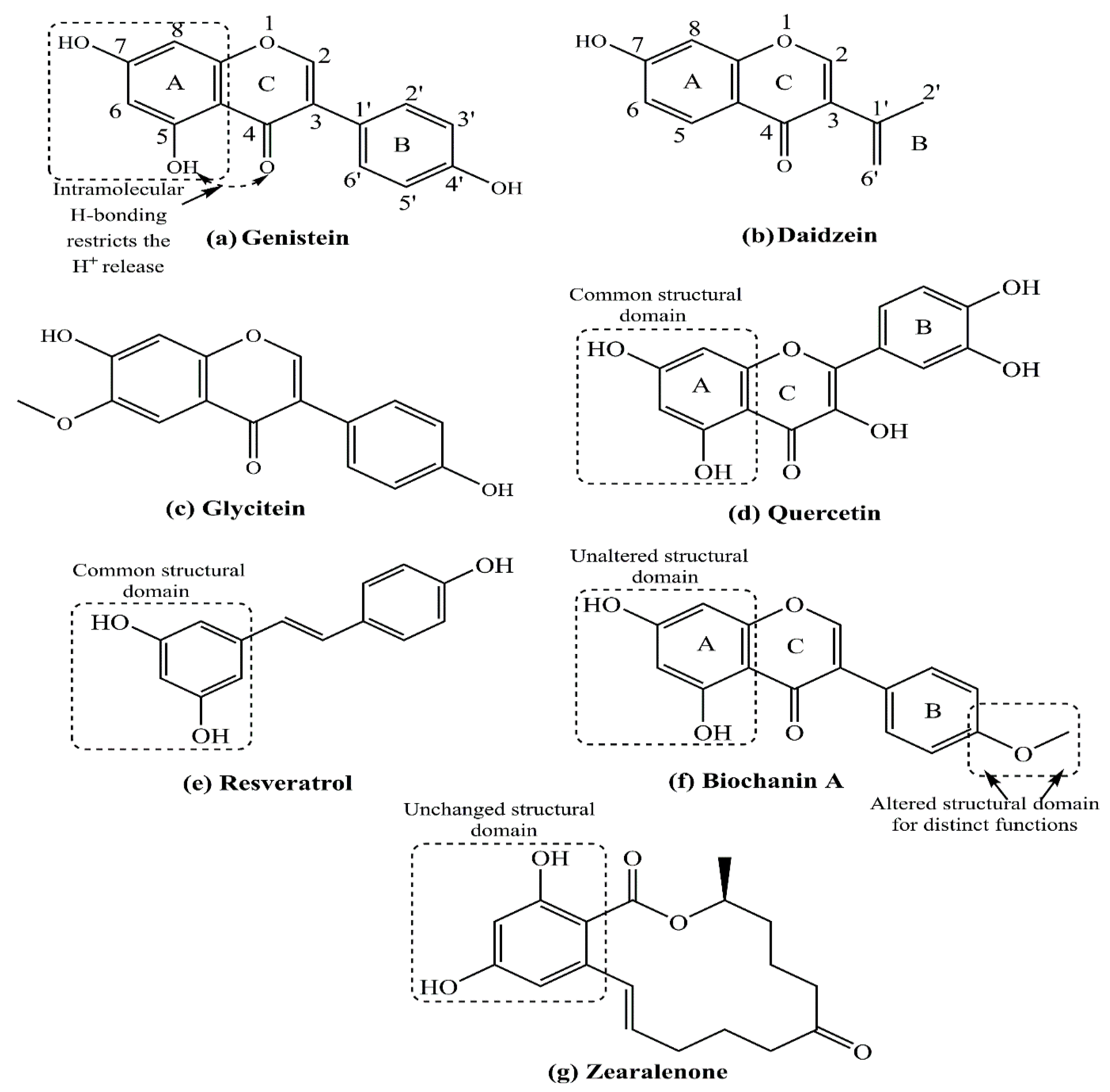
2. Structure–Function Relationship of Various Natural and Synthetic Estrogens
3. Pharmaceutical Estrogens as a Potential Risk Factor for Breast Cancer
3.1. Role of Hormone Replacement Therapy as a Potential Risk Factor for Breast Cancer
3.2. Oral Contraceptive Pills as a Potential Risk Factor for Breast Cancer
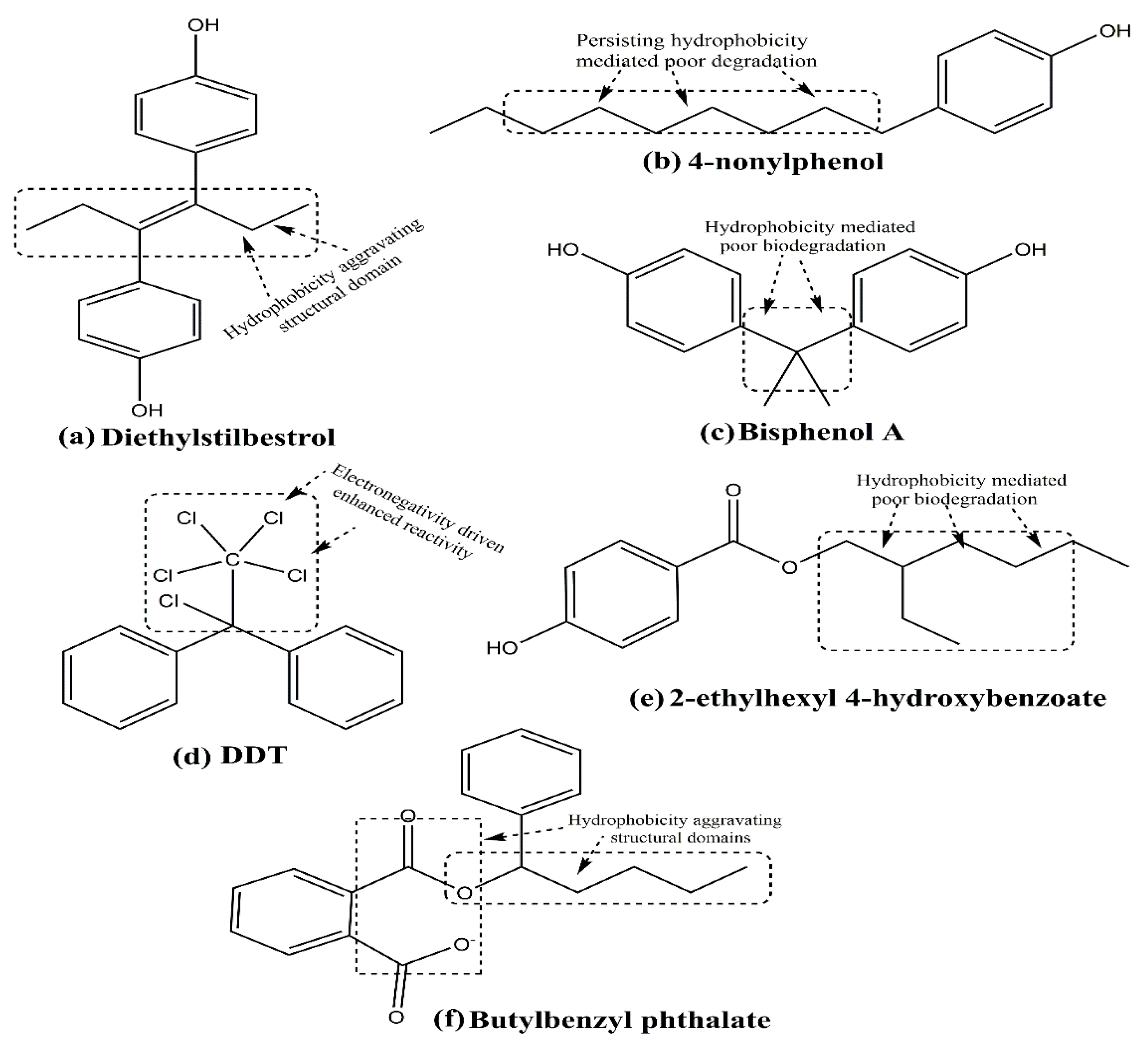
4. Environmental Xenoestrogens as a Potential Contributing Factor for Breast Cancer
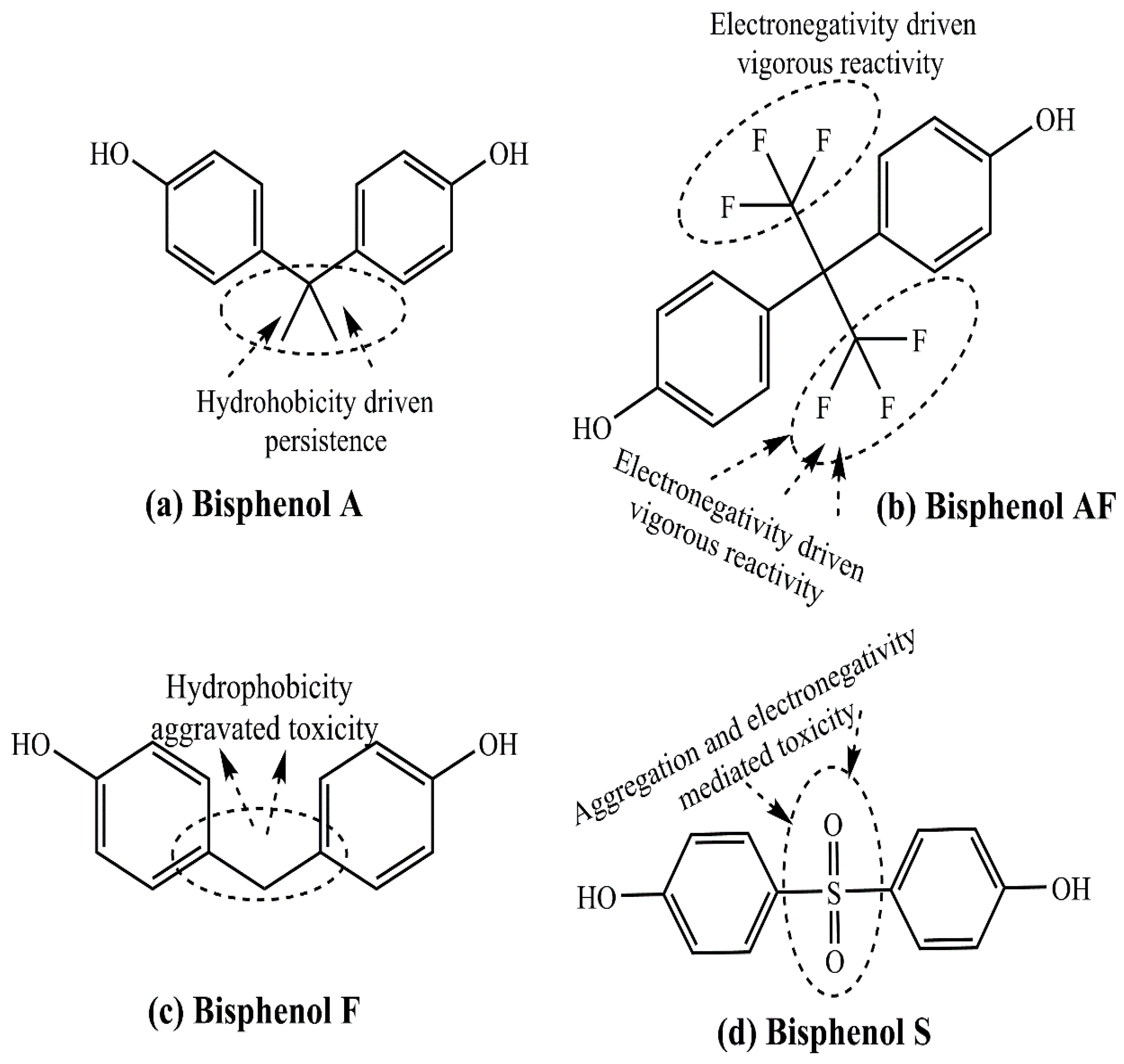
4.1. Bisphenol A
4.2. Dichloro-Diphenyl-Trichloroethane
4.3. Polychlorinated Biphenyls
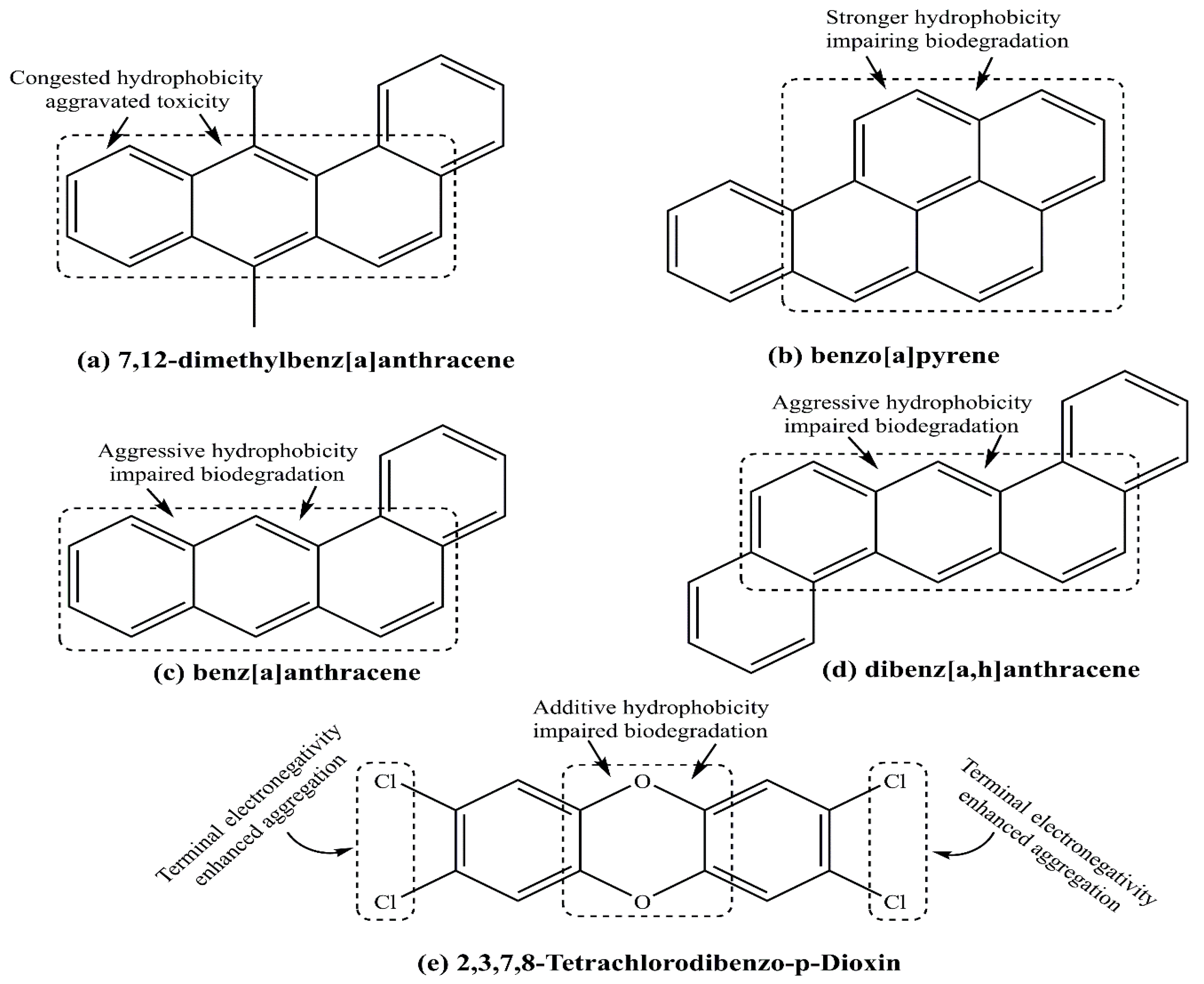
4.4. Polycyclic Aromatic Hydrocarbons
4.5. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin
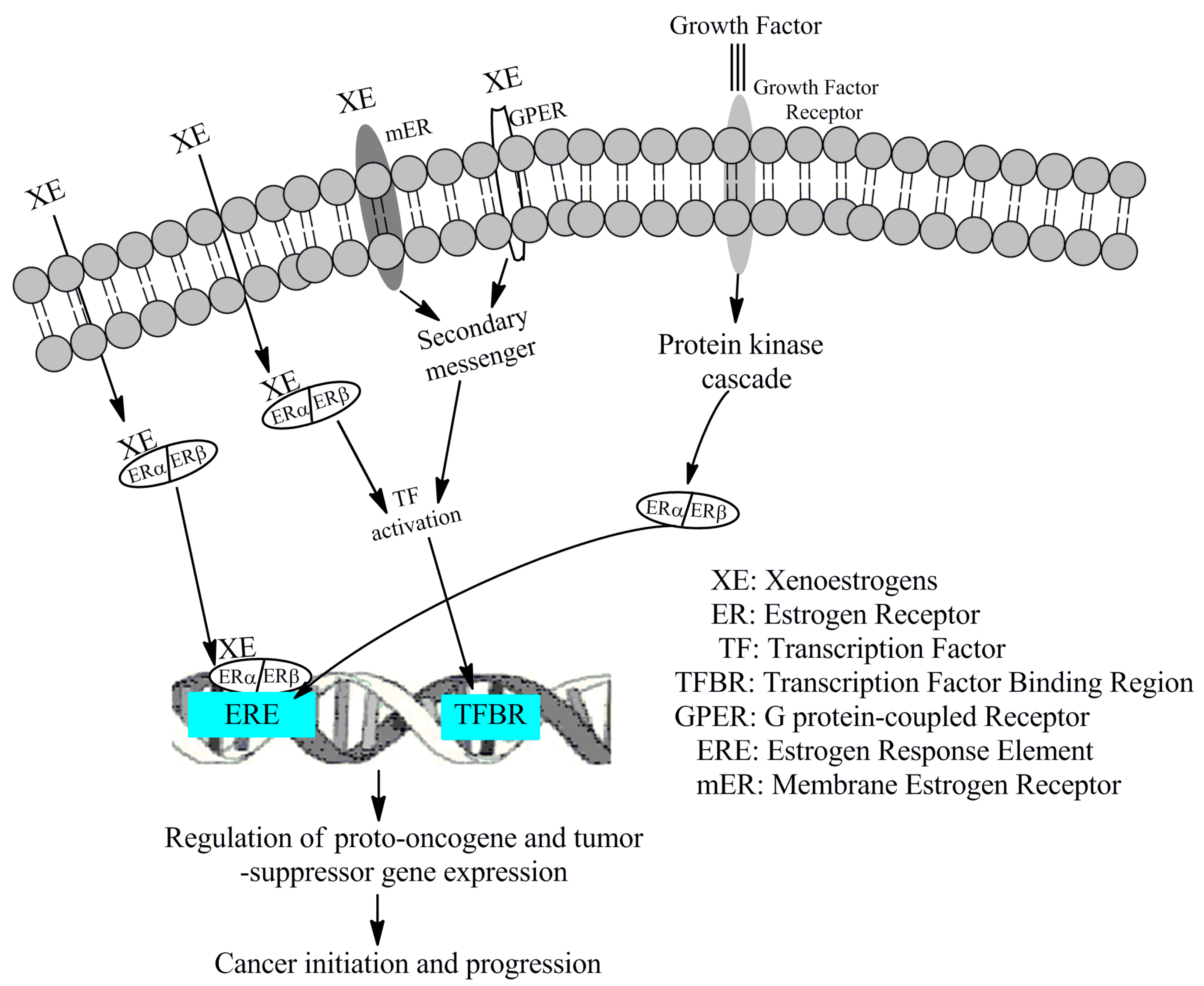
4.6. Mechanism of Action of Xenoestrogens in the Development of Cancers
5. Phytoestrogens as a Potential Preventive Factor for Breast Cancer
5.1. Role of Estrogen Receptors in Phytoestrogen-Dependent Breast Cancer Complications
5.2. Role of Menopausal Status in the Effects of Phytoestrogen-Dependent Breast Cancer Complications
5.3. Concentration-Dependent Response of Phytoestrogens on Breast Cancer Complications
5.4. Possible Protection Mechanisms of Phytoestrogen-Dependent Cancer Complications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Azcoitia, I.; Mendez, P.; Garcia-Segura, L.M. Aromatase in the human brain. Androg. Clin. Res. Ther. 2021, 2, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase-A brief overview. Ann. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef]
- Delgado, B.J.; Lopez-Ojeda, W. Estrogen; Stat Pearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Clemons, M.; Goss, P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001, 344, 276–285. [Google Scholar] [CrossRef]
- Joo, B.S.; Park, S.H.; An, B.M.; Kim, K.S.; Moon, S.E.; Moon, H.S. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil. Steril. 2010, 93, 442–446. [Google Scholar] [CrossRef] [PubMed]
- de Blok, C.J.M.; Wiepjes, C.M.; Nota, N.M.; van Engelen, K.; Adank, M.A.; Dreijerink, K.M.A.; Barbé, E.; Konings, I.R.H.M.; den Heijer, M. Breast cancer risk in transgender people receiving hormone treatment: Nationwide cohort study in the Netherlands. Biomed. J. 2019, 365, l1652. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Manson, J.E.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N. Engl. J. Med. 1991, 325, 756–762. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Yoshitake, R.; Chan, Y.S.; Sadava, D.; Chen, S. Exploring the biological activity and mechanism of xenoestrogens and phytoestrogens in cancers: Emerging methods and concepts. Int. J. Mol. Sci. 2021, 22, 8798. [Google Scholar] [CrossRef]
- Fucic, A.; Gamulin, M.; Ferencic, Z.; Katic, J.; von Kauss, M.K.; Bartonova, A.; Merlo, D.F. Environmental exposure to xenoestrogens and oestrogen related cancers: Reproductive system, breast, lung, kidney, pancreas, and brain. Environ. Health 2012, 11 (Suppl. 1), S8. [Google Scholar] [CrossRef]
- Maitra, R.; Malik, P.; Mukherjee, T.K. Targeting estrogens and various estrogen-related receptors against non-small cell lung cancers: A perspective. Cancers 2021, 14, 80. [Google Scholar] [CrossRef]
- Calaf, G.M.; Ponce-Cusi, R.; Aguayo, F.; Muñoz, J.P.; Bleak, T.C. Endocrine disruptors from the environment affecting breast cancer. Oncol. Lett. 2020, 20, 19–32. [Google Scholar] [CrossRef]
- Patra, S.; Gorai, S.; Pal, S.; Ghosh, K.; Pradhan, S.; Chakrabarti, S. A review on phytoestrogens: Current status and future direction. Phytother. Res. 2023, 37, 3097–3120. [Google Scholar] [CrossRef]
- Malik, P.; Singh, R.; Kumar, M.; Malik, A.; Mukherjee, T.K. Understanding the phytoestrogen genistein actions on breast cancer: Insights on estrogen receptor equivalence, pleiotropic essence and emerging paradigms in bioavailability modulation. Curr. Top. Med. Chem. 2023, 23, 1395–1413. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. Metabolism of endogenous and exogenous estrogens in women. J. Steroid Biochem. Mol. Biol. 2024, 242, 106539. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.V.; Russo, I.H.; Russo, J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol induce mutations in human breast epithelial cells. Int. J. Cancer 2006, 118, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y. New Insights into breast and endometrial cancers. Cancers 2020, 12, 2595. [Google Scholar] [CrossRef]
- Ulm, M.; Ramesh, A.V.; McNamara, K.M.; Ponnusamy, S.; Sasano, H.; Narayanan, R. Therapeutic advances in hormone-dependent cancers: Focus on prostate, breast and ovarian cancers. Endocr. Connect. 2019, 8, R10–R26. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Metaxas, G.; Harbach, C.A.J.; Savoy, L.A.; Darbre, P.D. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J. Appl. Toxicol. 2012, 32, 219–232. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals an Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Bjornstrom, L.; Sjoberg, M. Mechanism of estrogen receptor signaling: Convergence of genomic and nongenomic actions of target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Aggarwal, R. An overview of triple negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Goetz, M.P. Advances in systemic therapies for triple negative breast cancer. BMJ 2023, 381, e071674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lata, K.; Mukhopadhyay, S.; Mukherjee, T.K. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: A perspective. Biochim. Biophys. Acta BBA 2010, 1800, 1127–1135. [Google Scholar] [CrossRef]
- Cheng, Z.N.; Shu, Y.; Liu, Z.Q.; Wang, L.S.; Ou-Yang, D.S.; Zhou, H.H. Role of cytochrome p450 in estradiol metabolism in vitro. Acta Pharmacol. Sin. 2001, 22, 148–154. [Google Scholar]
- Helgason, S.; Damber, M.G.; von Schoultz, B.; Stigbrand, T. Estrogenic potency of oral replacement therapy estimated by the induction of pregnancy zone protein. Acta Obstet. Gynecol. Scand. 1982, 61, 75–79. [Google Scholar] [CrossRef]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef]
- Beatson, G. On the treatment of inoperable cases of carcinoma of the mamma suggestions for a new method of treatment with illustrative cases. Trans. Med. Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar] [PubMed]
- Jensen, E.V.; Jacobsonk, H.I. Basic guides to the mechanism of estrogen action. Recent. Prog. Horm. Res. 1962, 18, 387–414. [Google Scholar]
- Walter, P.; Green, S.; Greene, G.; Krust, A.; Bornert, J.M.; Jeltsch, J.M.; Staub, A.; Jensen, E.; Scrace, G.; Waterfield, M.; et al. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA 1985, 82, 7889–7893. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef] [PubMed]
- Mangani, S.; Piperigkou, Z.; Koletsis, N.E.; Ioannou, P.; Karamanos, N.K. Estrogen receptors and extracellular matrix: The critical interplay in cancer development and progression. FEBS J. 2025, 292, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Del Mastro, L.; Bruzzone, M.; Ceppi, M.; Razeti, M.G.; Fregatti, P.; Ruelle, T.; Pronzato, P.; Massarotti, C.; Franzoi, M.A.; et al. Safety of systemic hormone replacement therapy in breast cancer survivors: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2022, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Manson, J.E.; Anderson, G.L.; Cauley, J.A.; Aragaki, A.K.; Stefanick, M.L.; Lane, D.S.; Johnson, K.C.; Wactawski-Wende, J.; Chen, C.; et al. Estrogen plus progestin and breast cancer incidence and mortality in the women’s health initiative observational study. J. Natl. Cancer Inst. 2013, 105, 526–535. [Google Scholar] [CrossRef]
- Colditz, G.A.; Egan, K.M.; Stampfer, M.J. Hormone replacement therapy and risk of breast cancer: Results from epidemiologic studies. Am. J. Obs. Gynecol. 1993, 168, 1473–1480. [Google Scholar] [CrossRef]
- Gramling, R.; Eaton, C.B.; Rothman, K.J.; Cabral, H.; Silliman, R.A.; Lash, T.L. Hormone replacement therapy, family history, and breast cancer risk among postmenopausal women. Epidemiology 2009, 20, 752–756. [Google Scholar] [CrossRef]
- Ugras, S.K.; Layeequr Rahman, R. Hormone replacement therapy after breast cancer: Yes, No or maybe? Mol. Cell Endocrinol. 2021, 525, 111180. [Google Scholar] [CrossRef]
- Ito, K. Hormone replacement therapy and cancers: The biological roles of estrogen and progestin in tumorigenesis are different between the endometrium and breast. Tohoku J. Exp. Med. 2007, 212, 1–12. [Google Scholar] [CrossRef]
- Welnicka-Jaskiewicz, M.; Jassem, J. The risks and benefits of hormonal replacement therapy in healthy women and in breast cancer survivors. Cancer Treat. Rev. 2003, 29, 355–361. [Google Scholar] [CrossRef]
- Teede, H.J. Controversies in HRT. Aust. Fam. Physician 2002, 31, 413–418. [Google Scholar] [PubMed]
- Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. Oral-contraceptive use and the risk of breast cancer. N. Engl. J. Med. 1986, 315, 405–411. [Google Scholar] [CrossRef]
- Marchbanks, P.A.; McDonald, J.A.; Wilson, H.G.; Folger, S.G.; Mandel, M.G.; Daling, J.R.; Bernstein, L.; Malone, K.E.; Ursin, G.; Strom, B.L.; et al. Oral contraceptives and the risk of breast cancer. N. Engl. J. Med. 2002, 346, 2025–2032. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727. [Google Scholar] [CrossRef]
- Zhu, H.; Lei, X.; Feng, J.; Wang, Y. Oral contraceptive use and risk of breast cancer: A meta-analysis of prospective cohort studies. Eur. J. Contracept. Reprod. Health Care 2012, 17, 402–414. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Coeytaux, R.R.; Urrutia, R.P.; Havrilesky, L.J.; Moorman, P.G.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Hasselblad, V.; Sanders, G.D.; et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Kahlenborn, C.; Modugno, F.; Potter, D.M.; Severs, W.B. Oral contraceptive use as a risk factor for premenopausal breast cancer: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Kanadys, W.; Barańska, A.; Malm, M.; Błaszczuk, A.; Polz-Dacewicz, M.; Janiszewska, M.; Jędrych, M. Use f oral contraceptives as a potential risk factor for breast cancer: A systematic review and meta-analysis of case-control studies up to 2010. Int. J. Environ. Res. Public Health 2021, 18, 4638. [Google Scholar] [CrossRef]
- Lata, K.; Mukherjee, T.K. Knockdown of RAGE attenuate 17α-Ethinyl-estradiol dependent proliferation and survival of MCF-7 breast cancer cells. BBA-Gen. Subj. 2014, 1840, 1083–1091. [Google Scholar] [CrossRef]
- Chmielewski, J.; Nowak-Starz, G.; Łuszczki, J.; Gworek, B.; Czarny-Działak, M.; Dutkiewicz, E.; Król, H. Environmental exposition to xenoestrogens and related health effects. J. Elementol. 2021, 26, 717–730. [Google Scholar] [CrossRef]
- Blair, R.M.; Fang, H.; Branham, W.S.; Hass, B.S.; Dial, S.L.; Moland, C.L.; Tong, W.; Shi, L.; Perkins, R.; Sheehan, D.M. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: Structural diversity of ligands. Toxicol. Sci. 2000, 54, 138–153. [Google Scholar] [CrossRef]
- Pastor-Barriuso, R.; Fernández, M.F.; Castaño-Vinyals, G.; Whelan, D.; Pérez-Gómez, B.; Llorca, J.; Villanueva, C.M.; Guevara, M.; Molina-Molina, J.-M.; Artacho-Cordón, F.; et al. Total effective xenoestrogen burden in serum samples and risk for breast cancer in a population-based multicase-control study in Spain. Environ. Health Perspect. 2016, 124, 1575–1582. [Google Scholar] [CrossRef]
- Zeinomar, N.; Oskar, S.; Kehm, R.D.; Shamin Sahebzeda, S.; Terry, M.B. Environmental exposures and breast cancer risk in the context of underlying susceptibility: A systematic review of the epidemiological literature. Environ. Res. 2020, 187, 109346. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Ran, L.; Li, J.; Shi, Y.; Mu, C.; Hao, C. Endocrine-disrupting chemicals and breast cancer: A meta-analysis. Front. Oncol. 2023, 13, 1282651. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, J.; Wu, X.; Huang, B.; Pan, X. Nonmonotonic responses to low doses of xenoestrogens: A review. Environ. Res. 2017, 155, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Preindl, K.; Braun, D.; Aichinger, G.; Sieri, S.; Fang, M.; Marko, D.; Warth, B. A generic liquid chromatography-tandem mass spectrometry exposome method for determining xenoestrogens in biological matrices. Anal. Chem. 2019, 91, 11334–11342. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental endocrine-disrupting chemical exposure: Role in non-communicable diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- Gachowska, M.; Dąbrowska, A.; Wilczyński, B.; Kuźnicki, J.; Sauer, N.; Szlasa, W.; Kobierzycki, C.; Łapińska, Z.; Kulbacka, J. The influence of environmental exposure to xenoestrogens on the risk of cancer development. Int. J. Mol. Sci. 2024, 25, 12363. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Simonson, T.; Pisklak, D.M. Application of Various Molecular Modelling Methods in the Study of Estrogens and Xenoestrogens. Int. J. Mol. Sci. 2020, 21, 6411. [Google Scholar] [CrossRef]
- Amadasi, A.; Mozzarelli, A.; Meda, C.; Maggi, A.; Cozzini, P. Identification of xenoestrogens in food additives by an integrated in silico and in vitro approach. Chem. Res. Toxicol. 2009, 22, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Waddell, W.J. History of dose response. J. Toxicol. Sci. 2010, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, C.; Ormsby, J.N.; Gies, A.; Hass, U.; Heindel, J.J.; Holmer, M.L.; Nielsen, P.J.; Munn, S.; Schoenfelder, G. Low dose effects and non-monotonic dose responses for endocrine active chemicals: Science to practice workshop: Workshop summary. Chemosphere 2013, 93, 847–856. [Google Scholar] [CrossRef]
- Viñas, R.; Watson, C.S. Mixtures of xenoestrogens disrupt estradiol-induced nongenomic signaling and downstream functions in pituitary cells. Environ. Health 2013, 12, 26. [Google Scholar] [CrossRef]
- Moral, R.; Santucci-Pereira, J.; Wang, R.; Russo, I.H.; Lamartiniere, C.A.; Russo, J. In utero exposure to butyl benzyl phthalate induces modifications in the morphology and the gene expression profile of the mammary gland: An experimental study in rats. Environ. Health 2011, 10, 5. [Google Scholar] [CrossRef]
- Chen, M.; Ike, M.; Fujita, M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2002, 17, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Jhorar, R.; Mukherjee, T.K. Analysis of bisphenol A(BPA) migration inreusable polycarbonate containers and canned products available in the Indian market. Int. J. Adv. Res. 2016, 4, 1763–1767. [Google Scholar] [CrossRef]
- Brotons, J.A.; Olea-Serrano, M.F.; Villalobos, M.; Pedraza, V.; Olea, N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995, 103, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Pulgar, R.; Perez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef]
- Treviño, L.S.; Wang, Q.; Walker, C.L. Hypothesis: Activation of rapid signaling by environmental estrogens and epigenetic reprogramming in breast cancer. Reprod. Toxicol. 2015, 54, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Garg, V.K.; Tuli, H.S.; Kumar, G.; Kumar, M.; Mukherjee, T.K. Reactive oxygen species (ROS): An activator of apoptosis and autophagy in cancer. J. Biol. Chem. Sci. 2016, 3, 256–264. [Google Scholar]
- Kwon, Y. Potential pro-tumorigenic effect of Bisphenol A in breast cancer via altering the tumor microenvironment. Cancers 2022, 14, 3021. [Google Scholar] [CrossRef] [PubMed]
- Boszkiewicz, K.; Sawicka, E.; Piwowar, A. The impact of xenoestrogens on effectiveness of treatment for hormone-dependent breast cancer-current state of knowledge and perspectives for research. Ann. Agric. Environ. Med. 2020, 27, 526–534. [Google Scholar] [CrossRef]
- Deng, P.; Tan, M.; Zhou, W.; Chen, C.; Xi, Y.; Gao, P.; Ma, Q.; Liang, Y.; Chen, M.; Tian, L.; et al. Bisphenol A promotes breast cancer cell proliferation by driving miR-381-3p-PTTG1-dependent cell-cycle progression. Chemosphere 2021, 268, 129221. [Google Scholar] [CrossRef]
- Vafa, O.; Wade, M.; Kern, S.; Beeche, M.; Pandita, T.K.; Hampton, G.M.; Wahl, G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell 2002, 9, 1031–1044. [Google Scholar] [CrossRef]
- Pfeifer, D.; Chung, Y.M.; Hu, M.C. Effects of low-dose bisphenol A on DNA damage and proliferation of breast cells: The role of c-Myc. Environ. Health Perspect. 2015, 123, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P. How safe is bisphenol A? Fundamentals of toxicity: Metabolism, electron transfer and oxidative stress. Med. Hypotheses 2010, 75, 1–4. [Google Scholar] [CrossRef]
- Dumitrascu, M.C.; Mares, C.; Petca, R.C.; Sandru, F.; Popescu, R.I.; Mehedintu, C.; Petca, A. Carcinogenic effects of bisphenol A in breast and ovarian cancers. Oncol. Lett. 2020, 20, 282. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). DDT and associated compounds. IARC Monogr. Eval. Carcinog. Risk Hum. 1991, 53, 179–249. [Google Scholar]
- Eve, L.; Fervers, B.; Le Romancer, M.; Etienne-Selloum, N. Exposure to endocrine disrupting chemicals and risk of breast cancer. Int. J. Mol. Sci. 2020, 21, 9139. [Google Scholar] [CrossRef]
- Wolff, M.S.; Toniolo, P.G.; Lee, E.W.; Rivera, M.; Dubin, N. Blood levels of organochlorine residues and risk of breast cancer. J. Natl. Cancer Inst. 1993, 85, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L.; Bradlow, H.L.; Wolff, M.; Woodruff, T.; Hoel, D.G.; Anton-Culver, H. Medical hypothesis: Xenoestrogens as preventable causes of breast cancer. Environ. Health Perspect. 1993, 101, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Jaga, K. What are the implications of the interaction between DDT and estrogen receptors in the body? Med. Hypotheses 2000, 54, 18–25. [Google Scholar] [CrossRef]
- Scribner, J.D.; Mottet, N.K. DDT acceleration of mammary gland tumors induced in the male Sprague-Dawley rat by 2-acetamidophenanthrene. Carcinogenesis 1981, 2, 1235–1239. [Google Scholar] [CrossRef]
- Han, E.H.; Kim, H.G.; Hwang, Y.P.; Choi, J.H.; Im, J.H.; Park, B.; Yang, J.H.; Jeong, T.C.; Jeong, H.G. The role of cyclooxygenase-2-dependent signaling via cyclic AMP response element activation on aromatase up-regulation by o,p′-DDT in human breast cancer cells. Toxicol. Lett. 2010, 198, 331–341. [Google Scholar] [CrossRef]
- Thompson, L.A.; Ikenaka, Y.; Sobhy Darwish, W.; Nakayama, S.M.M.; Mizukawa, H.; Ishizuka, M. Effects of the organochlorine p,p′-DDT on MCF-7 cells: Investigating metabolic and immune modulatory transcriptomic changes. Environ. Toxicol. Pharmacol. 2019, 72, 103249. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, C.Y.; Lee, K.J.; Shin, D.W.; Jung, K.S.; Chung, Y.C.; Lee, S.S.; Shin, J.G.; Jeong, H.G. Induction of nitric oxide synthase and pro-inflammatory cytokines expression by o,p′-DDT in macrophages. Toxicol. Lett. 2004, 147, 261–269. [Google Scholar] [CrossRef]
- Harada, T.; Takeda, M.; Kojima, S.; Tomiyama, N. Toxicity and carcinogenicity of dichlorodiphenyltrichloroethane (DDT). Toxicol. Res. 2016, 32, 21–33. [Google Scholar] [CrossRef]
- Street, J.C.; Sharma, R.P. Alteration of induced cellular and humoral immune responses by pesticides and chemicals of environmental concern: Quantitative studies of immunosuppression by DDT, aroclor 1254, carbaryl, carbofuran, and methylparathion. Toxicol. Appl. Pharmacol. 1975, 32, 587–602. [Google Scholar] [CrossRef]
- Ardies, C.M.; Dees, C. Xenoestrogens significantly enhance risk for breast cancer during growth and adolescence. Med. Hypotheses 1998, 50, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Falck, F., Jr.; Ricci, A., Jr.; Wolff, M.S.; Godbold, J.; Deckers, P. Pesticides and polychlorinated biphenyl residues in human breast lipids and their relation to breast cancer. Arch. Environ. Health 1992, 47, 143–146. [Google Scholar] [PubMed]
- Wang, C.; Petriello, M.C.; Zhu, B.; Hennig, B. PCB 126 induces monocyte/macrophage polarization and inflammation through AhR and NF-κB pathways. Toxicol. Appl. Pharmacol. 2019, 367, 71–81. [Google Scholar] [CrossRef]
- Petriello, M.C.; Hoffman, J.B.; Vsevolozhskaya, O.; Morris, A.J.; Hennig, B. Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ. Pollut. 2018, 242 Pt A, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, W.; Meng, F.; Mi, J.; Peng, J.; Liu, J.; Zhang, X.; Hai, C.; Wang, X. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-κB signaling via downregulation of HNF1b. Redox. Biol. 2017, 12, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Kimbrough, R.D. Human health effects of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Ann. Rev. Pharmacol. Toxicol. 1987, 7, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Phillips, D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Bohácová, S.; Borská, L.; Fiala, Z.; Andrýs, C. Effect of polycyclic aromatic hydrocarbons on the immune system. Acta Med. Hradec Kral. Suppl. 1999, 42, 17–23. [Google Scholar] [PubMed]
- Yu, Y.-Y.; Jin, H.; Lu, Q. Effect of polycyclic aromatic hydrocarbons on immunity. J. Transl. Autoimmun. 2022, 5, 100177. [Google Scholar] [CrossRef]
- Smith, J.; Neupane, R.; McAmis, W.; Singh, U.; Chatterjee, S.; Raychoudhury, S. Toxicity of polycyclic aromatic hydrocarbons involves NOX2 activation. Toxicol. Rep. 2019, 6, 1176–1181. [Google Scholar] [CrossRef]
- De Jong, W.H.; Kroese, E.D.; Vos, J.G.; Van Loveren, H. Detection of immunotoxicity of benzo[a]pyrene in a sub-acute toxicity study after oral exposure in rats. Toxicol. Sci. 1999, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, C.A.; Gallo, M.E.; Hoffmann, E.J.; Fechner, J.H.; Schauer, J.J.; Bradfield, C.A.; Mezrich, J.D. Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive pro-inflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS ONE 2018, 13, e0209690. [Google Scholar] [CrossRef]
- Burchiel, S.W. Polycyclic Aromatic Hydrocarbons (PAHs) and the immune system. In Encyclopedic Reference of Immunotoxicology; Vohr, H.-W., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 515–518. [Google Scholar] [CrossRef]
- Salian, K.; Strezov, V.; Evans, T.J.; Taylor, M.; Nelson, P.F. Application of national pollutant inventories for monitoring trends on dioxin emissions from stationary industrial sources in Australia, Canada and European Union. PLoS ONE 2019, 14, e0224328. [Google Scholar] [CrossRef]
- Mathew, N.; Somanathan, A.; Tirpude, A.; Pillai, A.M.; Mondal, P.; Arfin, T. Dioxins and their impact: A review of toxicity, persistence, and novel remediation strategies. Anal. Meth. 2025, 17, 1698–1748. [Google Scholar] [CrossRef]
- Manz, A.; Berger, J.; Dwyer, J.H.; Flesch-Janys, D.; Nagel, S.; Waltsgott, H. Cancer mortality among workers in chemical plant contaminated with dioxin. Lancet 1991, 338, 959–964. [Google Scholar] [CrossRef]
- Warner, M.; Eskenazi, B.; Mocarelli, P.; Gerthoux, P.M.; Samuels, S.; Needham, L.; Patterson, D.; Brambilla, P. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s health study. Environ. Health Perspect. 2002, 110, 625–628. [Google Scholar] [CrossRef]
- Bekki, K.; Vogel, H.; Li, W.; Ito, T.; Sweeney, C.; Haarmann-Stemmann, T.; Matsumura, F.; Vogel, C.F. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic. Biochem. Physiol. 2015, 120, 5–13. [Google Scholar] [CrossRef]
- Ahn, N.S.; Hu, H.; Park, J.S.; Park, J.S.; Kim, J.S.; An, S.; Kong, G.; Aruoma, O.I.; Lee, Y.S.; Kang, K.S. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage-independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat. Res. 2005, 579, 189–199. [Google Scholar] [CrossRef]
- Brunnberg, S.; Andersson, P.; Poellinger, L.; Hanberg, A. The constitutively active Ah receptor (CA-AhR) mouse as a model for dioxin exposure-effects in reproductive organs. Chemosphere 2011, 85, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Danjou, A.M.; Fervers, B.; Boutron-Ruault, M.C.; Philip, T.; Clavel-Chapelon, F.; Dossus, L. Estimated dietary dioxin exposure and breast cancer risk among women from the French E3N prospective cohort. Breast Cancer Res. 2015, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Danjou, A.M.N.; Coudon, T.; Praud, D.; Lévêque, E.; Faure, E.; Salizzoni, P.; Le Romancer, M.; Severi, G.; Mancini, F.R.; Leffondré, K.; et al. Long-term airborne dioxin exposure and breast cancer risk in a case-control study nested within the French E3N prospective cohort. Environ. Int. 2019, 124, 236–248. [Google Scholar] [CrossRef]
- Bartkowiak-Wieczorek, J.; Jaros, A.; Gajdzińska, A.; Wojtyła-Buciora, P.; Szymański, I.; Szymaniak, J.; Janusz, W.; Walczak, I.; Jonaszka, G.; Bienert, A. The dual faces of oestrogen: The impact of exogenous oestrogen on the physiological and pathophysiological functions of tissues and organs. Int. J. Mol. Sci. 2024, 25, 8167. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.C.; Bennetau-Pelissero, C. Phytoestrogens and health effects. Nutrients 2023, 15, 317. [Google Scholar] [CrossRef] [PubMed]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef]
- Sabatier, C.V.; Bignon, Y.J.; Gallon, D.J.B. Effects of the phytoestrogens genistein and daidzein on BRCA2 tumor suppressor gene expression in breast cell lines. Nutr. Cancer 2003, 45, 247–255. [Google Scholar] [CrossRef]
- Duo, J.; Ying, G.-G.; Wang, G.-W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Apigenin induces apoptosis through a mitochondria/ caspase-pathway in human breast cancer MDA-MB-453 cells. J. Clin. Biochem. Nutr. 2009, 44, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Xie, K.P.; Huo, H.N.; Shang, F.; Zou, W.; Xie, M.J. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac. J. Cancer Prev. 2012, 13, 1431–1437. [Google Scholar] [CrossRef]
- Li, F.; Ye, L.; Lin, S.M.; Leung, L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011, 344, 51–58. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kiyozuka, Y.; Uemura, Y.; Senzaki, H.; Shikata, N.; Hioki, K.; Tsubura, A. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J. Cancer Res. Clin. Oncol. 2001, 127, 258–264. [Google Scholar] [CrossRef]
- Biedma, A.L.; Quesada, C.S.; Beltran, G.; Rodríguez, M.D.; Gaforio, J.J. Phytoestrogen (þ)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Wang, J.; Chung, M.H.; Xue, B.; Ma, H.; Ma, C.; Hattori, M. Estrogenic and anti-estrogenic activities of phloridzin. Biol. Pharm. Bull. 2010, 33, 592–597. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.-I.; Notas, G.; Nifli, A.-P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63. [Google Scholar] [CrossRef]
- Somasundaram, S.; Edmund, N.A.; Moore, D.T.; Small, G.W.; Shi, Y.Y.; Orlowski, R.Z. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002, 62, 3868–3875. [Google Scholar] [PubMed]
- Indap, M.; Radhika, S.; Motiwale, L.; Rao, K. Anticancer activity of phenolic antioxidants against breast cancer cells and a spontaneous mammary tumor. Ind. J. Pharm. Sci. 2006, 68, 470–474. [Google Scholar] [CrossRef]
- Lirdprapamongkol, K.; Kramb, J.-P.; Suthiphongchai, T.; Surarit, R.; Srisomsap, C.; Dannhardt, G.; Svasti, J. Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo. J. Agric. Food Chem. 2009, 57, 3055–3063. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Liu, H.; He, S.; Wang, T.; Orang-Ojong, B.; Lu, Q.; Zhang, Z.; Pan, L.; Chai, X.; Wu, H.; Fan, G.; et al. Selected phytoestrogens distinguish roles of ERα Transactivation and ligand binding for anti-inflammatory activity free. Endocrinology 2018, 159, 3351–3364. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-function elucidation of antioxidative andprooxidative activities of polyphenolic compound curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Malik, P.; Maktedar, S.S.; Avashthi, G.; Mukherjee, T.K.; Singh, M. Robust curcumin-mustard oil emulsions for pro to antioxidant modulation of graphene oxide. Ar. J. Chem. 2020, 13, 4606–4628. [Google Scholar] [CrossRef]
- Low, Y.L.; Taylor, J.I.; Grace, P.B.; Dowsett, M.; Scollen, S.; Dunning, A.M.; Mulligan, A.A.; Welch, A.A.; Luben, R.N.; Khaw, K.T.; et al. Phytoestrogen exposure correlation with plasma estradiol in postmenopausal women in European Prospective investigation of cancer and nutrition-norfolk may involve diet-gene interactions. Cancer Epidemiol. Biomark. Prev. 2005, 14, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.B.; Taylor, J.I.; Low, Y.L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.T.; Wareham, N.J.; et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Biomark. Prev. 2004, 13, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Fotsis, T.; Bannwart, C.; Wahala, K.; Makela, T.; Brunow, G.; Hase, T. Determination of urinary lignans and phytoestrogen metabolites potential antiestrogens and anticarcinogens in urine of women on various habitual diets. J. Steroid Biochem. 1986, 25, 791–797. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Verheus, M.; van Gils, C.H.; Keinan-Boker, L.; Grace, P.B.; Bingham, S.A.; Peeters, P.H.M. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007, 25, 648–655. [Google Scholar] [CrossRef]
- Lecomte, S.; Demay, F.; Ferrière, F.; Pakdel, F. Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int. J. Mol. Sci. 2017, 18, 1381. [Google Scholar] [CrossRef]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Rogero, M.M.; Fisberg, M.; Waitzberg, D. Health impact of childhood and adolescent soy consumption. Nutr. Rev. 2017, 75, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Thanos, J.; Cotterchio, M.; Boucher, B.A.; Kreiger, N.; Thompson, L.U. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control 2006, 17, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef]
- Ko, K.-P.; Kim, S.-W.; Ma, S.H.; Park, B.; Ahn, Y.; Lee, J.W.; Lee, M.H.; Kang, E.; Kim, L.S.; Jung, Y.; et al. Dietary intake and breast cancer among carriers and noncarriers of BRCA mutations in the Korean hereditary breast cancer study. Am. J. Clin. Nutr. 2013, 98, 1493–1501. [Google Scholar] [CrossRef]
- Mense, S.M.; Hei, T.K.; Ganju, R.K.; Bhat, H.K. Phytoestrogens and breast cancer prevention: Possible mechanisms of action. Environ. Health Perspect. 2008, 116, 426–433. [Google Scholar] [CrossRef]
- Guo, D.; Wang, J.; Wang, X.; Luo, H.; Zhang, H.; Cao, D.; Chen, L.; Huang, N. Double directional adjusting estrogenic effect of naringenin from Rhizoma drynariae (Gusuibu). J. Ethnopharmacol. 2011, 138, 451–457. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engstrom, O.; Ohman, L.; Greene, G.L.; Gustafsson, J.A. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Hugo, M. Phytoestrogens are weakly estrogenic and could compete with stronger human endogenous estrogens. J. Integr. Oncol. 2021, 10, 6. [Google Scholar]
- Zhu, B.T.; Han, G.Z.; Shim, J.Y.; Wen, Y.; Jiang, X.R. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology 2006, 147, 4132–4150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.; et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Nathan, L.; Dinh, H.; Reddy, S.T.; Chaudhuri, G. 17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression. J. Biol. Chem. 2003, 278, 11746–11752. [Google Scholar] [CrossRef]
- Zhang, F.F.; Haslam, D.E.; Terry, M.B.; Knight, J.A.; Andrulis, I.L.; Daly, M.B.; Buys, S.S.; John, E.M. Dietary isoflavone intake and all-cause mortality in breast cancer survivors: The Breast Cancer Family Registry. Cancer 2017, 123, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Fultz, J.; Allred, K.F.; Doerge, D.R.; Helferich, W.G. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis 2006, 27, 856–863. [Google Scholar] [CrossRef]
- Matsumura, A.; Ghosh, A.; Pope, G.S.; Darbre, P.D. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 431–443. [Google Scholar] [CrossRef]
- Shao, Z.M.; Wu, J.; Shen, Z.Z.; Barsky, S.H. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998, 58, 4851–4857. [Google Scholar] [PubMed]
- Foster, J.S.; Henley, D.C.; Ahamed, S.; Wimalasena, J. Oestrogens and cell-cycle regulation in breast cancer. Trends Endocrinol. Metabol. 2001, 12, 320–327. [Google Scholar] [CrossRef]
- Schmidt, S.; Michna, H.; Diel, P. Combinatory effects of phytoestrogens and 17β-oestradiol on proliferation and apoptosis in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 445–449. [Google Scholar] [CrossRef]
- Maggiolini, M.; Bonfiglio, D.; Marsico, S.; Panno, M.L.; Cenni, B.; Picard, D.; Ando, S. Oestrogen receptor ralpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol. Pharmacol. 2001, 60, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Allred, C.D.; Allred, K.F.; Ju, Y.H.; Virant, S.M.; Helferich, W.G. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001, 61, 5045–5050. [Google Scholar] [PubMed]
- Hsieh, C.Y.; Santell, R.C.; Haslam, S.Z.; Helferich, W.G. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998, 58, 3833–3838. [Google Scholar] [PubMed]
- Chen, J.; Ge, B.; Wang, Y.; Ye, Y.; Zeng, S.; Huang, Z. Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells. Cell. Physiol. Biochem. 2015, 35, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Prietsch, R.F.; Monte, L.G.; daSilva, F.A.; Beira, F.T.; DelPino, F.A.; Campos, V.F.; Collares, T.; Pinto, L.S.; Spanevello, R.M.; Gamaro, G.D.; et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol. Cell. Biochem. 2014, 390, 235–242. [Google Scholar] [CrossRef]
- Cotterchio, M.; Boucher, B.A.; Kreiger, N.; Mills, C.A.; Thompson, L.U. Dietary phytoestrogen intake-lignans and isoflavones-and breast cancer risk (Canada). Cancer Causes Control 2008, 19, 259–272. [Google Scholar] [CrossRef]
- Linseisen, J.; Piller, R.; Hermann, S.; Chang-Claude, J. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int. J. Cancer 2004, 110, 284–290. [Google Scholar] [CrossRef]
- Guha, N.; Kwan, M.L.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The life after cancer epidemiology study. Breast Cancer Res. Treat 2009, 118, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Q.; Wang, S.; Huang, X.; Jin, S. Effect of soy isoflavones on breast cancer recurrence and death for patients receiving adjuvant endocrine therapy. Can. Med. Assoc. J. 2010, 182, 1857–1862. [Google Scholar] [CrossRef]
- Woo, H.D.; Park, K.-S.; Ro, J.; Kim, J. Differential influence of dietary soy intake on the risk of breast cancer recurrence related to HER2 status. Nutr. Cancer 2012, 64, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Kang, H.-B.; Li, B.-L.; Zhang, R.-M. Positive effects of soy isoflavone food on survival of breast cancer patients in China. Asian Pac. J. Cancer Prev. 2012, 13, 479–482. [Google Scholar] [CrossRef]
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P.; et al. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Serrero, G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J. Cell. Physiol. 1999, 179, 297–304. [Google Scholar] [CrossRef]
- Balabhadrapathruni, S.; Thomas, T.J.; Yurkow, E.J.; Amenta, P.S.; Thomas, T. Effects of genistein and structurally related phytoestrogens on cell cycle kinetics and apoptosis in MDA-MB-468 human breast cancer cells. Oncol. Rep. 2000, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, H.; Veld, M.G.T.; Jacobs, N.; van der Saag, P.T.; Murk, A.J.; Rietjens, I.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef]
- Seo, H.S.; DeNardo, D.G.; Jacquot, Y.; Laïos, I.; Vidal, D.S.; Zambrana, C.R.; Leclercq, G.; Brown, P.H. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res. Treat. 2006, 99, 121–134. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose-response Meta-Analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Han, D.-H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2022, 66, 1479–1487. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Ruparelia, K.; Arroo, R.R.; Tsatsakis, A.M.; Spandidos, D.A. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology 2009, 264, 162–170. [Google Scholar] [CrossRef]
- Wang, K.L.; Hsia, S.M.; Chan, C.J.; Chang, F.Y.; Huang, C.Y.; Bau, D.T.; Wang, P.S. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Exp. Opin. Ther. Targets 2013, 17, 337–349. [Google Scholar] [CrossRef]
- Torrens-Mas, M.; Roca, P. Phytoestrogens for cancer prevention and treatment. Biology 2020, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, A.; Tang, N.; Zengin, G.; Li, M.-Y.; Liu, Y. Patient-derived xenograft models in pan-cancer: From bench to clinic. Interdiscip. Med. 2025, 3, e20250016. [Google Scholar] [CrossRef]
- Ma, P.; Wang, G.; Men, K.; Li, C.; Gao, N.; Li, L. Advances in clinical application of nanoparticle-based therapy for cancer treatment: A systematic review. Nano Trans. Med. 2024, 3, 100036. [Google Scholar] [CrossRef]
- Huang, M.; Gong, G.; Deng, Y.; Long, X.; Long, W.; Liu, Q.; Zhao, W.; Chen, R. Crosstalk between cancer cells and the nervous system. Med. Adv. 2023, 1, 173–189. [Google Scholar] [CrossRef]
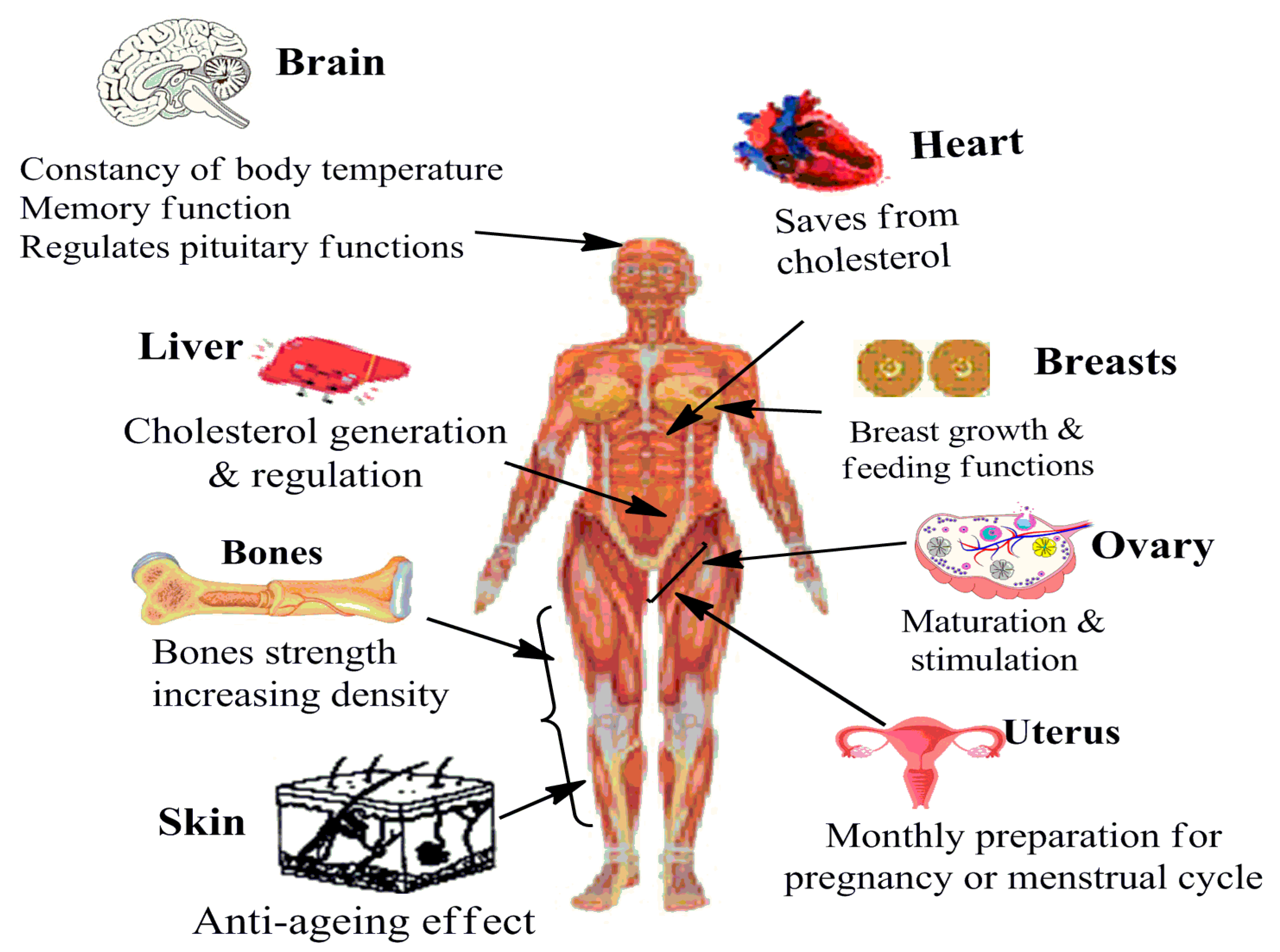



| Functional Specification | In Females | In Males |
|---|---|---|
| Reproductive |
|
|
| Non-reproductive |
|
|
| Sr. No. | Potential Compounds | Distinctive Class/ Family | Major Sources |
|---|---|---|---|
| 1 | Catechin, epicatechin, epigallocatechin 3-gallate | Flavanols | Green and black tea, cocoa |
| 2 | Genistein and daidzein | Isoflavones | Soy-based foods |
| 3 | Quercetin, kaemferol | Flavonols | Quercetin: Red wine, grapefruit, onions, apples and black tea Kaemferol: Broccoli, apples, strawberries, beans, tea and tomato |
| 4 | Resveratrol Pterostilbene | Stilbenoids | Resveratrol: Food and beverages derived from grapes, mulberries and peanuts Pterostilbenes: Blueberries |
| 5 | Hesperetin and naringenin | Flavanones | Citrus fruits |
| 6 | Curcumin | Curcuminoids | Rhizomes of the plant Curcuma longa Linn |
| 7 | Caffeic acid, ferulic acid | Hydroxycinnamic acids | Fruits, vegetables, tea, cocoa and wine |
| 8 | Pinoresinol | Lignans | Fiber-rich food, such as sesame seeds |
| 9 | Luteolin and apigenin | Flavones | Cereals and herbs |
| 10 | Esculetin, psoralen, esculin | Coumarins | Sweet woodruff, meadowsweet, sweet grass and Melilotus |
| 11 | Phenylacetic acids | Phenolic acids | Acidic tasting fruits |
| 12 | Phloridzin | Chalcones | Citrus fruits and apples |
| 13 | Vanillin | Benzoic aldehydes | Vanilla |
| Sr. No. | Screened Compound | IC50 Extent, Cell Line Studied Upon, Water Solubility | Prominent Findings | Limitations |
|---|---|---|---|---|
| 1 | Genistein, a phytoestrogen | 50 nM, MCF-7 <1 μg·mL−1 water solubility |
|
|
| 2 | Daidzein, a phytoestrogen | 10 nM, MCF-7 0.00831 mg·mL−1 water solubility at 25 °C |
|
|
| 3 | Biochanin A, a natural phytoestrogen | 10 μM, MCF-7 Not very soluble in water |
|
|
| 4 | Quercetin, a phytoestrogen | 5 μM, MCF-7 Water solubility of 0.00215 g·L−1 at 25 °C |
|
|
| 5 | 17β-estradiol (E2), a natural estrogen | 10−2 to 1 nM, MCF-7 3.1–12.96 mg·L−1 water solubility |
|
|
| 6 | Mestranol, a pharmaceutical estrogen | 40 nM, MCF-7 |
|
|
| 7 | Coumestrol, a phytoestrogen | 40 nM, MCF-7 Almost water insoluble |
|
|
| 8 | Diethylstilbestrol, a synthetic non-steroidal estrogen | 0.4 nM, MCF-7 BC cells Poor water solubility of 0.012 g·L−1 at 25 °C |
|
|
| 9 | Naringenin, a phytoestrogen | 0.3 μM, MCF-7 0.5 g·L−1 water solubility at 20 °C |
|
|
| 10 | Kaempferol, a phytoestrogens (173) | 50 μM, MCF-7 Low water solubility |
|
|
| 11 | Chrysin, a phytoestrogen | 50 nM, MCF-7 Almost water insoluble |
|
|
| 12 | Apigenin, a phytoestrogen | 1 μM, MCF-7 1.35 μg·mL−1 water solubility |
|
|
| 13 | Luteolin, a phytoestrogens (173) | 5 μM, MCF-7 Water solubility of 0.0064 mg·mL−1 |
|
|
| 14 | Genkwanin, a phytoestrogen | 1.6 μM for MDA-MB-468 BC and 75 μM MCF-10A (normal) cells <1 μg·mL−1 water solubility |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, P.; Mukherjee, T.K. Exogenous Estrogens as Breast Cancer Risk Factors: A Perspective. Cancers 2025, 17, 2680. https://doi.org/10.3390/cancers17162680
Malik P, Mukherjee TK. Exogenous Estrogens as Breast Cancer Risk Factors: A Perspective. Cancers. 2025; 17(16):2680. https://doi.org/10.3390/cancers17162680
Chicago/Turabian StyleMalik, Parth, and Tapan Kumar Mukherjee. 2025. "Exogenous Estrogens as Breast Cancer Risk Factors: A Perspective" Cancers 17, no. 16: 2680. https://doi.org/10.3390/cancers17162680
APA StyleMalik, P., & Mukherjee, T. K. (2025). Exogenous Estrogens as Breast Cancer Risk Factors: A Perspective. Cancers, 17(16), 2680. https://doi.org/10.3390/cancers17162680







