Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults
Simple Summary
Abstract
1. Introduction
2. Search Strategy
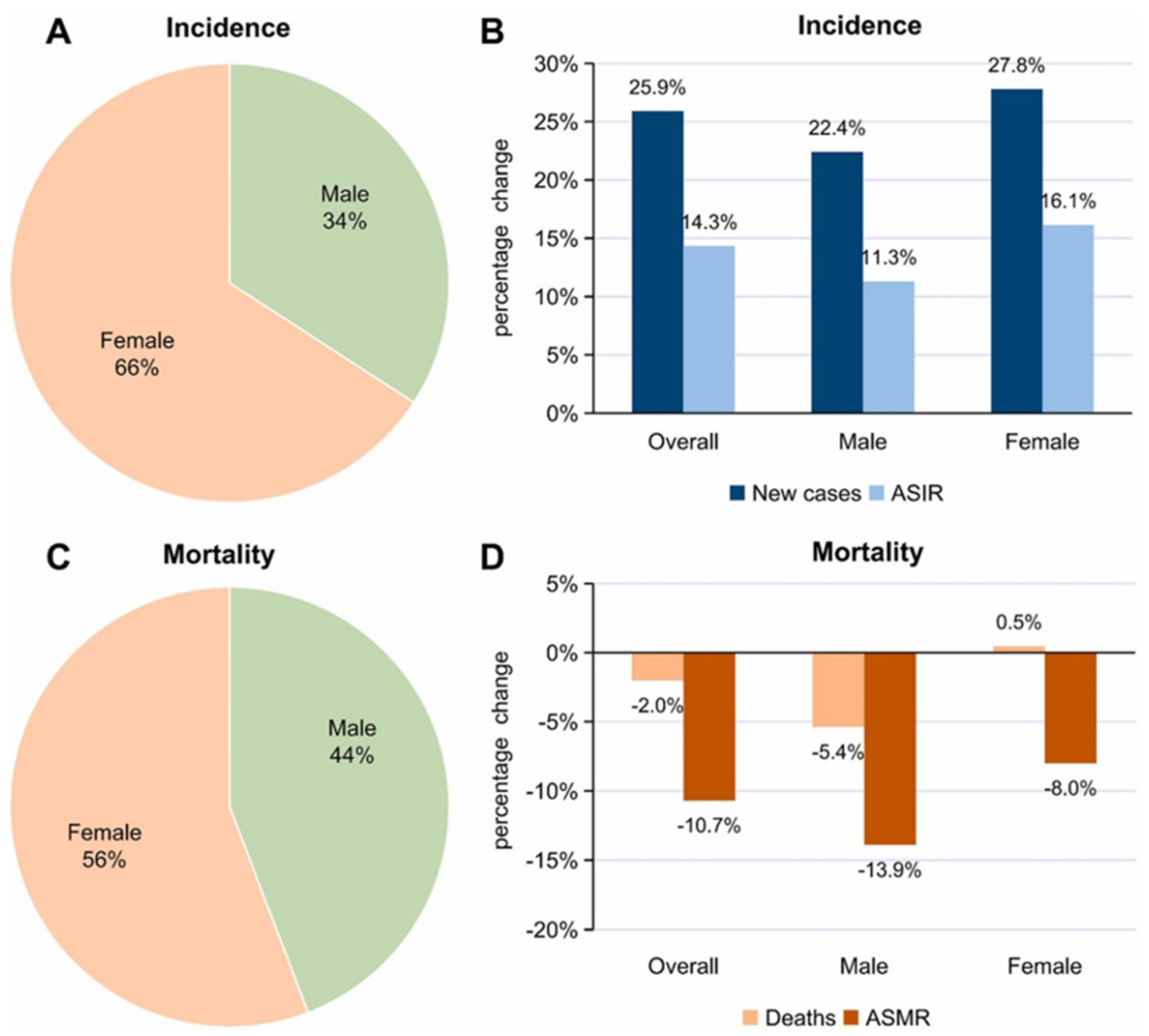
3. Cancer in the Young Population
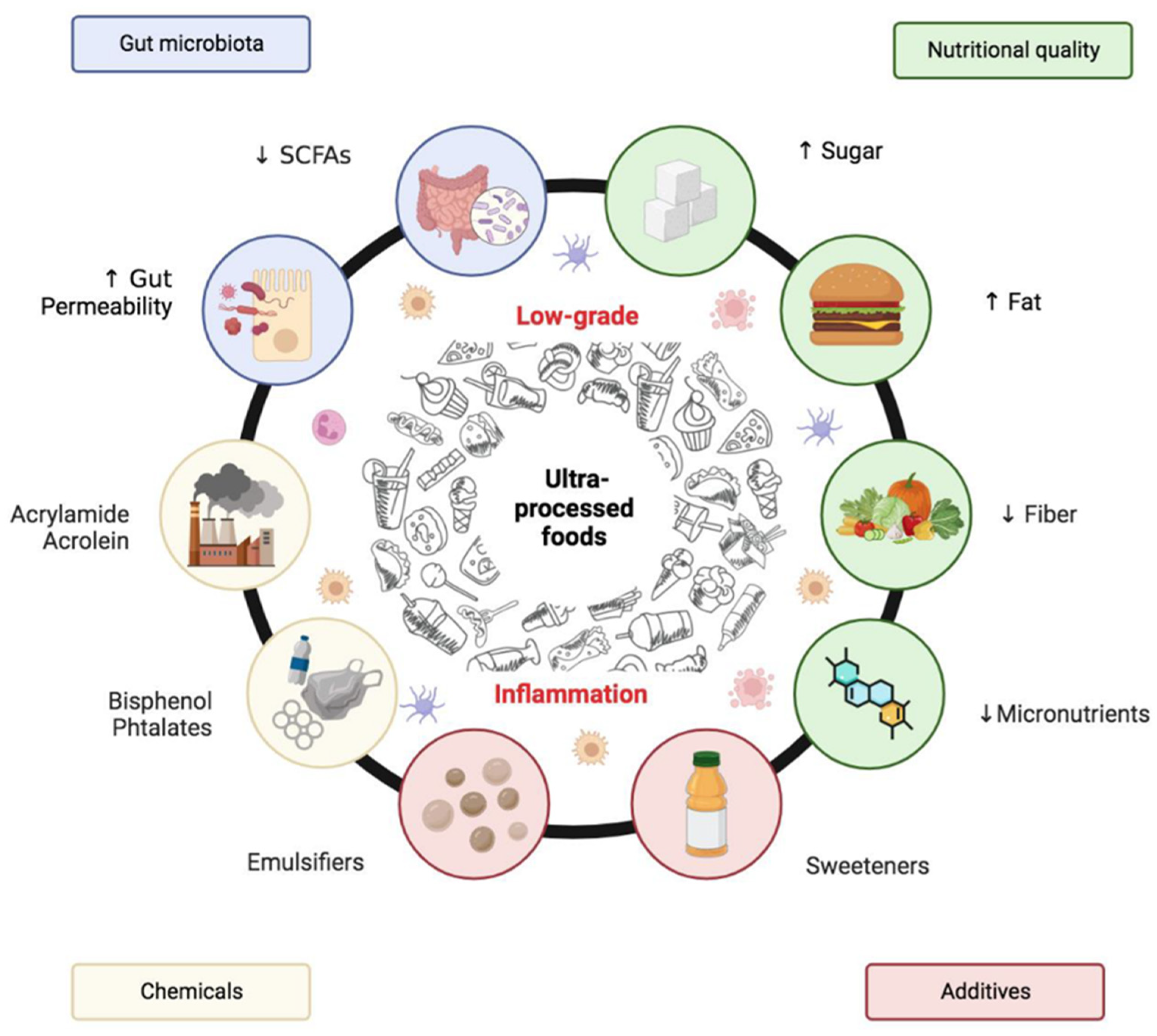
4. UPFs Are Beyond Poor Nutrition
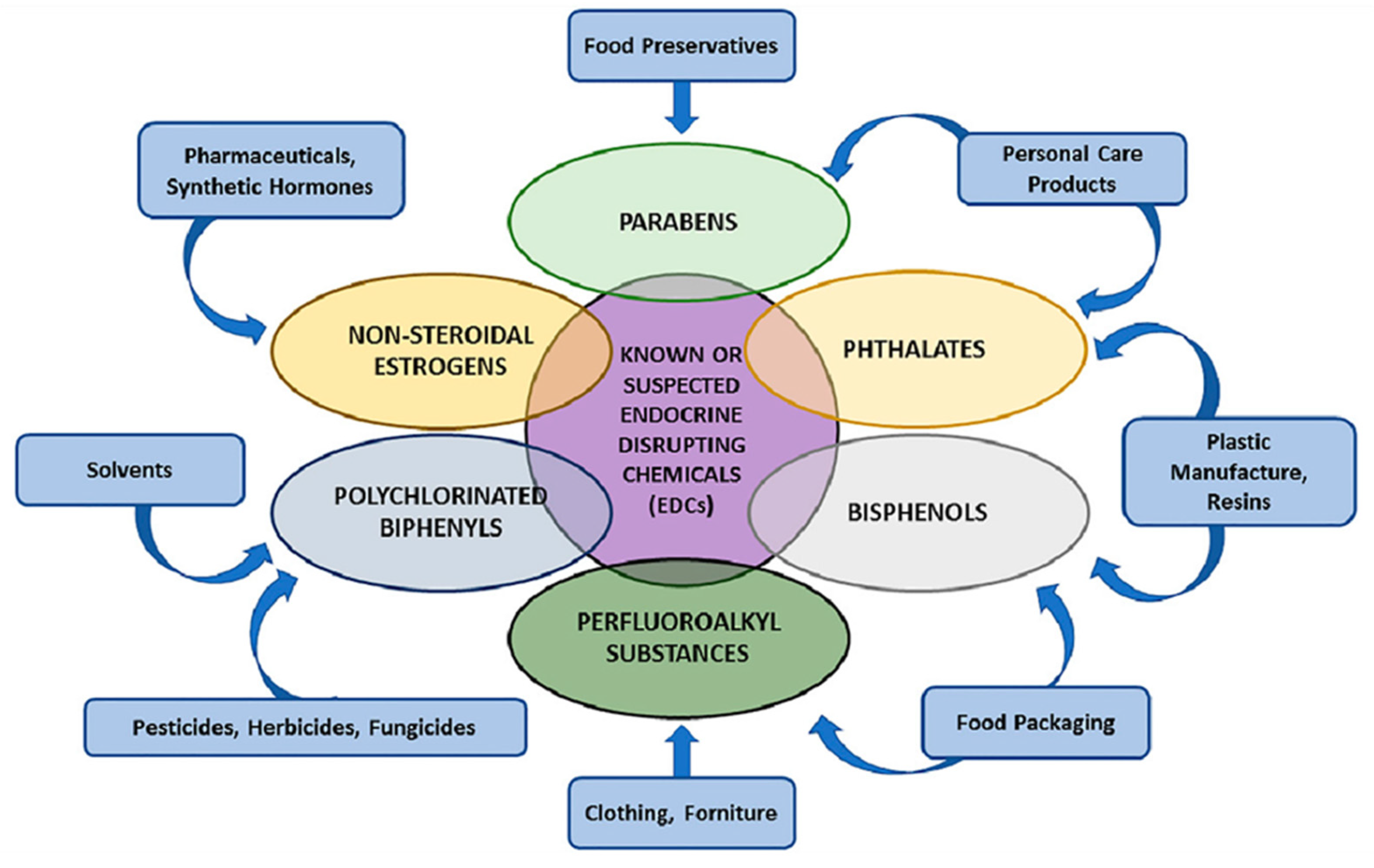
5. Endocrine Disruptors in UPFs and Their Systemic Impact
6. Mechanistic Pathways Linking Ultra-Processed Foods to Cancer
6.1. UPF-Induced Microbiome Disruption and Its Implications
6.2. Epigenetic Reprogramming, Silent Link Between UPFs and Cancer
6.3. Low-Grade Immune Activation in UPF-Driven Carcinogenesis
7. UPFs as a Trojan Horse for Cancer Development
8. Knowledge Gap and Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl Hydrocarbon Receptor |
| ASIR | Age-Standardised Incidence Rate |
| ASMR | Age-Standardised Mortality Rate |
| AYAs | Adolescents and Young Adults |
| BPA | Bisphenol A |
| CRC | Colorectal Cancer |
| CRP | C-Reactive Protein |
| CpG | Cytosine–phosphate–Guanine |
| DCA | Deoxycholic Acid |
| DEHP | Di(2-ethylhexyl) Phthalate |
| DINP | Diisononyl Phthalate |
| DII | Dietary Inflammatory Index |
| DNA | Deoxyribonucleic Acid |
| EDCs | Endocrine-Disrupting Chemicals |
| EMT | Epithelial–Mesenchymal Transition |
| EPA | Eicosapentaenoic Acid (if referenced) |
| GM | Gut Microbiota |
| HDAC | Histone Deacetylase |
| IL-6 | Interleukin-6 |
| LCA | Lithocholic Acid |
| LPS | Lipopolysaccharide |
| NNS | Non-Nutritive Sweeteners |
| NOVA | Not an acronym—name of food classification system |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| SCFA | Short-Chain Fatty Acids |
| TLR4 | Toll-Like Receptor 4 |
| TMAO | Trimethylamine N-oxide |
| TNF-α | Tumour Necrosis Factor-alpha |
| UPF | Ultra-Processed Food |
| UPP | Ultra-Processed Product |
References
- Gibney, M.J. Ultra-processed foods: Definitions and policy issues. Curr. Dev. Nutr. 2018, 3, nzy077. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F.; Magkos, F.; Fava, F.; Milani, G.P.; Agostoni, C.; Astrup, A.; Saguy, I.S. A multidisciplinary perspective of ultra-processed foods and associated food processing technologies: A view of the sustainable road ahead. Nutrients 2021, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Henney, A.E.; Gillespie, C.S.; Alam, U.; Hydes, T.J.; Boyland, E.; Cuthbertson, D.J. Ultra-processed food and non-communicable diseases in the United Kingdom: A narrative review and thematic synthesis of literature. Obes. Rev. 2024, 25, e13682. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Murugan, R.; Jeraud, M.; Dakkumadugula, A.; Periyasamy, R.; Arjunan, S. Additives in processed foods as a potential source of endocrine-disrupting chemicals: A review. J. Xenobiot. 2024, 14, 1697–1710. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Proietti, S.; Scazzocchio, B.; Masella, R. The detrimental impact of ultra-processed foods on the human gut microbiome and gut barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef]
- Bhatia, S.; Pappo, A.S.; Acquazzino, M.; Allen-Rhoades, W.A.; Barnett, M.; Borinstein, S.C.; Casey, R.; Choo, S.; Chugh, R.; Dinner, S.; et al. Adolescent and Young Adult (AYA) Oncology, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 851–880. [Google Scholar] [CrossRef]
- Hughes, T.; Harper, A.; Gupta, S.; Frazier, A.L.; van der Graaf, W.T.A.; Moreno, F.; Joseph, A.; Fidler-Benaoudia, M.M. The current and future global burden of cancer among adolescents and young adults: A population-based study. Lancet Oncol. 2024, 25, 1614–1624. [Google Scholar] [CrossRef]
- Li, J.; Kuang, X. Global cancer statistics of young adults and its changes in the past decade: Incidence and mortality from GLOBOCAN 2022. Public Health 2024, 237, 336–343. [Google Scholar] [CrossRef]
- di Martino, E.; Smith, L.; Bradley, S.H.; Hemphill, S.; Wright, J.; Renzi, C.; Bergin, R.; Emery, J.; Neal, R.D. Incidence trends for twelve cancers in younger adults—A rapid review. Br. J. Cancer 2022, 126, 1374–1386. [Google Scholar] [CrossRef]
- Toss, A.; Piombino, C.; Quarello, P.; Trama, A.; Mascarin, M.; Lambertini, M.; Canesi, M.; Incorvaia, L.; Milano, G.M.; Maruzzo, M.; et al. Risk factors behind the increase of early-onset cancer in Italian adolescents and young adults: An investigation from the Italian AYA Working Group. Eur. J. Cancer 2024, 212, 115042. [Google Scholar] [CrossRef]
- Lord, S.R.; Harris, A.L. Is it still worth pursuing the repurposing of metformin as a cancer therapeutic? Br. J. Cancer 2023, 128, 958–966. [Google Scholar] [CrossRef]
- Le, N.T.; Pham, Y.T.-H.; Le, L.T.; Dao, H.V.; Koriyama, C.; Ha, T.H.; Lichtveld, M.; Kuchipudi, S.V.; Huynh, N.Y.-N.; Nguyen, D.D.; et al. Factors affecting cancer mortality in young adults: Findings from a prospective cohort study. Cancers 2024, 16, 3853. [Google Scholar] [CrossRef]
- Rocha, L.L.; Gratão, L.H.A.; do Carmo, A.S.; Costa, A.B.P.; de Freitas Cunha, C.; de Oliveira, T.R.P.R.; Mendes, L.L. School Type, Eating Habits, and Screen Time are Associated with Ultra-Processed Food Consumption among Brazilian Adolescents. J. Acad. Nutr. Diet. 2021, 121, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Peter, N.B.; Muniz, L.C. Food consumption according to the level of processing among adolescents from the rural area of a municipality in the south of Brazil. Cienc. Saude Coletiva 2021, 26, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Khandpur, N.; da Costa Louzada, M.L.; Monteiro, C.A. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE 2020, 15, e0236738. [Google Scholar] [CrossRef]
- Sousa, R.D.S.; Bragança, M.L.B.M.; Oliveira, B.R.D.; Coelho, C.C.N.D.S.; Silva, A.A.M.D. Association between the Degree of Processing of Consumed Foods and Sleep Quality in Adolescents. Nutrients 2020, 12, 462. [Google Scholar] [CrossRef]
- Viola, P.C.D.A.F.; de Carvalho, C.A.; Bragança, M.L.B.M.; da Cunha França, A.K.T.; de Britto, M.T.S.S.; da Silva, A.A.M. High consumption of ultra-processed foods is associated with lower muscle mass in Brazilian adolescents in the RPS birth cohort. Nutrition 2020, 79–80, 110983. [Google Scholar] [CrossRef] [PubMed]
- Enes, C.C.; Camargo, C.M.D.; Justino, M.I.C. Ultra-processed food consumption and obesity in adolescents. Rev. Nutr. 2019, 32, e18170. [Google Scholar] [CrossRef]
- Melo, A.S.; Neves, F.S.; Batista, A.P.; Machado-Coelho, G.L.L.; Sartorelli, D.S.; de Faria, E.R.; Netto, M.P.; Oliveira, R.M.; Fontes, V.S.; Cândido, A.P.C. Percentage of energy contribution according to the degree of industrial food processing and associated factors in adolescents (EVA-JF study, Brazil). Public Health Nutr. 2021, 24, 4220–4229. [Google Scholar] [CrossRef]
- D’avila, H.F.; Kirsten, V.R. Energy intake from ultra-processed foods among adolescents. Rev. Paul. Pediatr. 2017, 35, 54–60. [Google Scholar] [CrossRef]
- Rauber, F.; Martins, C.A.; Azeredo, C.M.; Leffa, P.S.; da Costa Louzada, M.L.; Levy, R.B. Eating context and ultraprocessed food consumption among UK adolescents. Br. J. Nutr. 2022, 127, 112–122. [Google Scholar] [CrossRef]
- Wanjohi, M.N.; Asiki, G.; Wilunda, C.; Holdsworth, M.; Pradeilles, R.; Paulo, L.S.; Langat, N.; Amugsi, D.A.; Kimenju, S.; Kimani-Murage, E.W.; et al. Ultra-processed food consumption is associated with poor diet quality and nutrient intake among adolescents in urban slums, Kenya. Int. J. Public Health 2025, 69, 1607891. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gunter, M.J.; Rauber, F.; Millett, C.; Imamura, F.; Shi, Z.; Scarborough, P.; Monteiro, C.A.; Forouhi, N.G. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. EClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef] [PubMed]
- Aramburu, A.; Alvarado-Gamarra, G.; Cornejo, R.; Curi-Quinto, K.; Díaz-Parra, C.D.P.; Rojas- Limache, G.; Lanata, C.F. Ultra-processed foods consumption and health-related outcomes: A systematic review of randomised controlled trials. Front. Nutr. 2024, 11, 1421728. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. Ultra-processing should be understood as a holistic issue, from food matrix, to dietary patterns, food scoring, and food systems. J. Food Sci. 2024, 89, 4563–4573. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Braesco, V.; Souchon, I.; Sauvant, P.; Haurogné, T.; Maillot, M.; Féart, C.; Darmon, N. Ultra-processed foods: How functional is the NOVA system? Eur. J. Clin. Nutr. 2022, 76, 1245–1253. [Google Scholar] [CrossRef]
- Egnell, M.; Kesse-Guyot, E.; Galan, P.; Touvier, M.; Rayner, M.; Jewell, J.; Breda, J.; Hercberg, S.; Julia, C. Impact of Front-of-Pack Nutrition Labels on Portion Size Selection: An Experimental Study in a French Cohort. Nutrients 2018, 10, 1268. [Google Scholar] [CrossRef]
- McClements, D.J. Ultraprocessed plant-based foods: Designing the next generation of healthy and sustainable alternatives to animal-based foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3531–3559. [Google Scholar] [CrossRef]
- Levy, R.B.; Barata, M.F.; Leite, M.A.; Andrade, G.C. How and why ultra-processed foods harm human health. Proc. Nutr. Soc. 2024, 83, 1–8. [Google Scholar] [CrossRef]
- Hafner, E.; Hribar, M.; Pravst, I. Ultra-Processed Foods in the Food Supply: Prevalence, Nutritional Composition and Use of Voluntary Labelling Schemes. Nutrients 2025, 17, 1731. [Google Scholar] [CrossRef] [PubMed]
- Viennois, E.; Merlin, D.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifier-Induced Low-Grade Inflammation Promotes Colon Carcinogenesis. Cancer Res. 2017, 77, 27–40. [Google Scholar] [CrossRef]
- Dai, S.; Wellens, J.; Yang, N.; Li, D.; Wang, J.; Wang, L.; Yuan, S.; He, Y.; Song, P.; Munger, R.; et al. Ultra-processed foods and human health: An umbrella review and updated meta-analyses of observational evidence. Clin. Nutr. 2024, 43, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Cummings, J.R.; Gough, T.; Jones, A.; Evans, R. Consumer Awareness, Perceptions and Avoidance of Ultra-Processed Foods: A Study of UK Adults in 2024. Foods 2024, 13, 2317. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Bere, E. Ultra-processed foods—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr Res. 2024, 68, 10–29219. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Vasanth, K. Food contaminants: Impact of food processing, challenges and mitigation strategies for food security. Food Res. Int. 2024, 191, 114739. [Google Scholar] [CrossRef]
- Ubbink, J.; Levine, A.S. From Processed Foods to Ultraprocessed Foods: Evolution of an Industry Model and Impact on Dietary Quality, Health, and Society. Annu. Rev. Food Sci. Technol. 2025, 16, 1–24. [Google Scholar] [CrossRef]
- Esposito, S.; Gialluisi, A.; Di Castelnuovo, A.; Costanzo, S.; Pepe, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; et al. Ultra-processed food consumption is associated with the acceleration of biological aging in the Moli-sani Study. Am. J. Clin. Nutr. 2024, 120, 1432–1440. [Google Scholar] [CrossRef]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Env. Res. 2022, 211, 113049. [Google Scholar] [CrossRef]
- Zhao, H.; Gui, W.; Liu, S.; Zhao, F.; Fan, W.; Jing, F.; Sun, C. Ultra-processed foods intake and sex hormone levels among children and adolescents aged 6–19 years: A cross-sectional study. Front. Nutr. 2024, 11, 1451481. [Google Scholar] [CrossRef]
- Oviedo-Solís, C.I.; Monterrubio-Flores, E.A.; Cediel, G.; Denova-Gutiérrez, E.; Barquera, S. Trend of Ultraprocessed Product Intake Is Associated with the Double Burden of Malnutrition in Mexican Children and Adolescents. Nutrients 2022, 14, 4347. [Google Scholar] [CrossRef]
- Pearce, E.N. Endocrine Disruptors and Thyroid Health. Endocr. Pract. 2024, 30, 172–176. [Google Scholar] [CrossRef]
- Lagarde, F.; Beausoleil, C.; Belcher, S.M.; Belzunces, L.P.; Emond, C.; Guerbet, M.; Rousselle, C. Non-monotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment. Environ. Health 2015, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Hansel, M.C.; Rosenberg, A.M.; Kinkade, C.W.; Capurro, C.; Rivera-Núñez, Z.; Barrett, E.S. Exposure to synthetic endocrine-disrupting chemicals in relation to maternal and fetal sex steroid hormones: A scoping review. Curr. Environ. Health Rep. 2024, 11, 356–379. [Google Scholar] [CrossRef]
- Jalal, N.; Surendranath, A.R.; Pathak, J.L.; Yu, S.; Chung, C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 2017, 5, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kurşunoğlu, N.E.; Sarer Yurekli, B.P. Endocrine disruptor chemicals as obesogen and diabetogen: Clinical and mechanistic evidence. World J. Clin. Cases 2022, 10, 11226–11239. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Gény, B.; Talha, S. The endocrine function of the heart: Physiology and involvements of natriuretic peptides and cyclic nucleotide phosphodiesterases in heart failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar] [CrossRef]
- Zeng, B.; Wu, Y.; Huang, Y.; Zhang, Y.; Li, X.; Liu, Y.; Wang, Y.; Chen, L.; Zhou, Y.; Tang, S. Carcinogenic health outcomes associated with endocrine disrupting chemicals exposure in humans: A wide-scope analysis. J. Hazard. Mater. 2024, 476, 135067. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Vandenberg, L.N.; Demeneix, B.A.; Porta, M.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Economic, regulatory, and policy implications. Lancet Diabetes Endocrinol. 2020, 8, 719–730. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-disrupting chemicals’ (EDCs) effects on tumour microenvironment and cancer progression: Emerging contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Wang, Y.F.; Gao, J.; Yu, M.L.; Yu, Y.Y.; Yao, Y.Q. In vitro evidence for endocrine-disrupting chemical (EDC)'s inhibition of drug metabolism. Afr. Health Sci. 2014, 14, 185–188. [Google Scholar] [PubMed]
- Sneha, S.; Baker, S.C.; Green, A.; Storr, S.; Aiyappa, R.; Martin, S.; Pors, K. Intratumoural Cytochrome P450 Expression in Breast Cancer: Impact on Standard of Care Treatment and New Efforts to Develop Tumour-Selective Therapies. Biomedicines 2021, 9, 290. [Google Scholar] [CrossRef]
- Nebert, D.W. Aryl hydrocarbon receptor (AHR): “Pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 2017, 67, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.R.; Pimpão, A.B.; Correia, M.J.; Rodrigues, T.C.; Monteiro, E.C.; Morello, J.; Pereira, S.A. Pharmacological blockage of the AHR-CYP1A1 axis: A call for in vivo evidence. J. Mol. Med. 2022, 100, 215–243. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, J.S.; Ahn, C.; Jeung, E.B. Endocrine-Disrupting Chemicals and Their Adverse Effects on the Endoplasmic Reticulum. Int. J. Mol. Sci. 2022, 23, 1581. [Google Scholar] [CrossRef]
- Kazzaz, S.A.; Tawil, J.; Harhaj, E.W. The aryl hydrocarbon receptor-interacting protein in cancer and immunity: Beyond a chaperone protein for the dioxin receptor. J. Biol. Chem. 2024, 300, 107157. [Google Scholar] [CrossRef]
- Pappas, B.; Yang, Y.; Wang, Y.; Kim, K.; Chung, H.J.; Cheung, M.; Ngo, K.; Shinn, A.; Chan, W.K. p23 protects the human aryl hydrocarbon receptor from degradation via a heat shock protein 90-independent mechanism. Biochem. Pharmacol. 2018, 152, 34–44. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Baker, B.H.; Melough, M.M.; Paquette, A.G.; Barrett, E.S.; Day, D.B.; Kannan, K.; Nguyen, R.H.; Bush, N.R.; LeWinn, K.Z.; Carroll, K.N.; et al. Ultra-processed and fast food consumption, exposure to phthalates during pregnancy, and socioeconomic disparities in phthalate exposures. Environ. Int. 2024, 183, 108427. [Google Scholar] [CrossRef]
- Tagne-Fotso, R.; Riou, M.; Saoudi, A.; Zeghnoun, A.; Frederiksen, H.; Berman, T.; Montazeri, P.; Andersson, A.-M.; Rodriguez-Martin, L.; Akesson, A.; et al. Exposure to bisphenol A in European women from 2007 to 2014 using human biomonitoring data-The European Joint Programme HBM4EU. Environ. Int. 2024, 190, 108912. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The role of endocrine disruptors bisphenols and phthalates in obesity: Current evidence, perspectives and controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef] [PubMed]
- González-Casanova, J.E.; Bermúdez, V.; Caro Fuentes, N.J.; Angarita, L.C.; Caicedo, N.H.; Rivas Muñoz, J.; Rojas-Gómez, D.M. New evidence on BPA’s role in adipose tissue development of proinflammatory processes and its relationship with obesity. Int. J. Mol. Sci. 2023, 24, 8231. [Google Scholar] [CrossRef]
- Dong, Z.; He, L.; Wu, J.; Xie, C.; Geng, S.; Wu, J.; Zhong, C.; Li, X. Bisphenol A-Induced Cancer-Associated Adipocytes Promotes Breast Carcinogenesis via CXCL12/AKT Signaling. Mol. Cell. Endocrinol. 2025, 599, 112473. [Google Scholar] [CrossRef]
- Bokobza, E.; Hinault, C.; Tiroille, V.; Clavel, S.; Bost, F.; Chevalier, N. The adipose tissue at the crosstalk between EDCs and cancer development. Front. Endocrinol. 2021, 12, 691658. [Google Scholar] [CrossRef]
- Banerjee, O.; Paul, T.; Singh, S.; Maji, B.K.; Mukherjee, S. Individual and combined antagonism of aryl hydrocarbon receptor (AhR) and estrogen receptors (ERs) offers distinct level of protection against Bisphenol A (BPA)-induced pancreatic islet cell toxicity in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 3939–3954. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Licznerska, B.; Baer-Dubowska, W. The Aryl Hydrocarbon Receptor and Its Crosstalk: A Chemopreventive Target of Naturally Occurring and Modified Phytochemicals. Molecules 2024, 29, 4283. [Google Scholar] [CrossRef]
- Donini, C.F.; El Helou, M.; Wierinckx, A.; Győrffy, B.; Aires, S.; Escande, A.; Croze, S.; Clezardin, P.; Lachuer, J.; Diab-Assaf, M.; et al. Long-Term Exposure of Early-Transformed Human Mammary Cells to Low Doses of Benzo[a]pyrene and/or Bisphenol A Enhances Their Cancerous Phenotype via an AhR/GPR30 Interplay. Front. Oncol. 2020, 10, 712. [Google Scholar] [CrossRef]
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A review on immunomodulatory effects of BPA analogues. Arch. Toxicol. 2023, 97, 1831–1846. [Google Scholar] [CrossRef]
- Primost, M.A.; Chierichetti, M.A.; Castaños, C.; Bigatti, G.; Miglioranza, K.S.B. Persistent Organic Pollutants (POPs), Current Use Pesticides (CUPs) and Polycyclic Aromatic Hydrocarbons (PAHs) in edible marine invertebrates from a Patagonian harbor. Mar. Pollut. Bull. 2024, 207, 116940. [Google Scholar] [CrossRef]
- Ditchfield, C.; Kushida, M.M.; Mazalli, M.R.; Sobral, P.J.A. Can Chocolate Be Classified as an Ultra-Processed Food? A Short Review on Processing and Health Aspects to Help Answer This Question. Foods 2023, 12, 3070. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J.; Touvier, M.; Trasande, L.; Scheringer, M. Health impacts of exposure to synthetic chemicals in food. Nat. Med. 2025, 31, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary advanced glycation end products (dAGEs): An insight between modern diet and health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Nie, C.; Liu, H.; Ma, Q.; Peng, B.; Zhang, M.; Chen, Z.; Li, J. Comparison of metabolic fate, target organs, and microbiota interactions of free and bound dietary advanced glycation end products. Crit. Rev. Food Sci. Nutr. 2023, 63, 3612–3633. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western diet–microbiome–host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef]
- Aguilera, M.; Gálvez-Ontiveros, Y.; Rivas, A. Endobolome, a new concept for determining the influence of microbiota disrupting chemicals (MDC) in relation to specific endocrine pathogenesis. Front. Microbiol. 2020, 11, 578007. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Huang, G.; Zheng, Y.; Zhang, N.; Huang, G.; Zhang, W.; Li, Q.; Ren, X. Desulfovibrio vulgaris caused gut inflammation and aggravated DSS-induced colitis in C57BL/6 mice model. Gut Pathog. 2024, 16, 39. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary bile acids and tumorigenesis in colorectal cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dai, H.; Lu, Y.; Li, R.; Gao, C.; Pan, S. Trimethylamine N-oxide promotes cell proliferation and angiogenesis in colorectal cancer. J. Immunol. Res. 2022, 2022, 7043856. [Google Scholar] [CrossRef]
- Gubatan, J.; Boye, T.L.; Temby, M.; Sojwal, R.S.; Holman, D.R.; Sinha, S.R.; Rogalla, S.R.; Nielsen, O.H. Gut microbiome in inflammatory bowel disease: Role in pathogenesis, dietary modulation, and colitis-associated colon cancer. Microorganisms 2022, 10, 1371. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Speranza, B.; Racioppo, A.; Santillo, A.; Albenzio, M.; Derossi, A.; Caporizzi, R.; Francavilla, M.; Racca, D.; Flagella, Z.; et al. Ultra-Processed Food and Gut Microbiota: Do Additives Affect Eubiosis? A Narrative Review. Nutrients 2024, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Alcaire, F.; Giménez, A.; Ares, G. Food Additives Associated with Gut Dysbiosis in Processed and Ultra-Processed Products Commercialized in the Uruguayan Market. Food Res. Int. 2024, 191, 114721. [Google Scholar] [CrossRef]
- Gonza, I.; Goya-Jorge, E.; Douny, C.; Boutaleb, S.; Taminiau, B.; Daube, G.; Scippo, M.; Louis, E.; Delcenserie, V. Food Additives Impair Gut Microbiota from Healthy Individuals and IBD Patients in a Colonic In Vitro Fermentation Model. Food Res. Int. 2024, 182, 114157. [Google Scholar] [CrossRef]
- Li, P.; Qu, R.; Li, M.; Sheng, P.; Jin, L.; Huang, X.; Xu, Z.Z. Impacts of Food Additives on Gut Microbiota and Host Health. Food Res. Int. 2024, 196, 114998. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Rosés, C.; Nieto, J.A.; Viadel, B.; Gallego, E.; Romo-Hualde, A.; Streitenberger, S.; Milagro, F.I.; Barceló, A. An In Vitro Protocol to Study the Modulatory Effects of a Food or Biocompound on Human Gut Microbiome and Metabolome. Foods 2021, 10, 3020. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Aguilera, A.M.; Pérez-Burillo, S.; Navajas-Porras, B.; León, E.D.; Ruíz-Pérez, S.; Pastoriza, S.; Jiménez-Hernández, N.; Cämmerer, B.-M.; Rufián-Henares, J.Á.; Gosalbes, M.J.; et al. Effects of Different Foods and Cooking Methods on the Gut Microbiota: An In Vitro Approach. Front. Microbiol. 2024, 14, 1334623. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Armstrong, N.J.; Player, R.A.; Rood, J.C.; Soares, J.W.; McClung, H.L. The Fecal Metabolome Links Diet Composition, Foacidic Positive Ion Conditions, Chromatographicallyod Processing, and the Gut Microbiota to Gastrointestinal Health in a Randomized Trial of Adults Consuming a Processed Diet. J. Nutr. 2022, 152, 2343–2357. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bryden, K.; Chen, X.; Papachristou, E.; Verney, A.; Roig, M.; Hansen, R.; Nichols, B.; Papadopoulou, R.; Parrett, A. The Impact of Food Additives, Artificial Sweeteners and Domestic Hygiene Products on the Human Gut Microbiome and Its Fibre Fermentation Capacity. Eur. J. Nutr. 2020, 59, 3213–3230. [Google Scholar] [CrossRef]
- Hussain, S.; Tulsyan, S.; Dar, S.A.; Sisodiya, S.; Abiha, U.; Kumar, R.; Mishra, B.N.; Haque, S. Role of epigenetics in carcinogenesis: Recent advancements in anticancer therapy. Semin. Cancer Biol. 2022, 83, 441–451. [Google Scholar] [CrossRef]
- Barrero, M.J.; Cejas, P.; Long, H.W.; Ramirez de Molina, A. Nutritional epigenetics in cancer. Adv. Nutr. 2022, 13, 1748–1761. [Google Scholar] [CrossRef]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef]
- Isaksen, I.M.; Dankel, S.N. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 919–928. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-processed foods and human health: From epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Pértille, F.; Da Silva, V.H.; Johansson, A.M.; Lindström, T.; Wright, D.; Coutinho, L.L.; Jensen, P.; Guerrero-Bosagna, C. Mutation dynamics of CpG dinucleotides during a recent event of vertebrate diversification. Epigenetics 2019, 14, 685–707. [Google Scholar] [CrossRef]
- Bishop, K.S.; Ferguson, L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947. [Google Scholar] [CrossRef]
- Zhong, F.; Lin, Y.; Zhao, L.; Yang, C.; Ye, Y.; Shen, Z. Reshaping the tumour immune microenvironment in solid tumours via tumour cell and immune cell DNA methylation: From mechanisms to therapeutics. Br. J. Cancer 2023, 129, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bhootra, S.; Jill, N.; Shanmugam, G.; Rakshit, S.; Sarkar, K. DNA methylation and cancer: Transcriptional regulation, prognostic, and therapeutic perspective. Med. Oncol. 2023, 40, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Song, K.; Chen, H.; Dai, M. Advance and challenge of DNA methylation as cancer biomarkers for risk stratification, screening and early detection. J. Natl. Cancer Cent. 2025, 5, 108–112. [Google Scholar] [CrossRef]
- Zaib, S.; Rana, N.; Khan, I. Histone modifications and their role in epigenetics of cancer. Curr. Med. Chem. 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 2022, 83, 452–471. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of histone modification. Adv. Exp. Med. Biol. 2021, 1283, 1–16. [Google Scholar]
- Zhang, Z.; Zhang, J.; Diao, L.; Han, L. Small non-coding RNAs in human cancer: Function, clinical utility, and characterisation. Oncogene 2021, 40, 1570–1577. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Song, J.; Xu, Y.; Qian, H.; Jin, J.; Liang, Z.F. Non-coding RNAs’ function in cancer development, diagnosis and therapy. Biomed. Pharmacother. 2023, 167, 115527. [Google Scholar] [CrossRef]
- Romano, G.; Veneziano, D.; Acunzo, M.; Croce, C.M. Small non-coding RNA and cancer. Carcinogenesis 2017, 38, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Cristaldi, A.; Oliveri Conti, G. Oncogenic role of miRNA in environmental exposure to plasticisers: A systematic review. J. Pers. Med. 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Llauradó-Pont, J.; Stratakis, N.; Fiorito, G.; Handakas, E.; Neumann, A.; Barros, H.; Brantsæter, A.L.; Chang, K.; Chatzi, L.; Felix, J.F.; et al. A Meta-Analysis of Epigenome-Wide Association Studies of Ultra-Processed Food Consumption with DNA Methylation in European Children. Clin. Epigenetics 2025, 17, 3. [Google Scholar] [CrossRef]
- Edalati, S.; Bagherzadeh, F.; Asghari Jafarabadi, M.; Ebrahimi-Mamaghani, M. Higher Ultra-Processed Food Intake Is Associated with Higher DNA Damage in Healthy Adolescents. Br. J. Nutr. 2021, 125, 568–576. [Google Scholar] [CrossRef]
- Freitas, R.D.S.; da Silva, J. Impact of Ultra-Processed Foods on Human Health: A Comprehensive Review of Genomic Instability and Molecular Mechanisms. Nutrition 2025, 137, 112800. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C. Impact of Dietary Gut Microbial Metabolites on the Epigenome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170359. [Google Scholar] [CrossRef]
- Zhang, Y.; Giovannucci, E.L. Ultra-Processed Foods and Health: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10836–10848. [Google Scholar] [CrossRef]
- Milagro, F.I.; Mansego, M.L.; De Miguel, C.; Martínez, J.A. Dietary Factors, Epigenetic Modifications and Obesity Outcomes: Progresses and Perspectives. Mol. Asp. Med. 2013, 34, 782–812. [Google Scholar] [CrossRef]
- Liu, W.; Deng, Y.; Li, Z.; Chen, Y.; Zhu, X.; Tan, X.; Cao, G. Cancer Evo-Dev: A theory of inflammation-induced oncogenesis. Front. Immunol. 2021, 12, 768098. [Google Scholar] [CrossRef]
- Mella, C.; Tsarouhas, P.; Brockwell, M.; Ball, H.C. The role of chronic inflammation in pediatric cancer. Cancers 2025, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.M.; Abdelgawad, M.A.; Ghoneim, M.M.; Alhossan, A.; Al-Serwi, R.H.; Farouk, A. Impact of some natural and artificial sweeteners consumption on different hormonal levels and inflammatory cytokines in male rats: In vivo and in silico studies. ACS Omega 2024, 9, 30364–30380. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, S.; Jin, J.; Wang, J.; Chen, W.; Xue, Y.; Sheng, L.; Zhai, Y.; Yao, W. Sucralose triggers insulin resistance leading to follicular dysplasia in mice. Reprod. Toxicol. 2024, 128, 108644. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-grade inflammation and ultra-processed foods consumption: A review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Wang, Y.; Reddy, A.G.; Um, C.Y.; Hodge, R.A.; Lichtman, C.; Mitchell, D.; Nanavati, A.; Pollak, M.; Wang, Y.; et al. Nonnutritive sweetener consumption, metabolic risk factors, and inflammatory biomarkers among adults in the Cancer Prevention Study-3 Diet Assessment Sub-Study. J. Nutr. 2025, 155, 1952–1961. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Miller, A.W.; Granados-Portillo, O.; Tovar, A.R.; Torres, N. The development of metabolic endotoxemia is dependent on the type of sweetener and the presence of saturated fat in the diet. Gut Microbes 2020, 12, 1801301. [Google Scholar] [CrossRef]
- Bujtor, M. Can dietary intake protect against low-grade inflammation in children and adolescents? Brain Behav. Immun. Health 2021, 18, 100369. [Google Scholar] [CrossRef]
- Chavez-Dominguez, R.; Perez-Medina, M.; Aguilar-Cazares, D.; Galicia-Velasco, M.; Meneses-Flores, M.; Islas-Vazquez, L.; Camarena, A.; Lopez-Gonzalez, J.S. Old and new players of inflammation and their relationship with cancer development. Front. Oncol. 2021, 11, 722999. [Google Scholar] [CrossRef]
- Harris, H.R.; Willett, W.C.; Vaidya, R.L.; Michels, K.B. An adolescent and early adulthood dietary pattern associated with inflammation and the incidence of breast cancer. Cancer Res. 2017, 77, 1179–1187. [Google Scholar] [CrossRef]
- Suzuki, S.; Katagiri, R.; Yamaji, T.; Sawada, N.; Imatoh, T.; Ihira, H.; Inoue, M.; Tsugane, S.; Iwasaki, M.; Japan Public Health Center-Based Prospective Study Group. Association between C-reactive protein and risk of overall and 18 site-specific cancers in a Japanese case-cohort. Br. J. Cancer 2022, 126, 1481–1489. [Google Scholar] [CrossRef]
- Yang, K.; Song, X.; Cheng, C.; Shi, Q.; Li, X.; Long, J.; Yang, H.; Chen, S. Association between dietary inflammatory potential and liver cancer risk: A systematic review and dose-response meta-analysis. Nutr. Cancer 2025, 77, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ramal-Sanchez, M.; Bravo-Trippetta, C.; D’aNtonio, V.; Corvaglia, E.; Kämpfer, A.A.M.; Schins, R.P.F.; Serafini, M.; Angelino, D. Development and Assessment of an Intestinal Tri-Cellular Model to Investigate the Pro/Anti-Inflammatory Potential of Digested Foods. Front. Immunol. 2025, 16, 1545261. [Google Scholar] [CrossRef]
- Vahid, F.; Krischler, P.; Leners, B.; Bohn, T. Effect of Digested Selected Food Items on Markers of Oxidative Stress and Inflammation in a Caco-2-Based Human Gut Epithelial Model. Antioxidants 2024, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Le, N.P.K.; Altenburger, M.J.; Lamy, E. Development of an Inflammation-Triggered In Vitro “Leaky Gut” Model Using Caco-2/HT29-MTX-E12 Combined with Macrophage-like THP-1 Cells or Primary Human-Derived Macrophages. Int. J. Mol. Sci. 2023, 24, 7427. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.; Barbosa, J.; Faria, J.; Sá, S.I.; Cardoso, A.; Soares, R.; Fonseca, B.M.; Leal, S. Unhealthy Diets Induce Distinct and Regional Effects on Intestinal Inflammatory Signalling Pathways and Long-Lasting Metabolic Dysfunction in Rats. Int. J. Mol. Sci. 2022, 23, 10984. [Google Scholar] [CrossRef]
- Viennois, E.; Bretin, A.; Dubé, P.E.; Maue, A.C.; Dauriat, C.J.G.; Barnich, N.; Gewirtz, A.T.; Chassaing, B. Dietary Emulsifiers Directly Impact Adherent-Invasive E. coli Gene Expression to Drive Chronic Intestinal Inflammation. Cell Rep. 2020, 33, 108229. [Google Scholar] [CrossRef]
- Mignogna, C.; Costanzo, S.; Di Castelnuovo, A.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; Esposito, S.; De Curtis, A.; Persichillo, M.; Cerletti, C.; et al. The Inflammatory Potential of the Diet as a Link between Food Processing and Low-Grade Inflammation: An Analysis on 21,315 Participants to the Moli-sani Study. Clin. Nutr. 2022, 41, 2226–2234. [Google Scholar] [CrossRef]
- Vitale, M.; Costabile, G.; Testa, R.; D’Abbronzo, G.; Nettore, I.C.; Macchia, P.E.; Giacco, R. Ultra-processed foods and human health: A systematic review and meta-analysis of prospective cohort studies. Adv. Nutr. 2024, 15, 100121. [Google Scholar] [CrossRef]
- Forde, C.G. Beyond ultra-processed: Considering the future role of food processing in human health. Proc. Nutr. Soc. 2023, 82, 406–418. [Google Scholar] [CrossRef]
- Anastasiou, I.A.; Kounatidis, D.; Vallianou, N.G.; Skourtis, A.; Dimitriou, K.; Tzivaki, I.; Tsioulos, G.; Rigatou, A.; Karampela, I.; Dalamaga, M. Beneath the surface: The emerging role of ultra-processed foods in obesity-related cancer. Curr. Oncol. Rep. 2025, 27, 390–414. [Google Scholar] [CrossRef]
- Morys, F.; Kanyamibwa, A.; Fängström, D.; Tweedale, M.; Pastor-Bernier, A.; Azizi, H.; Liu, L.; Horstmann, A.; Dagher, A. Ultra-processed food consumption affects structural integrity of feeding-related brain regions independent of and via adiposity. NPJ Metab. Health Dis. 2025, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E.E.; Peñafiel, A.M.; Hernández Escalante, V.M.; Cabrera Araujo, Z.M. Ultra-processed diet, systemic oxidative stress, and breach of immunologic tolerance. Nutrition 2021, 91–92, 111419. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Ultraprocessed food: Addictive, toxic, and ready for regulation. Nutrients 2020, 12, 3401. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Ladányi, A.; Reszegi, A.; Kovalszky, I. Insights into the tumor microenvironment—Components, functions and therapeutics. Int. J. Mol. Sci. 2023, 24, 17536. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Harris, E. Ultraprocessed foods linked with 32 types of health problems. JAMA 2024, 331, 1265. [Google Scholar] [CrossRef]
- De Paula, L.C.P.; Alves, C. Food packaging and endocrine disruptors. J. Pediatr. 2024, 100 (Suppl. S1), S40–S47. [Google Scholar] [CrossRef]
- Valicente, V.M.; Peng, C.H.; Pacheco, K.N.; Lin, L.; Kielb, E.I.; Dawoodani, E.; Abdollahi, A.; Mattes, R.D. Ultraprocessed foods and obesity risk: A critical review of reported mechanisms. Adv. Nutr. 2023, 14, 718–738. [Google Scholar]
- Tobias, D.K.; Hall, K.D. Eliminate or reformulate ultra-processed foods? Biological mechanisms matter. Cell Metab. 2021, 33, 2314–2315. [Google Scholar] [CrossRef]
- Wang, L.; Du, M.; Wang, K.; Shen, W.; Lu, D.; Zheng, Y.; Song, M.; Cao, Y.; Smith-Warner, S.A.; Ogino, S.; et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: Results from three prospective US cohort studies. BMJ 2022, 378, e068921. [Google Scholar] [CrossRef]
- Jafari, F.; Yarmand, S.; Nouri, M.; Akbari, M.E.; Hadaegh, F.; Azizi, F.; Mirmiran, P. Ultra-processed food intake and risk of colorectal cancer: A matched case-control study. Nutr. Cancer 2023, 75, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.C.; Gu, H.T.; Peng, Y.; Wang, K.; Wu, Y.Q.; Hu, T.Y.; Jing, F.C.; Hao, F.B. Association of ultra-processed food consumption with cardiovascular mortality in the US population: Long-term results from a large prospective multicenter study. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]


| Author (Year) | Country | Sample Size | Age Group (Years) | UPF (%) |
|---|---|---|---|---|
| Rocha et al. (2021) [13] | Brazil | 71,533 | 12–17 | 28.0 |
| Oliveira et al. (2021) [14] | US | 462 | 13.1 ± 1.5 | 31.9 |
| Martinez Steele et al. (2020) [15] | Brazil | 9416 | 12–19 | 66.9 |
| Sousa et al. (2020) [16] | Brazil | 2499 | 18–19 | 35.8 |
| Viola et al. (2020) [17] | Brazil | 1525 | 18–19 | 37.0 |
| Enes et al. (2019) [18] | Brazil | 200 | 10–18 | 50.6 |
| Melo et al. (2021) [19] | Brazil | 804 | 16.1 ± 1.2 | 45.9 |
| D’Avila et al. (2017) [20] | Brazil | 784 | 15.2 ± 1.3 | 49.2 |
| Rauber et al. (2021) [21] | UK | 542 | 11–18 | 67.8 |
| Wanjohi et al. (2025) [22] | Kenya | 621 | 10–19 | 25.2 |
| Biomarker | Microbial Source | Host Effect | Role in CRC |
|---|---|---|---|
| Butyrate | Faecalibacterium, Roseburia spp. | Energy for colonocytes, HDAC inhibition, anti-inflammatory | Deficiency promotes epigenetic instability and inflammation [77,78] |
| Propionate | Bacteroides, Veillonella spp. | Anti-inflammatory, maintains the mucosal barrier | Reduction impairs immune regulation [77] |
| Hydrogen sulphide | Desulfovibrio spp. | Mitochondrial damage, oxidative stress, stem cell disruption | Genotoxic and proinflammatory [82] |
| DCA, LCA | Clostridium, Bacteroides spp. | DNA damage, oxidative stress, pathway activation | Promote Wnt/β-catenin, EGFR signalling [83] |
| TMAO | Microbial metabolism of choline/carnitine | Angiogenesis, cell proliferation via oncogenic signalling | Promotes tumour progression [84] |
| LPS | Gram-negative bacteria | Activates TLR4 and NF-κB, induces IL-6 and TNF-α | Drives chronic inflammation and cancer progression [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajkić, A.; Lepara, O.; Jahić, R.; Hadžović-Džuvo, A.; Belančić, A.; Chupin, A.; Pavković, D.; Sher, E.K. Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults. Cancers 2025, 17, 2196. https://doi.org/10.3390/cancers17132196
Fajkić A, Lepara O, Jahić R, Hadžović-Džuvo A, Belančić A, Chupin A, Pavković D, Sher EK. Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults. Cancers. 2025; 17(13):2196. https://doi.org/10.3390/cancers17132196
Chicago/Turabian StyleFajkić, Almir, Orhan Lepara, Rijad Jahić, Almira Hadžović-Džuvo, Andrej Belančić, Alexander Chupin, Doris Pavković, and Emina Karahmet Sher. 2025. "Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults" Cancers 17, no. 13: 2196. https://doi.org/10.3390/cancers17132196
APA StyleFajkić, A., Lepara, O., Jahić, R., Hadžović-Džuvo, A., Belančić, A., Chupin, A., Pavković, D., & Sher, E. K. (2025). Ultra-Processed Diets and Endocrine Disruption, Explanation of Missing Link in Rising Cancer Incidence Among Young Adults. Cancers, 17(13), 2196. https://doi.org/10.3390/cancers17132196










