Clinical Characteristics and Outcomes of SMARCA4-Mutated or Deficient Malignancies: A Systematic Review of Case Reports and Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Data Synthesis and Statistical Analysis
2.6. Ethical Considerations
3. Results
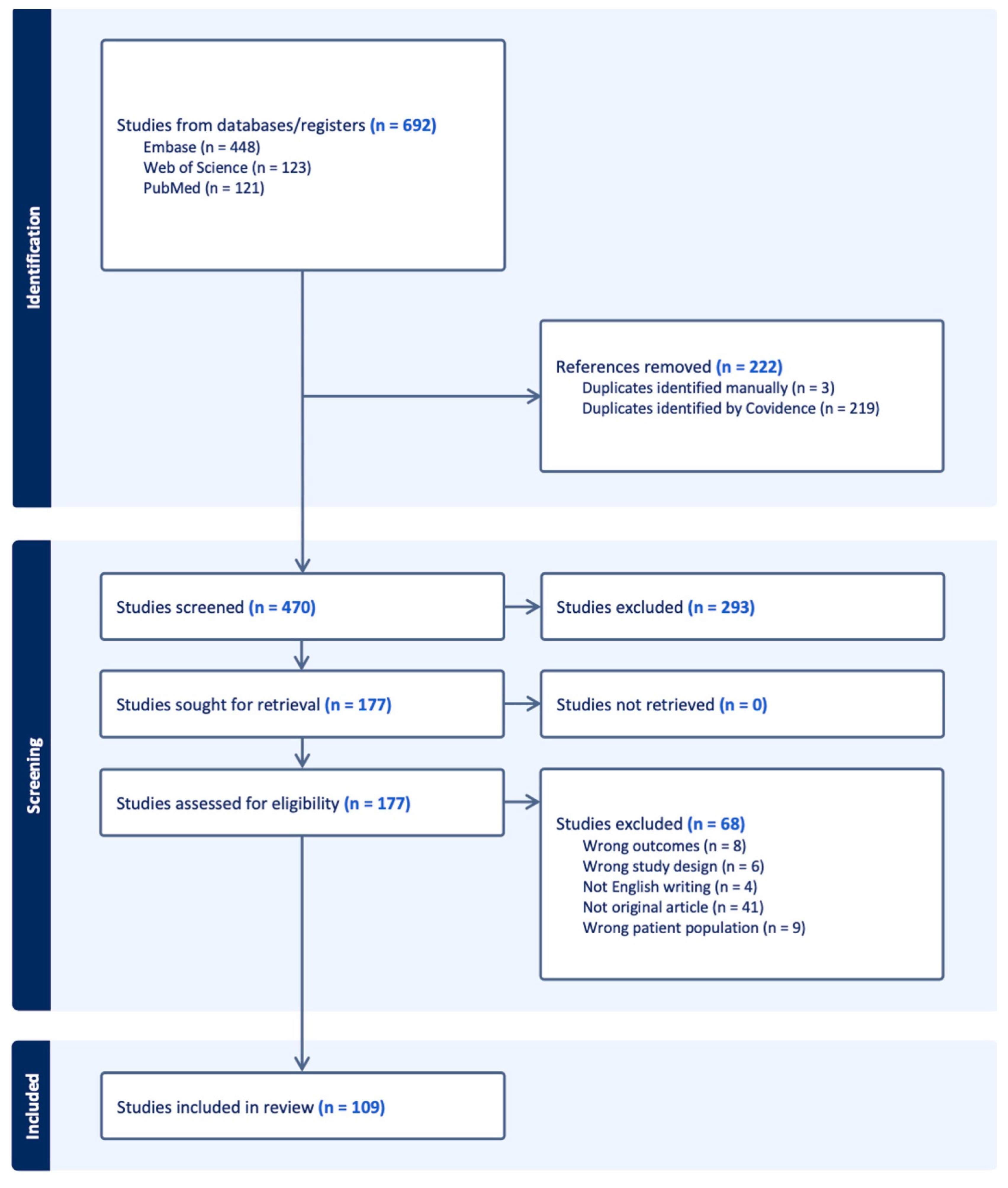
3.1. Study Selection
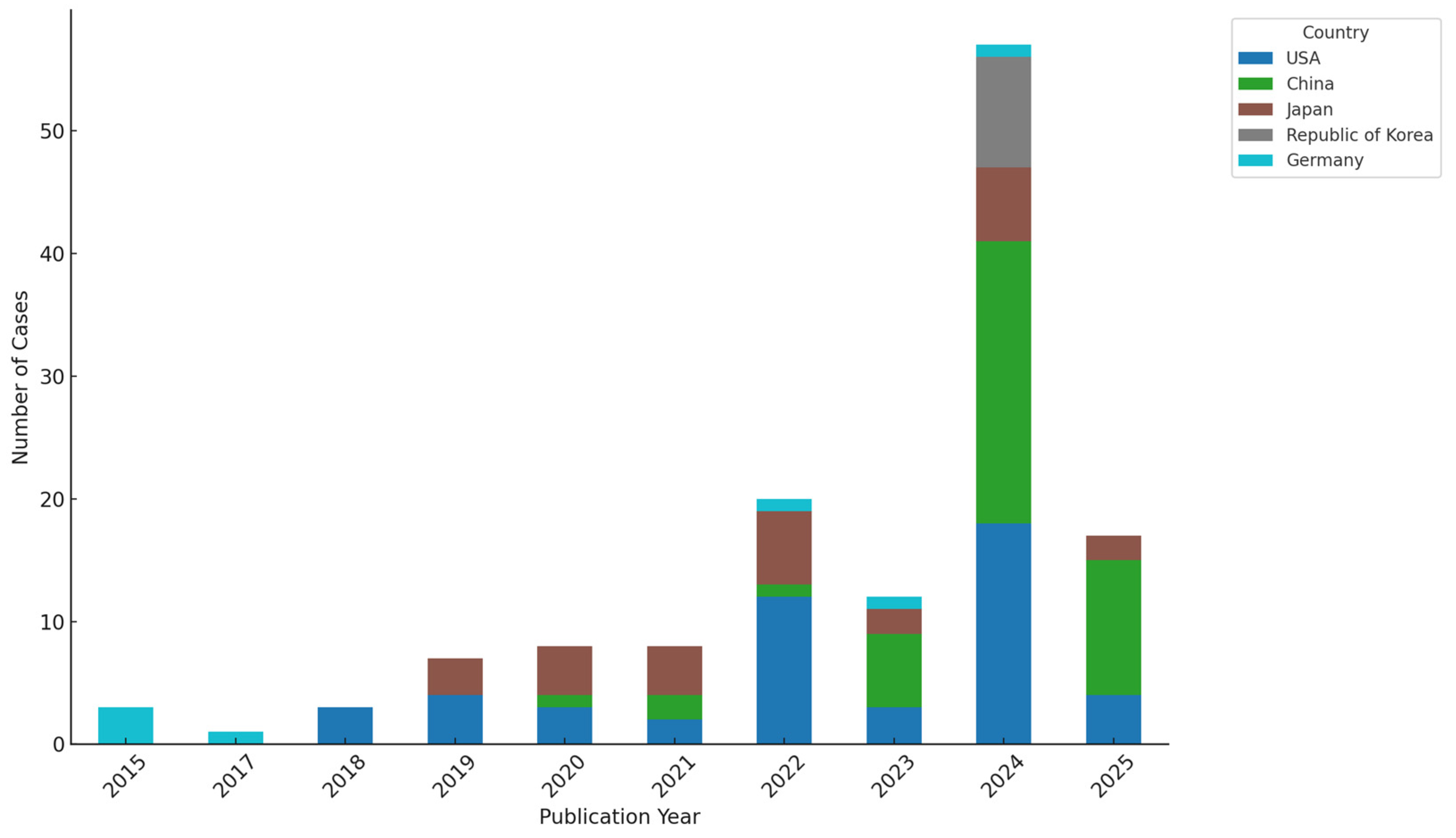
3.2. Study Characteristics
3.3. Demographic and Clinical Features
3.4. Pathological and Molecular Characteristics
3.5. Treatment Patterns and Outcomes
3.6. Risk of Bias Within Studies
4. Discussion
4.1. Summary of Main Findings
4.2. Comparison with the Previous Literature
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mardinian, K.; Adashek, J.J.; Botta, G.P.; Kato, S.; Kurzrock, R. SMARCA4: Implications of an Altered Chromatin-Remodeling Gene for Cancer Development and Therapy. Mol. Cancer Ther. 2021, 20, 2341–2351. [Google Scholar] [CrossRef]
- Fernando, T.M.; Piskol, R.; Bainer, R.; Sokol, E.S.; Trabucco, S.E.; Zhang, Q.; Sweeney, C.; Green, S.; Stetson, D.; Huw, L.Y.; et al. Functional Characterization of SMARCA4 Variants Identified by Targeted Exome-Sequencing of 131,668 Cancer Patients. Nat. Commun. 2020, 11, 5551. [Google Scholar] [CrossRef]
- Ye, W.; An, D.; Ou, W.-B. SMARCA4: Promises and Challenges in the Treatment of Cancers. Cancer Lett. 2025, 625, 217811. [Google Scholar] [CrossRef] [PubMed]
- Khanchel, F.; Hedhili, R.; Zenaidi, H.; Helal, I.; Yahmadi, A.; Ben Néji, H.; Ben Amar, M.; Hamdi, A.; Boudaya, M.S.; Bouzid, K. SMARCA4-Deficient Thoracic Sarcoma Revealed by Metastasis to the Small Intestine: A Diagnostic Dilemma. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1155–1158. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, L.; Li, X.; Li, H.; Zhao, M. SMARCA4: Current Status and Future Perspectives in Non-Small-Cell Lung Cancer. Cancer Lett. 2023, 554, 216022. [Google Scholar] [CrossRef] [PubMed]
- Al-Shbool, G.; Krishnan Nair, H. SMARCA4-Deficient Undifferentiated Tumor: A Rare Malignancy with Distinct Clinicopathological Characteristics. Cureus 2022, 14, e30708. [Google Scholar] [CrossRef]
- Liang, X.; Gao, X.; Wang, F.; Li, S.; Zhou, Y.; Guo, P.; Zhao, Q.; Zhang, M.; Liu, Y.; Chen, L. Clinical Characteristics and Prognostic Analysis of SMARCA4-Deficient Non-Small Cell Lung Cancer. Cancer Med. 2023, 12, 14171–14182. [Google Scholar] [CrossRef]
- Powell, F.L.; Haddad, P.A. Thoracic SMARCA4-Deficient Cancer Descriptors and Clinicopathologic Determinants of Survival: Analysis of a Pooled Database. J. Clin. Oncol. 2023, 41, 9049. [Google Scholar] [CrossRef]
- Longo, V.; Catino, A.; Montrone, M.; Montagna, E.S.; Pesola, F.; Marech, I.; Silvestris, N.; Santini, D.; Cives, M. Treatment of Thoracic SMARCA4-Deficient Undifferentiated Tumors: Where We Are and Where We Will Go. Int. J. Mol. Sci. 2024, 25, 63237. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Koneru, M.; Knapp, K.; Joneja, U.; Morrison, J.; Hong, Y.K. Identification and Treatment of SMARCA4-Deficient Poorly Differentiated Gastric Carcinoma. Am. Surg. 2022, 89, 4987–4989. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software ‘EZR’ for Medical Statistics. Bone Marrow Transplant. 2012, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Ararat, K.; Cutshall, H.; Gokden, M.; Rodriguez, A.; Rooper, L.; Lin, M.-T.; Chen, Y.; Kang, H.; Park, J.-Y.; et al. SMARCA4-Deficient Central Nervous System Metastases: A Case Series and Systematic Review. J. Neuropathol. Exp. Neurol. 2024, 83, 638–654. [Google Scholar] [CrossRef]
- Sheng, J.; Han, W.; Pan, H. Thoracic SMARCA4-Deficient Undifferentiated Tumor with ALK Fusion Treated with Alectinib Achieved Remarkable Tumor Regression: Case Report. JTO Clin. Res. Rep. 2023, 4, 100476. [Google Scholar] [CrossRef]
- Tamaki, I.; Kitagawa, K.; Kozai, H.; Yonenaga, Y.; Nitta, T. Mesenteric SMARCA2-Deficient Yet SMARCA4-Preserved Aggressive Undifferentiated Tumor: A Case Report. Surg. Case Rep. 2025, 11, 70. [Google Scholar] [CrossRef]
- Sauter, J.L.; Graham, R.P.; Larsen, B.T.; Jenkins, S.M.; Roden, A.C.; Boland, J.M. SMARCA4-Deficient Thoracic Sarcoma: A Distinctive Clinicopathological Entity with Undifferentiated Rhabdoid Morphology and Aggressive Behavior. Mod. Pathol. 2017, 30, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Field, N.R.; Dickson, K.-A.; Nassif, N.T.; Marsh, D.J. SMARCA4 and SMARCA2 Co-Deficiency: An Uncommon Molecular Signature Defining a Subset of Rare, Aggressive and Undifferentiated Malignancies Associated with Defective Chromatin Remodeling. Cancer Lett. 2024, 605, 217282. [Google Scholar] [CrossRef]
- Rekhtman, N.; Montecalvo, J.; Chang, J.C.; Alex, D.; Ptashkin, R.N.; Ai, N.; Liu, Y.; Wang, H.; Han, G.; Berry, L.D.; et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J. Thorac. Oncol. 2020, 15, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.S.; Qin, J. Switch/Sucrose Nonfermentable-Deficient Tumors—Morphology, Immunophenotype, Genetics, Epigenetics, Nosology, and Therapy. Lab. Investig. 2025, 105, 102185. [Google Scholar] [CrossRef]
- Manolakos, P.; Boccuto, L.; Ivankovic, D. A Critical Review of the Impact of SMARCA4 Mutations on Survival Outcomes in Non-Small Cell Lung Cancer. J. Pers. Med. 2024, 14, 684. [Google Scholar] [CrossRef]
- Shweikeh, F.; Hong, G.; Walter, J.; Hoscheit, M.; Lembo, A.; Mouchli, M.; Stauffer, J.A.; Nguyen, C.C.; Marshall, R.E.; Smoot, R.L.; et al. SMARCA4-Deficient Undifferentiated Esophageal Carcinoma: A Clinical Case Series and Literature Review. J. Gastrointest. Cancer 2024, 55, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Su, C.; Yao, J.; Li, X.; Lin, X. Retrospective Insights into the Clinicopathological Features and Treatment Outcomes of Thoracic SMARCA4-Deficient Tumors. Technol. Cancer Res. Treat. 2025, 24, 15330338251345377. [Google Scholar] [CrossRef] [PubMed]
- Gagné, A.; Alessi, J.V.M.; Ricciuti, B.; Lamberti, G.; Awad, M.M.; Sholl, L.M. Acquired SMARCA4 Alterations: An Uncommon Contributor to Cancer Progression in Lung Adenocarcinomas. Lung Cancer 2025, 206, 108644. [Google Scholar] [CrossRef]
- Cyrta, J.; Augspach, A.; De Filippo, M.R.; Prandi, D.; Thienger, P.; Benelli, M.; Cooley, V.; Kregel, S.; Rao, R.; Robinson, D.; et al. Role of Specialized Composition of SWI/SNF Complexes in Prostate Cancer Lineage Plasticity. Nat. Commun. 2020, 11, 5549. [Google Scholar] [CrossRef]
- Sood, R.; Tandon, A.; Khatoon, W.; Vasanthraman, J.; Nambirajan, A.; Mohan, A.; Malik, P.S.; Jain, D. Unravelling Switch/Sucrose Non-Fermentable (SWI-SNF) Complex-Deficient Thoracic Tumours: A Clinicopathological Comparative on Undifferentiated Tumours and Non-Small Cell Lung Carcinomas with BRG1 and BRM Deficiency. J. Clin. Pathol. 2024, 78, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Sewastjanow-Silva, M.; Yoshimura, K.; Rogers, J.E.; Vicentini, E.R.; Pizzi, M.P.; Fan, Y.; Zou, G.; Li, J.J.; Murphy, M.B.; et al. SMARCA4 Mutations in Gastroesophageal Adenocarcinoma: An Observational Study via a Next-Generation Sequencing Panel. Cancers 2024, 16, 1300. [Google Scholar] [CrossRef]
- Yatabe, Y.; Dacic, S.; Borczuk, A.C.; Warth, A.; Russell, P.A.; Lantuejoul, S.; Beasley, M.B.; Thunnissen, E.; Rekhtman, N.; Bubendorf, L.; et al. Best Practices Recommendations for Diagnostic Immunohistochemistry in Lung Cancer. J. Thorac. Oncol. 2019, 14, 377–407. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Q.; Wang, J.; Zhang, X.; Lv, Y. Efficacy of Immune Checkpoint Inhibitors in SMARCA4-Deficient and TP53 Mutant Undifferentiated Lung Cancer. Medicine 2024, 103, e36959. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Liu, J.; Li, J.; Sun, J.; Zehentmayr, F.; Gomez-Randulfe, I.; Liang, Y. SMARCA4-Deficient Undifferentiated Thoracic Tumor: A Case Report and Literature Review. J. Thorac. Dis. 2025, 17, 2730–2740. [Google Scholar] [CrossRef]
- Kotagiri, S.; Blazanin, N.; Xi, Y.; Han, Y.; Qudratullah, M.; Liang, X.; Wang, Y.; Pandey, P.; Mazhar, H.; Lam, T.N.; et al. Enhancer Reprogramming Underlies Therapeutic Utility of a SMARCA2 Degrader in SMARCA4 Mutant Cancer. Cell Chem. Biol. 2024, 31, 2069–2084.e2069. [Google Scholar] [CrossRef]
- Cantley, J.; Ye, X.; Rousseau, E.; Januario, T.; Hamman, B.D.; Rose, C.M.; Cheung, T.K.; Hinkle, T.; Soto, L.; Quinn, C.; et al. Selective PROTAC-Mediated Degradation of SMARCA2 Is Efficacious in SMARCA4 Mutant Cancers. Nat. Commun. 2022, 13, 6814. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ogiwara, H. Synthetic Lethal Therapy Based on Targeting the Vulnerability of SWI/SNF Chromatin Remodeling Complex-Deficient Cancers. Cancer Sci. 2020, 111, 774–782. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Age, median (range) | 58 years (18–88) |
| Sex—male | 112 (70.0%) |
| Sex—female | 48 (30.0%) |
| Smoking history documented | 71 (44.4%) |
| Most common primary tumor site | Thorax |
| Top 3 primary tumor sites | Thorax (59, 40.0%), gastrointestinal (28, 17.5%), Gynecologic (25, 15.6%) |
| Initial symptoms | Abdominal pain/discomfort, fatigue, weight loss, cough, neurologic symptoms |
| Cases without symptom description | 9 (5.6%) |
| Stage at diagnosis—metastatic (stage IV or stated as such) | 50 (31.3%) |
| Stage at diagnosis—unspecified | 110 (68.7%) |
| Most common metastatic sites | Liver (30), brain (18), lung, lymph nodes, none |
| Cases without metastasis at presentation | 34 (21.3%) |
| Cases without metastasis information | 2 (1.3%) |
| Category | Value |
|---|---|
| Detection method of SMARCA4 (IHC or NGS) | Reported in majority (≥86.9%) of cases |
| •Immunohistochemistry (IHC)-based confirmation | 139 cases (86.9%) |
| •Next-generation sequencing (NGS)-based analysis | 68 cases (42.5%) |
| Co-occurring genetic alterations (Top 5) | Reported in 60.6% of patients with available data |
| •TP53 | 12 cases |
| •ALK | 5 cases |
| •KRAS | 3 cases |
| •FAT1 | 3 cases |
| •PIK3CA | 3 cases |
| PD-L1 expression (n = 33 cases) | 33 cases reported |
| •PD-L1 ≥1% | 28 (84.8%) |
| •PD-L1 <1% | 4 (12.1%) |
| •Not quantifiable | 1 (3.0%) |
| Specific Agent or Procedure | No. of Patients | Median PFS (Months) | PFS IQR | Median OS (Months) | OS IQR |
|---|---|---|---|---|---|
| Chemotherapy (n = 74) | |||||
| Paclitaxel | 35 | 3.5 | 2.0–6 | 7.0 | 4.2–10.8 |
| Carboplatin | 34 | 3.5 | 2.2–5.8 | 7.0 | 4.0–11.5 |
| Cisplatin | 19 | 5.0 | 2.4–7.2 | 10.2 | 6.0–13.1 |
| Etoposide | 14 | 4.0 | 2.2–7.0 | 12.0 | 6.5–12.8 |
| Docetaxel | 9 | 2.0 | 2.0–2.0 | 4.0 | 3.0–5.0 |
| Gemcitabine | 9 | 7.0 | 4.5–9.5 | 6.0 | 5.0–6.0 |
| Nab-paclitaxel | 6 | 2.0 | 2.0–4.0 | 7.0 | 6.0–10.0 |
| Irinotecan | 1 | NR | NR | NR | NR |
| Immunotherapy (n = 18) | |||||
| Pembrolizumab | 16 | 4.5 | 3.2–5.8 | 6.0 | 4.6–9.0 |
| Nivolumab | 11 | 7.0 | 4.5–9.5 | 5.5 | 3.6–19.8 |
| Atezolizumab | 5 | 8.0 | 5.0–8.5 | 7.0 | 5.0–9.0 |
| Ipilimumab | 3 | 5.0 | NR | 14.8 | 9.1–20.4 |
| Durvalumab | 1 | NR | NR | 17.0 | NR |
| Surgery (n = 42) | |||||
| Tumor resection | 1 | NR | NR | 13 | NR |
| Lobectomy | 9 | 5.5 | 3.8–7.2 | 6.5 | 5.2–7.8 |
| Surgery (unspecified) | 38 | 4 | 2.1–5.5 | 6 | 5.0–12.0 |
| Excision | 2 | NR | NR | 5 | NR |
| Tumor Site | Treatment Modality | Number of Patients | Median OS (Months) | Median PFS (Months) |
|---|---|---|---|---|
| Thoracic | Immune Checkpoint Inhibitor | 21 | 8.0 | 7.0 |
| Thoracic | Chemotherapy | 33 | 9.0 | 7.0 |
| Thoracic | Surgery | 18 | 9.0 | 4.5 |
| Gastrointestinal | Immune Checkpoint Inhibitor | 2 | 15.5 | 7.0 |
| Gastrointestinal | Chemotherapy | 2 | 15.5 | 7.0 |
| Gastrointestinal | Surgery | 12 | 5.5 | 7.0 |
| Gynecologic | Immune Checkpoint Inhibitor | 3 | 7.0 | 5.0 |
| Gynecologic | Chemotherapy | 13 | 7.5 | 3.2 |
| Gynecologic | Surgery | 8 | 9.5 | 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, R.; Yamamoto, N.; Tanaka, K.; Sano, C.; Hayashi, H. Clinical Characteristics and Outcomes of SMARCA4-Mutated or Deficient Malignancies: A Systematic Review of Case Reports and Series. Cancers 2025, 17, 2675. https://doi.org/10.3390/cancers17162675
Ohta R, Yamamoto N, Tanaka K, Sano C, Hayashi H. Clinical Characteristics and Outcomes of SMARCA4-Mutated or Deficient Malignancies: A Systematic Review of Case Reports and Series. Cancers. 2025; 17(16):2675. https://doi.org/10.3390/cancers17162675
Chicago/Turabian StyleOhta, Ryuichi, Natsumi Yamamoto, Kaoru Tanaka, Chiaki Sano, and Hidetoshi Hayashi. 2025. "Clinical Characteristics and Outcomes of SMARCA4-Mutated or Deficient Malignancies: A Systematic Review of Case Reports and Series" Cancers 17, no. 16: 2675. https://doi.org/10.3390/cancers17162675
APA StyleOhta, R., Yamamoto, N., Tanaka, K., Sano, C., & Hayashi, H. (2025). Clinical Characteristics and Outcomes of SMARCA4-Mutated or Deficient Malignancies: A Systematic Review of Case Reports and Series. Cancers, 17(16), 2675. https://doi.org/10.3390/cancers17162675







