Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Studies
3.2. Oral Microbiome in HNSCC
3.2.1. Phylum-Level Differences Between HNSCC and Controls
3.2.2. Class, Order and Family Level Differences
3.2.3. Genus-Level Differences
3.2.4. Species-Level Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isayeva, T.; Li, Y.; Maswahu, D.; Brandwein-Gensler, M. Human papillomavirus in non-oropharyngeal head and neck cancers: A systematic literature review. Head. Neck. Pathol. 2012, 6 (Suppl. S1), S104–S120. [Google Scholar] [CrossRef]
- Stein, A.P.; Saha, S.; Kraninger, J.L.; Swick, A.D.; Yu, M.; Lambert, P.F.; Kimple, R.J. Prevalence of human papillomavirus in oropharyngeal cancer: A systematic review. Cancer J. 2015, 21, 138–146. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. Embo Rep. 2018, 19. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome–Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef]
- Pignatelli, P.; Nuccio, F.; Piattelli, A.; Curia, M.C. The Role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 2023, 11, 2358. [Google Scholar] [CrossRef]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Yang, F.; Huang, Z.-Q.; Li, Y.-Y.; Shi, H.-Y.; Sun, Q.; Ma, Y.; Wang, Y.; Zhang, Y.; Yang, S.; et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front. Immunol. 2023, 14, 1199173. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Bhat, S.G.; Sivaraman, K. Porphyromonas gingivalis adopts intricate and unique molecular mechanisms to survive and persist within the host: A critical update. J. Oral Microbiol. 2020, 12, 1801090. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Debusk, W.T.; Stepanov, I.; Gomez, A.; Khariwala, S.S. Oral Microbiome Profiling in Smokers with and without Head and Neck Cancer Reveals Variations Between Health and Disease. Cancer Prev. Res. 2020, 13, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, W.J.; Wang, K.; Zarins, K.; Bellile, E.; Blostein, F.; Argirion, I.; Taylor, J.M.G.; D’silva, N.J.; Chinn, S.B.; Rifkin, S.; et al. Oral Microbiome Community Composition in Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 2549. [Google Scholar] [CrossRef]
- Yan, K.; Auger, S.; Diaz, A.; Naman, J.; Vemulapalli, R.; Hasina, R.; Izumchenko, E.; Shogan, B.; Agrawal, N. Microbial Changes Associated With Oral Cavity Cancer Progression. Otolaryngol. Neck Surg. 2023, 168, 1443–1452. [Google Scholar] [CrossRef]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef]
- Wu, Z.; Han, Y.; Wan, Y.; Hua, X.; Chill, S.S.; Teshome, K.; Zhou, W.; Liu, J.; Wu, D.; Hutchinson, A.; et al. Oral microbiome and risk of incident head and neck cancer: A nested case-control study. Oral Oncol. 2023, 137, 106305. [Google Scholar] [CrossRef]
- Ganly, I.; Hao, Y.; Rosenthal, M.; Wang, H.; Migliacci, J.; Huang, B.; Katabi, N.; Brown, S.; Tang, Y.-W.; Pei, Z.; et al. Oral Microbiome in Nonsmoker Patients with Oral Cavity Squamous Cell Carcinoma, Defined by Metagenomic Shotgun Sequencing. Cancers 2022, 14, 6096. [Google Scholar] [CrossRef]
- Mougeot, J.-L.C.; Beckman, M.F.; Langdon, H.C.; Lalla, R.V.; Brennan, M.T.; Mougeot, F.K.B. Haemophilus pittmaniae and Leptotrichia spp. Constitute a Multi-Marker Signature in a Cohort of Human Papillomavirus-Positive Head and Neck Cancer Patients. Front. Microbiol. 2022, 12, 794546. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Funchain, P.; Bebek, G.; Altemus, J.; Zhang, H.; Niazi, F.; Peterson, C.; Lee, W.T.; Burkey, B.B.; Eng, C. Microbiomic differences in tumor and paired-normal tissue in head and neck squamous cell carcinomas. Genome Med. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Zhang, C.; Hua, H.; Hu, X. Compositional and functional changes in the salivary microbiota related to oral leukoplakia and oral squamous cell carcinoma: A case control study. BMC Oral Heal. 2023, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.K.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.-W.; Cai, L.; Lei, P.; Mou, Q.; Meehan, K.; Lau, E.H.L.; et al. Restoration of the Oral Microbiota After Surgery for Head and Neck Squamous Cell Carcinoma Is Associated With Patient Outcomes. Front. Oncol. 2021, 11, 737843. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Feng, Q.; Chen, B.; Li, M.; Liang, C.; Li, M.; Li, Z.; Xu, Q.; Zhang, L.; et al. Compositional and Functional Analysis of the Microbiome in Tissue and Saliva of Oral Squamous Cell Carcinoma. Front. Microbiol. 2019, 10, 1439. [Google Scholar] [CrossRef]
- Oyeyemi, B.F.; Kaur, U.S.; Paramraj, A.; Chintamani; Tandon, R.; Kumar, A.; Bhavesh, N.S. Microbiome analysis of saliva from oral squamous cell carcinoma (OSCC) patients and tobacco abusers with potential biomarkers for oral cancer screening. Heliyon 2023, 9, e21773. [Google Scholar] [CrossRef]
- Aparna, K.; Ravindra, J.; Chakraborty, G.; Ballamoole, K.K.; Kumar, J.V.; Chakraborty, A. 16S rRNA based metagenomic analysis unveils unique oral microbial signatures in oral squamous cell carcinoma cases from Coastal Karnataka, India. Acta Microbiol. Et Immunol. Hung. 2024, 71, 253–262. [Google Scholar] [CrossRef]
- Mäkinen, A.I.; Pappalardo, V.Y.; Buijs, M.J.; Brandt, B.W.; Mäkitie, A.A.; Meurman, J.H.; Zaura, E. Salivary microbiome profiles of oral cancer patients analyzed before and after treatment. Microbiome 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Unlu, O.; Demirci, M.; Paksoy, T.; Eden, A.B.; Tansuker, H.D.; Dalmizrak, A.; Aktan, C.; Senel, F.; Sunter, A.V.; Yigit, O.; et al. Oral microbial dysbiosis in patients with oral cavity cancers. Clin. Oral Investig. 2024, 28, 1–15. [Google Scholar] [CrossRef]
- Lessa, A.d.F.N.; Amâncio, A.M.T.d.S.; de Oliveira, A.C.R.; de Sousa, S.F.; Caldeira, P.C.; De Aguiar, M.C.F.; Bispo, P.J.M. Assessing the oral microbiome of head and neck cancer patients before and during radiotherapy. Support. Care Cancer 2024, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Szczesniak, M.; Maclean, J.; Yim, H.C.H.; Zhang, F.; Graham, P.; El-Omar, E.M.; Wu, P. Dysbiosis in Head and Neck Cancer: Determining Optimal Sampling Site for Oral Microbiome Collection. Pathogens 2022, 11, 1550. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Radcliff, F.J.; Taylor, M.W.; Douglas, R.G. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin. Exp. Dent. Res. 2018, 4, 255–262. [Google Scholar] [CrossRef]
- Yu, X.; Shi, Y.; Yuan, R.; Chen, Z.; Dong, Q.; Han, L.; Wang, L.; Zhou, J. Microbial dysbiosis in oral squamous cell carcinoma: A systematic review and meta-analysis. Heliyon 2023, 9, e13198. [Google Scholar] [CrossRef]
- Sahin, T.K.; Sonmezer, M.C. The role of the microbiome in head and neck squamous cell cancers. Eur. Arch. Oto-Rhino-L. 2024, 282, 623–637. [Google Scholar] [CrossRef]
- van Dijk, M.C.; Petersen, J.F.; Raber-Durlacher, J.E.; Epstein, J.B.; Laheij, A.M.G.A. Diversity and compositional differences in the oral microbiome of oral squamous cell carcinoma patients and healthy controls: A scoping review. Front. Oral Heal. 2024, 5, 1366153. [Google Scholar] [CrossRef]
- Chen, Z.; Wong, P.Y.; Ng, C.W.K.; Lan, L.; Fung, S.; Li, J.W.; Cai, L.; Lei, P.; Mou, Q.; Wong, S.H.; et al. The Intersection between Oral Microbiota, Host Gene Methylation and Patient Outcomes in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 3425. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18. [Google Scholar] [CrossRef]
- Niccolai, E.; Russo, E.; Baldi, S.; Ricci, F.; Nannini, G.; Pedone, M.; Stingo, F.C.; Taddei, A.; Ringressi, M.N.; Bechi, P.; et al. Significant and Conflicting Correlation of IL-9 With Prevotella and Bacteroides in Human Colorectal Cancer. Front. Immunol. 2021, 11, 573158. [Google Scholar] [CrossRef]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.-H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2020, 82, 1030–1046. [Google Scholar] [CrossRef]
- Healy, C.M.; Moran, G.P. The microbiome and oral cancer: More questions than answers. Oral Oncol. 2019, 89, 30–33. [Google Scholar] [CrossRef]
- Harrandah, A.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Progulske-Fox, A.; Chan, E.K.L. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J. Oral Microbiol. 2020, 13, 1849493. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.O.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral Sci. 2018, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, H.; Hirota, K.; Hirao, K.; Ninomiya, M.; Murakami, K.; Fujii, H.; Miyake, Y. The Pathogenic Factors from Oral Streptococci for Systemic Diseases. Int. J. Mol. Sci. 2019, 20, 4571. [Google Scholar] [CrossRef] [PubMed]
- Zaura, E.; Brandt, B.W.; de Mattos, M.J.T.; Buijs, M.J.; Caspers, M.P.M.; Rashid, M.-U.; Weintraub, A.; Nord, C.E.; Savell, A.; Hu, Y.; et al. Same Exposure but Two Radically Different Responses to Antibiotics: Resilience of the Salivary Microbiome versus Long-Term Microbial Shifts in Feces. mBio 2015, 6, e01693-15. [Google Scholar] [CrossRef]
- Han, Y.W.; Shi, W.; Huang, G.T.-J.; Haake, S.K.; Park, N.-H.; Kuramitsu, H.; Genco, R.J.; O’BRien, A.D. Interactions between Periodontal Bacteria and Human Oral Epithelial Cells: Fusobacterium nucleatum Adheres to and Invades Epithelial Cells. Infect. Immun. 2000, 68, 3140–3146. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and oral cancer: A critical review. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Hoppe, T.; Kraus, D.; Novak, N.; Probstmeier, R.; Frentzen, M.; Wenghoefer, M.; Jepsen, S.; Winter, J. Oral pathogens change proliferation properties of oral tumor cells by affecting gene expression of human defensins. Tumor Biol. 2016, 37, 13789–13798. [Google Scholar] [CrossRef]
- Eun, Y.-G.; Lee, J.-W.; Kim, S.W.; Hyun, D.-W.; Bae, J.-W.; Lee, Y.C. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.; Tangney, M. Bacteria and tumours: Causative agents or opportunistic inhabitants? Infect. Agents Cancer 2013, 8, 11. [Google Scholar] [CrossRef]
- Marsh, P. In Sickness and in Health—What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Gupta, R.; Gaur, S. Effect of diet and lifestyle on microbiome composition. Int. Rev. Cell Mol. Biol. 2025, 395, 157–174. [Google Scholar] [CrossRef]
- Zagury-Orly, I.; Khaouam, N.; Noujaim, J.; Desrosiers, M.Y.; Maniakas, A. The Effect of Radiation and Chemoradiation Therapy on the Head and Neck Mucosal Microbiome: A Review. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Zirk, M.; Wenzel, C.; Buller, J.; Zöller, J.E.; Zinser, M.; Peters, F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin. Oral Investig. 2018, 23, 2143–2151. [Google Scholar] [CrossRef]
- Pai, S.I.; Matheus, H.R.; Guastaldi, F.P.S. Effects of periodontitis on cancer outcomes in the era of immunotherapy. Am. J. Med Sci. 2023, 4, e166–e175. [Google Scholar] [CrossRef]
- Bruno, J.S.; Al-Qadami, G.H.; Laheij, A.M.G.A.; Bossi, P.; Fregnani, E.R.; Wardill, H.R. From Pathogenesis to Intervention: The Importance of the Microbiome in Oral Mucositis. Int. J. Mol. Sci. 2023, 24, 8274. [Google Scholar] [CrossRef]
- Amato, M.; Di Spirito, F.; D’ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Pacheco-Yanes, J.; Reynolds, E.; Li, J.; Mariño, E. Microbiome-targeted interventions for the control of oral–gut dysbiosis and chronic systemic inflammation. Trends Mol. Med. 2023, 29, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics 2023, 12, 868. [Google Scholar] [CrossRef]
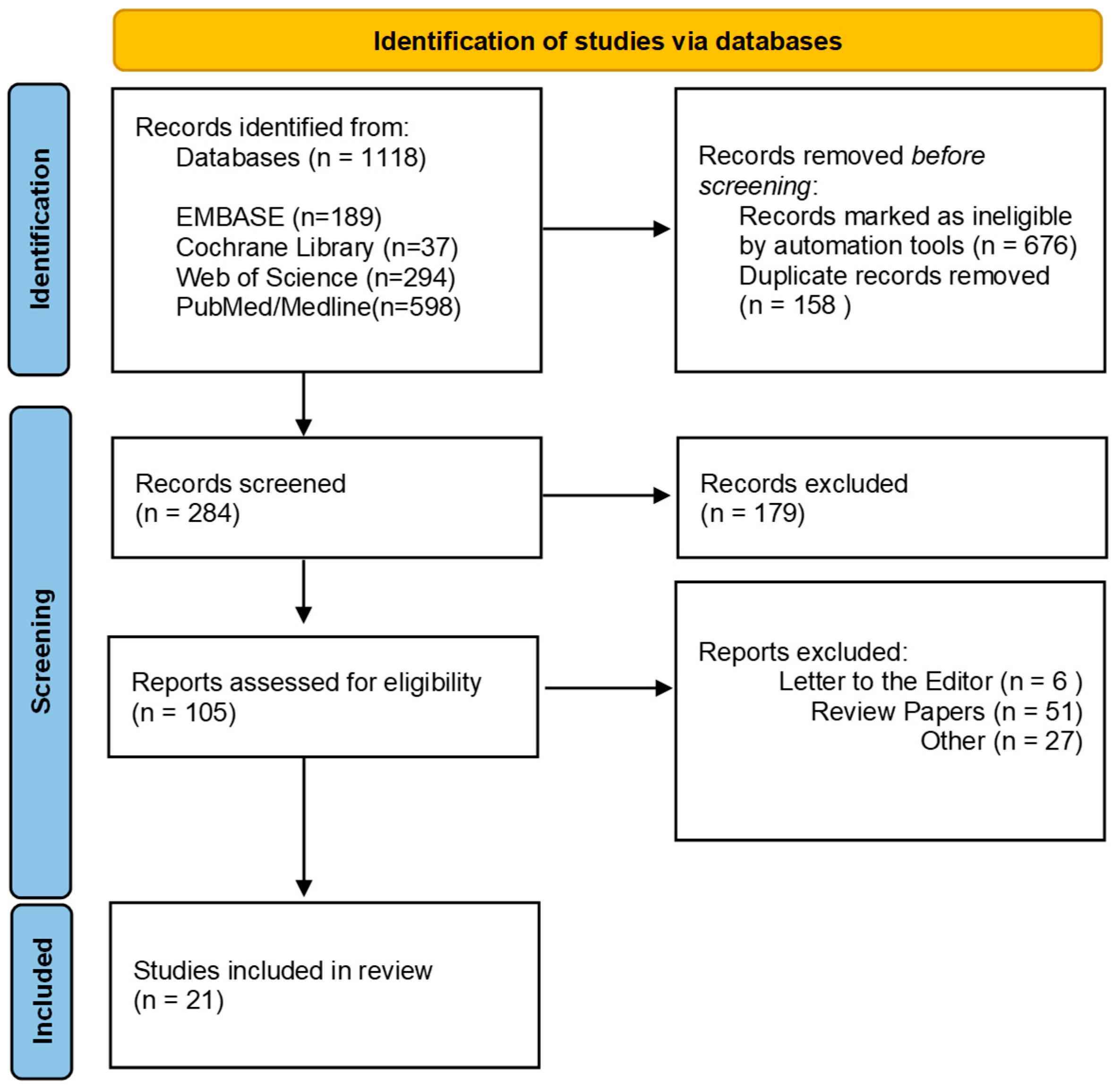
| First Author, Year, Country | Sample Size Age/Range | Treatment/Site | Stage | Lifestyle | Sample Collection | Sample Analysis | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Smoking | Alcohol | ||||||||
| de Freitas Neiva Lessa, A. et al., 2024, Brazil [32] | P (n= 49) C (n = 25) P (59 yrs) C (53 yrs) | CRT (n= 33) SRT (n = 7) SCRT (n = 5) RT alone (n = 4) | Oropharynx cancer (n = 20) Larynx cancer (n = 15) Oral cancer (n = 14) | Oropharynx cancer [stages IVA (n = 12), IVB (n = 6) and III (n = 2)], larynx cancer [stages IVA (n = 8), III (n = 4), I (n = 2), and IVB (n = 1)], oral cancer [stages IVA (n= 12), IVB (n =1), and III (n = 1)] | C (n = 8) P (n = 21) | C (n = 5) P (n = 7) | Oral Swab | 16S rRNA V1–V2 | Bacteroidetes were significantly more abundant in patients with HNSCC, whereas Firmicutes—particularly members of the genus Streptococcus—were depleted. Within the Bacteroidetes phylum, Prevotella and Porphyromonas were the predominant genera. |
| Unlu, O. et al., 2024, Turkey [31] | P (n= 10) C (n= 12) P (61 yrs) C (57 yrs) | Before Treatment | Oral cavity cancer | N/A | P (n = 10) C (n = 8) | P (n = 2) C (n = 4) | Saliva | 16S rRNA V3–V4 | Patients with oral cancer exhibited poorer oral health and a distinct oral microbiome composition influenced by daily personal habits, which may contribute to disease pathogenesis. Improved oral hygiene and management of periodontal disease may help limit oral cancer development and progression. |
| Aparna, K. et al., 2024, India [29] | P (n= 13) C (n= 13) P (55 yrs) | N/A | Stage II (pT2N0) Stage III (pT3N0) Stage IVA (pT4aN0) | N/A | N/A | Tumor Tissue, Normal Tissue | 16S rRNA V3–V4 | Fusobacterium, Prevotella, Capnocytophaga, Leptotrichia, Peptostreptococcus, Parvimonas, and members of the Bacteroidetes phylum were significantly enriched in oral squamous cell carcinoma (OSCC) lesions compared to adjacent non-cancerous tissues. | |
| Mäkinen, A. et al., 2023, Finland [30] | P (n= 99) C (n= 101) P (68 yrs) C (66 yrs) | RT (n = 44) CT (n = 5) | Oral cavity cancer of squamous cell origin | Stages I–II (n = 57) Stages III–IV (n = 42) | P (n = 43) C (n = 8) | P (n = 62) C (n = 59) | Saliva | 16S rRNA V4 | Salivary microbial profiles differed significantly between patients with oral squamous cell carcinoma (OSCC) and healthy controls. At baseline, OSCC patients exhibited ecologically adverse alterations—namely, increased proportions of aciduric taxa, reduced α-diversity, and elevated relative abundances of potentially pathogenic taxa. |
| Lan, Q. et al., 2023, China [24] | P (n= 18) C (n= 21) P (54 yrs) C (48 yrs) | Before Treatment | Oral cavity cancer | N/A | P (n = 5) C (n = 2) | P (n = 5) C (n = 2) | Saliva | Metagenomic sequencing | Salivary microbiota profiles differed significantly among patients with oral squamous cell carcinoma (OSCC), oral leukoplakia (OLK), and healthy controls (HCs). These compositional and functional alterations in the salivary microbiota may be linked to OSCC progression. |
| Benjamin, W.J. et al., 2023, USA [17] | P (n= 52) C (n= 102) P (59 yrs) C (59 yrs) | Before Treatment | Larynx Oral cavity Oropharynx Hypopharynx Nasal cavity, Sinus, or Skull Unknown primary | Stage 1 or 2 (34%) Stage 3 (17%) Stage 4 (48%) | P {(Never (n = 15) Former (n = 24) Current (n= 2)} C {(Never (n = 51) Former (n = 36) Current (n = 9)} | P {(Never (n = 3) Former (n = 12) Current (n = 26)} C {(Never (n = 5) Former (n = 18) Current (n = 72)} | Oral Wash | 16S rRNA V4 | Patients with HNSCC exhibited enrichment of the families Lachnospiraceae and Eikenella—taxa previously implicated in periodontitis—suggesting that preservation of a healthy oral microbiome may confer protection against HNSCC. A community type dominated by periodontitis-associated genera (Fusobacterium and Prevotella) was more frequently observed in older individuals and HNSCC patients, whereas a community enriched in commensal taxa (Streptococcus and Rothia) was more common in younger, cancer-free controls. |
| Yan, K. et al., 2023, USA [18] | P (n= 35) C (n= 31) P (66 yrs) C (64 yrs) | Before Treatment | Oral cavity | T1-T2 (n = 22) T3-T4 (n = 13) | P:{(Never (n = 13) Former (n = 14) Current (n = 8)} C:{(Never (n = 12) Former (n = 16) Current n = 3)} | P {(Yes (n = 23) No (n = 11)} C {(Yes (n = 15) No (n = 13)} | Oral Swabs | 16S rRNA N/A | Alterations in the relative abundance of bacterial genera have been associated with oral cavity carcinogenesis and disease progression. The abundance of several genera—including Fusobacterium, Peptostreptococcus, Parvimonas, Neisseria, and Treponema—was positively correlated with advancing tumor stage. |
| Oyeyemi, B.F. et al., 2023, India [28] | P (n= 10) C (n= 10) P (55 yrs) C (23 yrs) | N/A | Oral cavity | Stage I (n = 1) Sage II (n = 1) Stage III (n = 4) Stage IV (n = 4) | P (n = 9) C (n = 0) | P {(Yes (n = 7) No (n = 03)} C {(Yes (n = 00) No (n = 10)} | Saliva | 16S rRNA V3–V4 | The significant role of dysregulated microbial taxa in the development of oral squamous cell carcinoma (OSCC) and their association with smokeless tobacco use has been identified. Abnormal alterations in the oral microbiota may trigger chronic inflammatory responses, potentially leading to the activation of oncogenes and other tumor-promoting pathways. |
| Wu, Z. et al., 2023, USA [20] | P (n= 53) C (n= 110) P (72 yrs) C (71 yrs) | N/A | Oral cavity Pharynx | N/A | P {(Never (n = 15) Former (n = 32) Current (n = 06)} C {(Never (n = 29) Former (n = 65) Current (n = 16)} | P {(Yes (n = 46) No (n = 07)} C {(Yes (n = 90) No (n = 20)} | Oral Wash | Shotgun metagenomic sequencing | Alpha and beta diversity did not differ significantly between head and neck cancer (HNC) patients and controls. However, the presence of oral fungi and the relative abundance of several microbial species—including red and orange complex periodontal pathogens—were associated with a reduced risk of HNC. |
| Pandey, D. et al., 2022, Australia [34] | P (n= 21) C (n= 27) P (59 yrs) C (63 yrs) | Before Treatment | Mucosal squamous carcinoma of the tongue, buccal mucosa, tonsil, palate, hypopharynx, larynx | N/A | P (n = 38.1%) C (n = 22.2%) | P (n = 38.1%) C (n = 38.1%) | Saliva Tissue Oral Swab | 16S rRNA V3–V4 | Saliva, tissue, and oral swab samples were compared to evaluate their utility in oral microbiome analysis. Saliva microbiomes were found to be the most diverse and exhibited higher temporal stability. Moreover, salivary profiles effectively distinguished HNC patients from healthy controls. |
| Ganly, I. et al., 2022 USA [21] | P (n= 42) C (n= 45) P (63 yrs) C (63 yrs) | SRT (n = 24) SR post RT (n = 18) | Tongue 24 (57%) Floor of mouth 5 (12%) Upper gum 3 (7.2%) Lower gum 6 (14%) Buccal 2 (4.8%) Retromolar trigone 2 (4.8%) Lip | Stage I (n = 20) Stage II (n = 4) Stage III (n = 6) Stage IV (n = 12) | P {(Never (n = 22) Quit (n = 20)} C {(Never (n = 24) Quit (n = 21)} | P {(Yes (n = 27) No (n= 15)} C {(Yes (n = 30) No (n = 15)} | Oral Wash | Shotgun metagenomic sequencing | The taxonomic composition of the oral microbiome in patients with oral cavity squamous cell carcinoma (OC-SCC) is similarly altered in both smokers and non-smokers. |
| Mougeot, J.L.C. et al., 2022, USA [22] | P (n= 23) C (n= 20) P (49 yrs) C (66 yrs) | N/A | Base of tongue Nasopharynx Oral cavity Oropharynx Supraglottis Tongue Tonsil | N/A | N/A | N/A | Saliva, Oral Swab | 16S rRNA V3–V4 | The oral microbiome profiles of HNC patients with HPV-positive (HPV+) and HPV-negative (HPV−) status differed significantly, particularly in the abundance of periodontal-associated species. The findings suggest that certain oral bacterial species, such as Leptotrichia spp., which possess unique ecological niches and invasive capabilities, may coexist with HPV within HPV-induced oral lesions. |
| Chan, J.Y.K. et al., 2022, China [25] | P (n= 76) C (n= 76) P (≤ 60 = 29,> 60 = 47) C (≤ 60 = 31, >60 = 45) | Pre and Post Surgery | Oral cavity (n = 45) Larynx (n = 12) Oropharynx (n = 11) Hypopharynx (n = 5) Nasal cavity (n = 2) Paranasal sinus (n = 1) | T1 (n = 23) T2 (n = 20) T3 (n = 9) T4 (n = 24) N0 (n = 40) N1 (n = 14) N2 (n = 22) | P {(Yss n = 26, No(n= 50) C {(Yes (n= 26), No (n= 50)} | P {(Yes (n = 18), No (n = 58)} C {(Yes (n = 17) No (n = 59)} | Oral Rinse | 16S rRNA V3–V4 | Oral microbiome dysbiosis associated with HNSCC is dynamic, showing a post-treatment trend toward the re-establishment of microbial communities that resemble those of healthy individuals. These post-treatment microbiome shifts were also associated with patient outcomes and may serve as potential biomarkers for prognosis and clinical management in HNSCC. |
| Sharma, A.K. et al., 2020, USA [16] | P 27 C 24 P (58 yrs) C (48 yrs) | N/A | Oral cavity (n = 6) Oropharynx (n = 12) Larynx (n = 6) Hypopharynx (n = 2) N/A (n = 1) | Stage I = 3 Stage II = 5 Stage III = 4 Stage IV = 15 | C (18.8 mean Per day) Cases (13.9 mean per day) | (Never = Case7, Control 8) (Monthly = Case 2, Control 4) (2-4/month= Case 3, Control 5) (> 4/month = Case13, Control 7) (N/A= Case 2, Control 0) | Brushing Oral Mucosa | 16S rRNA V4 | Significant alterations in the oral microbiome were observed between smokers with oral and head and neck cancer (HNC) and cancer-free smokers. Specific bacterial taxa—most notably Stenotrophomonas—were positively associated with elevated DNA adduct levels and enhanced xenobiotic metabolism. Additionally, increased bacterial richness and diversity, along with the presence of these taxa, were linked to tobacco-related oral and head and neck carcinogenesis. |
| Zhang, L. et al., 2020, China [26] | P (n = 50) C (n = 50) P (61 yrs) C (61 yrs) | NA | Oral buccal mucosa | Stage I (n = 23) Sage II (n = 16) Stage III (n = 8) Stage IV (n = 3) | P {(Never (n = 24) Former (n = 17) Current (n = 09)} | P {(Never (n = 13) Former (n = 20) Current (n = 17) | Cancer Tissue, Normal Tissue | 16S rRNA V3–V4 | Oral bacterial profiles showed significant differences between cancer sites and normal tissue of OSCC patients, which might be considered diagnostic markers and treatment targets. |
| Zhang, Z. et al., 2019, China [27] | P (n= 30) C (n = 30) P (58 yrs) C (58 yrs) | Before Treatment | Cheek Gingiva Oropharynx Tongue Others | Stage I-II (n = 25) Stage III-IV (n = 5) | P {(Yess (n = 08), No (n = 22)} | P {(Yess (n = 07), No (n = 23)} | Tissue Saliva Mouth wash | 16S rRNA V1–V2 | The microbiota was compared with OSCC tissue, saliva, and mouthwash samples collected from the same subjects. In OSCC tissue, Acinetobacter and Fusobacterium were the most abundant taxa, particularly in late-stage OSCC. Their known roles in promoting infection and local inflammation suggest a potential contribution to OSCC progression. |
| Hayes, R.B. et al., 2018, USA [19] | P (n= 129) C (n= 254) P (71 yrs) C (71.0 yrs) | N/A | Oral (n = 41) Pharynx (n = 30) Larynx (n = 58) | N/A | P {(Never (n = 18) Former (n = 70) Current (n = 41)} C {(Never (n = 129) Former (n = 115) Current (n = 10)} | P {(Yess (n = 86), No (n = 20)} C {(Yess (n = 157), No (n = 68)} | Oral Rinse | 16S rRNA V3–V4 | An increased abundance of Corynebacterium, Kingella, and other selected genera and species was associated with an elevated risk of HNSCC. This study provides the first comprehensive evidence linking the oral microbiome to the subsequent risk of HNSCC, with the strongest associations observed in laryngeal cancer and among individuals with a history of tobacco use. |
| Vesty, A. et al., 2018, New Zealand [35] | P (n= 14) C (n= 07) P (49-81yrs) C (20-35 yrs) | N/A | Left parotid Buccal mucosa Right tongue Left palate Floor of mouth Lateral tongue Left tonsil Base of tongue | N/A | P {(Never (n = 05) Former (n = 06) Current (n = 02)} C:(Never (n = 07) | N/A | Saliva | 16S rRNA V3–V4 | This study reported that the utility of salivary bacterial communities as biomarkers for head and neck squamous cell carcinoma (HNSCC) is limited, due to their reduced capacity to distinguish HNSCC patients from dentally compromised individuals. |
| Yang, C.-Y. et al., 2018, Taiwan [6] | P (Stage 1, n= 41 Stage 2 and 3, n = 66 Stage 4, n = 90) C (n= 51) P (Stage 1= 53 yrs Stage 2,3 = 54 yrs Stage 4 = 52 yrs) C (31 yrs) | Before Treatment | Buccal mucosa; Tongue; Gingiva; Mouth floor; Others | Stage I (n = 41) Stage II (n = 49) Stage III (n = 17) Stage IV (n = 90) | P {Stage 1= Yes (n = 26), No (n = 15) Stage 2,3 = Yes (n = 50) No (n = 16) Stage 4 = Yes (n = 63), No (n = 27)} C (N/A) | P {(Stage 1= Yes (n = 23), No (n= 18) Stage 2,3 = Yes (n = 36), No (n = 30) Stage 4 = Yes (n = 51), No (n = 39)} C (N/A) | Oral Rinse | 16S rRNA V3–V4 | The oral microbiota community undergoes dynamic changes during the progression of oral cancer. A bacterial marker panel—characterized by upregulation of Fusobacterium periodonticum and downregulation of Streptococcus mitis and Prevotella pasteri—was able to discriminate stage IV oral squamous cell carcinoma (OSCC) patients from healthy controls. |
| Lim, Y. et al., 2018 Australia [33] | P (n= (HPV- =21 HPV⁺ =31) C (n= 20) P (HPV-ive >50 =20 yrs HPV⁺ive >50 = 31 yrs) | N/A | Oral cavity Oropharyngeal | HPV- (n = 21) Stage I (n = 3) Stage II (n = 3) Stage III (n = 6) Stage IV (n = 9) HPV⁺ (n = 31) Stage I (n = 1) Stage II (n = 1) Stage III (n = 4) Stage IV (n = 25) | P { HPV-ive (Never (n = 2) Former (n = 16) Current (n = 3) HPV⁺ive (Never (n = 8) Former (n = 30) Current (n = 03)} | P {(HPV-ive Yes (n = 14) No (n = 7) HPV⁺ive Yes (n = 12) No (n = 19) | Oral Rinse | 16S rRNA V6–V8 | An oral microbiome panel comprising Rothia, Haemophilus, Corynebacterium, Paludibacter, Porphyromonas, Oribacterium, and Capnocytophaga effectively distinguished age-matched healthy controls from patients with oral cavity cancer (OCC) and oropharyngeal cancer (OPC) with high accuracy. |
| Wang, H. et al., 2017 USA [23] | P (n= 121) C (n= 121) P (63 yrs) C (63 yrs) | SUR (n = 21) CT (n = 24) RT (n = 30) | Oral cavity Oropharynx Hypopharynx Larynx | Stage I–II (n = 24) Stage III–IV (n = 78) | P {(Never (n = 29) Former (n = 68) Current (n = 18)} | P {(Never (n= 37) Former (n = 10), Current (n = 67)} | Tumor Tissue, Normal Tissue | 16S rRNA V1–V4 | The microbiomes of HNSCC tumor microenvironments are largely similar in overall diversity and bacterial composition to those of histologically normal adjacent tissues. However, the study identified a decrease in the abundance of the genus Actinomyces and its higher-level taxa up to the phylum level, with this reduction being more pronounced in samples from higher T-stage tumors. |
| First Author, Year, Country | Diversity | Phyla | Class | Genus | Species | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alpha | Beta | HNSCC | HC | HNSCC | HC | HNSCC | HC | HNSCC | HC | |
| de Freitas Neiva Lessa, A. et al., 2024, Brazil [32] | S | NS | Prevotella↑ Porphyromonas↑ Fusobacterium↑ Streptococcus↓ Actinomyces↓ Leptotrichia↓ Corynebacterium↓ Rothia↓ | Prevotella↓ Porphyromonas↓ Fusobacterium↓ Streptococcus↑ Actinomyces↑ Leptotrichia↑ Corynebacterium↑ Rothia↑ | ||||||
| Unlu, O. et al., 2024, Turkey [31] | S | S | Firmicutes↑ Proteobacteria↓ | Proteobacteria↑ Firmicutes↓ | Streptococcus↑ Gemella↑ Peptostreptococcus↑ Fusobacterium↑ | Streptococcus↓ Gemella↓ Peptostreptococcus↓ Fusobacterium↓ | F. nucleatum↑ Lactobacillus spp↑ Rothia mucilaginosa↑ Granulicatella adiacens↑ Neisseria elongate↓ Aggregatibacter aphrophilus↓ Haemophilus sputorum↓ Actinomyces massiliensis↓ Veillonella spp↓ | Neisseria elongata↑ Aggregatibacter aphrophilus↑ Haemophilus sputorum↑ Veillonella spp↑ Actinomyces massiliensis↑ | ||
| Aparna, K. et al., 2024, India [29] | NS | NS | Firmicutes↑ Proteobacteria↓ Actinobacteria↓ | Proteobacteria↑ Actinobacteria↑ | Fusobacteria↑ Bacteroidetes↑ | Fusobacteria↓ Bacteroidetes↓ | Fusobacterium↑ Prevotella↑ Capnocytophaga↑ Leptotrichia↑ Peptostreptococcu↑ Parvimonas↑ Streptococcus↓ Haemophilus↓ | Streptococcus↑ Haemophilus↑ Fusobacterium↓ Prevotella↓ | Fusobacterium nucleatum↑ Prevotella intermedia↑ Streptococcus mitis↓ Haemophilus influenza↓ | Streptococcus mitis↑ Haemophilus influenza↑ Fusobacterium nucleatum↓ Prevotella intermedia↓ |
| Mäkinen, A. et al., 2023, Finland [30] | S | S | Veillonella↓ Actinomyces↓ | Veillonella↑ Actinomyces↑ Prevotella↑ | Streptococcus anginosus↑ Abiotrophia defectiva↑ Fusobacterium nucleatum↑ Streptococcus australis↓ | Streptococcus anginosus↓ Fusobacterium nucleatum↓ | ||||
| Lan, Q. et al., 2023, China [24] | NS | S | Actinobacteria↓ | Gemella↑ Lachnospira↑ Granulicatella↑ Fusobacterium↑ Streptococcus↑ Corynebacterium↓ Veillonella↓ | Veillonella↑ Corynebacterium↑ Gemella↓ Streptococcus↓ | |||||
| Benjamin, W.J. et al., 2023, USA [17] | NS | S | Fusobacterium↑ Eikenella↑ Lactobacillus↓ Bacillus↓ Acinetobacter↓ | Lactobacillus↑ Bacillus↑ Acinetobacter↑ Fusobacterium↓ | ||||||
| Yan, K. et al., 2023, USA [18] | NS | S | Fusobacterium↑ Peptostreptococcus↑ Neisseria↑ Parvimonas↑ Treponema↑ Streptococcus↓ Rothia↓ Actinomyces↓ Megasphaera↓ | Streptococcus↑ Rothia↑ Actinomyces↑ Megasphaera↑ Fusobacterium↓ Peptostreptococcus↓ Neisseria↓ | ||||||
| Oyeyemi, B.F. et al., 2023, India [28] | NS | NS | Firmicutes↑ Actinobacteria↓ Proteobacteria↓ | Bacteroidetes↑ | Neisseria↑ Leptotrichia↑ Campylobacter↓ | |||||
| Wu, Z. et al., 2023, USA [20] | NS | S | Corynebacterium↓ | Fusobacterium nucleatum↑ Porphyromonas gingivalis↑ Prevotella intermedia↑ Prevotella nigrescens↑ Red-complex bacteria↑ Kingella oralis↓ | Kingella oralis↑ Corynebacterium matruchotii↑ Fusobacterium nucleatum↓ Porphyromonas gingivalis↓ | |||||
| Pandey, D. et al., 2022, Australia [34] | S | S | Fusobacterium↑ Prevotella↑ Porphyromonas↑ Lactobacillus↑ Streptococcus↓ Veillonella↓ Corynebacterium↓ | Streptococcus↑ Veillonella↑ Corynebacterium↑ Fusobacterium↓ Prevotella↓ Porphyromonas↓ | ||||||
| Ganly, I. et al., 2022 USA [21] | NS | S | Synergistetes↑ Actinobacteria↓ Firmicutes↓ | Actinobacteria↑ Firmicutes↑ Synergistetes↓ | Bacteroidetes↑ | Bacteroidetes↓ | Fusobacterium↑ Corynebacterium↓ Streptococcus↓ Actinomyces↓ Cryptobacterium↓ Selenomonas↓ | Corynebacterium↑ Streptococcus↑ Actinomyces↑ Cryptobacterium↑ Selenomonas↑ Fusobacterium↓ | ||
| Mougeot, J.L.C. et al., 2022, USA [22] | S | Leptotrichia spp↑ Fusobacterium periodonticum↑ Haemophilus pittmania↑ Alloprevotella tannerae↑ Lachnoanaerobaulum orale↑ | Rothia mucilaginosa↑ Haemophilus parainfluenzae↑ | |||||||
| Chan, J.Y.K. et al., 2022, China [25] | S | S | Fusobacterium↑ Peptostreptococcus↑ Capnocytophaga↑ Parvimonas↑ Leptotrichia↑ Streptococcus↓ Rothia↓ | Streptococcus↑ Rothia↑ Fusobacterium↓ Peptostreptococcus↓ Capnocytophaga↓ | ||||||
| Sharma, A.K. et al., 2020, USA [16] | S | S | Fusobacterium nucleatum↑ Porphyromonas gingivalis↑ Gemella haemolysans↑ Lactobacillus spp↑ Tannerella forsythia↑ Prevotella intermedia↑ Neisseria subflava↓ Haemophilus parainfluenzae↓ Aggregatibacter actinomycetemcomitans↓ Veillonella dispar↓ | Neisseria subflava↑ Haemophilus parainfluenzae↑ Aggregatibacter actinomycetemcomitans↑ Veillonella dispar↑ Fusobacterium nucleatum↓ Porphyromonas gingivalis↓ Tannerella forsythia↓ Gemella haemolysans↓ | ||||||
| Zhang, L. et al., 2020, China [26] | NS | NS | Firmicutes↑ | Firmicutes↓ | Fusobacteria↑ Bacteroidetes↑ | Streptococcus↓ Veillonella↓ Rothia↓ | Streptococcus↑ Veillonella↑ Rothia↑ Fusobacterium↓ Prevotella↓ Porphyromonas↓ | Fusobacterium nucleatum↑ Prevotella intermedia↑ Peptostreptococcus stomatis↑ | ||
| Zhang, Z. et al., 2019, China [27] | S | S | Proteobacteria↑ Firmicutes↓ Actinobacteria↓ | Firmicutes↑ Actinobacteria↑ Proteobacteria↓ | Fusobacteria↑ | Fusobacteria↓ | Acinetobacter↑ Campylobacter↑ Fusobacterium↑ Streptococcus↓ Rothia↓ | Streptococcus↑ Rothia↑ Acinetobacter↓ Campylobacter↓ Fusobacterium↓ | Fusobacterium nucleatum↑ Acinetobacter baumannii↑ Streptococcus mitis↓ Rothia mucilaginosa↓ | Streptococcus mitis↑ Rothia mucilaginosa↑ Fusobacterium nucleatum↓ Acinetobacter baumannii↓ |
| Hayes, R.B. et al., 2018, USA [19] | NS | S | Corynebacterium↓ Kingella↓ | Corynebacterium↑ Kingella↑ | ||||||
| Vesty, A. et al., 2018, New Zealand [35] | S | S | Treponema↑ Actinomyces↓ Fusobacterium↓ | Actinomyces↑ Fusobacterium↑ Treponema↓ | Candida albicans↑ | |||||
| Yang, C.-Y. et al., 2018, Taiwan [6] | S | S | Actinobacteria↑ | Fusobacteria↑ Bacteroidetes↑ | Fusobacterium↑ Parvimonas↑ Streptococcus↓ Haemophilus↓ Porphyromonas↓ Actinomyces↓ | Streptococcus↑ Haemophilus↑ Porphyromonas↑ Actinomyces↑ Fusobacterium↓ | Streptococcus constellatus↑ Haemophilus influenza↑ Filifactor alocis↑ | |||
| Lim, Y. et al., 2018 Australia [33] | S | S | Firmicutes↑ | Bacteroidetes↑ | Fusobacterium↑ Prevotella↑ Porphyromonas↑ Streptococcus↓ Neisseria↓ | Streptococcus↑ Neisseria↑ Fusobacterium↓ Prevotella↓ Porphyromonas↓ | ||||
| Wang, H. et al., 2017 USA [23] | NS | S | Firmicutes↓ | Firmicutes↑ | Parvimonas↑ Actinomyces↓ | Actinomyces↑ Parvimonas↓ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Jayamanne, D.; Bergamin, S.; Lawless, A.; Guminski, A.; Lee, A.; Yuile, A.; Wheeler, H.; Eade, T.; Back, M.; et al. Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions. Cancers 2025, 17, 2667. https://doi.org/10.3390/cancers17162667
Ahmad S, Jayamanne D, Bergamin S, Lawless A, Guminski A, Lee A, Yuile A, Wheeler H, Eade T, Back M, et al. Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions. Cancers. 2025; 17(16):2667. https://doi.org/10.3390/cancers17162667
Chicago/Turabian StyleAhmad, Saad, Dasantha Jayamanne, Sarah Bergamin, Anna Lawless, Alexander Guminski, Adrian Lee, Alexander Yuile, Helen Wheeler, Thomas Eade, Michael Back, and et al. 2025. "Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions" Cancers 17, no. 16: 2667. https://doi.org/10.3390/cancers17162667
APA StyleAhmad, S., Jayamanne, D., Bergamin, S., Lawless, A., Guminski, A., Lee, A., Yuile, A., Wheeler, H., Eade, T., Back, M., Molloy, M., & Oh, B. (2025). Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions. Cancers, 17(16), 2667. https://doi.org/10.3390/cancers17162667








