Analysis of Metastases and Second Primary Malignancy Development in Patients with Invasive Transitional Cell Carcinoma of the Bladder
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
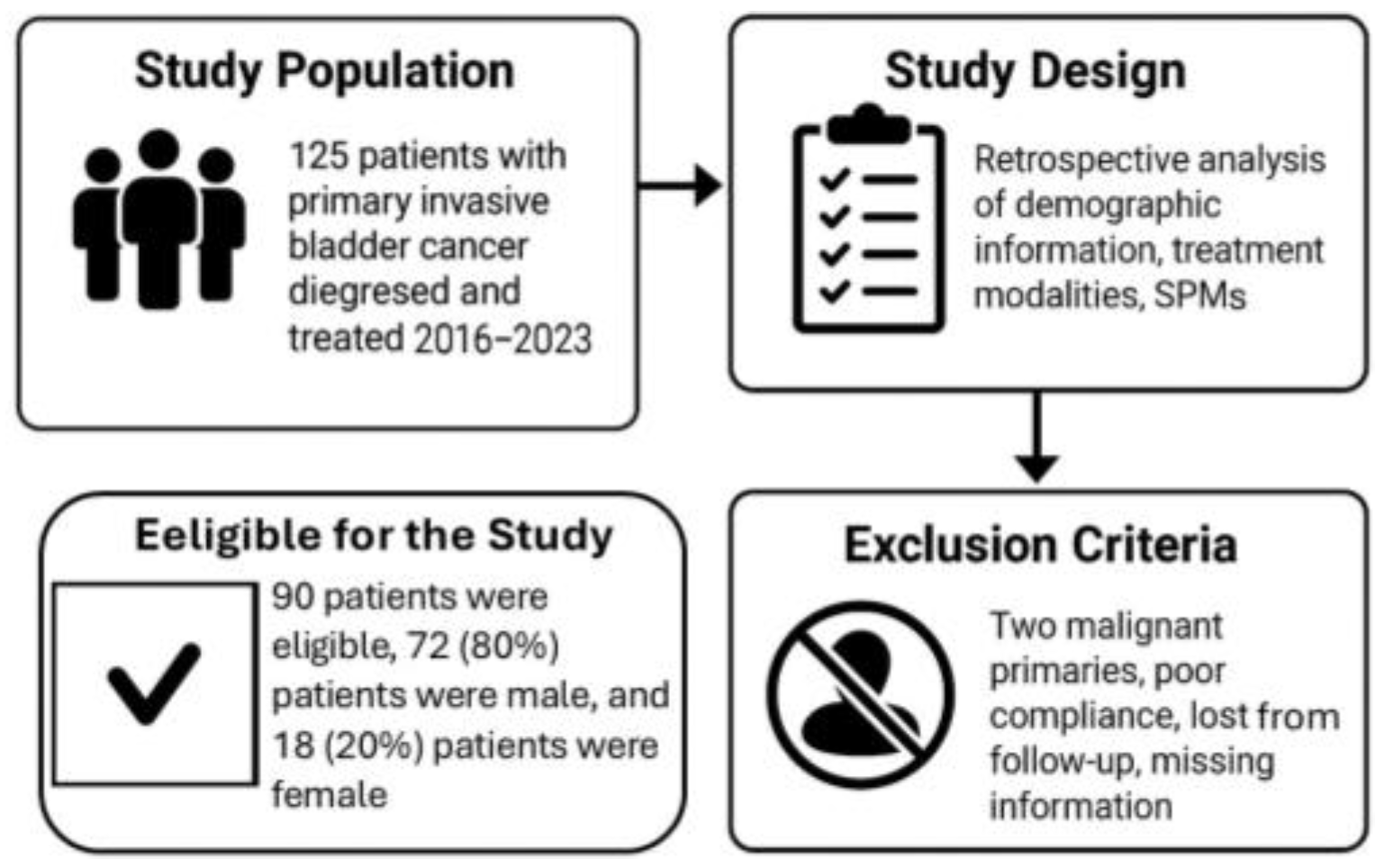
2.1. Study Population
2.2. Study Design
2.3. Exclusion Criteria
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kochanek, K.D.; Murphy, S.; Xu, J.; Arias, E. Mortality in the United States. NCHS Data Brief. 2016, 293, 1–8. [Google Scholar] [PubMed]
- Zheng, X.; Li, X.; Wang, M.; Shen, J.; Sisti, G.; He, Z.; Huang, J.; Li, Y.M.; Wu, A. Second primary malignancies among cancer patients. Ann. Transl. Med. 2020, 8, 638. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rumgay, H.; Li, M.; Yu, H.; Pan, H.; Ni, J. The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J. Glob. Health 2023, 13, 04109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afzal, A.; Liu, Y.Y.; Noureen, A.; Rehman, A.; Iftikhar, M.; Afzal, H.; Azam, F.; Saddozai, U.A.K.; Jan, T.; Asif, Z.; et al. Epidemiology of gall bladder cancer and its prevalence worldwide: A meta-analysis. Orphanet J. Rare Dis. 2025, 20, 143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Wéber, A.; Vignat, J.; Shah, R.; Morgan, E.; Laversanne, M.; Nagy, P.; Kenessey, I.; Znaor, A. Global burden of bladder cancer mortality in 2020 and 2040 according to GLOBOCAN estimates. World J. Urol. 2024, 42, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Husseini, M.J.; Kunbaz, A.; Saad, A.M.; Santos, J.V.; Salahia, S.; Iqbal, M.; Alahdab, F. Trends in the incidence and mortality of transitional cell carcinoma of the bladder for the last four decades in the USA: A SEER-based analysis. BMC Cancer 2019, 19, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wallerand, H.; Bernhard, J.-C.; Culine, S.; Ballanger, P.; Robert, G.; Reiter, R.E.; Ferrière, J.-M.; Ravaud, A. Targeted therapies in non-muscle-invasive bladder cancer according to the signaling pathways. Urol. Oncol. 2011, 29, 4–11. [Google Scholar] [CrossRef]
- Horstmann, M.; Witthuhn, R.; Falk, M.; Stenzl, A. Gender-specific differences in bladder cancer: A retrospective analysis. Gend. Med. 2008, 5, 385–394. [Google Scholar] [CrossRef]
- Mallin, K.; David, K.A.; Carroll, P.R.; Milowsky, M.I.; Nanus, D.M. Transitional cell carcinoma of the bladder: Racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007). J. Urol. 2011, 185, 1631–1636. [Google Scholar] [CrossRef]
- Patafio, F.M.; Robert Siemens, D.; Wei, X.; Booth, C.M. Is there a gender effect in bladder cancer? A population-based study of practice and outcomes. Can. Urol. Assoc. J. 2015, 9, 269–274. [Google Scholar] [CrossRef]
- Ghervan, L.; Zaharie, A.; Ene, B.; Elec, F.I. Small-cell carcinoma of the urinary bladder: Where do we stand? Clujul Med. 2017, 90, 13. [Google Scholar] [CrossRef]
- Dyrskjøt, L.; Hansel, D.E.; Efstathiou, J.A.; Knowles, M.A.; Galsky, M.D.; Teoh, J.; Theodorescu, D. Bladder cancer. Nat. Rev. Dis. Primers 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dobruch, J.; Oszczudłowski , M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745, Erratum in JAMA 2011, 306, 2220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ross, R.K.; A Jones, P.; Yu, M.C. Bladder cancer epidemiology and pathogenesis. Semin. Oncol. 1996, 23, 536–545. [Google Scholar] [PubMed]
- Samanic, C.M.; Kogevinas, M.; Silverman, D.T.; Tardón, A.; Serra, C.; Malats, N.; Real, F.X.; Carrato, A.; García-Closas, R.; Sala, M.; et al. Occupation and bladder cancer in a hospital-based case-control study in Spain. Occup. Environ. Med. 2008, 65, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Gao, F.; Xiao, Y.; Wu, J.; Wang, C.; Mo, X.; Zhang, Z.; Zheng, R.; Wu, D. Tobacco smoking exposure-mediated ELAVL1 regulates bladder cancer cell senescence via autophagy activation. Toxicology 2025, 516, 154193. [Google Scholar] [CrossRef] [PubMed]
- Talaska, G. Aromatic amines and human urinary bladder cancer: Exposure sources and epidemiology. J. Environ. Sci. Health Part C 2003, 21, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Merlo, F.; Pearce, N.; Puntoni, R. Bladder cancer and occupational exposure to polycyclic aromatic hydrocarbons. Int. J. Cancer 1989, 44, 648–651. [Google Scholar] [CrossRef] [PubMed]
- King, W.D.; Marrett, L.D. Case-control study of bladder cancer and chlorination by-products in treated water (Ontario, Canada). Cancer Causes Control. 1996, 7, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.W.; Zhao, L.G.; Yang, Y.; Ma, X.; Wang, Y.Y.; Xiang, Y.B. Obesity and risk of bladder cancer: A dose-response meta-analysis of 15 cohort studies. PLoS ONE 2015, 10, e0119313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayat, M.J.; Howlader, N.; Reichman, M.E.; Edwards, B.K. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007, 12, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Wallgren, A.; Bonetti, M.; Gelber, R.; Goldhirsch, A.; Castiglione-Gertsch, M.; Holmberg, S.; Lindtner, J.; Thürlimann, B.; Fey, M.; Werner, I.; et al. Risk factors for locoregional recurrence among breast cancer patients: Results from international breast Cancer study group trials I through VII. J. Clin. Oncol. 2003, 21, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Lawrence, W.F.; Clauser, S.; Davis, W.W.; Brown, M.L. Burden of illness in cancer survivors: Findings from a population-based national sample. J. Natl. Cancer Inst. 2004, 96, 1322–1330. [Google Scholar] [CrossRef]
- Schaapveld, M.; Visser, O.; Louwman, M.J.; de Vries, E.G.; Willemse, P.H.; Otter, R.; van der Graaf, W.T.A.; Coebergh, J.-W.W.; van Leeuwen, F.E. Risk of new primary nonbreast cancers after breast cancer treatment: A Dutch population-based study. J. Clin. Oncol. 2008, 26, 1239–1246. [Google Scholar] [CrossRef]
- Kranjac, D.; Kranjac, A.W. Age-period-cohort effects of adult cigarette smoking in the united States, 1971–2020. Sci. Rep. 2025, 15, 14341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thun, M.J.; Carter, B.D.; Feskanich, D.; Freedman, N.D.; Prentice, R.; Lopez, A.D.; Hartge, P.; Gapstur, S.M. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 351–364. [Google Scholar] [CrossRef]
- Tang, X.; Zhan, X.; Chen, X. Incidence, mortality and survival of transitional cell carcinoma in the urinary system: A population-based analysis. Medicine 2023, 102, e36063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barker, S.J.; Soylu, E.; Allen, B.C.; Auron, M.; Costa, D.N.; Gerena, M.; Lotan, Y.; Rose, T.L.; Solanki, A.; Surasi, D.S.; et al. ACR Appropriateness Criteria® Pretreatment Staging of Urothelial Cancer: 2024 Update. J. Am. Coll. Radiol. 2024, 21, S464–S489. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-Y.; Cheong, I.-S.; Lai, J.-N.; Hu, C.-Y.; Hung, K.-C.; Chen, Y.-T.; Chiu, L.-T.; Tsai, H.-T.; Jou, Y.-C.; Tzai, T.-S.; et al. Risk of secondary primary malignancies in survivors of upper tract urothelial carcinoma: A nationwide population-based analysis. Cancer Epidemiol. 2024, 89, 102536. [Google Scholar] [CrossRef] [PubMed]
- McCredie, M.; Macfarlane, G.J.; Stewart, J.; Coates, M. Second primary cancers following cancers of the kidney and prostate in New South Wales (Australia), 1972–1991. Cancer Causes Control. 1996, 7, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Nofal, S.; JOstrin, E.; Zhang, J.; Wu, J.; Scheet, P.; Antonoff, M.B.; VHeymach, J.; Toumazis, I. Risk of second primary lung cancer among cancer survivors stratified by the site of first primary cancer and the lung cancer screening eligibility status. Int. J. Cancer 2025, 157, 941–953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, T.M. Taiwan’s new national health insurance program: Genesis and experience so far. Health Aff. 2003, 22, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; van Rhijn, B.W.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Redorta, J.P.; et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef]
- Bellmunt, J.; Orsola, A.; Leow, J.J.; Wiegel, T.; De Santis, M.; Horwich, A.; on behalf of the ESMO Guidelines Working Group. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii40–iii48. [Google Scholar] [CrossRef]
- Saadh, M.J.; Saeed, T.N.; Al-Hussainy, A.F.; Bishoyi, A.K.; Ballal, S.; Singh, A.; Kavitha, V.; Aminov, Z.; Taher, S.G.; Alwan, M.; et al. Revolutionizing cancer diagnostics: The promise of exosome-based biosensors. Anal. Biochem. 2025, 705, 115912. [Google Scholar] [CrossRef] [PubMed]
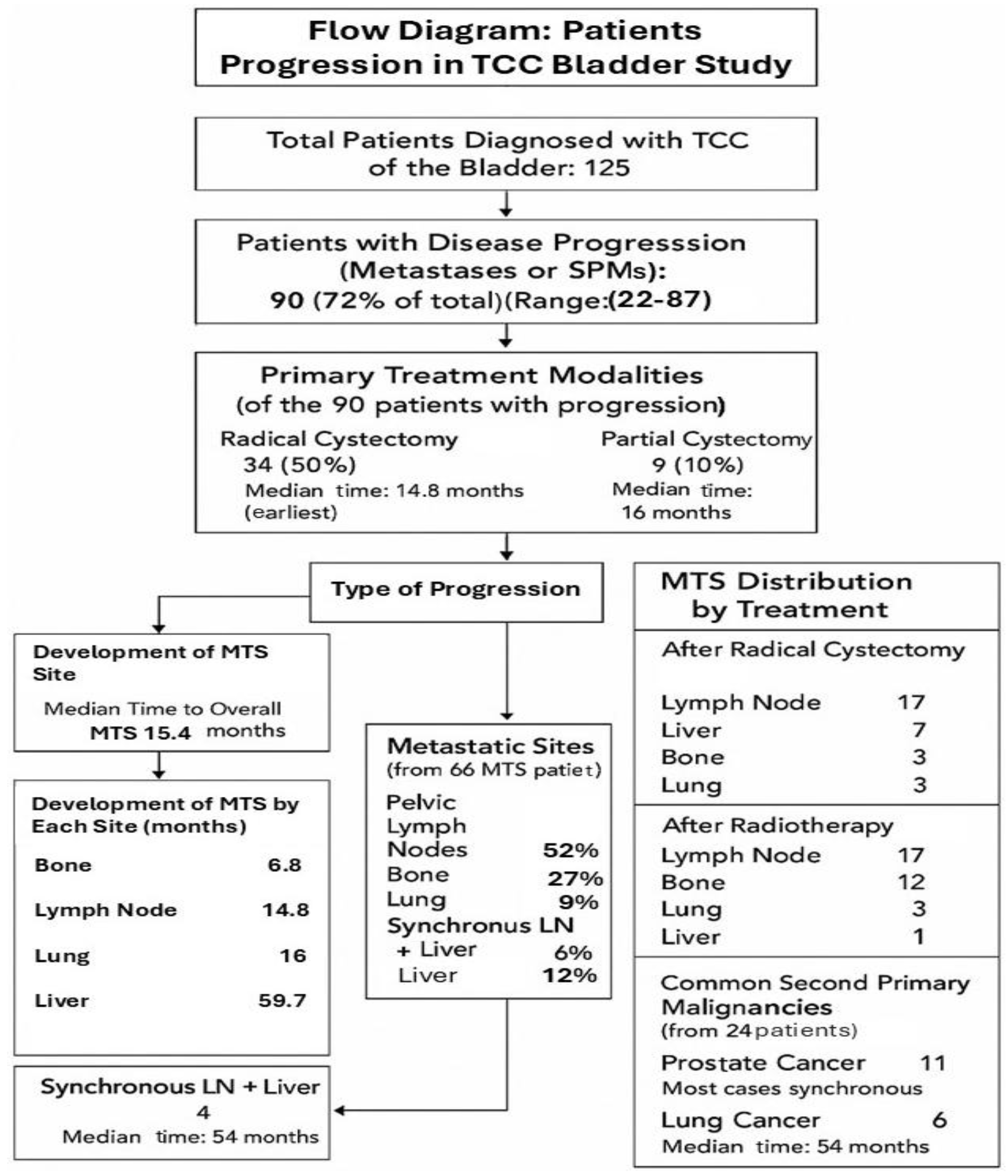
| Eligible Patients | 90 |
| Median age | 70 (range 22–87) |
| Primary treatments: | |
| Radical cystectomy | 58 patients (64%) |
| Median age | 66 (range 43–86) |
| Partial cystectomy * | 9 patients (10%) |
| Median age | 64 (range 22–73) |
| Radiotherapy | 23 patients (26%) |
| Median age | 74 (range 22–87) |
| Metastases Development | 66 Patients |
| Sites of MTS: | |
| Pelvic LNs | 34 patients (52%) |
| Bone | 18 patients (27%) |
| Liver | 8 patients (12%) |
| Lung | 6 patients (9%) |
| Synchronous LNs + Liver * | 4 patients (6%) |
| Average time from diagnosis to MTS | 14.3 months |
| Radical Cystectomy | Number of Patients |
|---|---|
| Lymph node | 17 |
| Liver | 7 |
| Bone | 6 |
| Lung | 3 |
| Radiotherapy (XRT) | |
| Lymph node | 17 |
| Bone | 12 |
| Lung | 3 |
| Liver | 1 |
| Site | Time (Months) | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|
| Bone | 6.8 | 3.25 (2.1–5.0) | <0.001 |
| Lymph node | 14.8 | 1.82 (1.1–3.0) | 0.015 |
| Lung | 16 | 1.70 (0.95–3.1) | 0.071 |
| Liver | 59.7 | Reference | — |
| Metastatic Site | Radical Cystectomy (%) | Radiotherapy (%) | p Value |
|---|---|---|---|
| Lymph node | 51.5% | 51.5% | 1.0 |
| Bone | 18.2% | 36.4% | 0.10 |
| Liver | 21.2% | 3.0% | 0.05 |
| Lung | 9.1% | 9.1% | 1.0 |
| Recommendation | GR |
|---|---|
| The follow-up is based on regular cystoscopy. | A |
| Patients with low-risk tumors should undergo cystoscopy at 3 months. If negative, subsequent cystoscopy is advised 9 months later, and then yearly for 5 years. | C |
| Patients with high-risk tumors should undergo cystoscopy and urinary cytology at 3 mo. If negative, subsequent cystoscopy and cytology should be repeated every 3 months for a period of 2 years, and every 6 months thereafter until 5 years, and then yearly. | C |
| Patients with intermediate-risk tumors should have an in-between follow-up scheme using cystoscopy and cytology, which is adapted according to personal and subjective factors. | C |
| Regular (yearly) upper tract imaging (CT-IVU or IVU) is recommended for high-risk tumors. | C |
| Endoscopy under anesthesia and bladder biopsies should be performed when office cystoscopy shows suspicious findings or if urinary cytology is positive. | B |
| During follow-up in patients with positive cytology and no visible tumor in the bladder, R-biopsies or biopsies with PDD (if equipment is available), and investigation of extravesical locations (CT urography, prostatic urethra biopsy) are recommended. | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouvinov, K.; Yakobson, A.; Tiganas, A.; Shani Shrem, N.; Chernomordikov, E.; Abu Jama, A.; Abu Yasin, N.; Brenner, R.; Ievko, A.; Abu Zeid, E.E.D.; et al. Analysis of Metastases and Second Primary Malignancy Development in Patients with Invasive Transitional Cell Carcinoma of the Bladder. Cancers 2025, 17, 2663. https://doi.org/10.3390/cancers17162663
Rouvinov K, Yakobson A, Tiganas A, Shani Shrem N, Chernomordikov E, Abu Jama A, Abu Yasin N, Brenner R, Ievko A, Abu Zeid EED, et al. Analysis of Metastases and Second Primary Malignancy Development in Patients with Invasive Transitional Cell Carcinoma of the Bladder. Cancers. 2025; 17(16):2663. https://doi.org/10.3390/cancers17162663
Chicago/Turabian StyleRouvinov, Keren, Alexander Yakobson, Angela Tiganas, Noa Shani Shrem, Elena Chernomordikov, Ashraf Abu Jama, Nashat Abu Yasin, Ronen Brenner, Anna Ievko, Ez El Din Abu Zeid, and et al. 2025. "Analysis of Metastases and Second Primary Malignancy Development in Patients with Invasive Transitional Cell Carcinoma of the Bladder" Cancers 17, no. 16: 2663. https://doi.org/10.3390/cancers17162663
APA StyleRouvinov, K., Yakobson, A., Tiganas, A., Shani Shrem, N., Chernomordikov, E., Abu Jama, A., Abu Yasin, N., Brenner, R., Ievko, A., Abu Zeid, E. E. D., Abu Juda, M., & Shalata, W. (2025). Analysis of Metastases and Second Primary Malignancy Development in Patients with Invasive Transitional Cell Carcinoma of the Bladder. Cancers, 17(16), 2663. https://doi.org/10.3390/cancers17162663







