The Significance of CEA and CA 19-9 Levels in Serum and Peritoneal Fluid in Colorectal Cancer Patients in the Context of Peritoneal Metastases and Cytology Results
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
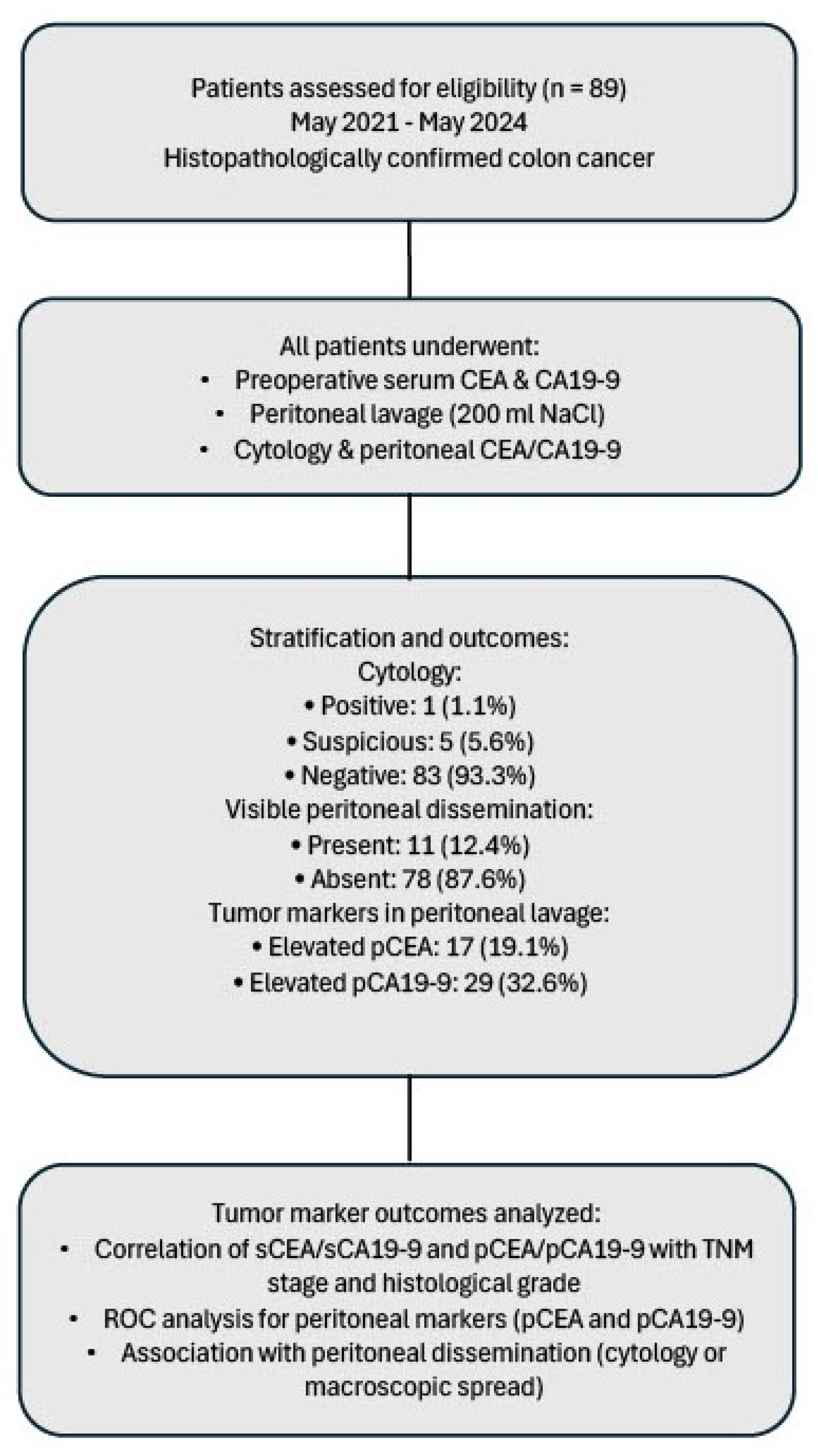
2.1. General Information
2.2. Research Methods
2.3. Statistical Analysis
3. Results
3.1. Features of the Patient Cohort
3.2. Serum and Peritoneal Tumor Markers
3.2.1. Serum and Peritoneal Marker Levels
3.2.2. Diagnostic Performance of Peritoneal Markers
3.2.3. Association with TNM Parameters and Other Features
3.2.4. Inter-Marker Correlation
3.3. Factors Affecting the Development of Peritoneal Carcinomatosis
3.4. Relationship Between Peritoneal Cytology Results and Clinicopathological Factors
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CA | Carbohydrate Antigen (część CA 19-9) |
| CEA | Carcinoembryonic Antigen |
| CRC | Colorectal Cancer |
| GFR | Glomerular Filtration Rate |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| PLT | Platelets |
| ROC | Receiver Operating Characteristic |
| WBC | White Blood Cells |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef]
- Feng, Y.; Jin, H.; Guo, K.; Wasan, H.S.; Ruan, S.; Chen, C. Causes of Death After Colorectal Cancer Diagnosis: A Population-Based Study. Front. Oncol. 2021, 11, 647179. [Google Scholar] [CrossRef]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, R.; Zhang, Z.; Xu, C.; Liu, J.; Zhang, J.; Wang, J.; Wang, Q.; Liu, C.; Feng, J.; et al. Prognostic significance of positive peritoneal cytology in endometrial carcinoma based on ESGO/ESTRO/ESP risk classification: A multicenter retrospective study. Gynecol. Oncol. 2023, 176, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Matsuzaki, S.; Nusbaum, D.J.; Machida, H.; Nagase, Y.; Grubbs, B.H.; Roman, L.D.; Wright, J.D.; Harter, P.; Klar, M. Malignant peritoneal cytology and decreased survival of women with stage I endometrioid endometrial cancer. Eur. J. Cancer 2020, 133, 33–46. [Google Scholar] [CrossRef]
- Deng, K.; Zhu, H.; Chen, M.; Wu, J.; Hu, R.; Tang, C.; Katoh, M. Prognostic Significance of Molecular Analysis of Peritoneal Fluid for Patients with Gastric Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0151608. [Google Scholar] [CrossRef] [PubMed]
- Pecqueux, M.; Fritzmann, J.; Adamu, M.; Thorlund, K.; Kahlert, C.; Reißfelder, C.; Weitz, J.; Rahbari, N.N. Free intraperitoneal tumor cells and outcome in gastric cancer patients: A systematic review and meta-analysis. Oncotarget 2015, 6, 35564–35578. [Google Scholar] [CrossRef]
- Gozalan, U.; Yasti, A.C.; Yuksek, Y.N.; Reis, E.; Kama, N.A. Peritoneal cytology in colorectal cancer: Incidence and prognostic value. Am. J. Surg. 2007, 193, 672–675. [Google Scholar] [CrossRef]
- Noura, S.; Ohue, M.; Seki, Y.; Yano, M.; Ishikawa, O.; Kameyama, M. Long-Term Prognostic Value of Conventional Peritoneal Lavage Cytology in Patients Undergoing Curative Colorectal Cancer Resection. Dis. Colon Rectum 2009, 52, 1312–1320. [Google Scholar] [CrossRef]
- Mohan, H.M.; O’Connor, D.B.; O’Riordan, J.M.; Winter, D.C. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: A systematic review. Surg. Oncol. 2013, 22, e1–e6. [Google Scholar] [CrossRef]
- Nishikawa, T.; Sunami, E.; Tanaka, T.; Tanaka, J.; Kiyomatsu, T.; Kawai, K.; Hata, K.; Kazama, S.; Nozawa, H.; Ishihara, S.; et al. Incidence and prognostic significance of positive peritoneal lavage in colorectal cancer. Surg. Today 2014, 45, 1073–1081. [Google Scholar] [CrossRef]
- Passot, G. Intra-operative peritoneal lavage for colorectal cancer. World J. Gastroenterol. 2014, 20, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Bąk, M.; Wojciech, M.; Pielech, A.; Holka, S.; Zawadzki, M.; Murawa, D. The Advancement Stage of Gastric Cancer and the Levels of CEA and Ca19-9 in Serum and Peritoneal Lavage. Biomedicines 2024, 12, 2584. [Google Scholar] [CrossRef]
- Mustafa, H.; Fu, M.; Enver, K.; Mehmet, G.; Nurcan, U. Use of serum and peritoneal CEA and CA19-9 in prediction of peritoneal dissemination and survival of gastric adenocarcinoma patients: Are they prognostic factors? Ind. Mark. Manag. 2018, 100, 257–266. [Google Scholar] [CrossRef]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; Kagawa, Y.; Denda, T.; Satoh, T.; Yamazaki, K.; et al. Effects of Metastatic Sites on Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100535. [Google Scholar] [CrossRef]
- Crisafulli, G. Liquid Biopsy and Challenge of Assay Heterogeneity for Minimal Residual Disease Assessment in Colon Cancer Treatment. Genes 2025, 16, 71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 11 December 2023).
- Pepe, M.S. The Statistical Evaluation of Medical Tests for Classification and Prediction; Oxford University Press (OUP): Oxford, UK, 2003; ISBN 9781383022209. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer: New York, NY, USA, 2009. [Google Scholar]
- Hase, K.; Ueno, H.; Kuranaga, N.; Utsunomiya, K.; Kanabe, S.; Mochizuki, H. Intraperitoneal exfoliated cancer cells in patients with colorectal cancer. Dis. Colon Rectum 1998, 41, 1134–1140. [Google Scholar] [CrossRef]
- Baskaranathan, S.; Philips, J.; McCredden, P.; Solomon, M.J. Free Colorectal Cancer Cells on the Peritoneal Surface: Correlation With Pathologic Variables and Survival. Dis. Colon Rectum 2004, 47, 2076–2079. [Google Scholar] [CrossRef]
- Yamamoto, S.; Akasu, T.; Fujita, S.; Moriya, Y. Long-term Prognostic Value of Conventional Peritoneal Cytology after Curative Resection for Colorectal Carcinoma. Ultrasound Med. Biol. 2003, 33, 33–37. [Google Scholar] [CrossRef]
- Horattas, M.C.; Evasovich, M.R.; Topham, N. Colorectal carcinoma and the relationship of peritoneal cytology. Am. J. Surg. 1997, 174, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, D.C.; Harris, D.A.; Evans, M.D.; Beynon, J. Systematic review and meta-analysis of intraoperative peritoneal lavage for colorectal cancer staging. Br. J. Surg. 2013, 100, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.; Castelino-Prabhu, S.; Cobb, C.; Raza, A. Role of cytopathology in the diagnosis and management of gastrointestinal tract cancers. J. Gastrointest. Oncol. 2012, 3, 285–298. [Google Scholar] [CrossRef]
- Hasbahceci, M.; Akcakaya, A.; Guler, B.; Kunduz, E.; Malya, F.U.; Muslumanoglu, M. Use of peritoneal washing cytology for the detection of free peritoneal cancer cells before and after surgical treatment of gastric adenocarcinoma. J. Cancer Res. Ther. 2018, 14, 1225–1229. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baba, H.; Kakeji, Y.; Endo, K.; Ikeda, Y.; Toh, Y.; Kohnoe, S.; Okamura, T.; Maehara, Y. Prognostic Significance of Tumor Markers in Peritoneal Lavage in Advanced Gastric Cancer. Oncology 2004, 67, 19–26. [Google Scholar] [CrossRef]
- Li, J.-K.; Zheng, M.; Miao, C.-W.; Zhang, J.-H.; Ding, G.-H.; Wu, W.-S. Peritoneal lavage cytology and carcinoembryonic antigen determination in predicting peritoneal metastasis and prognosis of gastric cancer. World J. Gastroenterol. 2005, 11, 7374–7377. [Google Scholar] [CrossRef]
- Tan, Y.; Zhu, Y.; Wang, F.; Song, Z.; Zhang, P.; Wang, L.; Cao, C.; Sun, S. Diagnostic Value of Tumor Markers in Peritoneal Lavage Fluid for Peritoneal Metastasis from Colorectal Cancer. Ann. Clin. Lab. Sci. 2022, 52, 95–100. [Google Scholar]
- Lee, I.K.; Kim, D.H.; Gorden, D.L.; Lee, Y.S.; Sung, N.Y.; Park, G.-S.; Kim, H.J.; Kang, W.K.; Park, J.K.; Ahn, C.H.; et al. Prognostic Value of CEA and CA 19-9 Tumor Markers Combined with Cytology from Peritoneal Fluid in Colorectal Cancer. Ann. Surg. Oncol. 2009, 16, 861–870. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Dou, Q.; Ye, J.; Xu, D.; Shang, H.; Xu, K.; Song, Y. Evaluation of tumor markers for the differential diagnosis of benign and malignant ascites. Ann. Hepatol. 2014, 13, 357–363. [Google Scholar] [CrossRef]
- Koh, J.-L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of Preoperative Computed Tomography in Estimating Peritoneal Cancer Index in Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef] [PubMed]

| Marker | AUC | Youden Index | Youden Index p-Value | Cut-Off Value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| pCEA | 0.912 | 0.839 | 0.002 | 8.95 | 91.7 | 92.2 |
| pCA 19-9 | 0.741 | 0.49 | <0.001 | 2.25 | 75 | 74 |
| Variable | CEA | CA 19-9 | ||
|---|---|---|---|---|
| Serum | Peritoneal | Serum | Peritoneal | |
| Cytology | 0.483 | 0.191 | 0.193 | 0.326 |
| Neoadjuvant radiotherapy | 0.002 | 0.34 | 1 | 1 |
| Neoadjuvant chemotherapy | 0.074 | 0.727 | 0.501 | 0.449 |
| Mucinous characteristic component | 0.341 | 0.322 | 1 | 0.721 |
| Tumor necrosis | 1 | 1 | 0.327 | 1 |
| Histopathological stage | 0.022 | 0.002 | 0.689 | 0.668 |
| Tumor location | 0.065 | 0.246 | 0.274 | 0.655 |
| T | 0.002 | <0.001 | 0.026 | 0.015 |
| N | 0.014 | <0.001 | <0.001 | 0.028 |
| M | <0.001 | <0.001 | <0.001 | <0.001 |
| L | <0.001 | <0.001 | 0.005 | 0.024 |
| V | 0.037 | <0.001 | 0.018 | 0.029 |
| Pn | <0.001 | <0.001 | 0.002 | 0.021 |
| WBC | 0.559 | 0.108 | 0.681 | 0.123 |
| HGB | 0.035 | 0.426 | 0.597 | 0.667 |
| HCT | 0.041 | 0.057 | 0.184 | 0.107 |
| PLT | 0.092 | 0.872 | 0.389 | 0.883 |
| Creatinine | 0.849 | 1 | 1 | 0.505 |
| GFR | 0.761 | 0.874 | 0.516 | 0.255 |
| Marker | CA 19-9 in Abdominal Cavity | CA 19-9 in Serum | CEA in Abdominal Cavity | CEA in Serum |
|---|---|---|---|---|
| CA 19-9 in abdominal cavity | - | |||
| CA 19-9 in serum | 0.612 (<0.001) | - | ||
| CEA in abdominal cavity | 0.398 (<0.001) | 0.394 (<0.001) | - | |
| CEA in serum | 0.321 (0.002) | 0.371 (<0.001) | 0.653 (<0.001) | - |
| Clinicopathologic Factors | Peritoneal Carcinomatosis | p-Value | |
|---|---|---|---|
| Negative, n (%) | Positive, n (%) | ||
| Sex | 0.905 | ||
| Female | 37 (47.4%) | 6 (54.5%) | |
| Male | 41 (52.6%) | 5 (45.5%) | |
| Cytology | 1.00 | ||
| Negative | 77 (98.7%) | 11 (100.0%) | |
| Positive | 1 (1.3%) | 0 (0.0%) | |
| Distant Metastases | 0.024 | ||
| Negative | 62 (79.5%) | 5 (45.5%) | |
| Positive | 16 (20.5%) | 6 (54.5%) | |
| CEA in Serum | 0.159 | ||
| Beyond the norm | 35 (44.9%) | 8 (72.7%) | |
| Norm | 43 (55.1%) | 3 (27.3%) | |
| CEA in Abdominal Cavity | <0.001 | ||
| Beyond the norm | 7 (9.0%) | 10 (90.9%) | |
| Norm | 71 (91.0%) | 1 (9.1%) | |
| CA 19-9 in Serum | 0.006 | ||
| Beyond the norm | 11 (14.3%) | 6 (54.5%) | |
| Norm | 66 (85.7%) | 5 (45.5%) | |
| CA 19-9 in Abdominal Cavity | 0.005 | ||
| Beyond the norm | 21 (26.9%) | 8 (72.7%) | |
| Norm | 57 (73.1%) | 3 (27.3%) | |
| Neoadjuvant Radiotherapy | 0.444 | ||
| Negative | 59 (75.6%) | 10 (90.9%) | |
| Positive | 19 (24.4%) | 1 (9.1%) | |
| Neoadjuvant Chemotherapy | 0.681 | ||
| Negative | 63 (80.8%) | 10 (90.9%) | |
| Positive | 15 (19.2%) | 1 (9.1%) | |
| Mucinous Characteristic Component | 0.223 | ||
| Negative | 64 (82.1%) | 7 (63.6%) | |
| Positive | 14 (17.9%) | 4 (36.4%) | |
| Tumor Necrosis | 0.558 | ||
| Negative | 73 (93.6%) | 10 (90.9%) | |
| Positive | 5 (6.4%) | 1 (9.1%) | |
| Histopathological Stage | 0.002 | ||
| G1 | 52 (66.7%) | 3 (27.3%) | |
| G2 | 15 (19.2%) | 1 (9.1%) | |
| G3 | 11 (14.1%) | 7 (63.6%) | |
| Tumor Location | 0.085 | ||
| Ascending colon | 11 (14.1%) | 2 (18.2%) | |
| Cecum | 13 (16.7%) | 4 (36.4%) | |
| Descending colon | 2 (2.6%) | 1 (9.1%) | |
| Intraperitoneal rectum | 43 (55.1%) | 2 (18.2%) | |
| Sigmoid colon | 5 (6.4%) | 1 (9.1%) | |
| Transverse colon | 4 (5.1%) | 1 (9.1%) | |
| T Stage | <0.001 | ||
| 0 | 1 (1.3%) | 0 (0.0%) | |
| 1 | 2 (2.6%) | 0 (0.0%) | |
| 2 | 21 (26.9%) | 0 (0.0%) | |
| 3 | 43 (55.1%) | 1 (9.1%) | |
| 4 | 11 (14.1%) | 10 (90.9%) | |
| N Stage | <0.001 | ||
| 0 | 40 (51.3%) | 0 (0.0%) | |
| 1 | 23 (29.5%) | 1 (9.1%) | |
| 2 | 15 (19.2%) | 10 (90.9%) | |
| M Stage | <0.001 | ||
| 0 | 63 (80.8%) | 0 (0.0%) | |
| 1 | 15 (19.2%) | 11 (100.0%) | |
| L Stage | <0.001 | ||
| 0 | 59 (75.6%) | 0 (0.0%) | |
| 1 | 19 (24.4%) | 11 (100.0%) | |
| V Stage | <0.001 | ||
| 0 | 63 (80.8%) | 1 (9.1%) | |
| 1 | 15 (19.2%) | 10 (90.9%) | |
| Pn Stage | <0.001 | ||
| 0 | 61 (78.2%) | 1 (9.1%) | |
| 1 | 17 (21.8%) | 10 (90.9%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bąk, M.; Wojciech, M.; Monczak, R.; Zawadzki, M.; Murawa, D. The Significance of CEA and CA 19-9 Levels in Serum and Peritoneal Fluid in Colorectal Cancer Patients in the Context of Peritoneal Metastases and Cytology Results. Cancers 2025, 17, 2661. https://doi.org/10.3390/cancers17162661
Bąk M, Wojciech M, Monczak R, Zawadzki M, Murawa D. The Significance of CEA and CA 19-9 Levels in Serum and Peritoneal Fluid in Colorectal Cancer Patients in the Context of Peritoneal Metastases and Cytology Results. Cancers. 2025; 17(16):2661. https://doi.org/10.3390/cancers17162661
Chicago/Turabian StyleBąk, Michał, Magdalena Wojciech, Roman Monczak, Marek Zawadzki, and Dawid Murawa. 2025. "The Significance of CEA and CA 19-9 Levels in Serum and Peritoneal Fluid in Colorectal Cancer Patients in the Context of Peritoneal Metastases and Cytology Results" Cancers 17, no. 16: 2661. https://doi.org/10.3390/cancers17162661
APA StyleBąk, M., Wojciech, M., Monczak, R., Zawadzki, M., & Murawa, D. (2025). The Significance of CEA and CA 19-9 Levels in Serum and Peritoneal Fluid in Colorectal Cancer Patients in the Context of Peritoneal Metastases and Cytology Results. Cancers, 17(16), 2661. https://doi.org/10.3390/cancers17162661






