Off-Clamp Robotic-Assisted Partial Nephrectomy: Retrospective Comparative Analysis from a Large Italian Multicentric Series
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
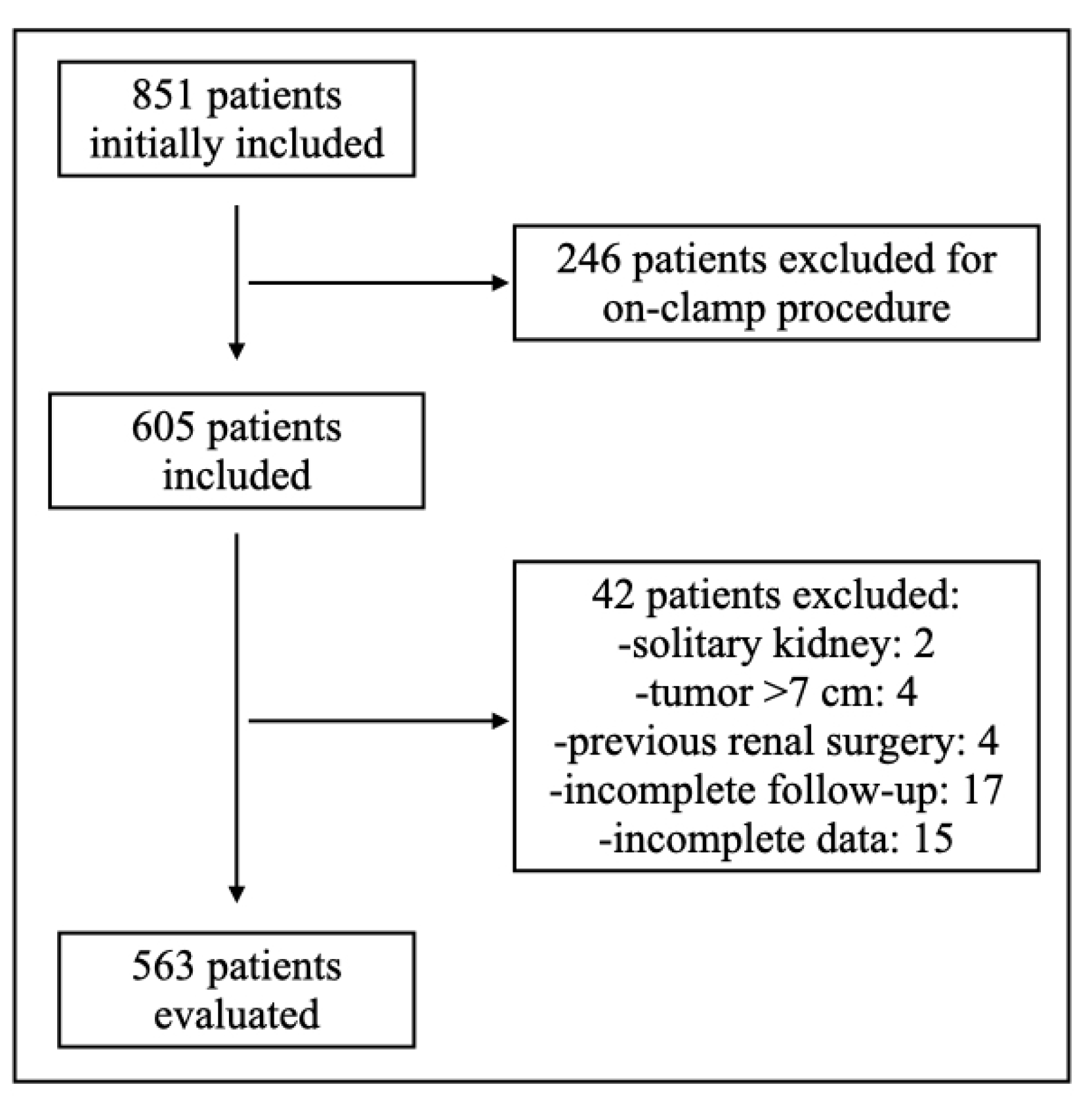
2.1. Study Design and Setting
2.2. Objective
2.3. Patient Selection Criteria
- Multifocal tumors or bilateral renal masses,
- Patients with solitary kidney or previous ipsilateral renal surgery,
- Conversion to hilar clamping or open surgery intraoperatively,
- Tumors >7 cm or locally advanced disease (≥T2),
- Lack of complete perioperative or follow-up data.
2.4. Preoperative Assessment
2.5. Surgical Technique
2.6. Perioperative Management
2.7. Data Collection
- Total operative time (from incision to skin closure),
- Console time (robot docking to undocking),
- Estimated blood loss (EBL),
- Need for intraoperative transfusion,
- Intraoperative complications,
- Conversion to hilar clamping or open surgery.
- Length of hospital stay,
- Complications graded by the Clavien–Dindo classification system,
- Readmission within 30 days,
- Pathological data including tumor histology, Fuhrman grade, surgical margin status,
- Imaging follow-up to assess recurrence.
2.8. Functional Outcome Assessment
2.9. Oncologic Follow-Up
2.10. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
- Demographics and comorbidities: no statistically significant differences were noted between groups in the following:
- Age at diagnosis (58 vs. 62 years)
- Gender distribution (approx. 70:30 male:female in both)
- ASA score (median 2 in both)
- Age-adjusted Charlson Comorbidity Index (AACCI; median 3 vs. 4)
- Smoking status (comparable proportions)
- Baseline renal function (eGFR median 87.4 vs. 85.9 mL/min/1.73 m2).
3.2. Pre-Operative Tumor Characteristics
- Tumor features were statistically similar across both groups:
- Focality: Predominantly monofocal in both (82% vs. 86%),
- Number of lesions: Median of 1 in both groups,
- Size of the largest lesion: 3.7 cm (off-clamp) vs. 4.2 cm (on-clamp),
- Complexity scores:
- RENAL: Median 6 vs. 7,
- Padua: Median 6 vs. 7.
3.3. Surgical Characteristics
- Surgical approach: Robotic-assisted surgery predominated in both groups (97% vs. 86%), though the on-clamp group had slightly more laparoscopic/open cases.
- Length of surgery: Longer in the on-clamp group (118 vs. 140 min, p = 0.03).
- Estimated blood loss: Greater in the on-clamp group (150 vs. 200 mL, p = 0.03).
- Ischemia time: Only applicable to on-clamp (median 21 min).
- Complication rates:
- Intraoperative: 0% (off-clamp) vs. 3.3% (on-clamp), p = 0.00.
- Perioperative: 9.2% (off-clamp) vs. 13.9% (on-clamp), p = 0.04.
- The median length of hospital stay for off-clamp cases was 3 days (range: 2–7 days), reflecting a rapid recovery in most patients. The median length of stay in the on-clamp group was 4 days (range: 3–9 days), which was significantly longer compared to the off-clamp cohort (p < 0.05), likely reflecting higher complication burden.
3.4. Functional Outcomes
3.5. Oncologic Outcomes
- pTNM stage: Comparable distribution (majority pT1a in both),
- Histology: Final pathology revealed clear cell RCC in 72%, papillary RCC in 18%, chromophobe RCC in 6%, and benign lesions (e.g., oncocytoma, angiomyolipoma) in 4%. Histological variants/aspects occurred in ~9% of both groups.
- Local recurrence (LR):
- Rate: Low in both groups (0.36% vs. 1.2%, ns),
- Time to LR: Median 15 vs. 18 months.
- Positive surgical margins (PSM): Slightly higher in on-clamp group (3.7% vs. 2.3%, not significant).
3.6. Survival Outcomes
- Overall survival (OS): 92.1% (off-clamp) vs. 87.8% (on-clamp),
- Cancer-specific survival (CSS): 95.4% vs. 93.3%,
- Both survival metrics were high and statistically comparable (p > 0.05).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, I.S.; Aron, M.; Gervais, D.A.; Jewett, M.A. Clinical practice. Small renal mass. N. Engl. J. Med. 2010, 362, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Derweesh, I.H.; Gill, I.S.; Jewett, M.A.; et al. Renal mass and localized renal cancer: AUA guideline. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology guidelines on renal cell carcinoma: The 2022 update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Patel, M.N.; Allaf, M.E.; Rogers, C.G.; Sukumar, S.; Ruel, N.; Menon, M.; Gill, I.S.; Pierorazio, P.M. Robotic-assisted partial nephrectomy: Contemporary practice. J. Robot. Surg. 2020, 14, 639–646. [Google Scholar]
- Pierorazio, P.M.; Patel, H.D.; Feng, T.; Yohannan, J.; Hyams, E.S.; Allaf, M.E. Five-year oncologic outcomes after RAPN. Eur. Urol. 2017, 72, 90–97. [Google Scholar]
- Khalifeh, A.; Kaouk, J.H.; Hillyer, S.R.; Autorino, R.; Hegarty, N.; Akca, O.; Ouzaid, I.; Haber, G.P.; Stein, R.; Gill, I.S.; et al. Comparative outcomes of robotic versus open partial nephrectomy: A matched pair analysis. J. Urol. 2013, 189, 860–866. [Google Scholar]
- Yu, H.-Y.; Cellini, C.; Nguyen, T.; Trinh, Q.; Moore, R.; Derweesh, I.; Finelli, A.; Wong, Y.Y.; Kiemeney, L.A.; De La Rosette, J. Trends in the use of partial nephrectomy in the United States. JAMA 2010, 303, 1151–1156. [Google Scholar]
- Nguyen, M.M.; Gill, I.S.; Ellison, L.M. Effect of renal ischemia on kidney function after partial nephrectomy. J. Urol. 2008, 179, 794–799. [Google Scholar]
- Thompson, R.H.; Boorjian, S.A.; Lohse, C.M.; Leibovich, B.C.; Kwon, E.D.; Cheville, J.C.; Blute, M.L. Chronic kidney disease after nephrectomy for renal cortical tumors. J. Am. Soc. Nephrol. 2008, 19, 146–153. [Google Scholar]
- Porpiglia, F.; Fiori, C.; Bertolo, R.; Amparore, D.; Checcucci, E.; De Cillis, S.; Verri, P.; Mottrie, A.; Carini, M. Zero ischemia laparoscopic partial nephrectomy: A multi-institutional study. Urology 2012, 80, 569–574. [Google Scholar]
- Spana, G.; Larcher, A.; Rodriguez-Fernandez, F.J.; Campi, R.; Pecoraro, A.; Marchioni, M.; Volpe, A.; Ficarra, V.; Minervini, A.; Serni, S.; et al. Selective suturing strategies in robotic partial nephrectomy. Eur. Surg. Oncol. 2018, 44, 1494–1499. [Google Scholar]
- Bertolo, R.; Porpiglia, F.; Amparore, D.; Bertini, R.; Simeone, C.; Verri, P.; Volpe, A.; Ficarra, V.; Carini, M. Clampless and sutureless robotic partial nephrectomy: Functional and oncologic results. Eur. Urol. Focus 2019, 5, 722–728. [Google Scholar]
- Kaouk, J.H.; Khalifeh, A.; Hillyer, S.; Haber, G.P.; Stein, R.J.; Autorino, R. Robot-assisted laparoscopic partial nephrectomy: Step-by-step contemporary technique and surgical outcomes at a single high-volume institution. Eur. Urol. 2012, 62, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Gan, V.H.L.; Lim, B.J.H.; Chan, Y.H.; Castellani, D.; Chen, K.; Tay, K.J.; Ho, H.S.S.; Yuen, J.S.P.; Aslim, E.; et al. Off-clamp vs on-clamp robot-assisted partial nephrectomy: A systematic review and meta-analysis. BJU Int. 2024, 133, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Blute, M.L.; Ficarra, V.; Gill, I.S.; Kutikov, A.; Porpiglia, F.; Rogers, C.; Touijer, K.A.; Van Poppel, H.; Thompson, R.H. Renal Ischemia and Function After Partial Nephrectomy: A Collaborative Review of the Literature. Eur. Urol. 2015, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Buffi, N.; Lista, G.; Larcher, A.; Lughezzani, G.; Ficarra, V.; Cestari, A.; Lazzeri, M.; Guazzoni, G. Margin, ischemia, and complications (MIC) score in partial nephrectomy: A new system for evaluating achievement of optimal outcomes in nephron-sparing surgery. Eur. Urol. 2012, 62, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Zabell, J.R.; Wu, J.; Suk-Ouichai, C.; Campbell, S.C. Renal Ischemia and Functional Outcomes Following Partial Nephrectomy. Urol Clin. N. Am. 2017, 44, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, J.; Ren, Y.; Shen, C.; Ying, X.; Pan, S. Robot-assisted versus laparoscopic partial nephrectomy for localized renal tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 4770–4779. [Google Scholar] [PubMed] [PubMed Central]
- Zhou, Z.; Li, Z.; Ning, K.; Xiong, L.; Liu, H.; Huang, Y.; Luo, X.; Peng, Y.; Chen, L.; Ma, B.; et al. Long-term effect of acute ischemic injury on the kidney underwent clamped partial nephrectomy. iScience 2023, 26, 107610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haase, V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am. J. Physiol. Renal Physiol. 2010, 299, F1–F13. [Google Scholar] [CrossRef]
- Nangaku, M.; Eckardt, K.U. Hypoxia and the HIF system in kidney disease. J. Mol. Med. 2007, 85, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Chen, G.; Dong, G.; Kunzendorf, U.; Krautwald, S.; Dong, Z. Regulated cell death in AKI. J. Am. Soc. Nephrol. 2014, 25, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Fine, L.G.; Norman, J.T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 2008, 74, 867–872. [Google Scholar] [CrossRef]
- Parikh, C.R.; Devarajan, P. New biomarkers of acute kidney injury. Crit. Care Med. 2008, 36 (Suppl. 4), S159–S165. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Jonathan, B.; Prasad, D. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Tublin, M.E.; Bude, R.O.; Platt, J.F. The resistive index in renal Doppler sonography: Where do we stand? AJR Am. J. Roentgenol. 2003, 180, 885–892. [Google Scholar] [CrossRef]
- Schneider, A.G.; Goodwin, M.D.; Bellomo, R. Measurement of kidney perfusion in critically ill patients. Crit. Care 2013, 17, 220. [Google Scholar] [CrossRef]
- Minervini, A.; Campi, R.; Spana, A.; Pecoraro, A.; Mercadante, C.; Simone, G.; Ficarra, V.; Volpe, A.; Carini, M.; Brunelli, M. Advances in suturing techniques for nephron-sparing surgery. Eur. Urol. 2019, 75, 809–810. [Google Scholar]
- Autorino, R.; Cacciamani, G.E.; Proietti, S.; Brandao, S.; Zargar, H.; Laydner, H.; Gill, I.S.; Kaouk, J.H. Off-clamp robotic partial nephrectomy: A systematic review of the literature. BJU Int. 2012, 110, E1237–E1247. [Google Scholar]
- Aboumarzouk, O.M.; Stein, R.J.; Eyraud, R.; Haber, G.P.; Chlosta, P.L.; Somani, B.K.; Autorino, R.; Gill, I.S. Zero-ischemia partial nephrectomy: A meta-analysis. Can. Urol. Assoc. J. 2014, 8, E637–E643. [Google Scholar]
- Komninos, C.; Lopez-Corona, E.; Rehman, J.; Rogers, C.G.; Sukumar, S.; Su, L.M.; Menon, M. Off-clamp RAPN for complex tumors. World J. Urol. 2014, 32, 999–1005. [Google Scholar]
- Desai, M.M.; Gill, I.S.; Ramani, A.; Rogers, C.; Kaouk, J.H.; Zhang, X.; Sung, G.; Hobart, M.; Strohmeier, D.; Sutton, C.; et al. Robotic partial nephrectomy for renal tumors: Phase I clinical trial. Urology 2011, 78, 815–821. [Google Scholar]
- Gill, I.S.; Kavoussi, L.R.; Lane, B.R.; Blute, M.L.; Babineau, D.; Colombo, J.R.; Frank, I.; Permpongkosol, S.; Weight, C.J.; Kaouk, J.H.; et al. Outcomes of 1,800 robotic partial nephrectomies: Experience from a high-volume center. Eur. Urol. 2015, 68, 735–742. [Google Scholar]
- Kutikov, A.; Uzzo, R.G. The RENAL nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef]
- Simmons, M.N.; Fergany, A.F.; Campbell, S.C. Effect of parenchymal volume preservation on kidney function after partial nephrectomy. J. Urol. 2011, 186, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Hayn, M.H.; Schwaab, T.; Underwood, W.; Kim, H.L. RENAL nephrometry score predicts surgical outcomes of laparoscopic partial nephrectomy. BJU Int. 2011, 108, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sun, J.; Zhang, Z.; Zhang, H.; Zhou, Q.; Xu, J.; Ling, Z.; Ouyang, J. Parallel comparison of R.E.N.A.L.; PADUA, and C-index scoring systems in predicting outcomes after partial nephrectomy: A systematic review and meta-analysis. Cancer Med. 2021, 10, 5062–5077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mir, M.C.; Campbell, R.A.; Sharma, N.; Remer, E.M.; Li, J.; Demirjian, S.; Kaouk, J.H.; Campbell, S.C. Parenchymal volume preservation and ischemia during partial nephrectomy: Functional and volumetric analysis. Urology 2013, 82, 263–268. [Google Scholar] [CrossRef]
- Dubeux, V.; Zanier, J.F.C.; Chantong, C.G.C.; Carrerette, F.; Gabrich, P.N.; Damião, R. Nephrometry scoring systems: Their importance for the planning of nephron-sparing surgery and the relationships among them. Radiol. Bras. 2022, 55, 242–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ficarra, V.; Novara, G.; Secco, S.; Macchi, V.; Porzionato, A.; De Caro, R.; Artibani, W. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumors in patients who are candidates for nephron-sparing surgery. Eur. Urol. 2009, 56, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in partial nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Grivas, N.; Kalampokis, N.; Larcher, A.; Tyritzis, S.; Rha, K.H.; Ficarra, V.; Buffi, N.; Ploumidis, A.; Autorino, R.; Porpiglia, F.; et al. Robot-assisted versus open partial nephrectomy: Comparison of outcomes. A systematic review. Minerva Urol. Nefrol. 2019, 71, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Sharma, G.; Ahluwalia, P.; Gautam, G.; Erdem, S.; Amparore, D.; Marchioni, M.; Pavan, N.; Marandino, L.; Roussel, E.; et al. Off-clamp Versus On-clamp Robot-assisted Partial Nephrectomy: A Systematic Review and Quantitative Synthesis by the European Association of Urology Young Academic Urologists Renal Cancer Study Group. Eur. Urol. Open Sci. 2023, 58, 10–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rod, X.; Peyronnet, B.; Seisen, T.; Pradere, B.; Gomez, F.D.; Verhoest, G.; Vaessen, C.; De La Taille, A.; Bensalah, K.; Roupret, M. Impact of ischaemia time on renal function after partial nephrectomy: A systematic review. BJU Int. 2016, 118, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.A.; Salamone, V.; Di Maida, F.; Giudici, S.; Cadenar, A.; Lambertini, L.; Conte, F.L.; Bacchiani, M.; Mazzola, L.; Crisci, A.; et al. Robot-assisted partial nephrectomy for renal cell carcinoma: A narrative review of different clinical scenarios. Asian J. Urol. 2025, 12, 210–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Off-Clamp | On-Clamp | p-Value | |
|---|---|---|---|
| Patients’ characteristics | >0.05 | ||
| Number of patients | 563 | 244 | |
| Age at diagnosis, years | 58 (35–80) | 62 (55–73) | |
| Gender, m:f, % | 70.9:29.1 | 73.3:26.7 | |
| ASA score, median (range) | 2 (2–3) | 2 (2–3) | |
| AACCI, median (range) | 3 (3–5) | 4 (4–5) | |
| Smoking status, no smoker: active/former smoker, % | 64.5:35.5 | 69.7:30.3 | |
| Pre-operative eGFR, mL/min/1.73 m2, median (IQR) | 87.4 (57.9–108.3) | 85.9 (38–98) | |
| Pre-operative tumour characteristics | >0.05 | ||
| Focality, monofocal:multifocal, % | 81.9:18.1 | 85.7:14.3 | |
| N° of lesions, median (range) | 1 (1–2) | 1 (1–3) | |
| Median size of the largest lesion, cm (IQR) | 3.7 (1.0–5.8) | 4.2 (2.3–5.3) | |
| Median RENAL score, median value (IQR) | 6 (5–8) | 7 (6–8) | |
| Median Padua score, median (IQR) | 6 (5–8) | 7 (6–9) | |
| Surgical characteristics | |||
| Approach, robotic:laparoscopic:open | 96.8:3.2:0 | 86.1:9:4.9 | >0.05 |
| Median length of surgery, minutes (IQR) | 118 (100–140) | 140 (120–182) | 0.03 |
| Median blood loss, mL (IQR) | 150 (50–400) | 200 (180–300) | 0.03 |
| Median ischemia time, minutes (IQR) | - | 21 (15.5–24) | - |
| Intraoperative complications, % | 0 | 3.3 | 0.00 |
| Perioperative complications, % | 9.2 | 13.9 | 0.04 |
| Pathological characteristics | >0.05 | ||
| pTNM | |||
| pT1a | 78.9% | 81.5% | |
| pT1b | 21.1% | 18.5% | |
| Histology | |||
| Clear cell RCC | 85.7% | 83.6% | |
| Papillary RCC | 12.3% | 11.9% | |
| Chromophobe RCC | 2.1% | 4.5% | |
| Variants/aspects, % | 8.9% | 9.8% | |
| Rate of LR, % | 0.36 | 1.2 | >0.05 |
| Median time to LR, months (IQR) | 15 (13–19) | 18 (12–39) | >0.05 |
| Rate of PSM, % | 2.3 | 3.7 | >0.05 |
| OS, % | 92.1 | 87.8 | >0.05 |
| CSS, % | 95.4 | 93.3 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porreca, A.; Marino, F.; De Marchi, D.; Giampaoli, M.; Simonetti, F.; Amodeo, A.; Corsi, P.; Claps, F.; Romagnoli, D.; Crestani, A.; et al. Off-Clamp Robotic-Assisted Partial Nephrectomy: Retrospective Comparative Analysis from a Large Italian Multicentric Series. Cancers 2025, 17, 2645. https://doi.org/10.3390/cancers17162645
Porreca A, Marino F, De Marchi D, Giampaoli M, Simonetti F, Amodeo A, Corsi P, Claps F, Romagnoli D, Crestani A, et al. Off-Clamp Robotic-Assisted Partial Nephrectomy: Retrospective Comparative Analysis from a Large Italian Multicentric Series. Cancers. 2025; 17(16):2645. https://doi.org/10.3390/cancers17162645
Chicago/Turabian StylePorreca, Angelo, Filippo Marino, Davide De Marchi, Marco Giampaoli, Francesca Simonetti, Antonio Amodeo, Paolo Corsi, Francesco Claps, Daniele Romagnoli, Alessandro Crestani, and et al. 2025. "Off-Clamp Robotic-Assisted Partial Nephrectomy: Retrospective Comparative Analysis from a Large Italian Multicentric Series" Cancers 17, no. 16: 2645. https://doi.org/10.3390/cancers17162645
APA StylePorreca, A., Marino, F., De Marchi, D., Giampaoli, M., Simonetti, F., Amodeo, A., Corsi, P., Claps, F., Romagnoli, D., Crestani, A., & Di Gianfrancesco, L. (2025). Off-Clamp Robotic-Assisted Partial Nephrectomy: Retrospective Comparative Analysis from a Large Italian Multicentric Series. Cancers, 17(16), 2645. https://doi.org/10.3390/cancers17162645







