An In Silico Feasibility Study of Dose-Escalated Hypofractionated Proton Therapy for Rectal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. Treatment Planning
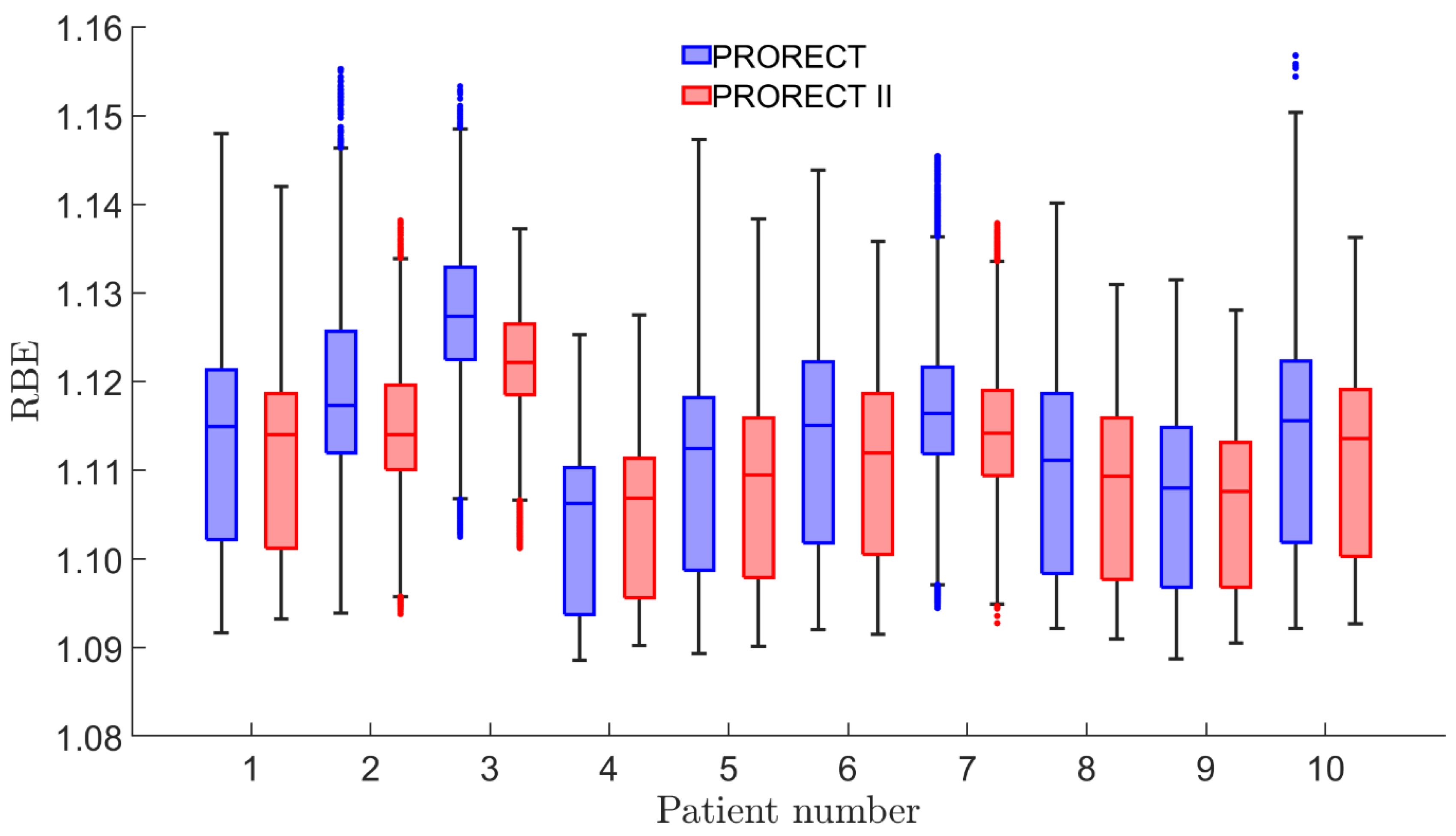
2.3. RBE and Integral Dose
2.4. Plan and Feasibility Evaluation
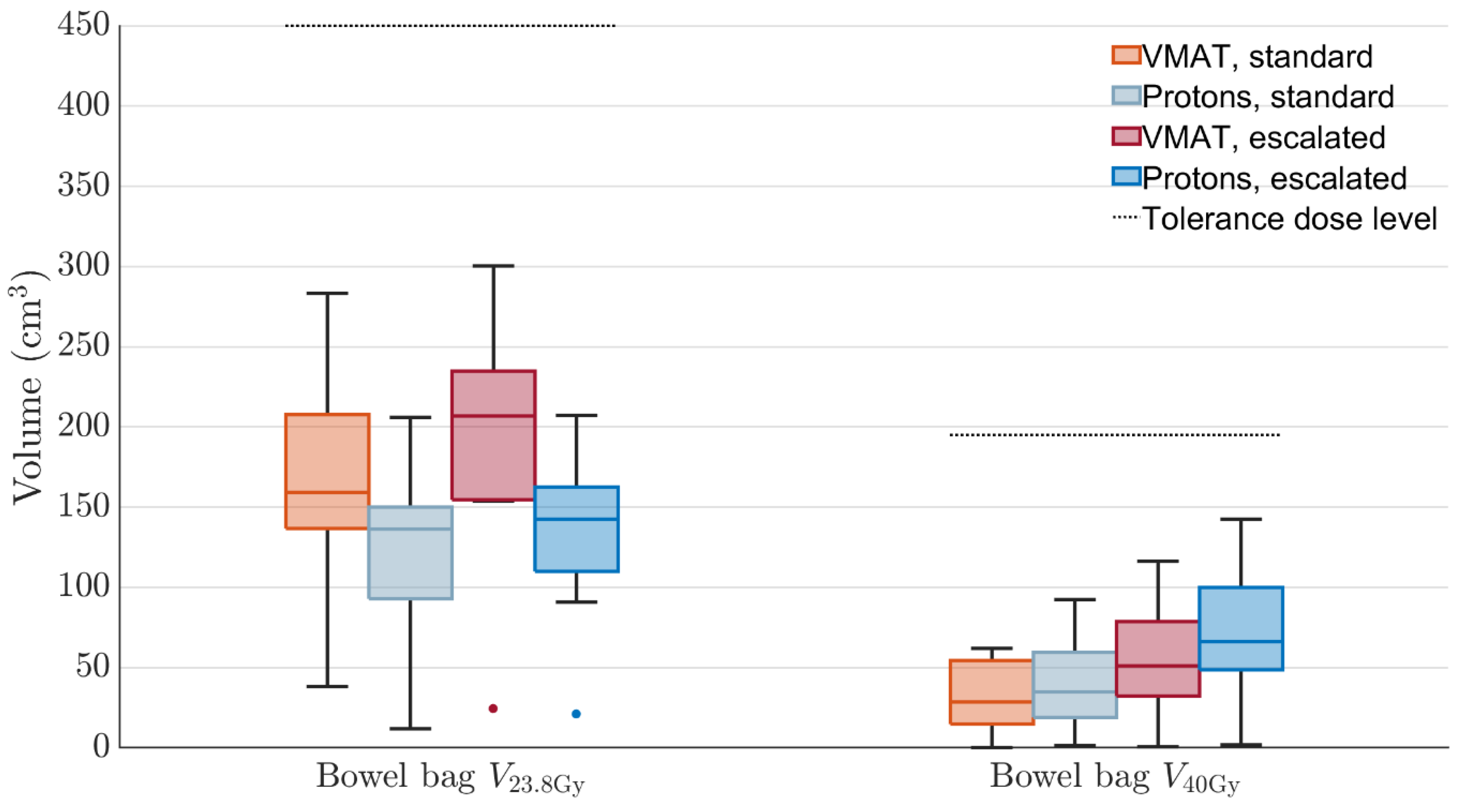
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cCR | Clinical complete response |
| CT | Computed tomography |
| CTV | Clinical target volume |
| DVH | Dose volume histogram |
| GTV | Gross tumor volume |
| HU | Hounsfield unit |
| ID | Integral dose |
| LARC | Locally advanced rectal cancer |
| LCRT | Long-course radiotherapy |
| LET | Linear energy transfer |
| MFO | Multifield optimization |
| OAR | Organ at risk |
| pCR | Pathological complete response |
| PT | Proton therapy |
| PTV | Planning target volume |
| RBE | Relative biological effectiveness |
| RT | Radiotherapy |
| SCRT | Short course radiotherapy |
| SFUD | Single field uniform dose |
| TME | Total mesorectal excision |
| xRT | Photon radiotherapy |
References
- Ystgaard, M.F.; Myklebust, T.Å.; Smeby, J.; Larsen, I.K.; Guren, T.K.; Kure, E.H.; Tveit, K.M.; Glimelius, B.; Guren, M.G.; Hamfjord, J. Early-onset colorectal cancer incidence in Norway: A national registry-based study (1993-2022) analyzing subsite and morphology trends. ESMO Gastrointest. Oncol. 2025, 7, 100065. [Google Scholar] [CrossRef]
- Suresh, R.S.; Garcia, L.E.; Gearhart, S.L. Young-Onset Rectal Cancer: Is It for Real? Adv. Surg. 2024, 58, 275–291. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Cancer of colon, rectum and anus: The rising burden of disease worldwide from 1990 to 2019. J. Public Health 2024, 46, 20–29. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Fokas, E.; Benhaim, L.; Price, T.J.; Arnold, D.; Beets-Tan, R.; Guren, M.G.; Hospers, G.A.P.; Lonardi, S.; Nagtegaal, I.D.; et al. Localised rectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B. Recent advances in rectal cancer treatment—Are we on the right track? Ups. J. Med. Sci. 2024, 129, e10537. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, 40, 2546–2556. [Google Scholar] [CrossRef] [PubMed]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- van der Heijden, J.A.G.; Koëter, T.; Smits, L.J.H.; Sietses, C.; Tuynman, J.B.; Maaskant-Braat, A.J.G.; Klarenbeek, B.R.; de Wilt, J.H.W. Functional complaints and quality of life after transanal total mesorectal excision: A meta-analysis. Br. J. Surg. 2020, 107, 489–498. [Google Scholar] [CrossRef]
- Lauricella, S.; Brucchi, F.; Carrano, F.M.; Cassini, D.; Cirocchi, R.; Sylla, P. Quality of life and functional outcomes after laparoscopic total mesorectal excision (LaTME) and transanal total mesorectal excision (taTME) for rectal cancer. an updated meta-analysis. Int. J. Color. Dis. 2024, 39, 129. [Google Scholar] [CrossRef]
- Feeney, G.; Sehgal, R.; Sheehan, M.; Hogan, A.; Regan, M.; Joyce, M.; Kerin, M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019, 25, 4850–4869. [Google Scholar] [CrossRef]
- Vendrely, V.; Rivin Del Campo, E.; Modesto, A.; Jolnerowski, M.; Meillan, N.; Chiavassa, S.; Serre, A.A.; Gérard, J.P.; Créhanges, G.; Huguet, F.; et al. Rectal cancer radiotherapy. Cancer Radiother. 2022, 26, 272–278. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Braendengen, M.; Tveit, K.M.; Berglund, A.; Birkemeyer, E.; Frykholm, G.; Påhlman, L.; Wiig, J.N.; Byström, P.; Bujko, K.; Glimelius, B. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J. Clin. Oncol. 2008, 26, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Gama-Rodrigues, J.; São Julião, G.P.; Proscurshim, I.; Sabbagh, C.; Lynn, P.B.; Perez, R.O. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Ruby, J.A.; Goodman, K.A.; Saltz, L.B.; Guillem, J.G.; Weiser, M.R.; Temple, L.K.; Nash, G.M.; Paty, P.B. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann. Surg. 2012, 256, 965–972. [Google Scholar] [CrossRef]
- Teste, B.; Rouanet, P.; Tuech, J.J.; Valverde, A.; Lelong, B.; Rivoire, M.; Faucheron, J.L.; Jafari, M.; Portier, G.; Meunier, B.; et al. Early and late morbidity of local excision after chemoradiotherapy for rectal cancer. BJS Open 2021, 5, zrab043. [Google Scholar] [CrossRef]
- Wo, J.Y.; Anker, C.J.; Ashman, J.B.; Bhadkamkar, N.A.; Bradfield, L.; Chang, D.T.; Dorth, J.; Garcia-Aguilar, J.; Goff, D.; Jacqmin, D.; et al. Radiation Therapy for Rectal Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 13–25. [Google Scholar] [CrossRef]
- Bach, S.P.; Gilbert, A.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.G.; Khan, J.; et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): A randomised, open-label feasibility study. Lancet Gastroenterol. Hepatol. 2021, 6, 92–105. [Google Scholar] [CrossRef]
- Hearn, N.; Atwell, D.; Cahill, K.; Elks, J.; Vignarajah, D.; Lagopoulos, J.; Min, M. Neoadjuvant Radiotherapy Dose Escalation in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis of Modern Treatment Approaches and Outcomes. Clin. Oncol. (R. Coll. Radiol.) 2021, 33, e1–e14. [Google Scholar] [CrossRef]
- Lomax, A.J.; Bortfeld, T.; Goitein, G.; Debus, J.; Dykstra, C.; Tercier, P.A.; Coucke, P.A.; Mirimanoff, R.O. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother. Oncol. 1999, 51, 257–271. [Google Scholar] [CrossRef]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; MacDonald, S.M.; Yock, T.I.; Tarbell, N.J. Secondary Malignancy Risk Following Proton Radiation Therapy. Front. Oncol. 2015, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Fok, M.; Toh, S.; Easow, J.; Fowler, H.; Clifford, R.; Parsons, J.; Vimalachandran, D. Proton beam therapy in rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2021, 38, 101638. [Google Scholar] [CrossRef] [PubMed]
- Vaios, E.J.; Wo, J.Y. Proton beam radiotherapy for anal and rectal cancers. J. Gastrointest. Oncol. 2020, 11, 176–186. [Google Scholar] [CrossRef]
- Radu, C.; Norrlid, O.; Brændengen, M.; Hansson, K.; Isacsson, U.; Glimelius, B. Integrated peripheral boost in preoperative radiotherapy for the locally most advanced non-resectable rectal cancer patients. Acta Oncol. 2013, 52, 528–537. [Google Scholar] [CrossRef]
- Colaco, R.J.; Nichols, R.C.; Huh, S.; Getman, N.; Ho, M.W.; Li, Z.; Morris, C.G.; Mendenhall, W.M.; Mendenhall, N.P.; Hoppe, B.S. Protons offer reduced bone marrow, small bowel, and urinary bladder exposure for patients receiving neoadjuvant radiotherapy for resectable rectal cancer. J. Gastrointest. Oncol. 2014, 5, 3–8. [Google Scholar] [CrossRef]
- Ling, T.C.; Kang, J.I.; Slater, J.D.; Yang, G.Y. Proton therapy for gastrointestinal cancers. Transl. Cancer Res. 2012, 1, 150–158. [Google Scholar] [CrossRef]
- Santos, A.; Penfold, S.; Gorayski, P.; Le, H. The Role of Hypofractionation in Proton Therapy. Cancers 2022, 14, 2271. [Google Scholar] [CrossRef]
- Glimelius, B.; Khan, T.; Adolfsson, K.; Angenete, E.; Berglund, Å.; Bonde, K.; Elander, N.; Fokstuen, T.; Haux, J.; Imam, I.; et al. Total neoadjuvant treatment using short-course radiotherapy and four CAPOX cycles in locally advanced rectal cancer with high-risk criteria for recurrence: A Swedish nationwide cohort study (LARCT-US). EClinicalMedicine 2024, 75, 102771. [Google Scholar] [CrossRef]
- Erlandsson, J.; Fuentes, S.; Radu, C.; Frödin, J.E.; Johansson, H.; Brandberg, Y.; Holm, T.; Glimelius, B.; Martling, A. Radiotherapy regimens for rectal cancer: Long-term outcomes and health-related quality of life in the Stockholm III trial. BJS Open 2021, 5, zrab137. [Google Scholar] [CrossRef]
- Suwinski, R.; Wzietek, I.; Tarnawski, R.; Namysl-Kaletka, A.; Kryj, M.; Chmielarz, A.; Wydmanski, J. Moderately low alpha/beta ratio for rectal cancer may best explain the outcome of three fractionation schedules of preoperative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 793–799. [Google Scholar] [CrossRef]
- Zhang, M.X.; Li, X.B.; Guan, B.J.; Guan, G.X.; Lin, X.Y.; Wu, X.D.; Chi, P.; Xu, B.H. Dose escalation of preoperative short-course radiotherapy followed by neoadjuvant chemotherapy in locally advanced rectal cancer: Protocol for an open-label, single-centre, phase I clinical trial. BMJ Open 2019, 9, e025944. [Google Scholar] [CrossRef]
- Pedone, C.; Sorcini, B.; Staff, C.; Färlin, J.; Fokstuen, T.; Frödin, J.E.; Nilsson, P.J.; Martling, A.; Valdman, A. Preoperative short-course radiation therapy with PROtons compared to photons in high-risk RECTal cancer (PRORECT): Initial dosimetric experience. Clin. Transl. Radiat. Oncol. 2023, 39, 100562. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.I.; Roy, A.; Pedersen, K.S.; Huang, Y.; Hunt, S.R.; Glasgow, S.C.; Tan, B.R.; Wise, P.E.; Silviera, M.L.; Smith, R.K.; et al. Clinical Complete Response in Patients With Rectal Adenocarcinoma Treated With Short-Course Radiation Therapy and Nonoperative Management. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Pedersen, K.; Olsen, J.R.; Mutch, M.G.; Chin, R.I.; Glasgow, S.C.; Wise, P.E.; Silviera, M.L.; Tan, B.R.; Wang-Gillam, A.; et al. Nonoperative Rectal Cancer Management With Short-Course Radiation Followed by Chemotherapy: A Nonrandomized Control Trial. Clin. Color. Cancer 2021, 20, e185–e193. [Google Scholar] [CrossRef] [PubMed]
- PRORECT Radiotherapy Appendix, Version 3 (September). Available online: https://prorect.se/ws/media-library/f577f9c145804282bd319e893ef2cf46/appendix-1_radiotherapy_appendix_v3_sep2024.pdf (accessed on 3 June 2025).
- Parzen, J.S.; Zheng, W.; Li, X.; Ding, X.; Kabolizadeh, P. Optimization of Field Design in the Treatment of Rectal Cancer with Intensity Modulated Proton Beam Radiation Therapy: How Many Fields Are Needed to Account for Rectal Distension Uncertainty? Adv. Radiat. Oncol. 2021, 6, 100749. [Google Scholar] [CrossRef]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef]
- Friedrich, T. Proton RBE dependence on dose in the setting of hypofractionation. Br. J. Radiol. 2020, 93, 20190291. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef]
- McNamara, A.L.; Schuemann, J.; Paganetti, H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys. Med. Biol. 2015, 60, 8399–8416. [Google Scholar] [CrossRef]
- Hanna, G.G.; Murray, L.; Patel, R.; Jain, S.; Aitken, K.L.; Franks, K.N.; van As, N.; Tree, A.; Hatfield, P.; Harrow, S.; et al. UK Consensus on Normal Tissue Dose Constraints for Stereotactic Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 5–14. [Google Scholar] [CrossRef]
- Ratnakumaran, R.; Hinder, V.; Brand, D.; Staffurth, J.; Hall, E.; van As, N.; Tree, A. The Association between Acute and Late Genitourinary and Gastrointestinal Toxicities: An Analysis of the PACE B Study. Cancers 2023, 15, 1288. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Poenisch, F.; Li, H.; Zhang, X.; Sahoo, N.; Wu, R.Y.; Li, X.; Lee, A.K.; Chang, E.L.; Choi, S.; et al. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat. Oncol. 2014, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, A.M.; Burbach, J.P.M.; Berbee, M.; Lacle, M.M.; Arensman, R.; Raicu, M.G.; Wessels, F.J.; Verdult, J.; Roodhart, J.; Reerink, O.; et al. Efficacy of Dose-Escalated Chemoradiation on Complete Tumor Response in Patients with Locally Advanced Rectal Cancer (RECTAL-BOOST): A Phase 2 Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1008–1018. [Google Scholar] [CrossRef]
- Xiang, M.; Chang, D.T.; Pollom, E.L. Second cancer risk after primary cancer treatment with three-dimensional conformal, intensity-modulated, or proton beam radiation therapy. Cancer 2020, 126, 3560–3568. [Google Scholar] [CrossRef]
- Berman, A.T.; Both, B.; Sharkoski, T.; Goldrath, K.; Tochner, Z.; Apisamthanarax, S.; Metz, J.M.; Plastaras, J.P. Proton Reirradiation of Recurrent Rectal Cancer: Dosimetric Comparison, Toxicities, and Preliminary Outcomes. Int. J. Part. Ther. 2014, 1, 2–13. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Ishikawa, H.; Murakami, M.; Nakamura, M.; Shimizu, S.; Enomoto, T.; Oda, T.; Mizumoto, M.; Nakai, K.; Okumura, T.; et al. Proton Beam Therapy for Local Recurrence of Rectal Cancer. Anticancer Res. 2021, 41, 3589–3595. [Google Scholar] [CrossRef]
- Koroulakis, A.; Molitoris, J.; Kaiser, A.; Hanna, N.; Bafford, A.; Jiang, Y.; Bentzen, S.; Regine, W.F. Reirradiation for Rectal Cancer Using Pencil Beam Scanning Proton Therapy: A Single Institutional Experience. Adv. Radiat. Oncol. 2021, 6, 100595. [Google Scholar] [CrossRef]
- Rønde, H.S.; Kallehauge, J.F.; Kronborg, C.J.S.; Nyvang, L.; Rekstad, B.L.; Hanekamp, B.A.; Appelt, A.L.; Guren, M.G.; Spindler, K.L.S. Intensity modulated proton therapy planning study for organ at risk sparing in rectal cancer re-irradiation. Acta Oncol. 2021, 60, 1436–1439. [Google Scholar] [CrossRef]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Ma, J.; Dragojevic, S.; Remmes, N.B.; Mendelson, N.L.; Kloeber, J.A.; Ebner, D.K.; Wu, Z.; Gunn, H.J.; Merrell, K.W.; Hallemeier, C.L.; et al. Linear energy transfer optimized proton therapy for rectal cancer. Radiother. Oncol. 2025, 207, 110850. [Google Scholar] [CrossRef]
- Carabe-Fernandez, A.; Dale, R.G.; Jones, B. The incorporation of the concept of minimum RBE (RbEmin) into the linear-quadratic model and the potential for improved radiobiological analysis of high-LET treatments. Int. J. Radiat. Biol. 2007, 83, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.L.; O’Connor, J.D.; McMahon, S.J. Benchmarking proton RBE models. Phys. Med. Biol. 2024, 69, 085022. [Google Scholar] [CrossRef]
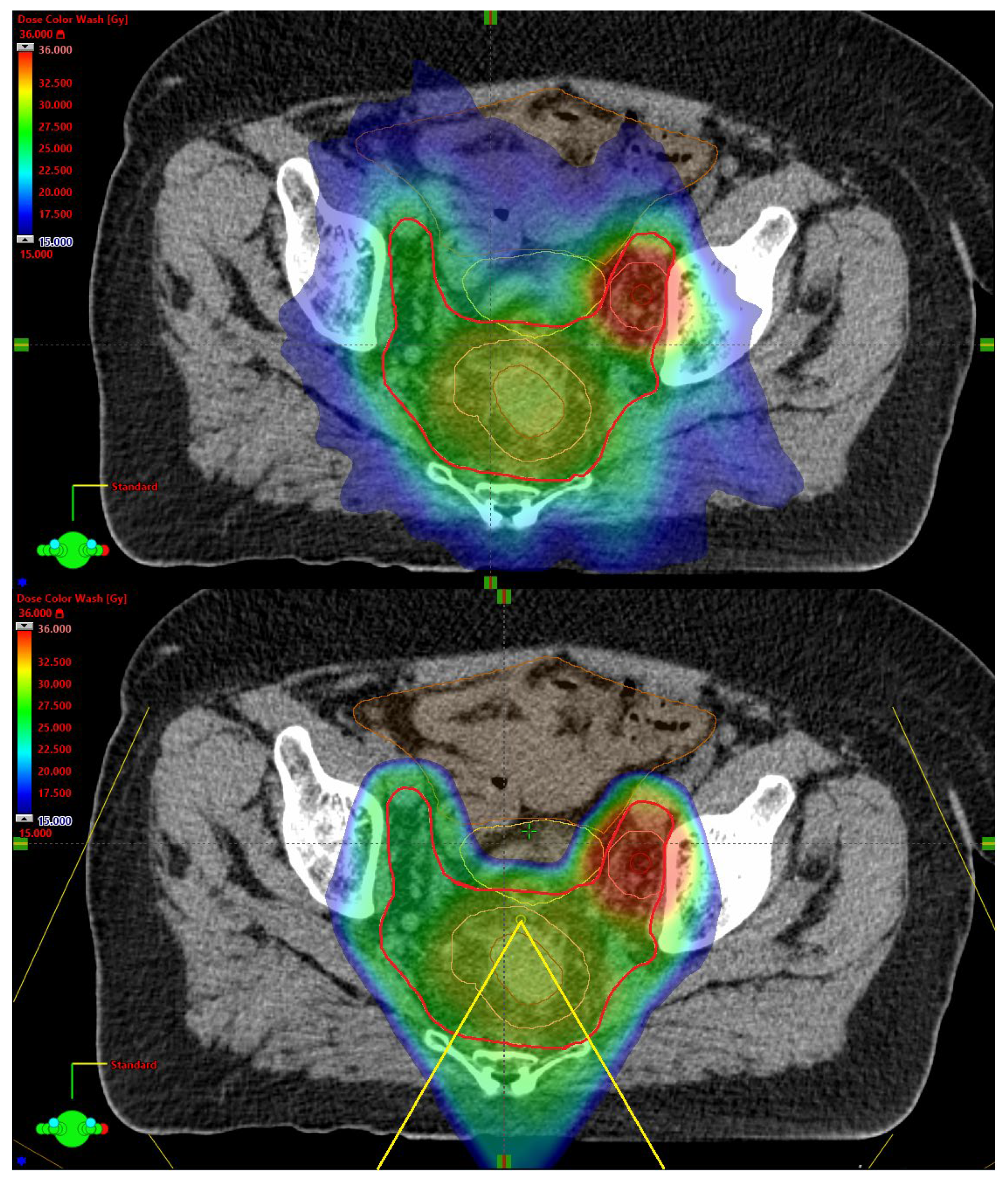
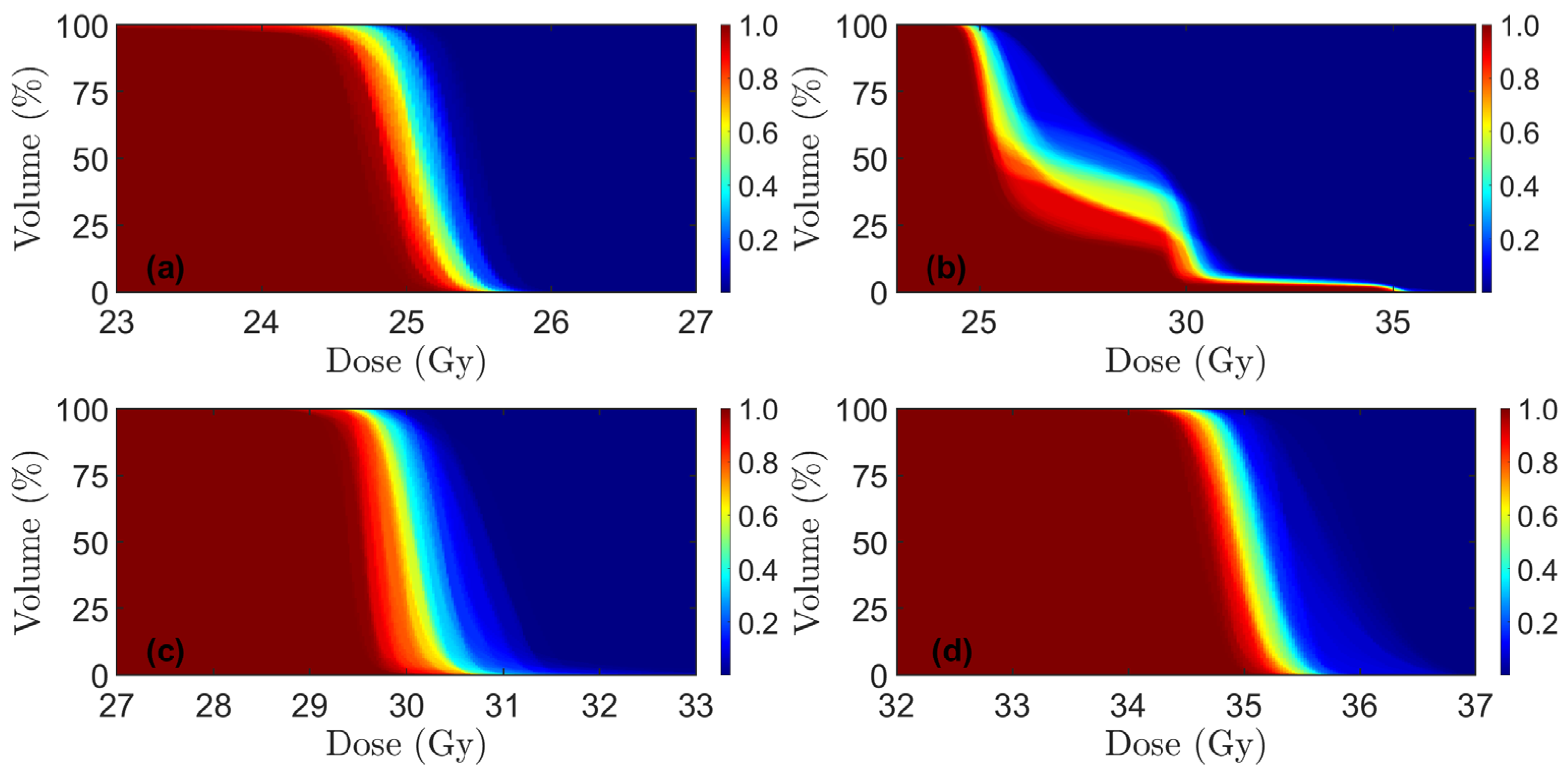
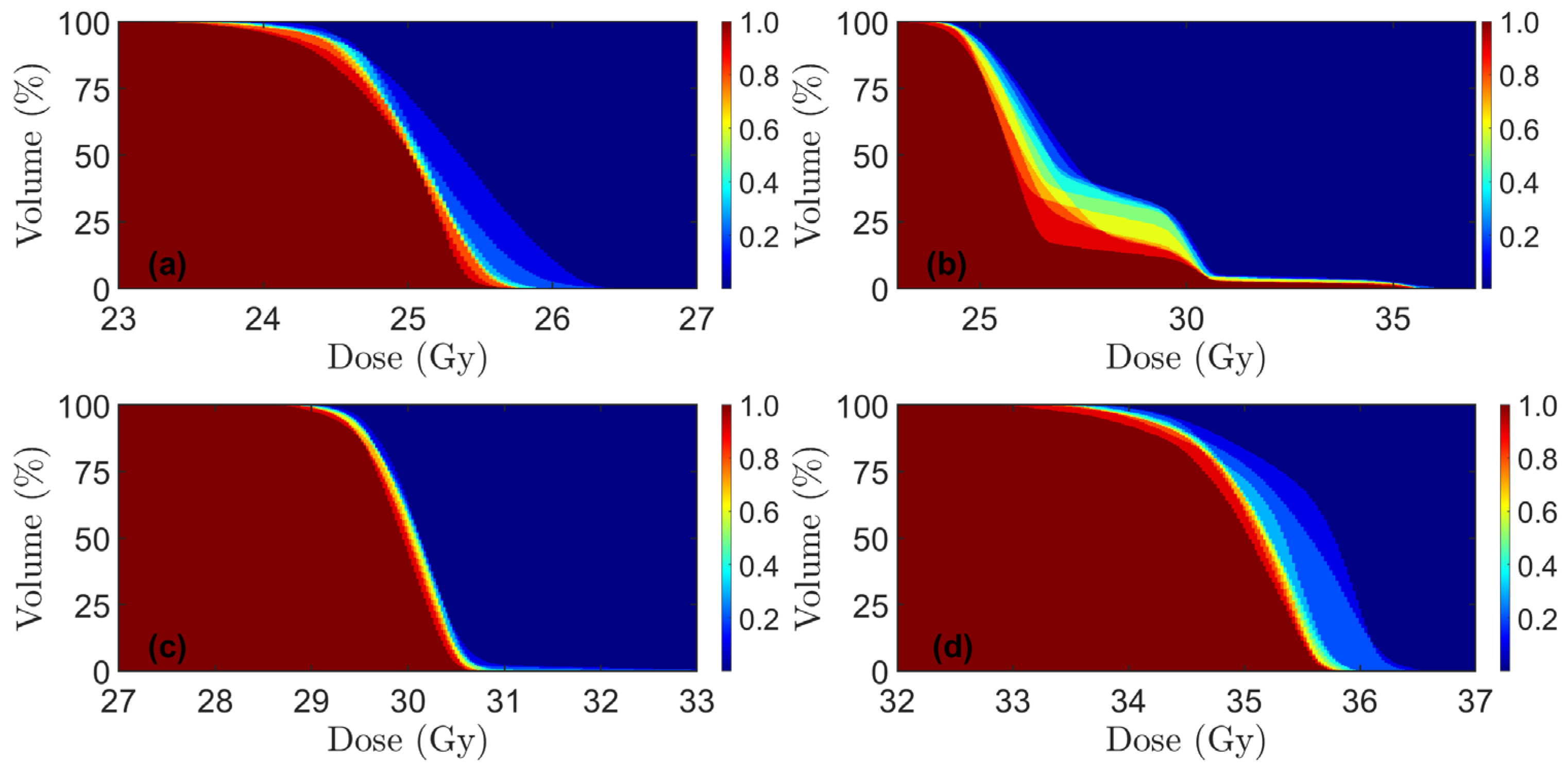
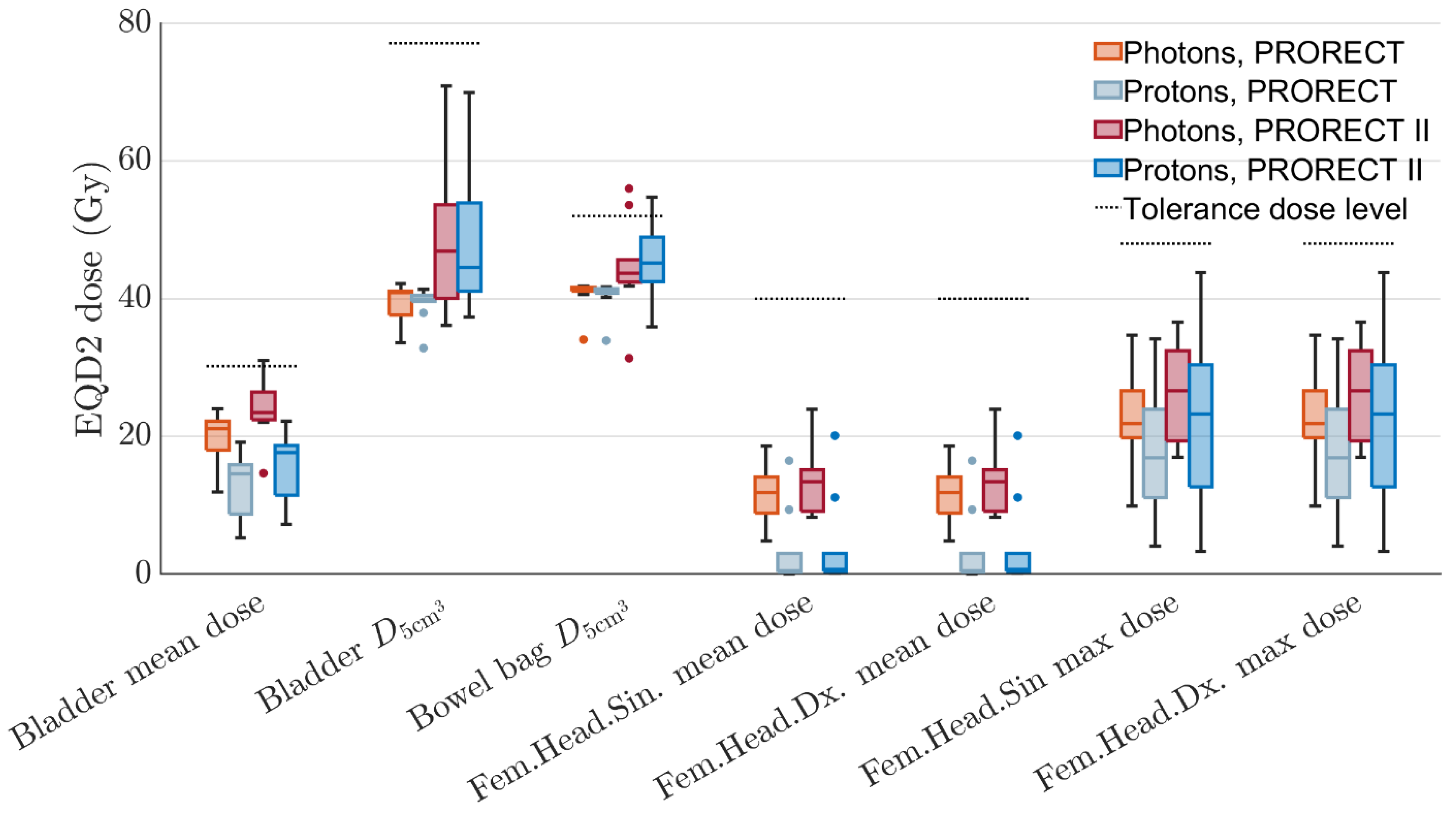

| Patient | Sex | Age | Tumor Height, mm | Lateral LN+ | mr T-Stage | mr N-Stage | MRF+ | EMVI+ | LN + Compartment * | Inguinal Lymphatic Nodes in CTV | Iliaca Externa Subsite in CTV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 47 | 90 | 0 | T4a | N2 | 1 | 0 | 1 | 0 | 0 |
| 2 | Female | 51 | 85 | 0 | T3b | N1 | 0 | 1 | 2 | 0 | 0 |
| 3 | Male | 62 | 50 | 1 | T2 | N1 | 0 | 0 | 2 | 0 | 0 |
| 4 | Male | 48 | 30 | 0 | T4b | N2 | 1 | 1 | 1 | 1 | 0 |
| 5 | Female | 73 | 60 | 0 | T4b | N2 | 1 | 1 | 2 | 0 | 1 |
| 6 | Male | 61 | 100 | 0 | T3c | N2 | 1 | 1 | 2 | 0 | 0 |
| 7 | Male | 51 | 120 | 0 | T3b | N1 | 0 | 1 | 1 | 0 | 0 |
| 8 | Male | 60 | 70 | 1 | T3a | N2 | 0 | 0 | 1 | 0 | 0 |
| 9 | Female | 58 | 20 | 0 | T4b | N1 | 1 | 1 | 1 | 1 | 1 |
| 10 | Male | 68 | 60 | 0 | T3c | N2 | 0 | 0 | 2 | 0 | 0 |
| Modality | Structure | DVH Criteria |
|---|---|---|
| PT | All CTV volumes | for all robustness scenarios |
| PT | All CTV volumes | for 12/14 robustness scenarios |
| PT | All CTV volumes | for 12/14 robustness scenarios |
| xRT | All PTVs | |
| xRT | All PTVs | |
| Both | Bowel bag | |
| Both | Bowel bag | |
| Both | Bowel bag | |
| Both | Bladder | |
| Both | Bladder | |
| Both | Femoral head | |
| Both | Femoral head |
| Metric | PRORECT (xRT vs. PT) | PRORECT II (xRT vs. PT) |
|---|---|---|
| Bladder mean dose | 0.002 | 0.004 |
| 0.492 | ||
| 0.193 | ||
| Fem. head left, mean dose | 0.002 | 0.002 |
| Fem. head right, mean dose | 0.002 | 0.002 |
| Fem. head left, max dose | 0.037 | |
| Fem. head right, max dose | 0.037 | |
| 0.002 | 0.002 | |
| 0.322 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almhagen, E.; Alkhiat, A.; Sorcini, B.; Alpsten, F.; Kronborg, C.J.S.; Rønde, H.S.; Guren, M.G.; Pilskog, S.; Valdman, A. An In Silico Feasibility Study of Dose-Escalated Hypofractionated Proton Therapy for Rectal Cancer. Cancers 2025, 17, 2627. https://doi.org/10.3390/cancers17162627
Almhagen E, Alkhiat A, Sorcini B, Alpsten F, Kronborg CJS, Rønde HS, Guren MG, Pilskog S, Valdman A. An In Silico Feasibility Study of Dose-Escalated Hypofractionated Proton Therapy for Rectal Cancer. Cancers. 2025; 17(16):2627. https://doi.org/10.3390/cancers17162627
Chicago/Turabian StyleAlmhagen, Erik, Ali Alkhiat, Bruno Sorcini, Freja Alpsten, Camilla J. S. Kronborg, Heidi S. Rønde, Marianne G. Guren, Sara Pilskog, and Alexander Valdman. 2025. "An In Silico Feasibility Study of Dose-Escalated Hypofractionated Proton Therapy for Rectal Cancer" Cancers 17, no. 16: 2627. https://doi.org/10.3390/cancers17162627
APA StyleAlmhagen, E., Alkhiat, A., Sorcini, B., Alpsten, F., Kronborg, C. J. S., Rønde, H. S., Guren, M. G., Pilskog, S., & Valdman, A. (2025). An In Silico Feasibility Study of Dose-Escalated Hypofractionated Proton Therapy for Rectal Cancer. Cancers, 17(16), 2627. https://doi.org/10.3390/cancers17162627






