Gene Expression in Muscle-Invasive and Non-Muscle-Invasive Bladder Cancer Cells Exposed to Hypoxia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Cells
2.3. Hypoxia Treatment
2.4. Cell Harvesting
2.5. RNA Extraction
2.6. Data Normalisation and Batch Correction
2.7. Differentially Expressed Gene (DEG) Analysis
2.8. Gene Expression Analyses
2.9. Hypoxia Sensitivity in a Published Bladder Cancer Xenograft
2.10. Hypoxia Scores
2.11. Analysis of the Bladder Cancer TCGA Data Base and BCON
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- West, C.M.L.; Slevin, F. Tumour hypoxia. Clin. Oncol. R. Coll. Radiol. 2019, 31, 595–599. [Google Scholar] [CrossRef]
- Flaig, T.W. NCCN Guidelines Updates: Management of Muscle-Invasive Bladder Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 591–593. [Google Scholar]
- Flegar, L.; Kraywinkel, K.; Zacharis, A.; Aksoy, C.; Koch, R.; Eisenmenger, N.; Groeben, C.; Huber, J. Treatment trends for muscle-invasive bladder cancer in Germany from 2006 to 2019. World J. Urol. 2022, 40, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Mistry, H.; Irlam, J.; Valentine, H.; Yang, L.; Lane, B.; West, C.; Choudhury, A.; Hoskin, P.J. Long-Term Outcomes of Radical Radiation Therapy with Hypoxia Modification with Biomarker Discovery for Stratification: 10-Year Update of the BCON (Bladder Carbogen Nicotinamide) Phase 3 Randomized Trial (ISRCTN45938399). Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Taylor, J.; Eustace, A.; Irlam, J.J.; Denley, H.; Hoskin, P.J.; Alsner, J.; Buffa, F.M.; Harris, A.L.; Choudhury, A.; et al. A Gene Signature for Selecting Benefit from Hypoxia Modification of Radiotherapy for High-Risk Bladder Cancer Patients. Clin. Cancer Res. 2017, 23, 4761–4768. [Google Scholar] [CrossRef]
- Smith, T.A.D.; Lane, B.; More, E.; Valentine, H.; Lunj, S.; Abdelkarem, O.A.; Irlam, J.J.; Shabbir, R.; Vora, S.; Denley, H.; et al. Comparison of multiple gene expression platforms for measuring a bladder cancer hypoxia signature. Mol. Med. Rep. 2022, 26, 261. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; West, C.M.L. Hypoxia gene expression signatures as predictive biomarkers for personalising radiotherapy. Br. J. Radiol. 2018, 92, 20180036. [Google Scholar] [CrossRef]
- Lane, B.; Khan, M.T.; Choudhury, A.; Salem, A.; West, C.M.L. Development and validation of a hypoxia-associated signature for lung adenocarcinoma. Sci. Rep. 2022, 12, 1290. [Google Scholar] [CrossRef]
- Calvo Tardón, M.; Marinari, E.; Migliorini, D.; Bes, V.; Tankov, S.; Charrier, E.; McKee, T.A.; Dutoit, V.; Dietrich, P.Y.; Cosset, E.; et al. An Experimentally Defined Hypoxia Gene Signature in Glioblastoma and Its Modulation by Metformin. Biology 2020, 9, 264. [Google Scholar] [CrossRef]
- Trong, P.D.; Rösch, S.; Mairbäurl, H.; Pusch, S.; Unterberg, A.; Herold-Mende, C.; Warta, R. Identification of a Prognostic Hypoxia-Associated Gene Set in IDH-Mutant Glioma. Int. J. Mol. Sci. 2018, 19, 2903. [Google Scholar] [CrossRef]
- Yang, L.; Forker, L.; Irlam, J.J.; Pillay, N.; Choudhury, A.; West, C.M.L. Validation of a hypoxia related gene signature in multiple soft tissue sarcoma cohorts. Oncotarget 2017, 9, 3946–3955. [Google Scholar] [CrossRef] [PubMed]
- Boegemann, M.; Krabbe, L.M. Prognostic Implications of Immunohistochemical Biomarkers in Non-muscle-invasive Blad Cancer and Muscle-invasive Bladder Cancer. Mini. Rev. Med. Chem. 2020, 20, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jia, X.; Duan, Y.; Xiao, H.; Sundqvist, K.G.; Permert, J.; Wang, F. Excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol. Ther. 2013, 14, 428–435. [Google Scholar] [CrossRef][Green Version]
- Carvalho, B.S.; Irizarry, R.A. A Framework for Oligonucleotide Microarray Preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Iimma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Shabbir, R.; Telfer, B.A.; Dickie, B.; Reardon, M.; Babur, M.; Williams, K.; West, C.M.L.; Choudhury, A.; Smith, T.A.D. Implementation of Oxygen Enhanced Magnetic Resonance Imaging (OE-MRI) and a Pilot Genomic Study of Hypoxia in Bladder Cancer Xenografts. Cancer Genom. Proteom. 2024, 21, 380–387. [Google Scholar] [CrossRef]
- Song, M.; Schnettler, E.; Venkatachalam, A.; Wang, Y.; Feldman, L.; Argenta, P.; Rodriguez-Rodriguez, L.; Ramakrishnan, S. Increased expression of collagen prolyl hydroxylases in ovarian cancer is associated with cancer growth and metastasis. Am. J. Cancer Res. 2023, 13, 6051–6062. [Google Scholar]
- Aggarwal, V.; Sahoo, S.; Donnenberg, V.S.; Chakraborty, P.; Jolly, M.K.; Sant, S. P4HA2: A link between tumor-intrinsic hypoxia, partial EMT and collective migration. Adv. Cancer Biol. Metastasis 2022, 5, 100057. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, D.; Huang, K.; Liang, M. Hypoxia-induced P4HA1 overexpression promotes post-ischemic angiogenesis by enhancing endothelial glycolysis through downregulating FBP1. J. Transl. Med. 2024, 22, 74. [Google Scholar] [CrossRef]
- Liu, Y.; Murazzi, I.; Fuller, A.M.; Pan, H.; Irizarry-Negron, V.M.; Devine, A.; Katti, R.; Skuli, N.; Ciotti, G.E.; Pak, K.; et al. Sarcoma Cells Secrete Hypoxia-Modified Collagen VI to Weaken the Lung Endothelial Barrier and Promote Metastasis. Cancer Res. 2024, 84, 977–993. [Google Scholar] [CrossRef]
- Wu, X.; Xie, W.; Gong, B.; Fu, B.; Chen, W.; Zhou, L.; Luo, L. Development and validation of a combined hypoxia- and metabolism-related prognostic signature to predict clinical prognosis and immunotherapy responses in clear cell renal cell carcinoma. Front. Oncol. 2023, 13, 1162846. [Google Scholar] [CrossRef]
- Gong, X.; Wang, A.; Song, W. Clinicopathological significances of PLOD2, epithelial-mesenchymal transition markers, and cancer stem cells in patients with esophageal squamous cell carcinoma. Medicine 2022, 101, e30112. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Du, Y.; Ning, X.; Liu, N.; Huang, D.; Liang, J.; Xue, Y.; Fan, D. Dual-specificity phosphatase DUSP1 protects overactivation of hypoxia-inducible factor 1 through inactivating ERK MAPK. Exp. Cell Res. 2005, 309, 410–418. [Google Scholar] [CrossRef]
- Yu, J.; Li, H.; Huang, C.; Chen, H. Identification and characterization of ferroptosis-related genes in therapy-resistant gastric cancer. Medicine 2024, 103, e38193. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, Y. Development of a Comprehensive Gene Signature Linking Hypoxia, Glycolysis, Lactylation, and Metabolomic Insights in Gastric Cancer through the Integration of Bulk and Single-Cell RNA-Seq Data. Biomedicines 2023, 11, 2948. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.N.; Albert, G.P.; Halterman, M.W. Expression Profiling of the MAP Kinase Phosphatase Family Reveals a Role for DUSP1 in the Glioblastoma Stem Cell Niche. Cancer Microenviron. 2017, 10, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Sun, J.; Xie, S.; Chi, C.; Zhu, Y.; Pan, J.; Dong, B.; Huang, Y.; Xia, W.; Sha, J.; et al. PRKAR2B-HIF-1α loop promotes aerobic glycolysis and tumour growth in prostate cancer. Cell Prolif. 2020, 53, e12918. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Jiang, H.; He, W. Targeting CAMK2N1/CAMK2 inhibits invasion, migration and angiogenesis of non-small cell lung cancer by promoting autophagy and apoptosis via AKT/mTOR signaling pathway. Gene 2024, 913, 148375. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Ren, X.; Ren, Q. NcRNA-mediated upregulation of CAMK2N1 is associated with poor prognosis and tumor immune infiltration of gastric cancer. Front. Genet. 2022, 13, 888672. [Google Scholar] [CrossRef]
- Carneiro, I.; Quintela-Vieira, F.; Lobo, J.; Moreira-Barbosa, C.; Menezes, F.D.; Martins, A.T.; Oliveira, J.; Silva, R.; Jerónimo, C.; Henrique, R. Expression of EMT-related genes CAMK2N1 and WNT5A is increased in locally invasive and metastatic prostate cancer. J. Cancer 2019, 10, 5915. [Google Scholar] [CrossRef]
- Mekkawy, A.H.; Pourgholami, M.H.; Morris, D.L. Human Sprouty1 suppresses growth, migration, and invasion in human breast cancer cells. Tumour. Biol. 2014, 35, 5037–5048. [Google Scholar] [CrossRef]
- Kwabi-Addo, B.; Wang, J.; Erdem, H.; Vaid, A.; Castro, P.; Ayala, G.; Ittmann, M. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res. 2004, 64, 4728–4735. [Google Scholar] [CrossRef]
- Celik-Selvi, B.E.; Stütz, A.; Mayer, C.E.; Salhi, J.; Siegwart, G.; Sutterlüty, H. Sprouty3 and Sprouty4, Two Members of a Family Known to Inhibit FGF-Mediated Signaling, Exert Opposing Roles on Proliferation and Migration of Glioblastoma-Derived Cells. Cells 2019, 8, 808. [Google Scholar] [CrossRef]
- Tamura, S.; Kaike, M.; Kunisaki, C.; Tanaka, K.; Masuda, M.; Imada, T. Clinical significance of STC1 gene expression in patients with colorectal cancer. Anticancer Res. 2011, 31, 325–329. [Google Scholar]
- Chang, A.C.; Doherty, J.; Huschtscha, L.I.; Redvers, R.; Restall, C.; Reddel, R.R.; Anderson, R.L. STC1 expression is associated with tumor growth and metastasis in breast cancer. Clin. Exp. Metastasis 2015, 32, 15–27. [Google Scholar] [CrossRef]
- Xiao, Q.; Ge, G. Lysyl, Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis Cancer. Microenviron 2012, 5, 261–273. [Google Scholar] [CrossRef]
- Said, H.M.; Staab, A.; Hagemann, C.; Vince, G.H.; Katzer, A.; Flentje, M.; Vordermark, D. Distinct patterns of hypoxic expression of carbonic anhydrase IX (CA IX) in human malignant glioma cell lines. J. Neuro-Oncol. 2007, 81, 27–38. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Simopoulos, K.; Pastorek, J.; Wykoff, C.C.; Gatter, K.C.; Harris, A.L. Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin. Cancer Res. 2001, 7, 3399–3403. [Google Scholar] [PubMed]
- Tanaka, N.; Kato, H.; Inose, T.; Kimura, H.; Faried, A.; Sohda, M.; Nakajima, M.; Fukai, Y.; Miyazaki, T.; Masuda, N.; et al. Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br. J. Cancer 2008, 99, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Willam, C.; Warnecke, C.; Schefold, J.C.; Kügler, J.; Koehne, P.; Frei, U.; Wiesener, M.; Eckardt, K.U. Inconsistent effects of acidosis on HIF-α protein and its target genes. Pflügers Arch. 2006, 451, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.G.; Overgaard, J. Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with known modifiable hypoxia: An evaluation of the DAHANCA 5 study. Radiother. Oncol. 2007, 83, 383–388. [Google Scholar] [CrossRef]
- Douglas, C.M.; Bernstein, J.M.; Ormston, V.E.; Hall, R.C.; Merve, A.; Swindell, R.; Valentine, H.R.; Slevin, N.J.; West, C.M.L.; Homer, J.J. Lack of prognostic effect of carbonic anhydrase-9, hypoxia inducible factor-1α and bcl-2 in 286 patients with early squamous cell carcinoma of the glottic larynx treated with radiotherapy. Clin. Oncol. R. Coll. Radiol. 2013, 25, 59–65. [Google Scholar] [CrossRef]
- Jonathan, R.A.; Wijffels, K.I.; Peeters, W.; de Wilde, P.C.; Marres, H.A.; Merkx, M.A.; Oosterwijk, E.; van der Kogel, A.J.; Kaanders, J.H. The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother. Oncol. 2006, 79, 288–297. [Google Scholar] [CrossRef]
- Jubb, A.M.; Pham, T.Q.; Hanby, A.M.; Frantz, G.D.; Peale, F.V.; Wu, T.D.; Koeppen, H.W.; Hillan, K.J. Expression of vascular endothelial growth factor, hypoxia inducible factor 1α, and carbonic anhydrase IX in human tumours. J. Clin. Pathol. 2004, 57, 504–512. [Google Scholar] [CrossRef]
- Miller, K.W. A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. Proc. Am. Soc. Clin. Oncol. 2005, 23, 4. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003, 349, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Pellerin, È.; Caneparo, C.; Ringuette-Goulet, C.; Pouliot, F.; Bolduc, S. Bladder cancer cell lines adapt their aggressiveness profile to oxygen tension. Oncol. Lett. 2022, 24, 220. [Google Scholar] [CrossRef]
- Smith, T.A.D.; AbdelKarem, O.A.; Irlam, J.J.; Lane, B.; Valentine, H.; Bibby, B.A.S.; Denley, H.; Choudhury, A.; West, C.M.L. Selection of endogenous control genes for normalising gene expression data derived from formalin-fixed paraffin-embedded tumour tissue. Sci. Rep. 2020, 10, 17258. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Sun, Y.; Jin, T.; Zhu, P.; Wan, X.; Hou, Y.; Tu, G. SLC6A8-mediated intracellular creatine accumulation enhances hypoxic breast cancer cell survival via ameliorating oxidative stress. J. Exp. Clin. Cancer Res. 2021, 40, 168. [Google Scholar] [CrossRef]
- Kurth, I.; Yamaguchi, N.; Andreu-Agullo, C.; Tian, H.S.; Sridhar, S.; Takeda, S.; Gonsalves, F.C.; Loo, J.M.; Barlas, A.; Manova-Todorova, K.; et al. Therapeutic targeting of SLC6A8 creatine transporter suppresses colon cancer progression and modulates human creatine levels. Sci. Adv. 2021, 7, eabi7511. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Liu, X.; Yan, N.; Li, S.; Cao, G.; Cheng, Q.; Xia, Q.; Wang, H. Hypoxia-inducible transcription factor-1alpha promotes hypoxia-induced A549 apoptosis via a mechanism that involves the glycolysis pathway. BMC Cancer 2006, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Schwab, L.P.; Peacock, D.L.; Majumdar, D.; Ingels, J.F.; Jensen, L.C.; Smith, K.D.; Cushing, R.C.; Seagroves, T.N. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012, 14, R6. [Google Scholar] [CrossRef] [PubMed]
- Tátrai, E.; Bartal, A.; Gacs, A.; Paku, S.; Kenessey, I.; Garay, T.; Hegedűs, B.; Molnár, E.; Cserepes, M.T.; Hegedűs, Z. Cell type-dependent HIF1 α-mediated effects of hypoxia on proliferation, migration and metastatic potential of human tumor cells. Oncotarget 2017, 8, 44498–44510. [Google Scholar] [CrossRef]
- Olbryt, M.; Habryka, A.; Student, S.; Jarząb, M.; Tyszkiewicz, T.; Lisowska, K.M. Global gene expression profiling in three tumor cell lines subjected to experimental cycling and chronic hypoxia. PLoS ONE 2014, 9, e105104. [Google Scholar] [CrossRef]
- Ord, J.J.; Streeter, E.H.; Roberts, I.S.; Cranston, D.; Harris, A.L. Comparison of hypoxia transcriptome in vitro with in vivo gene expression in human bladder cancer. Br. J. Cancer 2005, 93, 346–354. [Google Scholar] [CrossRef][Green Version]

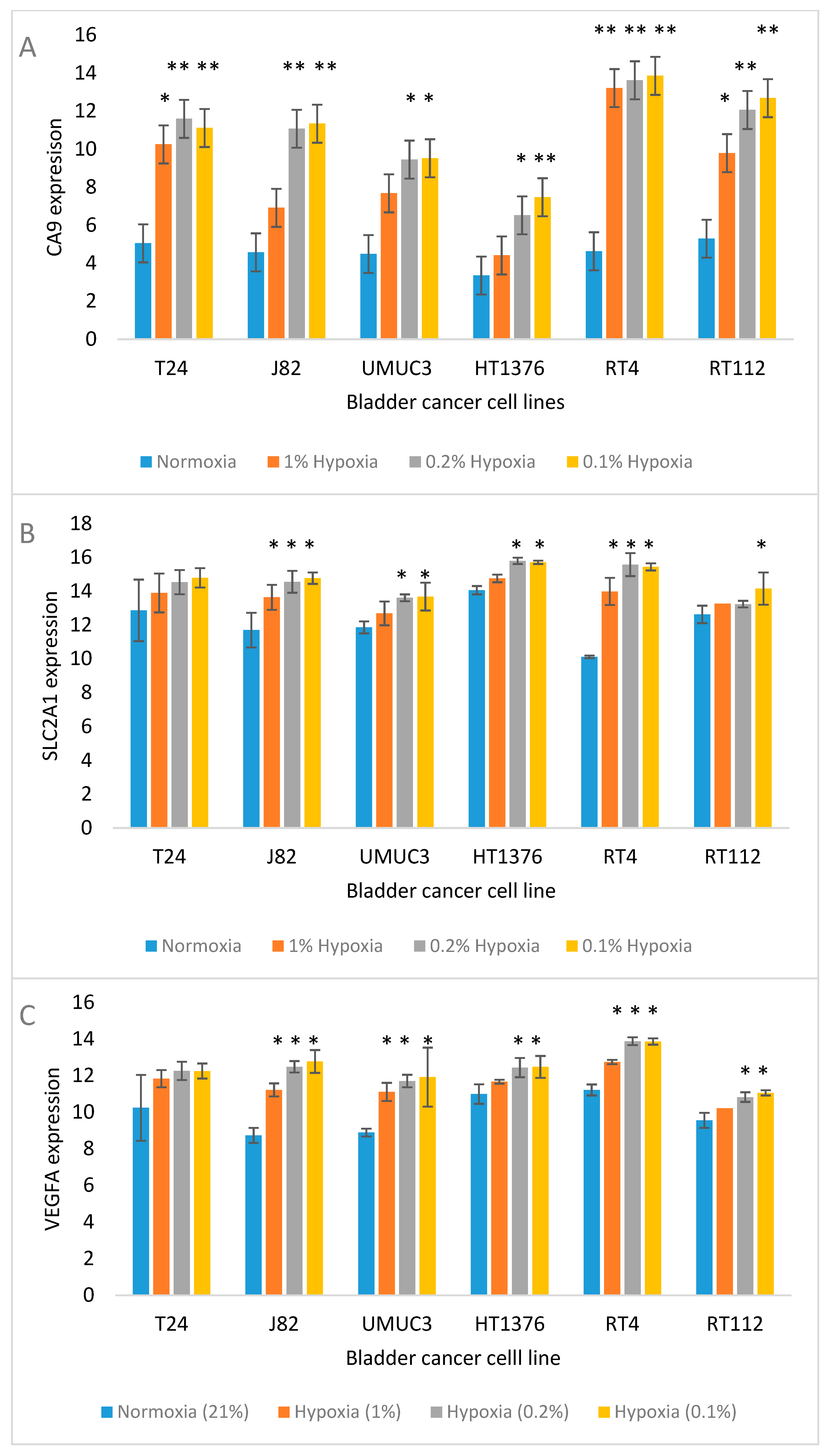
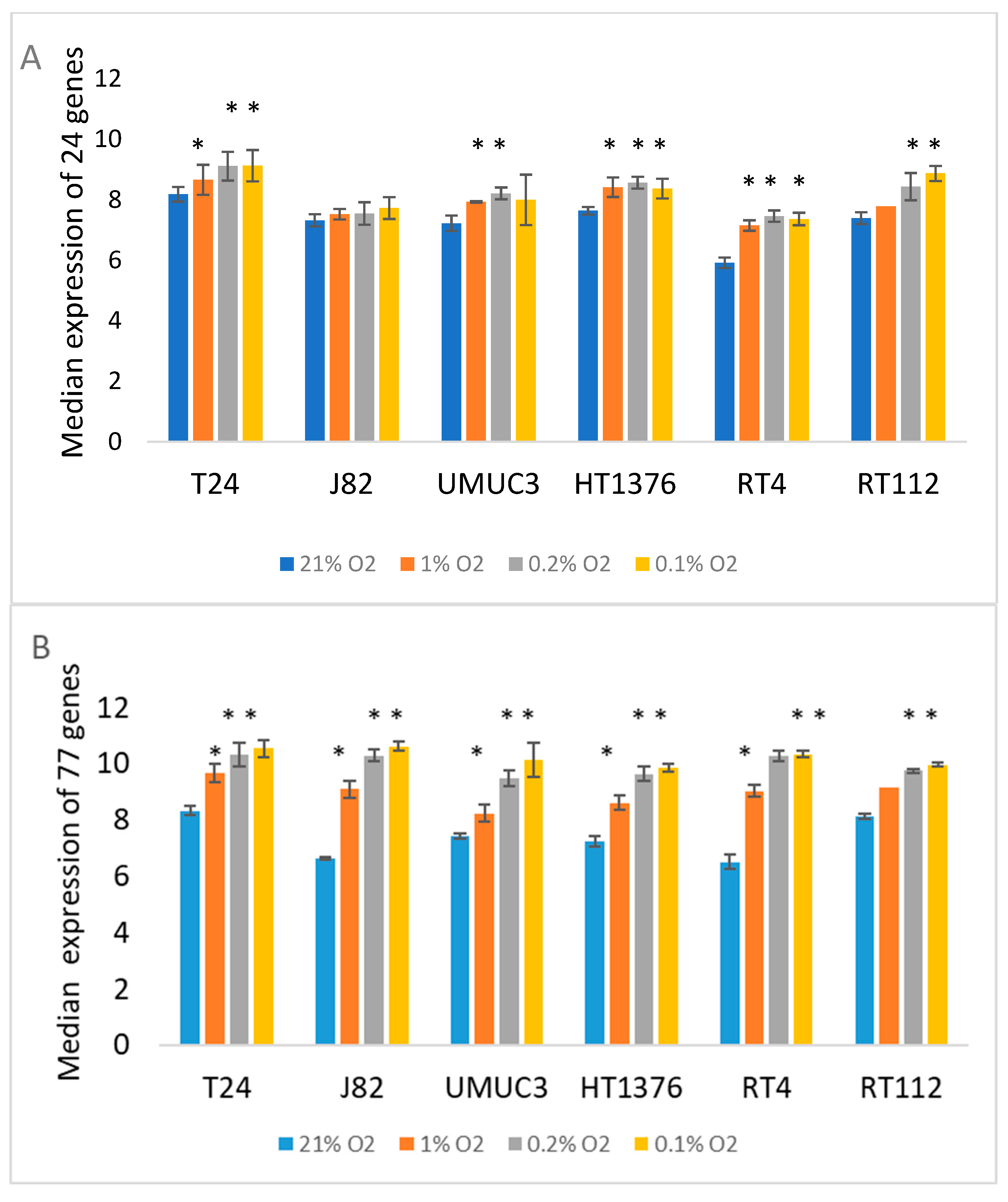
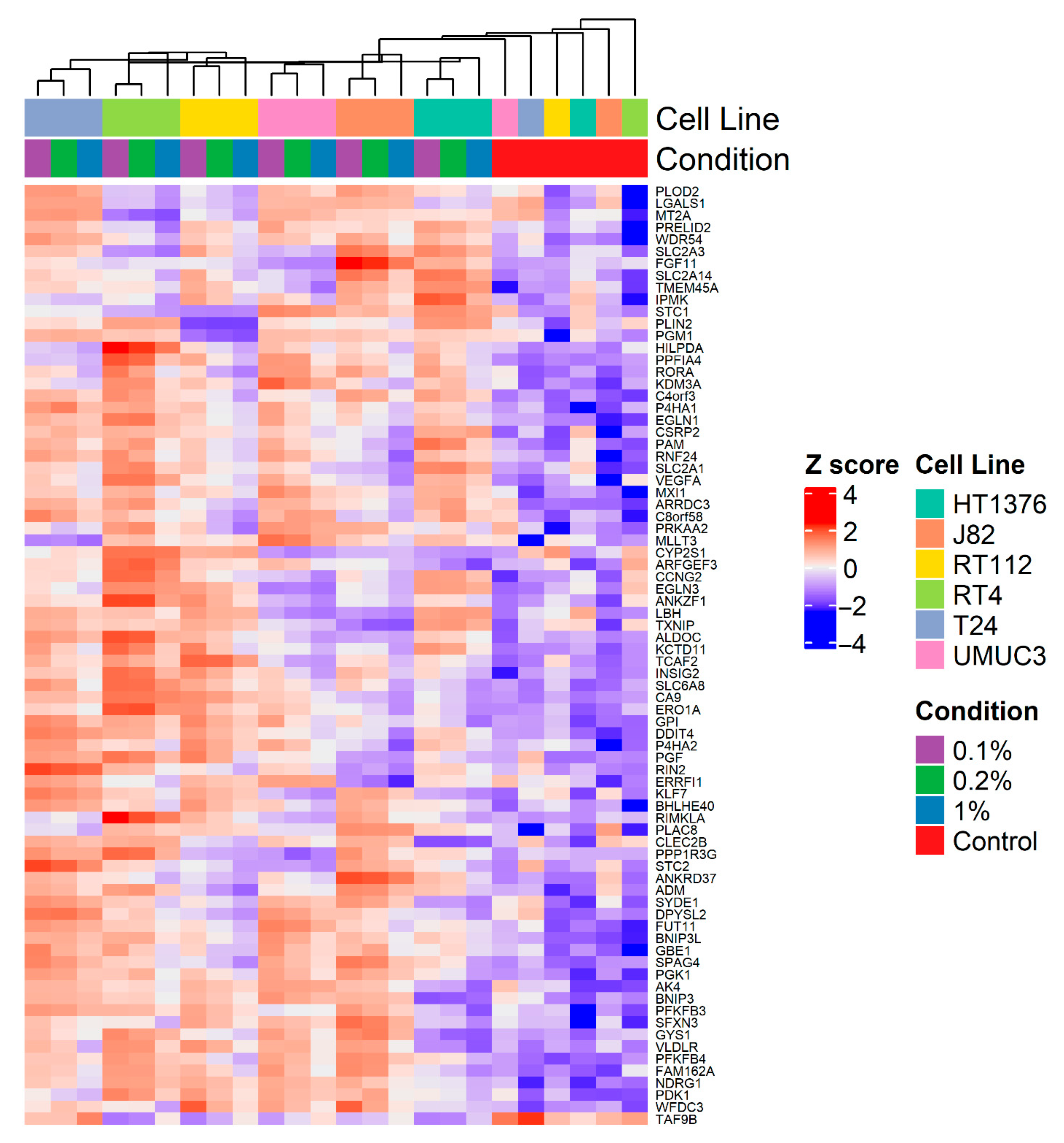
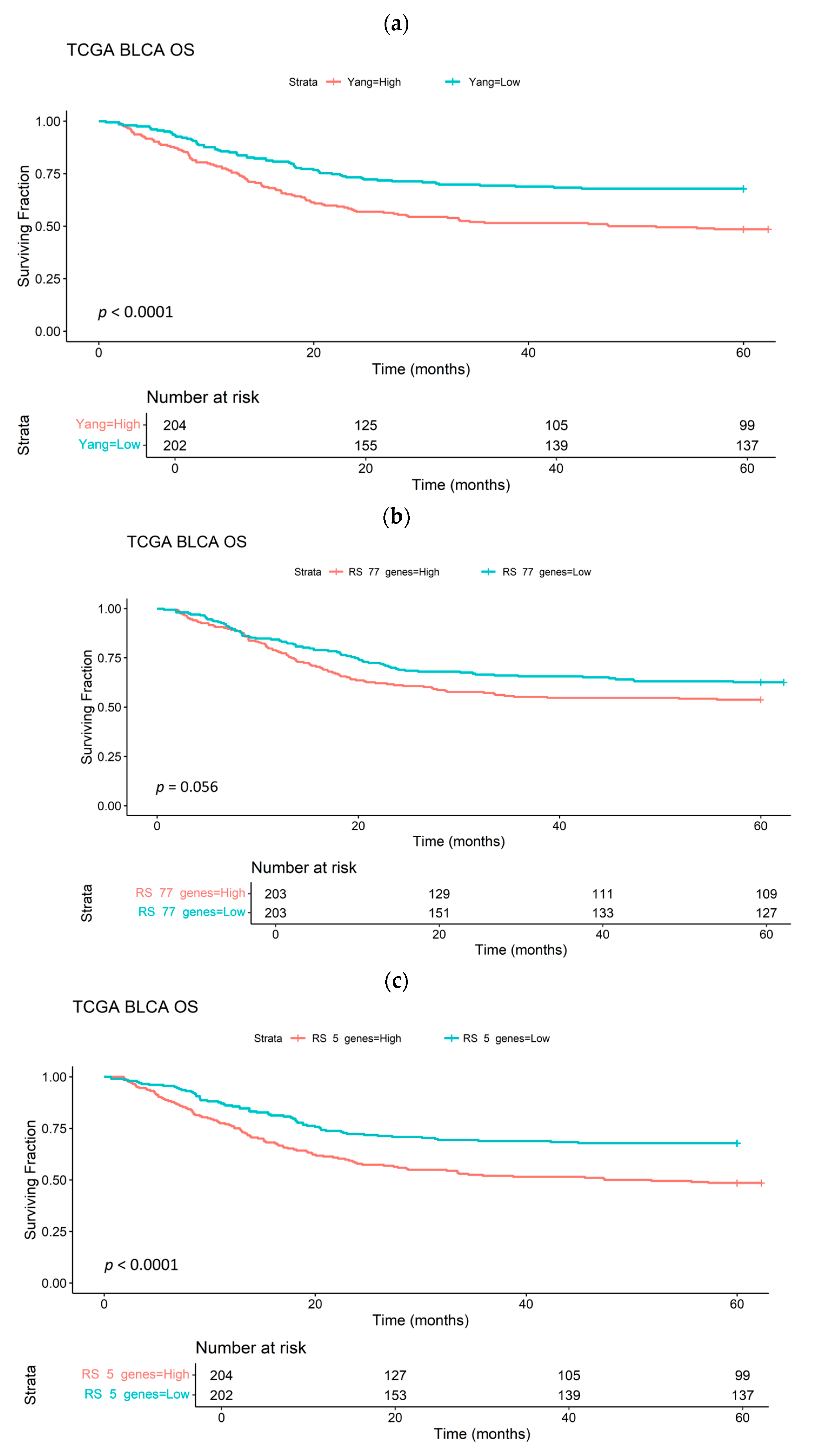
| Cell Line | Tissue Origin/Grade | Donor Age and Sex | Common Mutations | Overexpressed Receptors/Markers |
|---|---|---|---|---|
| RT4 | Papilloma, non-invasive/grade I | 63 M | Wild-type TP53/PTEN; FGFR3-driven luminal TERT and TSC1 promoter mutations; CDKN2A | FGFR; HER2; EN2 EGF; P2X1; P2X7; HRAS; PSCA |
| RT112 | Non-muscle-invasive carcinoma grade 2 | FGFR3 fusion/amplification; PIK3CA; wild-type PTEN; SRC; TERT promoter mutation | FGFR; EN2 CKs; FGFR3; PSCA | |
| HT-1376 | Invasive transitional cell carcinoma/ grade 3 | 58 F | Loss of CDKN2A/2B (9p21), likely TP53 alteration, typical invasive type; HRAS | EGFR; CD46; GPR87; CD74 |
| T24 | Muscle-invasive carcinoma/grade 3 | 81 F | TP53 mutant; PTEN mutant; TERT promoter mutation; HRAS | GRβ; EN2; ZEB1/2; EGFR; TRPM7 |
| UMUC-3 | Muscle-invasive carcinoma/grade 3 | M | TP53 mutant; PTEN mutant + partial deletion; ATM mutation; KRAS | EN2; GRβ; ZEB1/2; TPβ |
| J82 | Muscle-invasive carcinoma/grade 3 | 58 M | TP53 mutant; PTEN mutant + partial deletion; PI3KCA | EN2; ZEB1/2; EGFR; RON |
| Oxygen Level vs. Control | Number of Gene Affected in 1–6 Cell Lines | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Up- and downregulated | ||||||
| 1% | 239 | 42 | 11 | 4 | 0 | 0 |
| 0.2% | 1014 | 145 | 47 | 20 | 8 | 2 |
| 0.1% | 1264 | 216 | 78 | 33 | 18 | 3 |
| Upregulated | ||||||
| 1% | 178 | 42 | 11 | 4 | 0 | 0 |
| 0.2% | 589 | 131 | 47 | 20 | 8 | 2 |
| 0.1% | 739 | 178 | 77 | 33 | 18 | 3 |
| Downregulated | ||||||
| 1% | 61 | 0 | 0 | 0 | 0 | 0 |
| 0.2% | 425 | 14 | 0 | 0 | 0 | 0 |
| 0.1% | 525 | 38 | 1 | 0 | 0 | 0 |
| Gene | ||||||
|---|---|---|---|---|---|---|
| ADM | CA9 * | FGF11 | LGALS1 | PGK1 | RORA | TMEM45A * |
| AK4 * | CCNG2 | FUT11 | MLLT3 | PGM1 | SFXN3 | TXNIP |
| ALDOC | CLEC2B | GBE1 | MT2A | PLAC8 | SLC2A1 | VEGFA |
| ANKRD37 | CSRP2 | GPI | MXI1 * | PLIN2 | SLC2A14 | VLDLR |
| ANKZF1 | CYP2S1 | GYS1 | NDRG1 * | PLOD2 | SLC2A3 | WDR54 |
| ARFGEF3 | DDIT4 * | HILPDA | P4HA1 | PPFIA4 | SLC6A8 | WFDC3 |
| ARRDC3 | DPYSL2 | INSIG2 | P4HA2 | PPP1R3G | SPAG4 | |
| BHLHE40 | EGLN1 * | IPMK | PAM | PRELID2 | STC1 | |
| BNIP3 * | EGLN3 | KCTD11 | PDK1 | PRKAA2 | STC2 | |
| BNIP3L | ERO1A | KDM3A | PFKFB3 * | RIMKLA | SYDE1 | |
| C4orf3 | ERRFI1 | KLF7 | PFKFB4 * | RIN2 | TAF9B | |
| C8orf58 | FAM162A | LBH | PGF | RNF24 | TCAF2 * | |
| O2 Level | PANTHER GO-Slim Biological Process | Fold Enrichment | FDR |
|---|---|---|---|
| 1% | Cellular response to hypoxia | 58 | 1.8 × 10−3 |
| 0.2% | Protein hydroxylation (GO: 0018126) | >100 | 3.9 × 10−5 |
| 0.2% | Glucose transmembrane transport (GO: 1904659) | >100 | 0.0027 |
| 0.2% | Response to hypoxia (GO: 0001666) | 71.46 | 0.005 |
| 0.2% | Hexose biosynthetic process (GO: 0019319) | 66 | 0.023 |
| 0.2% | Vitamin transport (GO: 0051180) | 64.3 | 0.004 |
| 0.2% | Glycolytic process (GO: 0006096) | 55.9 | 0.0048 |
| 0.2% | Apoptotic mitochondrial changes (GO: 0008637) | 45.1 | 0.04 |
| 0.2% | Mitochondrial membrane organization (GO: 0007006) | 45.1 | 0.039 |
| 0.2% | Mitochondrial transport (GO: 0006839) | 19.5 | 0.027 |
| 0.2% | L-amino acid metabolic process (GO: 0170033) | 18.9 | 0.029 |
| 0.2% | Cellular modified amino acid metabolic process (GO: 0006575) | 16.3 | 0.039 |
| 0.2% | Organic anion transport (GO: 0015711) | 10.9 | 0.01 |
| 0.1% | Protein hydroxylation (GO: 0018126) | 98.9 | 7 × 10−7 |
| 0.1% | Response to hypoxia (GO: 0001666) | 85.8 | 1 × 10−7 |
| 0.1% | Sprouting angiogenesis (GO: 0002040) | 73.5 | 0.026 |
| 0.1% | Positive regulation of epithelial cell proliferation (GO: 0050679) | 64.3 | 0.03 |
| 0.1% | Vascular endothelial growth factor receptor signaling pathway (GO: 0048010) | 64.3 | 0.03 |
| 0.1% | Glucose transmembrane transport (GO: 1904659) | 64.31 | 0.007 |
| 0.1% | Positive regulation of leukocyte chemotaxis (GO: 0002690) | 46.8 | 0.039 |
| 0.1% | Hexose biosynthetic process (GO: 0019319) | 39.6 | 0.043 |
| 0.1% | Vitamin transport (GO: 0051180) | 38.6 | 0.01 |
| 0.1% | Glycolytic process (GO: 0006096) | 33.6 | 0.012 |
| 0.1% | L-amino acid metabolic process (GO: 0170033) | 15.1 | 0.017 |
| 0.1% | Cellular modified amino acid metabolic process (GO: 0006575) | 13.0 | 0.026 |
| 0.1% | Apoptotic process (GO: 0006915) | 12.4 | 0.027 |
| 0.1% | Regulation of multicellular organismal development (GO: 2000026) | 9.03 | 0.04 |
| 0.1% | Organic anion transport | 6.5 | 0.04 |
| 0.1% | Peptidyl-amino acid modification (GO: 0018193) | 5.72 | 0.03 |
| Upregulated at 1% | ||||||
|---|---|---|---|---|---|---|
| AK4 | BNIP3 | DDIT4 | EGLN3 | NDRG1 | PFKFB4 | TMEM45A |
| WFDC3 | ||||||
| Upregulated at 0.2% | ||||||
| ADM | BNIP3L | DPYSL2 | KCTD11 | PAM | PLAC8 | SLC2A14 |
| AK4 | C4orf3 | EGLN3 | KDM3A | PDK1 | PLOD2 | SLC2A3 |
| ALDOC | CA9 | FAM162A | MXI1 | PFKFB3 | PPFIA4 | SLC6A8 |
| ANKZF1 | CCNG2 | FUT11 | NDRG1 | PFKFB4 | RIMKLA | SPAG4 |
| ARID3A | CSRP2 | GPI | P4HA1 | PGF | RIN2 | SYDE1 |
| BNIP3 | DDIT4 | INSIG2 | P4HA2 | PGK1 | SFXN3 | TCAF2 |
| VLDLR | WFDC3 | |||||
| Upregulated at 0.1% | ||||||
| ADM | BNIP3L | EGLN3 | INSIG2 | P4HA2 | PNRC1 | SNX33 |
| AK4 | C4orf3 | ERO1A | KCTD11 | PAM | RIN2 | SPAG4 |
| ALDOC | CA9 | FAM162A | KDM3A | PFKFB3 | RNF24 | SYDE1 |
| ANKRD37 | CCNG2 | FOSL2 | LRP1 | PFKFB4 | RORA | TCAF2 |
| ANKZF1 | CSRP2 | FUT11 | MRPL23 | PGF | SFXN3 | TMEM45A |
| ARRDC3 | DDIT4 | GBE1 | MXI1 | PGK1 | SLC2A14 | VLDLR |
| BHLHE40 | DPYSL2 | GPI | NDRG1 | PLAC8 | SLC2A3 | WFDC3 |
| BNIP3 | EGLN1 | HILPDA | P4HA1 | PLOD2 | SLC6A8 | YEATS2 |
| (A). | |||
|---|---|---|---|
| O2 Level | PANTHER GO-Slim Biological Process | Fold Enrichment | FDR |
| 1% | Response to hypoxia (GO: 0001666) | >100 | 0.043 |
| 0.2% | Protein hydroxylation (GO: 0018126) | >100 | 0.000033 |
| 0.2% | Response to hypoxia (GO: 0001666) | 99.42 | 0.00007 |
| 0.2% | Glucose transmembrane transport (GO: 1904659) | 74.57 | 0.02 |
| 0.2% | Hexose biosynthetic process (GO: 0019319) | 68.83 | 0.02 |
| 0.2% | Glycolytic process (GO: 0006096) | 58.4 | 0.006 |
| 0.2% | Apoptotic mitochondrial changes (GO: 0008637) | 47 | 0.03 |
| 0.2% | Mitochondrial membrane organization (GO: 0007006) | 47 | 0.03 |
| 0.2% | Vitamin transport (GO: 0051180) | 44.7 | 0.04 |
| 0.2% | mitochondrial transport (GO: 0006839) | 20.3 | 0.021 |
| 0.2% | L-amino acid metabolic process (GO: 0170033) | 19.74 | 0.022 |
| 0.2% | Cellular modified amino acid metabolic process (GO: 0006575) | 17 | 0.03 |
| 0.2% | Organic anion transport (GO: 0015711) | 9 | 0.035 |
| 0.2% | Peptidyl-amino acid modification (GO: 0018193) | 8.29 | 0.02 |
| 0.1% | Protein hydroxylation (GO: 0018126) | >100 | 3.7 × 10−7 |
| 0.1% | Response to hypoxia (GO: 0001666) | >100 | 1.2 × 10−6 |
| 0.1% | Glucose transmembrane transport (GO: 1904659) | 60.2 | 0.026 |
| 0.1% | Hexose biosynthetic process (GO: 0019319) | 55.55 | 0.028 |
| 0.1% | Glycolytic process (GO: 0006096) | 47.09 | 0.01 |
| 0.1% | Apoptotic mitochondrial changes (GO: 0008637) | 38 | 0.047 |
| 0.1% | Mitochondrial membrane organization (GO: 0007006) | 38.01 | 0.047 |
| 0.1% | L-amino acid metabolic process (GO: 0170033) | 21.24 | 0.009 |
| 0.1% | Cellular modified amino acid metabolic process (GO: 0006575) | 18.28 | 0.01 |
| 0.1% | Mitochondrial transport (GO: 0006839) | 16.41 | 0.037 |
| 0.1% | Peptidyl-amino acid modification (GO: 0018193) | 6.69 | 0.038 |
| (B). | |||
| O2 Level | PANTHER GO-Slim Biological Process | Fold Enrichment | FDR |
| 0.2% | Carbohydrate metabolic process (GO:0005975) | 13.65 | 0.011 |
| 0.1% | Negative regulation of protein kinase activity (GO:0006469) | 28.50 | 0.0009 |
| 0.1% | Glycolytic process (GO:0006096) | 28.3 | 0.018 |
| 0.1% | Negative regulation of MAPK cascade (GO:0043409) | 23.21 | 0.03 |
| 0.1% | Regulation of protein serine/threonine kinase activity (GO:0071900) | 14.94 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, R.; Quiles, C.G.; Lane, B.; Zeef, L.; Hoskin, P.J.; Choudhury, A.; West, C.M.L.; Smith, T.A.D. Gene Expression in Muscle-Invasive and Non-Muscle-Invasive Bladder Cancer Cells Exposed to Hypoxia. Cancers 2025, 17, 2624. https://doi.org/10.3390/cancers17162624
Shabbir R, Quiles CG, Lane B, Zeef L, Hoskin PJ, Choudhury A, West CML, Smith TAD. Gene Expression in Muscle-Invasive and Non-Muscle-Invasive Bladder Cancer Cells Exposed to Hypoxia. Cancers. 2025; 17(16):2624. https://doi.org/10.3390/cancers17162624
Chicago/Turabian StyleShabbir, Rekaya, Conrado G. Quiles, Brian Lane, Leo Zeef, Peter J. Hoskin, Ananya Choudhury, Catharine M. L. West, and Tim A. D. Smith. 2025. "Gene Expression in Muscle-Invasive and Non-Muscle-Invasive Bladder Cancer Cells Exposed to Hypoxia" Cancers 17, no. 16: 2624. https://doi.org/10.3390/cancers17162624
APA StyleShabbir, R., Quiles, C. G., Lane, B., Zeef, L., Hoskin, P. J., Choudhury, A., West, C. M. L., & Smith, T. A. D. (2025). Gene Expression in Muscle-Invasive and Non-Muscle-Invasive Bladder Cancer Cells Exposed to Hypoxia. Cancers, 17(16), 2624. https://doi.org/10.3390/cancers17162624






