Activation of G Protein-Coupled Estrogen Receptor Induces p53 and ADAMTS1 to Inhibit Tumor Growth and Suppress Liver Cancer Metastasis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cytotoxicity
2.3. Flow Cytometry Analysis
2.4. Western Blotting
2.5. RNA Sequencing
2.6. Proteomics
2.7. Protein–Protein Interaction
2.8. Animal Studies
2.9. Immunohistochemistry (IHC) and Hematoxylin Staining
2.10. Invasion Assay
2.11. Overall Survival Analysis of Differentially Expressed Genes
2.12. Statistical Analysis
3. Results
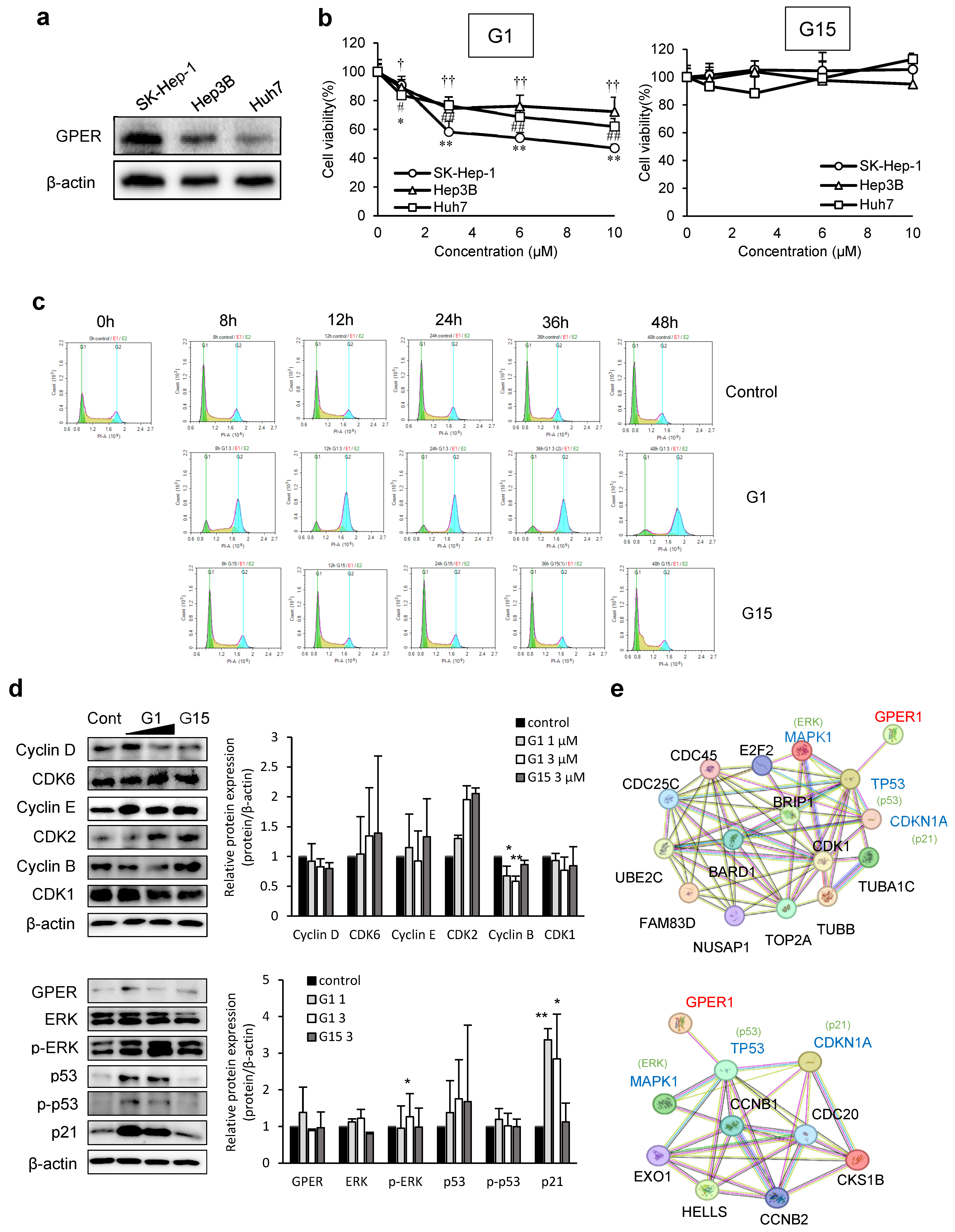
3.1. GPER Activation in Liver Cancer Cell Proliferation and Signaling
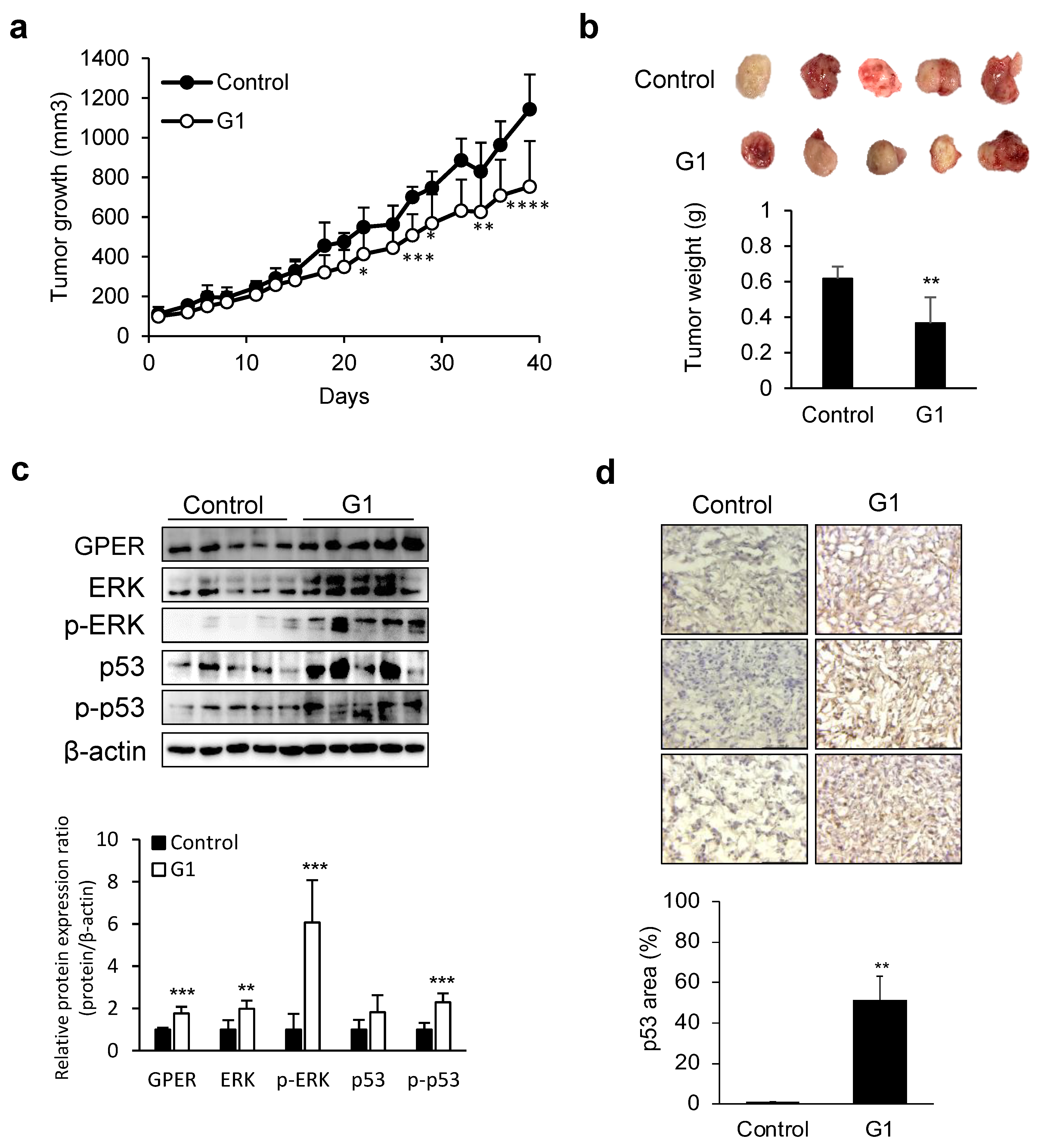
3.2. Tumor Growth Suppression by GPER Agonists in Liver Cancer Xenograft Models
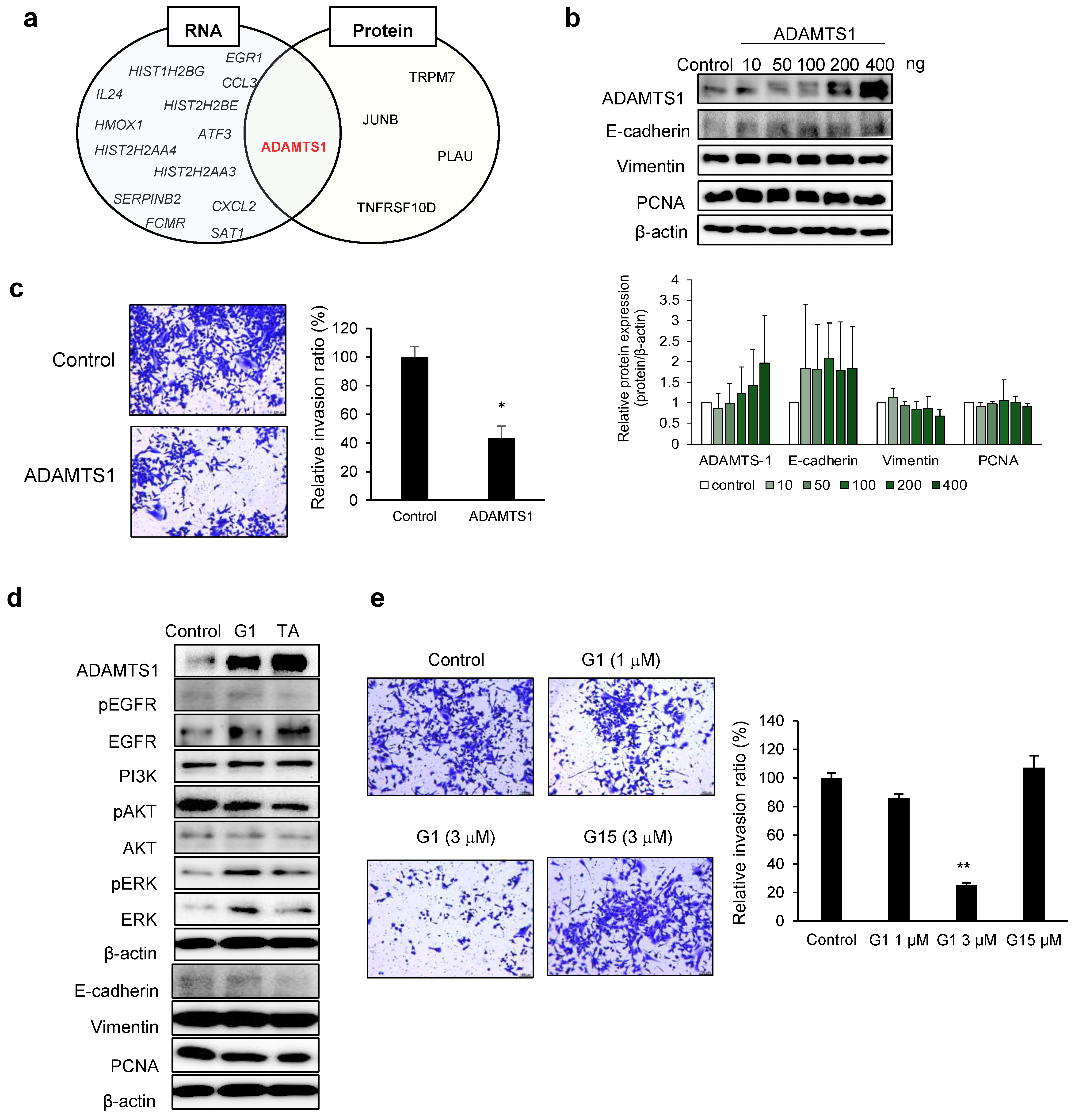
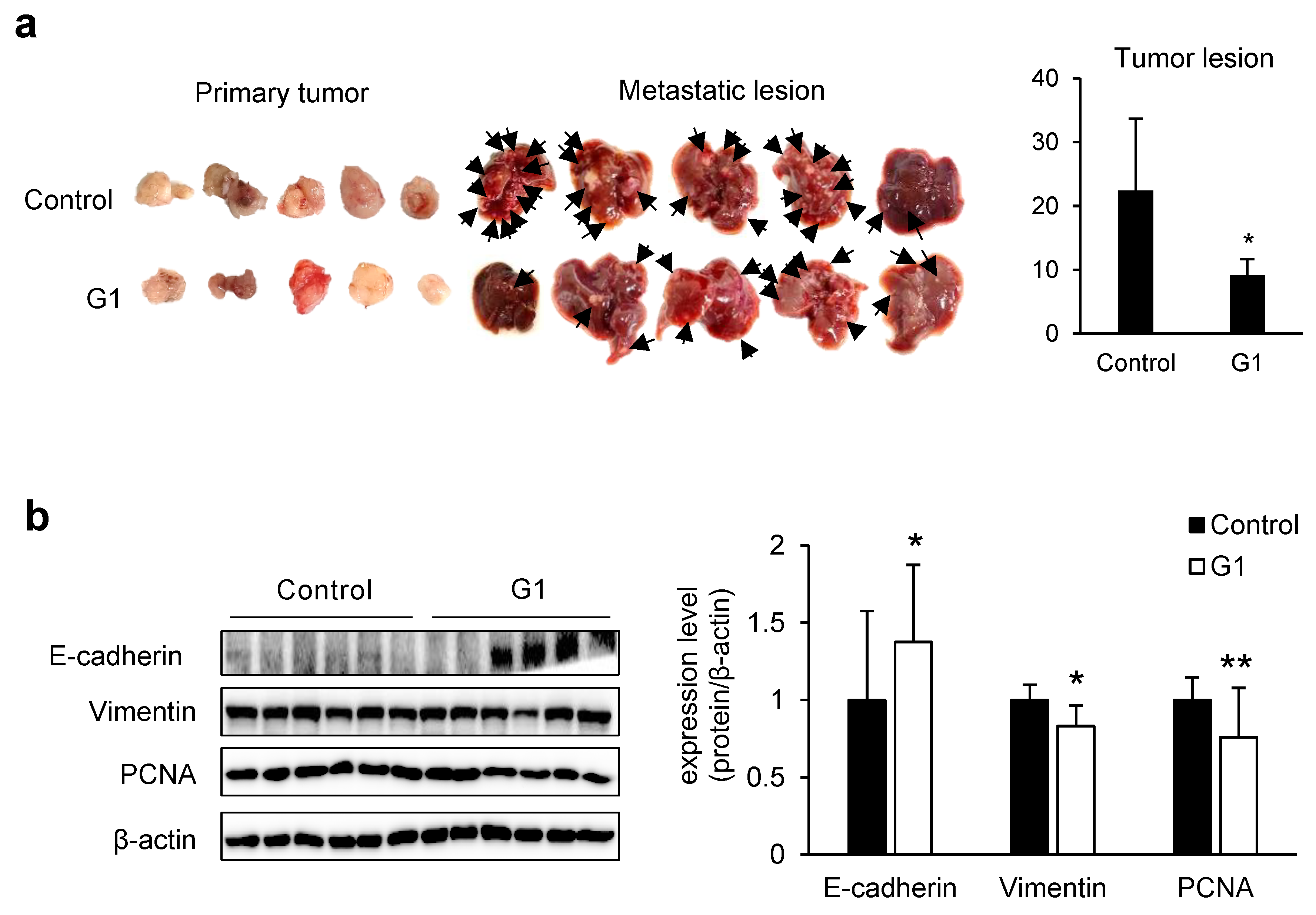
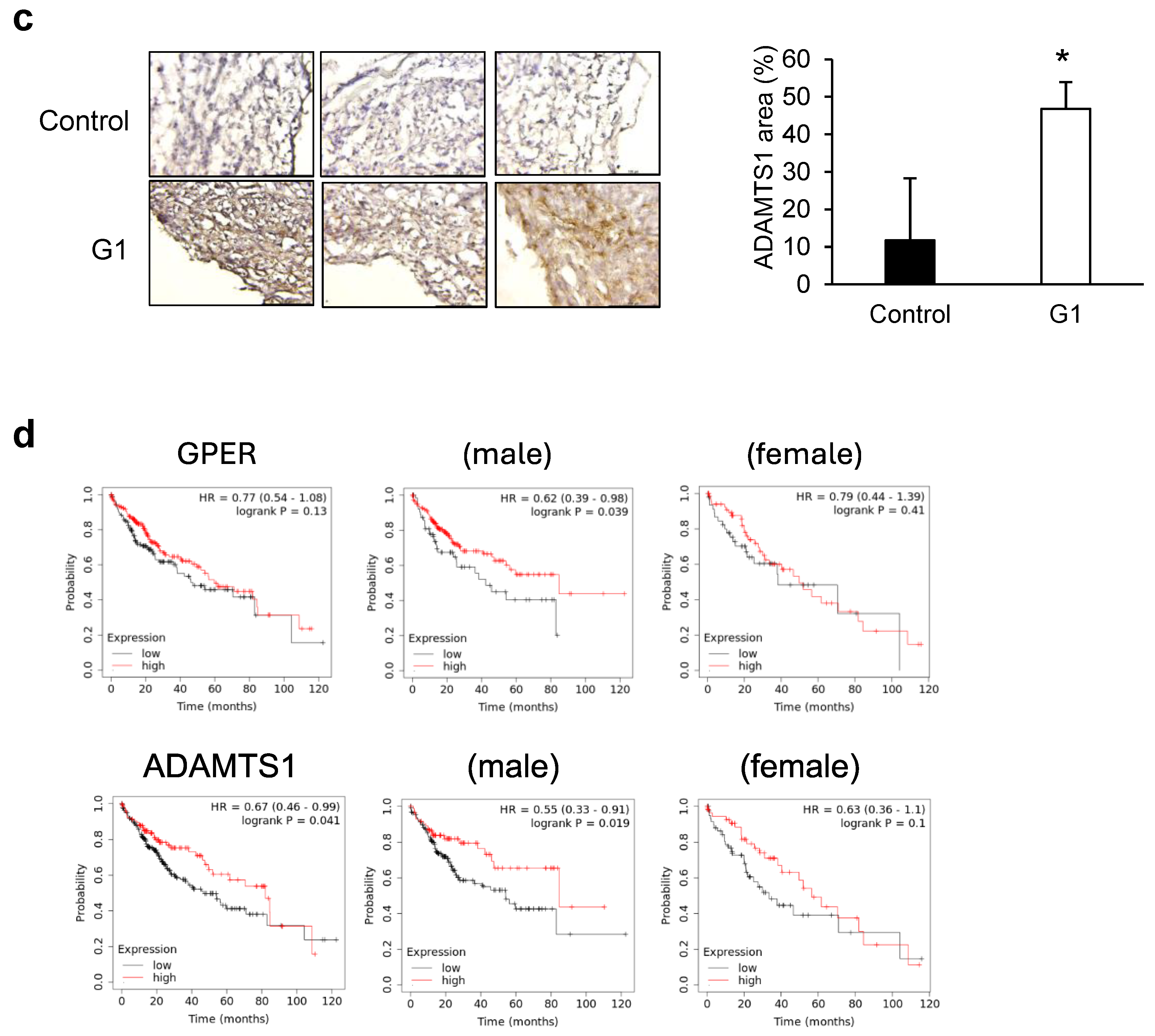
3.3. ADAMTS1-Mediated Inhibition of EMT and Metastasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BSA | bovine serum albumin |
| CDK | cyclin-dependent kinase |
| DEG | differentially expressed gene |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| FBS | fetal bovine serum |
| GPER | G protein-coupled estrogen receptor |
| IHC | immunohistochemistry |
| OS | overall survival |
| PCNA | proliferating cell nuclear antigen |
| TA | tamoxifen |
| VEGF | vascular endothelial growth factor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kur, P.; Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Wiszniewska, B. Sex hormone-dependent physiology and diseases of liver. Int. J. Environ. Res. Public Health 2020, 17, 2620. [Google Scholar] [CrossRef]
- Qiu, Y.A.; Xiong, J.; Fu, Q.; Dong, Y.; Liu, M.; Peng, M.; Jin, W.; Zhou, L.; Xu, X.; Huang, X.; et al. GPER-induced ERK signaling decreases cell viability of hepatocellular carcinoma. Front. Oncol. 2021, 11, 638171. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G protein-coupled oestrogen receptor GPER in health and disease: An update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef]
- Jung, J. Role of G protein-coupled estrogen receptor in cancer progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef]
- Wang, C.; Lv, X.; He, C.; Hua, G.; Tsai, M.Y.; Davis, J.S. The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 2013, 4, e869. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Kwon, H.J.; Lee, G.S.; Moon, J.H.; Jung, J. Chrysin-induced G protein-coupled estrogen receptor activation suppresses pancreatic cancer. Int. J. Mol. Sci. 2022, 23, 9673. [Google Scholar] [CrossRef]
- Wei, T.; Chen, W.; Wen, L.; Zhang, J.; Zhang, Q.; Yang, J.; Liu, H.; Chen, B.W.; Zhou, Y.; Feng, X.; et al. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 2016, 382, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, J.; Pan, F.; Zhang, B.; Yao, Y. The activation of GPER inhibits cells proliferation, invasion and EMT of triple-negative breast cancer via CD151/miR-199a-3p bio-axis. Am. J. Transl. Res. 2020, 12, 32–44. [Google Scholar] [PubMed]
- Deng, Y.; Miki, Y.; Nakanishi, A. Estradiol/GPER affects the integrity of mammary duct-like structures in vitro. Sci. Rep. 2020, 10, 1386. [Google Scholar] [CrossRef]
- Tang, B.L.; Hong, W. ADAMTS: A novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats. FEBS Lett. 1999, 445, 223–225. [Google Scholar] [CrossRef]
- Chien, M.-H.; Yang, Y.-C.; Ho, K.-H.; Ding, Y.-F.; Chen, L.-H.; Chiu, W.-K.; Chen, J.-Q.; Tung, M.-C.; Hsiao, M.; Lee, W.-J. Cyclic increase in the ADAMTS1-L1CAM-EGFR axis promotes the EMT and cervical lymph node metastasis of oral squamous cell carcinoma. Cell Death Dis. 2024, 15, 82. [Google Scholar] [CrossRef]
- Wen, Y.C.; Lin, Y.W.; Chu, C.Y.; Yang, Y.C.; Yang, S.F.; Liu, Y.F.; Hsiao, M.; Lee, W.J.; Chien, M.H. Melatonin-triggered post-transcriptional and post-translational modifications of ADAMTS1 coordinately retard tumorigenesis and metastasis of renal cell carcinoma. J. Pineal Res. 2020, 69, e12668. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Frewin, K.M.; Tan, I.; Williams, E.D.; Opeskin, K.; Pritchard, M.A.; Ingman, W.V.; Russell, D.L. The ADAMTS1 protease gene is required for mammary tumor growth and metastasis. Am. J. Pathol. 2011, 179, 3075–3085. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, R.; Li, L.; Wang, Y.; Du, P.; Ou, Y.; Zhao, Z.; Liu, X.; Xiao, W.; Dong, D.; et al. The chromosome 11q13.3 amplification associated lymph node metastasis is driven by miR-548k through modulating tumor microenvironment. Mol. Cancer 2018, 17, 125. [Google Scholar] [CrossRef]
- Link, F.; Li, Y.; Zhao, J.; Munker, S.; Fan, W.; Nwosu, Z.C.; Yao, Y.; Wang, S.; Huang, C.; Liebe, R.; et al. ECM1 attenuates hepatic fibrosis by interfering with mediators of latent TGF-β1 activation. Gut 2025, 74, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, J.W.; Chung, H.K.; Jung, J. Comprehensive model for simultaneous monitoring of primary tumor to metastatic cancer utilizing Prkdc and Il2rg double knockout mice. Sci. Rep. 2024, 14, 23531. [Google Scholar] [CrossRef]
- Smith, H.O.; Leslie, K.K.; Singh, M.; Qualls, C.R.; Revankar, C.M.; Joste, N.E.; Prossnitz, E.R. GPR30: A novel indicator of poor survival for endometrial carcinoma. Am. J. Obstet. Gynecol. 2007, 196, 386.e1–386.e11. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Arias-Pulido, H.; Kuo, D.Y.; Howard, T.; Qualls, C.R.; Lee, S.J.; Verschraegen, C.F.; Hathaway, H.J.; Joste, N.E.; Prossnitz, E.R. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol. 2009, 114, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xu, Y.; Li, J.; Zheng, S.; Sun, P.; Wang, T. Prognostic role of GPER/Ezrin in triple-negative breast cancer is associated with menopausal status. Endocr. Connect. 2019, 8, 661–671. [Google Scholar] [CrossRef]
- Wei, W.; Chen, Z.J.; Zhang, K.S.; Yang, X.L.; Wu, Y.M.; Chen, X.H.; Huang, H.B.; Liu, H.L.; Cai, S.H.; Du, J.; et al. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Dis. 2014, 5, e1428. [Google Scholar] [CrossRef]
- Weißenborn, C.; Ignatov, T.; Ochel, H.-J.; Costa, S.D.; Zenclussen, A.C.; Ignatova, Z.; Ignatov, A. GPER functions as a tumor suppressor in triple-negative breast cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 713–723. [Google Scholar] [CrossRef]
- Chan, Q.K.Y.; Lam, H.M.; Ng, C.F.; Lee, A.Y.Y.; Chan, E.S.Y.; Ng, H.K.; Ho, S.M.; Lau, K.M. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of ERK1/2, c-Jun/c-fos-dependent upregulation of p21, and induction of G2 cell-cycle arrest. Cell Death Differ. 2010, 17, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Modl, S.; Thulig, M.; Weißenborn, C.; Treeck, O.; Ortmann, O.; Zenclussen, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. GPER-1 acts as a tumor suppressor in ovarian cancer. J. Ovarian Res. 2013, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, X.; Jiang, C.; Davis, J.S. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am. J. Transl. Res. 2012, 4, 390–402. [Google Scholar] [PubMed]
- Hirtz, A.; Bailly, Y.; Rech, F.; Pierson, J.; Dumond, H.; Dubois-pot-Schneider, H. Molecular characterization of the dual effect of the GPER agonist G-1 in glioblastoma. Int. J. Mol. Sci. 2022, 23, 14309. [Google Scholar] [CrossRef]
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and hydroxytyrosol activate GPER/ GPR30-dependent pathways leading to apoptosis of ER-negative SKBR3 breast cancer cells. Mol. Nutr. Food Res. 2014, 58, 478–489. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef]
- Dulić, V.; Stein, G.H.; Far, D.F.; Reed, S.I. Nuclear accumulation of p21Cip1 at the onset of mitosis: A role at the G2/M-phase transition. Mol. Cell. Biol. 1998, 18, 546–557. [Google Scholar] [CrossRef]
- Choi, Y.H.; Zhang, L.; Lee, W.H.; Park, K.Y. Genistein-induced G2/M arrest is associated with the inhibition of cyclin B1 and hte induction of p21 in human breast carcinoma cells. Int. J. Oncol. 1998, 13, 391–396. [Google Scholar] [CrossRef]
- Rehn, A.P.; Birch, M.A.; Karlström, E.; Wendel, M.; Lind, T. ADAMTS-1 increases the three-dimensional growth of osteoblasts through type I collagen processing. Bone 2007, 41, 231–238. [Google Scholar] [CrossRef]
- Luque, A.; Carpizo, D.R.; Iruela-Arispe, M.L. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003, 278, 23656–23665. [Google Scholar] [CrossRef]
- de Assis Lima, M.; da Silva, S.V.; Serrano-Garrido, O.; Hülsemann, M.; Santos-Neres, L.; Rodríguez-Manzaneque, J.C.; Hodgson, L.; Freitas, V.M. Metalloprotease ADAMTS-1 decreases cell migration and invasion modulating the spatiotemporal dynamics of Cdc42 activity. Cell. Signal. 2021, 77, 109827. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Wei, W.; Jiang, G.-M.; Liu, H.; Wei, W.-D.; Yang, X.; Wu, Y.-M.; Liu, H.; Wong, C.K.C.; Du, J.; et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-κB signals. Mol. Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef]
- Cortes, E.; Sarper, M.; Robinson, B.; Lachowski, D.; Chronopoulos, A.; Thorpe, S.D.; Lee, D.A.; Del Río Hernández, A.E. GPER is a mechanoregulator of pancreatic stellate cells and the tumor microenvironment. EMBO Rep. 2019, 20, e46556. [Google Scholar] [CrossRef]
- Gallaher, J.A.; Brown, J.S.; Anderson, A.R.A. The impact of proliferation-migration tradeoffs on phenotypic evolution in cancer. Sci. Rep. 2019, 9, 2425. [Google Scholar] [CrossRef]
- Obika, M.; Ogawa, H.; Takahashi, K.; Li, J.; Hatipoglu, O.F.; Cilek, M.Z.; Miyoshi, T.; Inagaki, J.; Ohtsuki, T.; Kusachi, S.; et al. Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci. 2012, 103, 1889–1897. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, X.; Xu, Y.; Fan, Z.; Kang, L.; Li, L.; Liang, Y.; Guo, J.; Hong, T.; Li, Z.; et al. miR-105/Runx2 axis mediates FGF2-induced ADAMTS expression in osteoarthritis cartilage. J. Mol. Med. 2016, 94, 681–694. [Google Scholar] [CrossRef]
- Suga, A.; Hikasa, H.; Taira, M. Xenopus ADAMTS1 negatively modulates FGF signaling independent of its metalloprotease activity. Dev. Biol. 2006, 295, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Rocks, N.; Paulissen, G.; Quesada-Calvo, F.; Munaut, C.; Gonzalez, M.-L.A.; Gueders, M.; Hacha, J.; Gilles, C.; Foidart, J.-M.; Noel, A.; et al. ADAMTS-1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivo. Cancer Res. 2008, 68, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sakai, T. Biological significance of local TGF-β activation in liver diseases. Front. Physiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.A.; Ahmed, E.K.; Mahmoud, A.A.; Atef, A.A. G protein-coupled estrogen receptor (GPER) selective agonist G1 attenuates the neurobehavioral, molecular and biochemical alterations induced in a valproic acid rat model of autism. Life Sci. 2023, 328, 121860. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.J.; Lee, G.S.; Moon, J.H.; Jung, J. Activation of G Protein-Coupled Estrogen Receptor Induces p53 and ADAMTS1 to Inhibit Tumor Growth and Suppress Liver Cancer Metastasis. Cancers 2025, 17, 2623. https://doi.org/10.3390/cancers17162623
Kwon HJ, Lee GS, Moon JH, Jung J. Activation of G Protein-Coupled Estrogen Receptor Induces p53 and ADAMTS1 to Inhibit Tumor Growth and Suppress Liver Cancer Metastasis. Cancers. 2025; 17(16):2623. https://doi.org/10.3390/cancers17162623
Chicago/Turabian StyleKwon, Hee Jung, Ga Seul Lee, Jeong Hee Moon, and Joohee Jung. 2025. "Activation of G Protein-Coupled Estrogen Receptor Induces p53 and ADAMTS1 to Inhibit Tumor Growth and Suppress Liver Cancer Metastasis" Cancers 17, no. 16: 2623. https://doi.org/10.3390/cancers17162623
APA StyleKwon, H. J., Lee, G. S., Moon, J. H., & Jung, J. (2025). Activation of G Protein-Coupled Estrogen Receptor Induces p53 and ADAMTS1 to Inhibit Tumor Growth and Suppress Liver Cancer Metastasis. Cancers, 17(16), 2623. https://doi.org/10.3390/cancers17162623






