The Multifaceted Role of Autophagy in Nasopharyngeal Carcinoma: Translational Perspectives on Pathogenesis, Biomarkers, Treatment Resistance, and Emerging Therapies
Simple Summary
Abstract
1. Introduction
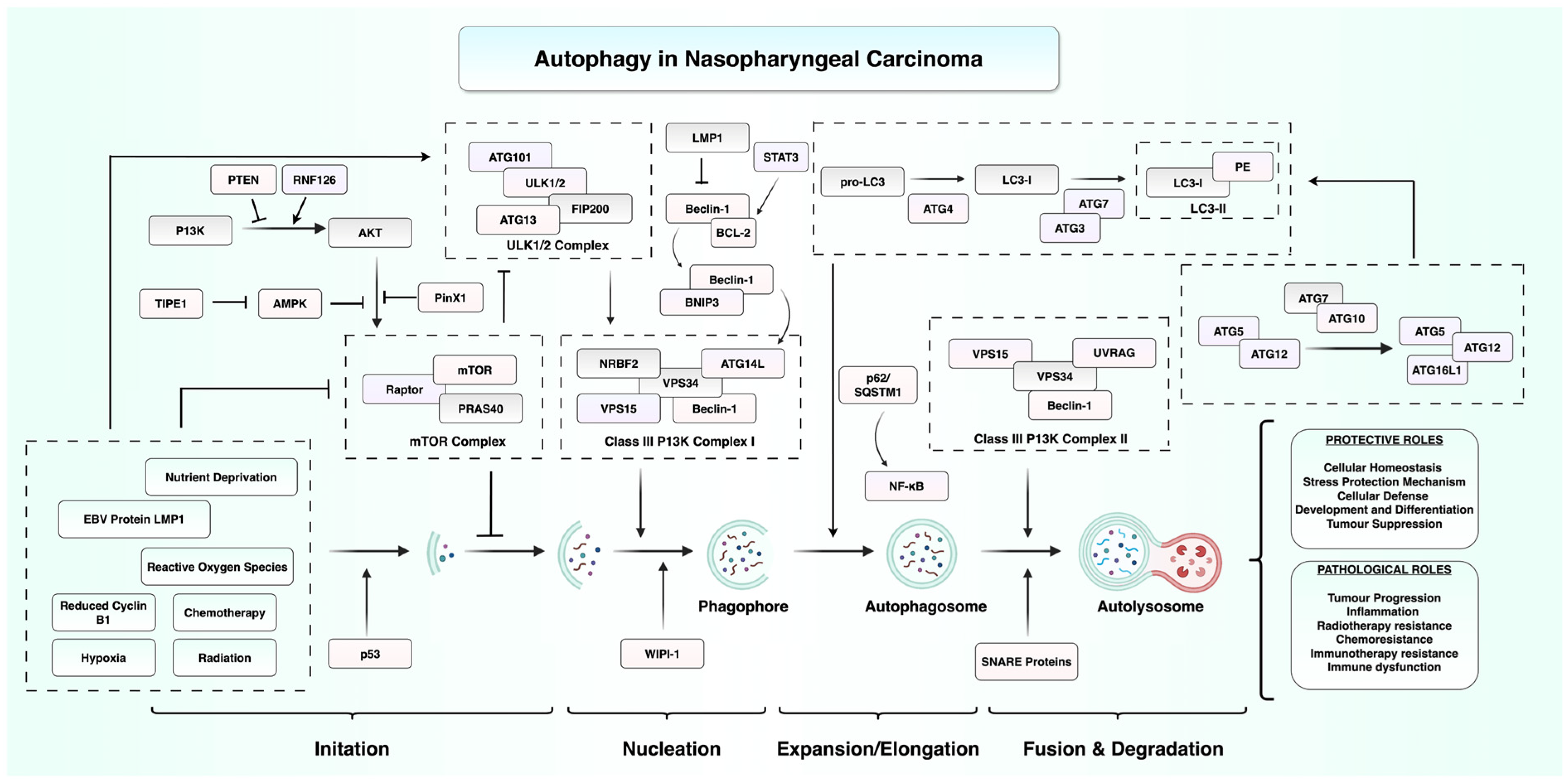
2. Autophagy in the Initiation and Progression of Nasopharyngeal Carcinoma
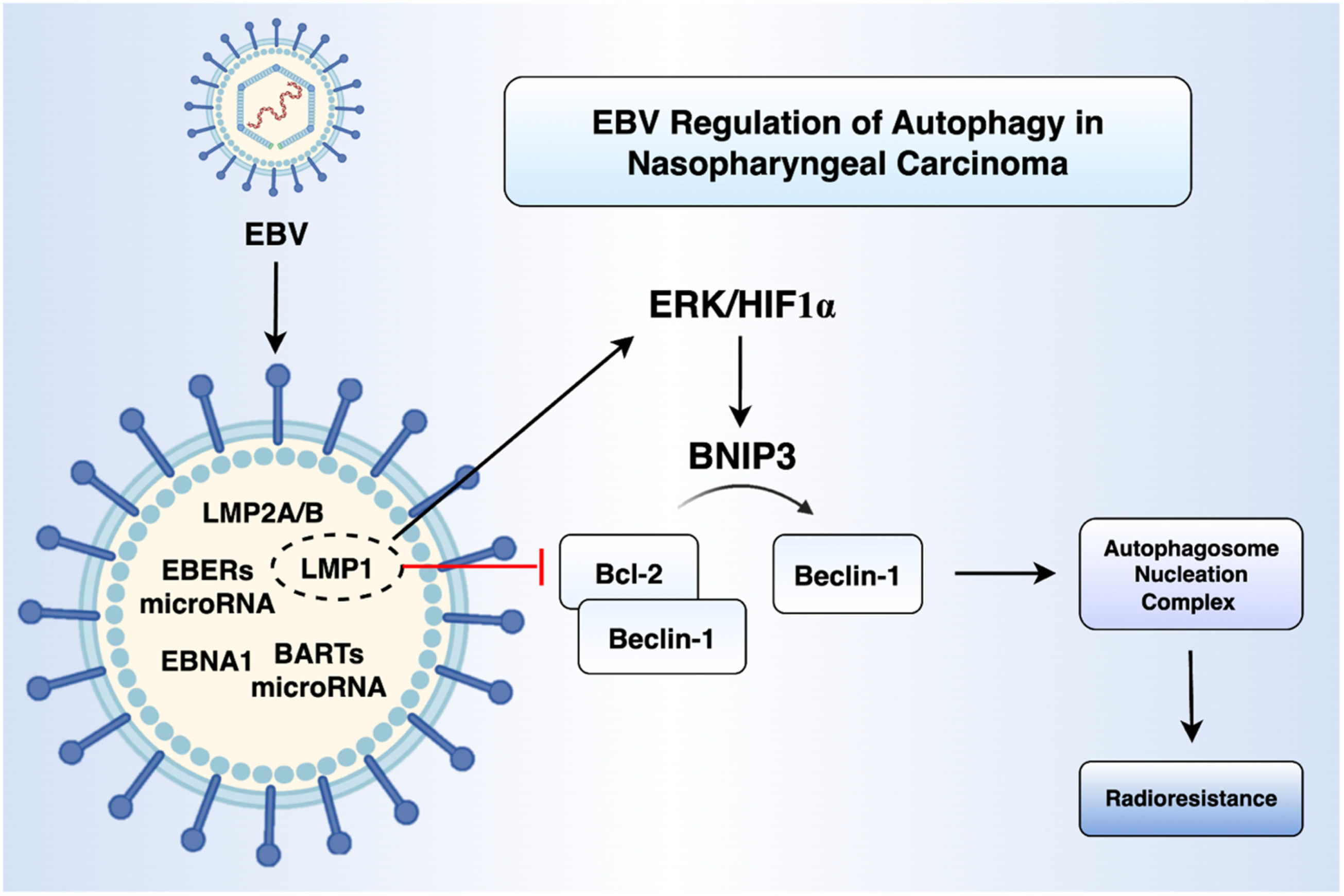
2.1. EBV Infection and Autophagy
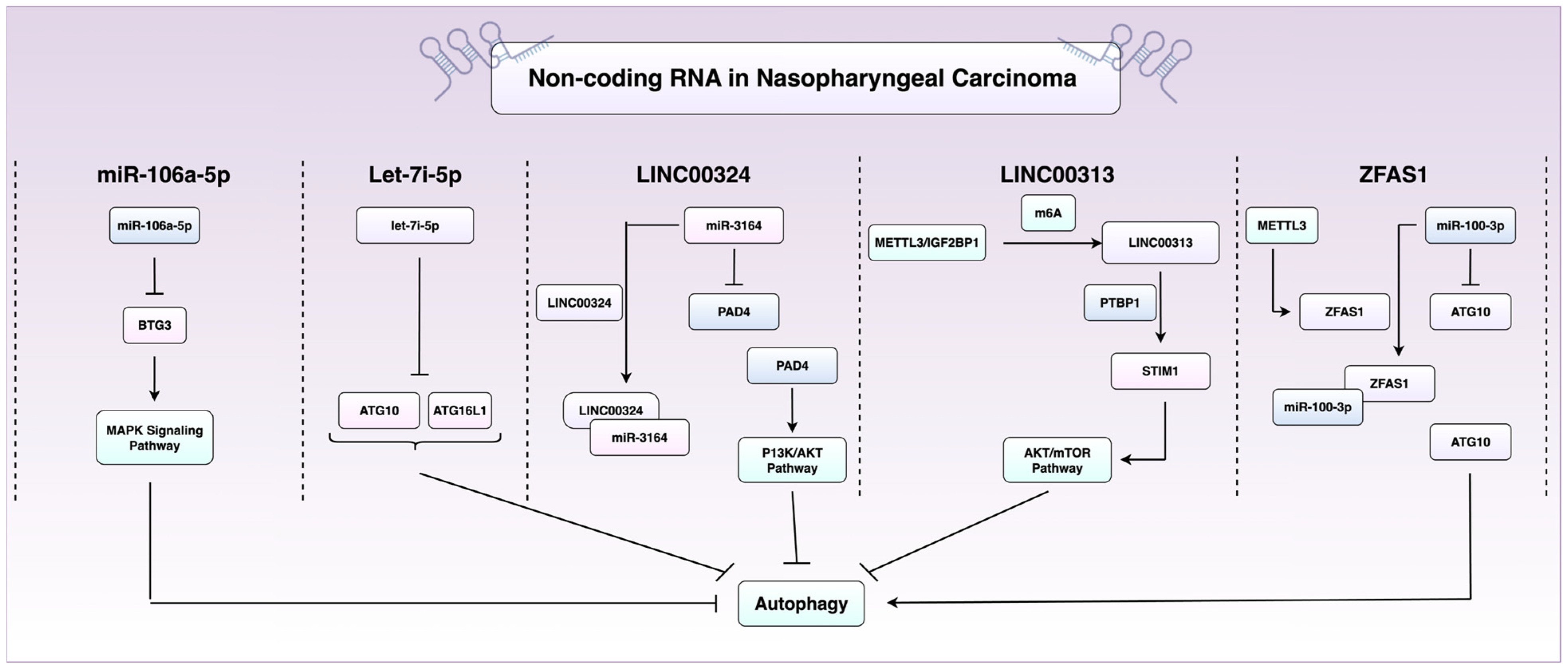
2.2. RNA-Mediated and Epigenetic Control of Autophagy in NPC
2.3. Immune Regulation by Autophagy
3. Therapeutic Resistance: The Role of Autophagy
3.1. Radioresistance
3.2. Chemoresistance
3.3. Resistance to Immunotherapy
4. Autophagy-Related Proteins as NPC Biomarkers and Predictors of Therapeutic Resistance
4.1. SQSTM1/p62
4.2. Beclin-1
4.3. Aurora Kinase A
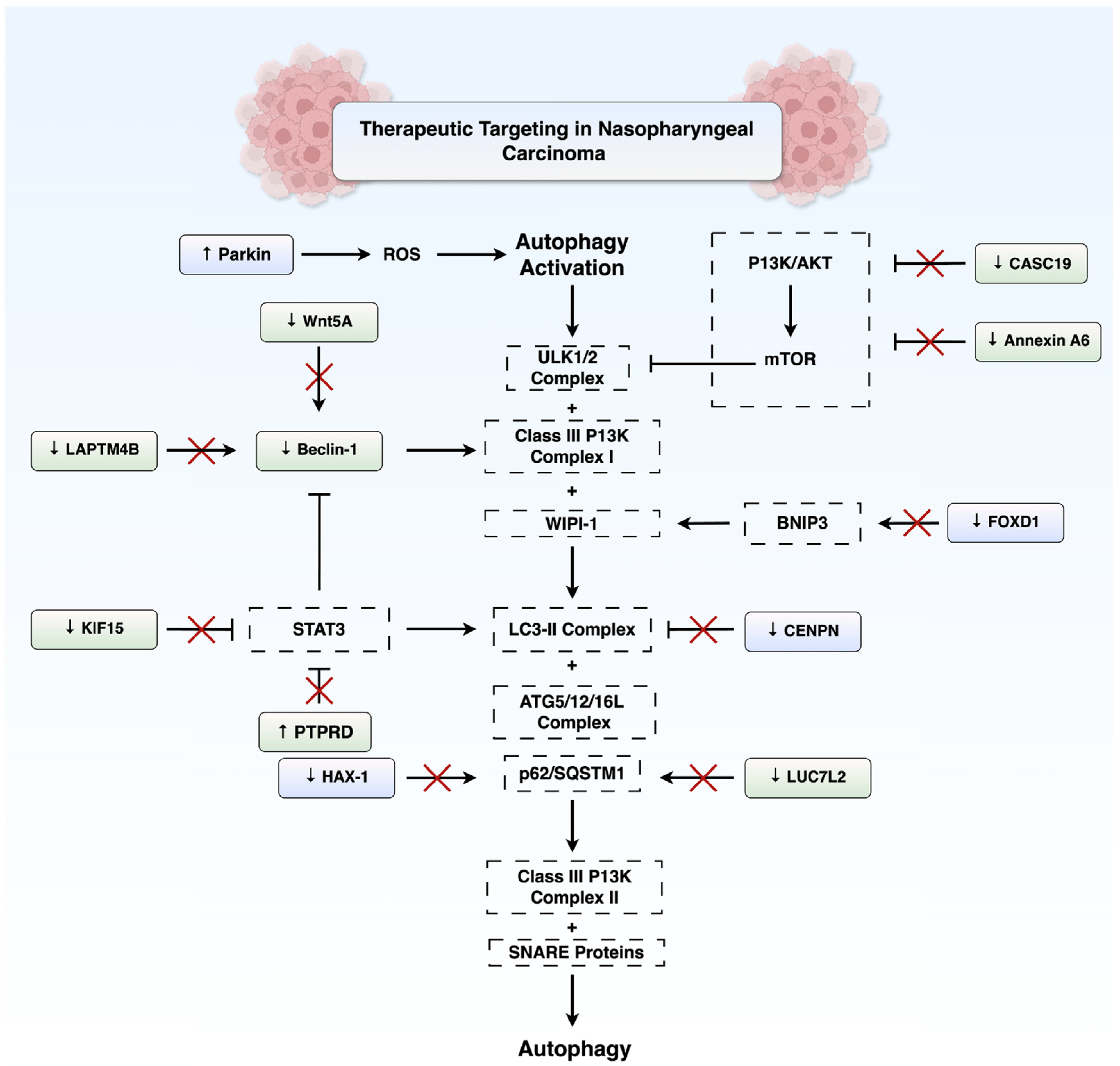
5. Therapeutic Targeting of Autophagy in NPC
5.1. Autophagy Activation
5.2. Autophagy Inhibition
5.3. Targeting Autophagy Using Natural Compounds
5.4. Barriers to the Clinical Translation of Autophagy Modulators
6. Future Perspectives and Ongoing Research
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jicman Stan, D.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Vesa, D.; Bezman, L.; Bujoreanu, F.C.; Sarbu, M.I.; Mihailov, R.; et al. Nasopharyngeal carcinoma: A new synthesis of literature data (Review). Exp. Ther. Med. 2022, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.J. Nasopharyngeal carcinoma as a paradigm of cancer genetics. Chin. J. Cancer 2011, 30, 79–84. [Google Scholar] [CrossRef]
- van Velsen, J.S.; van der Vegt, B.; Plaat, B.E.C.; Langendijk, J.A.; Epskamp-Kuijpers, C.; van Dijk, B.A.C.; Oosting, S.F. Nasopharyngeal carcinoma: Nationwide trends in subtype-specific incidence and survival over 3 decades in a non-endemic area. J. Cancer Res. Clin. Oncol. 2024, 150, 49. [Google Scholar] [CrossRef]
- Thompson, L.D.R. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Ear Nose Throat J. 2006, 85, 74. [Google Scholar] [CrossRef]
- Su, Z.Y.; Siak, P.Y.; Leong, C.O.; Cheah, S.C. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 2023, 14, 1116143. [Google Scholar] [CrossRef]
- Fung, S.Y.; Lam, J.W.; Chan, K.C. Clinical utility of circulating Epstein-Barr virus DNA analysis for the management of nasopharyngeal carcinoma. Chin. Clin. Oncol. 2016, 5, 18. [Google Scholar] [CrossRef]
- Niedobitek, G.; Hansmann, M.L.; Herbst, H.; Young, L.S.; Dienemann, D.; Hartmann, C.A.; Finn, T.; Pitteroff, S.; Welt, A.; Anagnostopoulos, I.; et al. Epstein-Barr virus and carcinomas: Undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J. Pathol. 1991, 165, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Wen, W.N.; Chen, J.Y.; Hsu, M.M.; Hsu, H.C. Detection of Epstein-Barr virus genome in nasopharyngeal carcinoma by in situ DNA hybridization. Intervirology 1993, 36, 91–98. [Google Scholar] [CrossRef]
- Hørding, U.; Nielsen, H.W.; Albeck, H.; Daugaard, S. Nasopharyngeal carcinoma: Histopathological types and association with Epstein-Barr Virus. Eur. J. Cancer B Oral Oncol. 1993, 29b, 137–139. [Google Scholar] [CrossRef]
- Stepan, K.O.; Mazul, A.L.; Skillington, S.A.; Paniello, R.C.; Rich, J.T.; Zevallos, J.P.; Jackson, R.S.; Pipkorn, P.; Massa, S.; Puram, S.V. The prognostic significance of race in nasopharyngeal carcinoma by histological subtype. Head. Neck 2021, 43, 1797–1811. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, G.; Geara, F.B.; Garden, A.S.; Tucker, S.L.; Ang, K.K.; Morrison, W.H.; Peters, L.J. Carcinoma of the nasopharynx treated by radiotherapy alone: Determinants of local and regional control. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 985–996. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Kondo, S.; Dochi, H.; Kobayashi, E.; Mizokami, H.; Komura, S.; Endo, K. Recent Advances in Assessing the Clinical Implications of Epstein-Barr Virus Infection and Their Application to the Diagnosis and Treatment of Nasopharyngeal Carcinoma. Microorganisms 2024, 12, 14. [Google Scholar] [CrossRef]
- Paiar, F.; Di Cataldo, V.; Zei, G.; Pasquetti, E.M.; Cecchini, S.; Meattini, I.; Mangoni, M.; Agresti, B.; Iermano, C.; Bonomo, P.; et al. Role of chemotherapy in nasopharyngeal carcinoma. Oncol. Rev. 2012, 6, e1. [Google Scholar] [CrossRef]
- Tsao, S.W.; Yip, Y.L.; Tsang, C.M.; Pang, P.S.; Lau, V.M.; Zhang, G.; Lo, K.W. Etiological factors of nasopharyngeal carcinoma. Oral. Oncol. 2014, 50, 330–338. [Google Scholar] [CrossRef]
- Okekpa, S.I.; Mydin, R.B.S.M.N.; Mangantig, E.; Azmi, N.S.A.; Zahari, S.N.S.; Kaur, G.; Musa, Y. Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac. J. Cancer Prev. 2019, 20, 3505–3514. [Google Scholar] [CrossRef]
- Wang, Y.; Koh, K.K.; Chua, E.; Kiong, K.L.; Kwan, Y.H.; Charn, T.C. The association between chronic sinonasal inflammation and nasopharyngeal carcinoma—A systematic review and meta-analysis. Am. J. Otolaryngol. 2024, 45, 104206. [Google Scholar] [CrossRef]
- Jiromaru, R.; Nakagawa, T.; Yasumatsu, R. Advanced Nasopharyngeal Carcinoma: Current and Emerging Treatment Options. Cancer Manag. Res. 2022, 14, 2681–2689. [Google Scholar] [CrossRef]
- Spratt, D.E.; Lee, N. Current and emerging treatment options for nasopharyngeal carcinoma. Onco Targets Ther. 2012, 5, 297–308. [Google Scholar] [CrossRef][Green Version]
- Nazeer, F.; Poulose, J.V.; Kainickal, C.T. Induction chemotherapy in nasopharyngeal carcinoma—A systematic review of phase III clinical trials. Cancer Treat. Res. Commun. 2022, 32, 100589. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 2018, 36, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Yang, Y.; Ma, Y.; Hong, S.; Lin, L.; He, X.; Xiong, J.; Li, P.; Zhao, H.; Huang, Y.; et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol. 2018, 19, 1338–1350. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.-G. Anti-Cancer Strategy Based on Changes in the Role of Autophagy Depending on the Survival Environment and Tumorigenesis Stages. Molecules 2024, 29, 5134. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Hunt, D.K.; Acosta-Jaquez, H.A.; Fingar, D.C.; Tee, A.R. ULK1 inhibits mTORC1 signaling, promotes multisite Raptor phosphorylation and hinders substrate binding. Autophagy 2011, 7, 737–747. [Google Scholar] [CrossRef]
- Russell, R.C.; Yuan, H.-X.; Guan, K.-L. Autophagy regulation by nutrient signaling. Cell Res. 2014, 24, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, D.M.; Kraft, C. Autophagosomes are formed at a distinct cellular structure. Curr. Opin. Cell Biol. 2020, 65, 50–57. [Google Scholar] [CrossRef]
- Pyo, J.-O.; Nah, J.; Jung, Y.-K. Molecules and their functions in autophagy. Exp. Mol. Med. 2012, 44, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y. Class III phosphatidylinositol 3-kinase complex I subunit NRBF2/Atg38—From cell and structural biology to health and disease. Autophagy 2021, 17, 3897–3907. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.Y. Molecular Mechanism of Autophagosome-Lysosome Fusion in Mammalian Cells. Cells 2024, 13, 500. [Google Scholar] [CrossRef]
- Thoresen, S.B.; Pedersen, N.M.; Liestøl, K.; Stenmark, H. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp. Cell Res. 2010, 316, 3368–3378. [Google Scholar] [CrossRef] [PubMed]
- Iershov, A.; Nemazanyy, I.; Alkhoury, C.; Girard, M.; Barth, E.; Cagnard, N.; Montagner, A.; Chretien, D.; Rugarli, E.I.; Guillou, H.; et al. The class 3 PI3K coordinates autophagy and mitochondrial lipid catabolism by controlling nuclear receptor PPARα. Nat. Commun. 2019, 10, 1566. [Google Scholar] [CrossRef]
- Li, W.; He, P.; Huang, Y.; Li, Y.F.; Lu, J.; Li, M.; Kurihara, H.; Luo, Z.; Meng, T.; Onishi, M.; et al. Selective autophagy of intracellular organelles: Recent research advances. Theranostics 2021, 11, 222–256. [Google Scholar] [CrossRef] [PubMed]
- Johansen, T.; Lamark, T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J. Mol. Biol. 2020, 432, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Kim, I.S.; Baek, S.H. Decoding the dual role of autophagy in cancer through transcriptional and epigenetic regulation. FEBS Lett. 2025. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Fediuk, M.; Smolle-Juettner, F.M. Autophagy, Oxidative Stress and Cancer Development. Cancers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.-F.; Li, Q.-J.; He, S.-W.; He, Q.-M.; Long, L.-F.; Liu, N.; Ma, J. WIPI-1 inhibits metastasis and tumour growth via the WIPI-1-TRIM21 axis and MYC regulation in nasopharyngeal carcinoma. Oral. Oncol. 2021, 122, 105576. [Google Scholar] [CrossRef]
- Shen, C.; Chen, F.; Wang, H.; Li, G.; Yu, C.; Wang, X.; Wen, Z. The Pinx1 Gene Downregulates Telomerase and Inhibits Proliferation of CD133+ Cancer Stem Cells Isolated from a Nasopharyngeal Carcinoma Cell Line by Regulating Trfs and Mad1/C-Myc/p53 Pathways. Cell. Physiol. Biochem. 2018, 49, 282–294. [Google Scholar] [CrossRef]
- Yang, M.; Chen, F.; Yu, C.; Cai, Z.; Zhong, Q.; Feng, J.; Li, G.; Shen, C.; Wen, Z. PinX1-Promoted Autophagy Inhibits Cell Proliferation and Induces Cell Apoptosis by Inhibiting the NF-κB/p65 Signaling Pathway in Nasopharyngeal Carcinoma. Front. Biosci. 2023, 28, 170. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Zhao, Z.; Song, D.; Wang, L.; Zhai, Q.; Zhang, X.; Zhao, P.; Xiang, X. TIPE1-mediated autophagy suppression promotes nasopharyngeal carcinoma cell proliferation via the AMPK/mTOR signalling pathway. J. Cell Mol. Med. 2020, 24, 9135–9144. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, W.; Zheng, W.; Chen, T.; Yang, H.; Sun, L.; Huang, F.; Wang, Z.; Lin, H.; Chen, L.; et al. Downregulation of G2/mitotic-specific cyclinB1 triggers autophagy via AMPK-ULK1-dependent signal pathway in nasopharyngeal carcinoma cells. Cell Death Dis. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, W.; Chen, Q.; Zhou, Y.; Pan, Y.; Zhang, J.; Bai, Y.; Shao, C. lncRNA CASC19 Contributes to Radioresistance of Nasopharyngeal Carcinoma by Promoting Autophagy via AMPK-mTOR Pathway. Int. J. Mol. Sci. 2021, 22, 1407. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, M.-X.; Zou, X.; Liu, Y.-P.; You, R.; Yu, T.; Jiang, R.; Zhang, Y.-N.; Cao, J.-Y.; Hong, M.-H.; et al. A Prognostic Bio-Model Based on SQSTM1 and N-Stage Identifies Nasopharyngeal Carcinoma Patients at High Risk of Metastasis for Additional Induction Chemotherapy. Clin. Cancer Res. 2018, 24, 648–658. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Maruo, S.; Takada, K. EBV lytic infection enhances transformation of B-lymphocytes infected with EBV in the presence of T-lymphocytes. J. Med. Virol. 2012, 84, 504–510. [Google Scholar] [CrossRef]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef]
- Siciliano, M.C.; Tornambè, S.; Cevenini, G.; Sorrentino, E.; Granai, M.; Giovannoni, G.; Marrelli, D.; Biviano, I.; Roviello, F.; Yoshiyama, H.; et al. EBV persistence in gastric cancer cases conventionally classified as EBER-ISH negative. Infect. Agent. Cancer 2022, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Sam, C.K.; Brooks, L.A.; Niedobitek, G.; Young, L.S.; Prasad, U.; Rickinson, A.B. Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int. J. Cancer 1993, 53, 957–962. [Google Scholar] [CrossRef]
- Marquitz, A.R.; Raab-Traub, N. The role of miRNAs and EBV BARTs in NPC. Semin. Cancer Biol. 2012, 22, 166–172. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Peng, X.; Tao, X.; Zhou, P.; Zhang, K.; Peng, J.; Li, D.; Shen, L.; Yang, L. EBV-LMP1 promotes radioresistance by inducing protective autophagy through BNIP3 in nasopharyngeal carcinoma. Cell Death Dis. 2021, 12, 344. [Google Scholar] [CrossRef]
- Yiu, S.P.T.; Hui, K.F.; Münz, C.; Lo, K.W.; Tsao, S.W.; Kao, R.Y.T.; Yang, D.; Chiang, A.K.S. Autophagy-Dependent Reactivation of Epstein-Barr Virus Lytic Cycle and Combinatorial Effects of Autophagy-Dependent and Independent Lytic Inducers in Nasopharyngeal Carcinoma. Cancers 2019, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Chen, H.; Wei, L.; Luo, M.; Wang, X.; Zhu, C.; Huang, H.; Liu, X.; Lu, H.; Zhong, Y. LINC00324 suppresses apoptosis and autophagy in nasopharyngeal carcinoma through upregulation of PAD4 and activation of the PI3K/AKT signaling pathway. Cell Biol. Toxicol. 2022, 38, 995–1011. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Yang, Y.; Shu, L.; Sun, Y. LINC00313 suppresses autophagy and promotes stemness of nasopharyngeal carcinoma cells through PTBP1/STIM1 axis. Radiother. Oncol. 2024, 196, 110310. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, H.; Liu, F.; Wu, Q.; Liu, S. The m6A methyltransferase METTL3 affects autophagy and progression of nasopharyngeal carcinoma by regulating the stability of lncRNA ZFAS1. Infect. Agent. Cancer 2022, 17, 1. [Google Scholar] [CrossRef]
- You, B.; Zhang, P.; Gu, M.; Yin, H.; Fan, Y.; Yao, H.; Pan, S.; Xie, H.; Cheng, T.; Liu, H.; et al. Let-7i-5p promotes a malignant phenotype in nasopharyngeal carcinoma via inhibiting tumor-suppressive autophagy. Cancer Lett. 2022, 531, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, Q.; Gu, M.; Zhang, K.; Xia, T.; Zhang, S.; Chen, W.; Yin, H.; Yao, H.; Fan, Y.; et al. MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy 2021, 17, 1667–1683. [Google Scholar] [CrossRef]
- Yu, C.; Xue, B.; Li, J.; Zhang, Q. Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes nasopharyngeal carcinoma progression by regulating PTEN ubiquitination. Apoptosis 2022, 27, 590–605. [Google Scholar] [CrossRef]
- Alvarez-Guaita, A.; Vilà de Muga, S.; Owen, D.M.; Williamson, D.; Magenau, A.; García-Melero, A.; Reverter, M.; Hoque, M.; Cairns, R.; Cornely, R.; et al. Evidence for annexin A6-dependent plasma membrane remodelling of lipid domains. Br. J. Pharmacol. 2015, 172, 1677–1690. [Google Scholar] [CrossRef]
- Cao, J.; Wan, S.; Chen, S.; Yang, L. ANXA6: A key molecular player in cancer progression and drug resistance. Discov. Oncol. 2023, 14, 53. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, W.; Zhu, L.; Yao, D.; Wang, C.; Song, Y.; Hu, S.; Liu, H.; Bai, Y.; Pan, Y.; et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2020, 8, 232. [Google Scholar] [CrossRef]
- Wang, W.J.; Guo, C.A.; Li, R.; Xu, Z.P.; Yu, J.P.; Ye, Y.; Zhao, J.; Wang, J.; Wang, W.A.; Zhang, A.; et al. Long non-coding RNA CASC19 is associated with the progression and prognosis of advanced gastric cancer. Aging 2019, 11, 5829–5847. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Wu, C.; Shen, H.; Yi, J.; Liu, L. LncRNA CASC19 promotes the growth and glycolysis of colorectal cancer cells and tumor metastasis in mice. BMC Cancer 2025, 25, 829. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, C.; Fang, R.; Yang, S.; Zhao, G.; Liu, S.; Li, P. LncRNA CASC19: A novel oncogene involved in human cancer. Clin. Transl. Oncol. 2023, 25, 2841–2851. [Google Scholar] [CrossRef]
- Chu, C.; Niu, X.; Ou, X.; Hu, C. LAPTM4B knockdown increases the radiosensitivity of EGFR-overexpressing radioresistant nasopharyngeal cancer cells by inhibiting autophagy. Onco Targets Ther. 2019, 12, 5661–5677. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, Y.; Jing, Q. Wnt5a-mediated autophagy promotes radiation resistance of nasopharyngeal carcinoma. J. Cancer 2022, 13, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, W.; Zhu, L.; Liu, H.; Song, Y.; Hu, S.; Bai, Y.; Pan, Y.; Zhang, J.; Guan, J.; et al. LACTB2 renders radioresistance by activating PINK1/Parkin-dependent mitophagy in nasopharyngeal carcinoma. Cancer Lett. 2021, 518, 127–139. [Google Scholar] [CrossRef]
- Shen, L.; Li, C.; Chen, F.; Shen, L.; Li, Z.; Li, N. CRISPR/Cas9 genome-wide screening identifies LUC7L2 that promotes radioresistance via autophagy in nasopharyngeal carcinoma cells. Cell Death Discov. 2021, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, X.; Yang, K.; Chen, Y.; Wang, L.; Luo, W.; Li, Y.; Liao, J.; Zhou, Y.; Lei, Y.; et al. Protein tyrosine phosphatase receptor type D gene promotes radiosensitivity via STAT3 dephosphorylation in nasopharyngeal carcinoma. Oncogene 2021, 40, 3101–3117. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Zhang, L.; Wu, X.; Tian, L.; Zou, J.; Pi, G. METTL3 methylated KIF15 promotes nasopharyngeal carcinoma progression and radiation resistance by blocking ATG7-mediated autophagy through the activation of STAT3 pathway. Transl. Oncol. 2025, 51, 102161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Li, Y.; Yang, L.; Ma, Y.; Peng, X.; Yang, S.; Liu, J.; Li, H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gu, M.; Yin, H.; Pan, S.; Xie, H.; Chen, W.; Gul, S.; Zhao, Y.; Wang, Z.; Zheng, W.; et al. IGF2BP1-HAX-1 positive feedback loop-mediated HAX-1 overexpression blocks autophagic flux and promotes chemoresistance in nasopharyngeal carcinoma. Cell Mol. Life Sci. 2025, 82, 105. [Google Scholar] [CrossRef] [PubMed]
- Chittori, S.; Hong, J.; Saunders, H.; Feng, H.; Ghirlando, R.; Kelly, A.E.; Bai, Y.; Subramaniam, S. Structural mechanisms of centromeric nucleosome recognition by the kinetochore protein CENP-N. Science 2018, 359, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.R.; Han, J.B.; Jiang, Y.; Xu, S.; Yang, R.; Kong, Y.G.; Tao, Z.Z.; Hua, Q.Q.; Zou, Y.; Chen, S.M. CENPN suppresses autophagy and increases paclitaxel resistance in nasopharyngeal carcinoma cells by inhibiting the CREB-VAMP8 signaling axis. Autophagy 2024, 20, 329–348. [Google Scholar] [CrossRef]
- Ni, H.; Liu, R.; Zhou, Z.; Jiang, B.; Wang, B. Parkin enhances sensitivity of paclitaxel to nasopharyngeal carcinoma by activating BNIP3/NIX-mediated mitochondrial autophagy. Chin. J. Physiol. 2023, 66, 503–515. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Y.; He, D.; Zhu, Y.; Xiao, M.; Liu, X.; Cheng, Y.; Cao, K. Targeting ALG3/FOXD1/BNIP3 Axis Prevents Mitophagy and Gemcitabine Resistance of Nasopharyngeal Carcinoma. Int. J. Biol. Sci. 2025, 21, 1894–1913. [Google Scholar] [CrossRef]
- You, B.; Xia, T.; Gu, M.; Zhang, Z.; Zhang, Q.; Shen, J.; Fan, Y.; Yao, H.; Pan, S.; Lu, Y.; et al. AMPK-mTOR-Mediated Activation of Autophagy Promotes Formation of Dormant Polyploid Giant Cancer Cells. Cancer Res. 2022, 82, 846–858. [Google Scholar] [CrossRef]
- Kam, N.W.; Lau, C.Y.; Lau, J.Y.H.; Dai, X.; Liang, Y.; Lai, S.P.H.; Chung, M.K.Y.; Yu, V.Z.; Qiu, W.; Yang, M.; et al. Cell-associated galectin 9 interacts with cytotoxic T cells confers resistance to tumor killing in nasopharyngeal carcinoma through autophagy activation. Cell Mol. Immunol. 2025, 22, 260–281. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, H.; Cao, J.; Chen, Y.; Huang, J.; Weng, Y. AURKA, as a potential prognostic biomarker, regulates autophagy and immune infiltration in nasopharyngeal carcinoma. Immunobiology 2023, 228, 152314. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.B.; Fan, X.J.; Chen, M.Y.; Xiang, J.; Huang, P.Y.; Guo, L.; Wu, X.Y.; Xu, J.; Long, Z.J.; Zhao, Y.; et al. Elevated Beclin 1 expression is correlated with HIF-1alpha in predicting poor prognosis of nasopharyngeal carcinoma. Autophagy 2010, 6, 395–404. [Google Scholar] [CrossRef]
- Okano, M.; He, F.; Ma, N.; Kobayashi, H.; Oikawa, S.; Nishimura, K.; Tawara, I.; Murata, M. Taurine induces upregulation of p53 and Beclin1 and has antitumor effect in human nasopharyngeal carcinoma cells in vitro and in vivo. Acta Histochem. 2023, 125, 151978. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, K.; Chen, J.; Deng, H.; Weng, K.; Wu, H.; Li, K.; Xiao, B.; Luo, S.; Hao, W. Preclinical efficacy and involvement of mTOR signaling in the mechanism of Orf virus against nasopharyngeal carcinoma cells. Life Sci. 2022, 291, 120297. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.N.; Liu, S.L.; Yang, Y.; Shu, L.; Sun, Y. CircLASP1 silence strengthens the therapeutic effects of MK-2206 on nasopharyngeal cancer through upregulating miR-625. Cancer Sci. 2023, 114, 2123–2138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.; Ji, D.; Bao, X.; Tan, G.; Liang, X.; Deng, P.; Pi, H.; Lu, Y.; Chen, C.; et al. BIX-01294, a G9a inhibitor, suppresses cell proliferation by inhibiting autophagic flux in nasopharyngeal carcinoma cells. Invest. New Drugs 2021, 39, 686–696. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A Natural Isoquinoline Alkaloid with Antitumor Activity: Studies of the Biological Activities of Berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Cheung, Y.C.; Lui, V.W.-Y.; Yip, Y.L.; Zhang, G.; Lin, V.W.; Cheung, K.C.-P.; Feng, Y.; Tsao, S.W. Berberine suppresses tumorigenicity and growth of nasopharyngeal carcinoma cells by inhibiting STAT3 activation induced by tumor associated fibroblasts. BMC Cancer 2013, 13, 619. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, Q.; Tang, Q.; Chen, L.; Deng, H.; He, Y.; Tang, F. A specific super-enhancer actuated by berberine regulates EGFR-mediated RAS–RAF1–MEK1/2–ERK1/2 pathway to induce nasopharyngeal carcinoma autophagy. Cell. Mol. Biol. Lett. 2024, 29, 92. [Google Scholar] [CrossRef]
- Guan, X.; Yu, D.; Huangfu, M.; Huang, Z.; Dou, T.; Liu, Y.; Zhou, L.; Li, X.; Wang, L.; Liu, H.; et al. Curcumol inhibits EBV-positive Nasopharyngeal carcinoma migration and invasion by targeting nucleolin. Biochem. Pharmacol. 2021, 192, 114742. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Han, M.; Li, X.; Zhou, L.; Dou, T.; Liu, Y.; Huangfu, M.; Guan, X.; Wang, Y.; et al. RNA-binding domain 2 of nucleolin is important for the autophagy induction of curcumol in nasopharyngeal carcinoma cells. Phytomedicine 2023, 115, 154833. [Google Scholar] [CrossRef]
- Zeng, Y.; Duan, T.; Huang, J.; Wang, X. Astragaloside IV inhibits nasopharyngeal carcinoma progression by suppressing the SATB2/Wnt signaling axis. Toxicol. Res. 2025, 14, tfaf047. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, S.; Liu, Y.; Cao, X.; Zhao, M.; Xiao, J.; Feng, C. Geraniin inhibits cell growth and promoted autophagy-mediated cell death in the nasopharyngeal cancer C666-1 cells. Saudi J. Biol. Sci. 2022, 29, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, X.; Sun, C.; Hou, Y.; Hu, Y.; Ma, S.; Huang, L.; Ma, L.; Zhang, Y.; Dai, X. Isogarcinol inhibits nasopharyngeal carcinoma growth through mitochondria-mediated autophagic cell death. Phytomedicine 2024, 130, 155745. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lin, C.H.; Chiu, W.C.; Hsieh, T.J.; Chang, S.J.; Chang, Y.C.; Lan, Y.Y. Treatment with autophagic inhibitors enhances oligonol-induced apoptotic effects in nasopharyngeal carcinoma cells. Biomed. Rep. 2024, 21, 143. [Google Scholar] [CrossRef]
- Fan, C.-W.; Tang, J.; Jiang, J.-C.; Zhou, M.-M.; Li, M.-S.; Wang, H.-S. Pentagalloylglucose suppresses the growth and migration of human nasopharyngeal cancer cells via the GSK3β/β-catenin pathway in vitro and in vivo. Phytomedicine 2022, 102, 154192. [Google Scholar] [CrossRef]
- Yang, C.-H.; Tung, K.-L.; Wu, Y.-T.; Liu, C.; Lin, S.-C.; Yang, C.-C.; Wu, C.-H.; Chang, H.-Y.; Wu, S.-Y.; Huang, B.-M.; et al. Qing Yan Li Ge Tang, a Chinese Herbal Formula, Induces Autophagic Cell Death through the PI3K/Akt/mTOR Pathway in Nasopharyngeal Carcinoma Cells In Vitro. Evid. Based Complement. Altern. Med. 2021, 2021, 9925684. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Bu, S.; Guo, X.; Ge, H. Theaflavin promoted apoptosis in nasopharyngeal carcinoma unexpectedly via inducing autophagy in vitro. Iran. J. Basic. Med. Sci. 2022, 25, 68–74. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y. Trifolirhizin targets PTK6 to induce autophagy and exerts antitumor effects in nasopharyngeal carcinoma. Drug Dev. Res. 2024, 85, e70000. [Google Scholar] [CrossRef]
- Wang, P.; Burikhanov, R.; Jayswal, R.; Weiss, H.L.; Arnold, S.M.; Villano, J.L.; Rangnekar, V.M. Neoadjuvant administration of hydroxychloroquine in a phase 1 clinical trial induced plasma Par-4 levels and apoptosis in diverse tumors. Genes Cancer 2018, 9, 190–197. [Google Scholar] [CrossRef]
- Arnaout, A.; Robertson, S.J.; Pond, G.R.; Lee, H.; Jeong, A.; Ianni, L.; Kroeger, L.; Hilton, J.; Coupland, S.; Gottlieb, C.; et al. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res. Treat. 2019, 178, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Rubinson, D.A.; Wang, X.; Chan, J.A.; Cleary, J.M.; Enzinger, P.C.; Fuchs, C.S.; McCleary, N.J.; Meyerhardt, J.A.; Ng, K.; et al. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist 2014, 19, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D.; et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014, 4, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Kettner, N.M.; Kwiatkowski, D.; Damodaran, S.; Wang, Y.; Ramirez, D.; Gombos, D.S.; Hunt, K.K.; Shen, Y.; Keyomarsi, K.; et al. Phase I trial of hydroxychloroquine to enhance palbociclib and letrozole efficacy in ER+/HER2− breast cancer. npj Breast Cancer 2025, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, R.; Leone, R.; Chang, Y.C.; Fecher, L.A.; Schuchter, L.M.; Kramer, A.; Tan, K.S.; Heitjan, D.F.; Rodgers, G.; Gallagher, M.; et al. Phase I trial of hydroxychloroquine with dose-intense temozolomide in patients with advanced solid tumors and melanoma. Autophagy 2014, 10, 1369–1379. [Google Scholar] [CrossRef]
- Vogl, D.T.; Stadtmauer, E.A.; Tan, K.-S.; Heitjan, D.F.; Davis, L.E.; Pontiggia, L.; Rangwala, R.; Piao, S.; Chang, Y.C.; Scott, E.C.; et al. Combined autophagy and proteasome inhibition. Autophagy 2014, 10, 1380–1390. [Google Scholar] [CrossRef]
- Roark, K.M.; Iffland, P.H., 2nd. Rapamycin for longevity: The pros, the cons, and future perspectives. Front. Aging 2025, 6, 1628187. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Goyal, A.; Cusick, A.T.; Lahan, S.; Dhaliwal, H.S.; Bhyan, P.; Bhattad, P.B.; Aslam, F.; Ranka, S.; Dalia, T.; et al. Hydroxychloroquine: A comprehensive review and its controversial role in coronavirus disease 2019. Ann. Med. 2021, 53, 117–134. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Charbel Issa, P.; Ahn, S.J. Hydroxychloroquine-induced Retinal Toxicity. Front. Pharmacol. 2023, 14, 1196783. [Google Scholar] [CrossRef]
- Aga, T.; Endo, K.; Tsuji, A.; Aga, M.; Moriyama-Kita, M.; Ueno, T.; Nakanishi, Y.; Hatano, M.; Kondo, S.; Sugimoto, H.; et al. Inhibition of autophagy by chloroquine makes chemotherapy in nasopharyngeal carcinoma more efficient. Auris Nasus Larynx 2019, 46, 443–450. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Li, L.; Nie, D.; Tao, Q.; Wu, J.; Fan, J.; Lin, C.; Zhao, S.; Ju, D. Inhibition of Autophagy Potentiated the Antitumor Effect of Nedaplatin in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. PLoS ONE 2015, 10, e0135236. [Google Scholar] [CrossRef]
- He, J.H.; Liao, X.L.; Wang, W.; Li, D.D.; Chen, W.D.; Deng, R.; Yang, D.; Han, Z.P.; Jiang, J.W.; Zhu, X.F. Apogossypolone, a small-molecule inhibitor of Bcl-2, induces radiosensitization of nasopharyngeal carcinoma cells by stimulating autophagy. Int. J. Oncol. 2014, 45, 1099–1108. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.-N.; Huang, X.-Y.; Zhu, J.-M.; Lin, F.; You, Q.; Lin, Y.-Z.; Cai, H.; Wei, Y.; Xue, X.-Y.; et al. Machine learning-based autophagy-related prognostic signature for personalized risk stratification and therapeutic approaches in bladder cancer. Int. Immunopharmacol. 2024, 138, 112623. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Autophagy-Regulating Mechanism | Prognostic Impact | Therapeutic Resistance | Patient Cohort Details | Detection Method | Ref. |
|---|---|---|---|---|---|---|
| AURKA | Activates mTOR/ULK1 pathway, thereby suppressing autophagy; linked to reduced immune infiltration and poor immune landscape in high-AURKA tumours. | High expression associated with poorer OS and DMFS | Not directly evaluated in study. | n = 208 NPC patients; validation in TCGA data | IHC and qRT-PCR | [79] |
| Beclin-1 | Core autophagy initiator protein. High Beclin-1 coexpresses with HIF-1α, possibly promoting survival under hypoxia and autophagy-mediated therapeutic resistance. | High expression associated with poorer OS, PFS, and DMFS | High Beclin-1 with high HIF-1α correlates with resistance to chemoradiotherapy. | n = 128 advanced NPC patients (RCT-derived); divided into training (n = 61) and testing set (n = 67) | IHC | [80] |
| CENPN | Suppresses autophagy by inhibiting the CREB-VAMP8 signalling axis, reducing autophagosome formation and increasing paclitaxel resistance. | High expression associated with poorer OS and DFS | Not directly evaluated in study. | n = 98 NPC patients; stages I–IV | IHC, Western blot, qRT-PCR | [74] |
| PTPRD | Promotes radiation-induced autophagy via STAT3 dephosphorylation → increased ATG5 transcription → autophagic flux enhancement. | Low expression associated with poorer OS | Low PTPRD associated with radioresistance; overexpression enhances radiosensitivity via STAT3 dephosphorylation leading to increased ATG5 and autophagy. | n = 107 NPC patient samples | IHC, qRT-PCR, bisulfite pyrosequencing | [69] |
| SQSTM1 | Downstream of impaired autophagic flux. Its accumulation activates NF-κB and induces EMT, linking defective autophagy to metastasis. | High expression associated with increased risk of distant metastasis | Inhibiting SQSTM1 enhanced sensitivity to cisplatin in NPC cells. | n = 116 NPC patients (retrospective); validation cohort n = 134 (prospective RCT) | IHC, Western blot, qRT-PCR | [44] |
| TIPE1 | Inhibits autophagy by blocking AMPK activation and enhancing mTOR signalling → leads to reduced LC3-II and increased p62 accumulation. | High expression associated with poorer OS | Not directly evaluated in study. | n = 108 NPC patients | IHC, qRT-PCR, Western blot | [41] |
| WIPI1 | Enhances starvation-induced autophagy via TRIM21 interaction; loss of WIPI1 decreases autophagic activity and may promote Myc-driven proliferation. | Low expression associated with poorer PFS | Not directly evaluated in study. | n = 17 NPC tissues vs. 14 normal tissues; in vitro and in vivo models | qRT-PCR, Western blot, RNA-seq | [38] |
| Compound | Source of Compound | Experimental Model | Primary Mechanism | Effect on Autophagy | Effect on NPC Cells | References |
|---|---|---|---|---|---|---|
| Berberine (BBR) | Coptis chinensis | NPC cells (S18, 5-8F, C666-1) | Activates EGFR → RAS/RAF/MEK/ERK; SE-driven autophagy. | Induces autophagy | Inhibits proliferation, migration, invasion. | [87] |
| Curcumol | Curcuma species | NPC C666-1 cells; xenografts | Targets nucleolin; inhibits PI3K/Akt/mTOR. | Induces autophagy | Suppresses proliferation. | [89] |
| Isogarcinol | Garcinia oblongifolia | NPC CNE1/CNE2; xenografts | Autophagy-regulating mechanism not fully elucidated. | Induces autophagy, blocks autophagic flux | Blocks proliferation, migration, invasion; induces mitochondrial apoptosis | [92] |
| Astragaloside IV (AS-IV) | Astragalus membranaceus | NPC C666-1/HK-1; xenografts | Suppresses SATB2/Wnt signalling. | Inhibits autophagy | Inhibits proliferation, migration; enhances apoptosis. | [90] |
| Pentagalloylglucose (PGG) | Paeonia lactiflora, Galla Rhois | NPC CNE1/CNE2 cells; xenografts | Upregulates p38 MAPK; downregulates Wnt/β-catenin and mTOR. | Induces autophagy | Reduces proliferation, migration; triggers apoptosis and autophagy. | [94] |
| Oligonol | Lychee fruit polyphenol | NPC TW01, HK1 cells | Autophagy-regulating mechanism not fully elucidated. | Induces autophagy | Decreases cell viability. | [93] |
| Qing Yan Li Ge Tang | Chinese herbal formula | NPC TW01 cells | Activates autophagy via PI3K/Akt/mTOR pathway. | Induces autophagy | Inhibits proliferation and colony formation; triggers autophagy-mediated cell death. | [95] |
| Theaflavin | Black tea (Camellia sinensis) | NPC CNE2 cells | Inhibits mTOR. | Induces autophagy | Suppresses proliferation; promotes apoptosis. | [96] |
| Geraniin | Phyllanthus species | NPC C666-1 cells | Modulates PI3K/Akt/mTOR. | Induces autophagy | Inhibits proliferation; enhances ROS generation. | [91] |
| Trifolirhizin | Sophora flavescens | NPC C666-1 cells | Targets PTK6. | Induces autophagy | Reduces proliferation; promotes apoptosis. | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakerdi, A.L.; Finnegan, E.; Sheng, Y.-Y.; Pidgeon, G.P. The Multifaceted Role of Autophagy in Nasopharyngeal Carcinoma: Translational Perspectives on Pathogenesis, Biomarkers, Treatment Resistance, and Emerging Therapies. Cancers 2025, 17, 2577. https://doi.org/10.3390/cancers17152577
Shakerdi AL, Finnegan E, Sheng Y-Y, Pidgeon GP. The Multifaceted Role of Autophagy in Nasopharyngeal Carcinoma: Translational Perspectives on Pathogenesis, Biomarkers, Treatment Resistance, and Emerging Therapies. Cancers. 2025; 17(15):2577. https://doi.org/10.3390/cancers17152577
Chicago/Turabian StyleShakerdi, Abdul L., Emma Finnegan, Yin-Yin Sheng, and Graham P. Pidgeon. 2025. "The Multifaceted Role of Autophagy in Nasopharyngeal Carcinoma: Translational Perspectives on Pathogenesis, Biomarkers, Treatment Resistance, and Emerging Therapies" Cancers 17, no. 15: 2577. https://doi.org/10.3390/cancers17152577
APA StyleShakerdi, A. L., Finnegan, E., Sheng, Y.-Y., & Pidgeon, G. P. (2025). The Multifaceted Role of Autophagy in Nasopharyngeal Carcinoma: Translational Perspectives on Pathogenesis, Biomarkers, Treatment Resistance, and Emerging Therapies. Cancers, 17(15), 2577. https://doi.org/10.3390/cancers17152577







