Application of Artificial Intelligence in Pancreatic Cyst Management: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
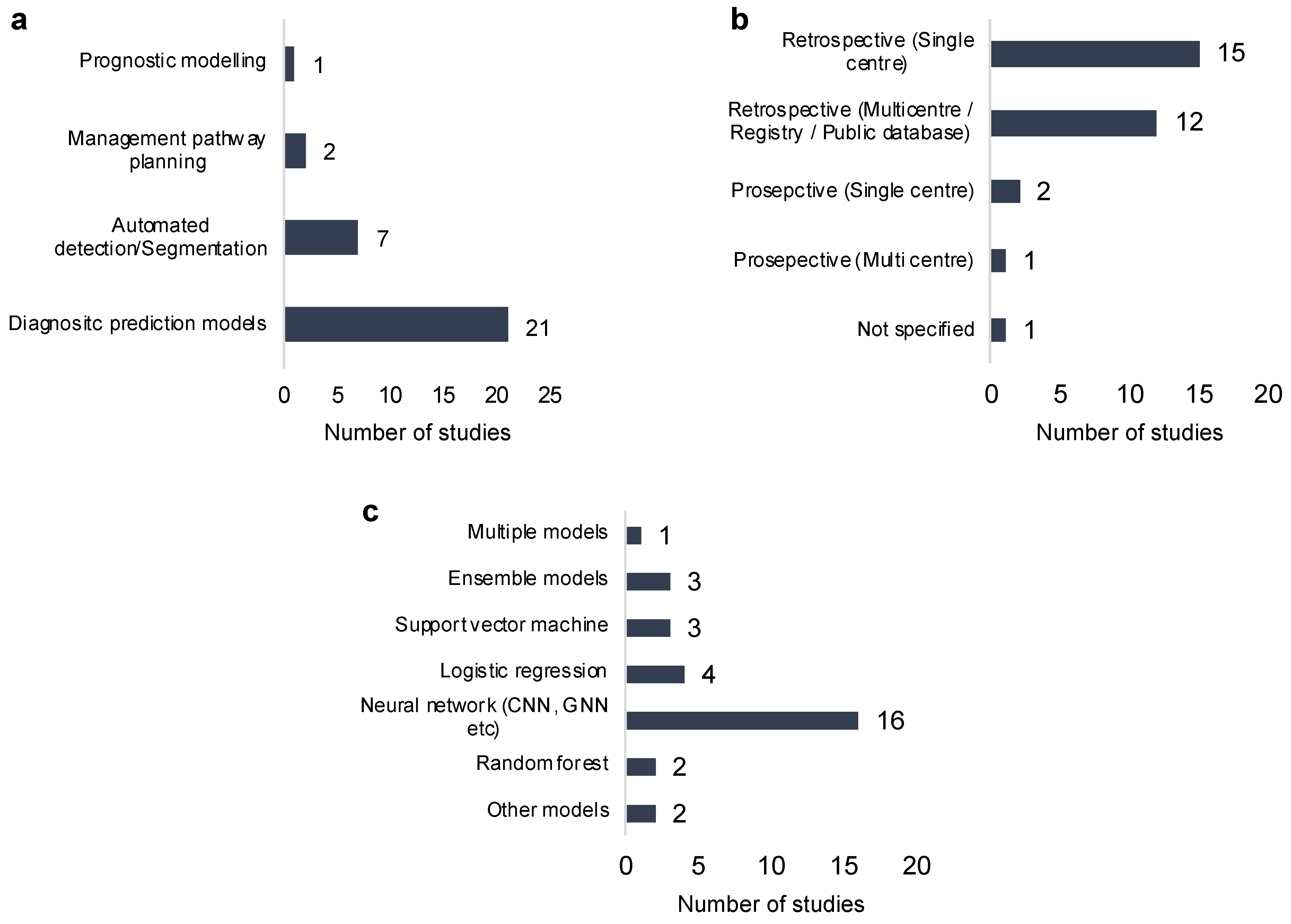
3. Results
3.1. Machine Learning Methods
3.2. Diagnosis and Subtyping
3.3. Management Support Models
3.4. Prognostic Models
3.5. Risk of Bias Assessment
3.6. High Quality Studies
4. Discussion
4.1. Clinical Applicability and Integration
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Author | Category | Type | Data Source | AI Model | Country | Imaging Method | Number of Patients | Parameter Used | Endpoint | Performance | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang et al. (2022) [26] | Preoperative diagnosis | Retrospective | Multicentre | Ensemble | China | CT | n = 363 | Radiological | Differentiating benign vs. malignant cysts | AUC = 0.91, Accuracy = 0.84, Sensitivity = 0.96, Specificity = 0.68 |
|
| Deng et al. (2024) [33] | Preoperative diagnosis | Retrospective | Multicentre | Logistic Regression | China | CT | n = 388 | Radiological and Clinical (sex, age, jaundice, pancreatitis, CEA and CA19-9 levels) | Differentiating benign vs. malignant cysts | AUC = 0.948, Accuracy = 0.900, Sensitivity = 0.963, Specificity = 0.826 |
|
| Saraiva et al. (2024) [62] | Preoperative Diagnosis | Retrospective | Multicentre | CNN | Portugal, USA | EUS | n = 378, EUS images = 126,000 | Endoscopic EUS images | Classification of PCLs and PSL | Accuracy = 99%, Sensitivity = 98.9%, Specificity = 99.1%, |
|
| Watson et al. (2021) [34] | Preoperative Diagnosis | Retrospective | Single centre | CNN | USA | CT | n = 27 | Radiological | Differentiating benign vs. malignant cysts | Accuracy = 88.9%, AUC = N/A |
|
| Author | Category | Type | Data Source | AI Model | Country | Imaging Method | Number of Patients | Parameter Used | Endpoint | Performance | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Awe et al., 2021 [60] | Preoperative diagnosis | Retrospective | Single centre | Ensemble | USA | CT | n = 99 | Radiological, clinical, and radiomics | To differentiate mucinous from non-mucinous pancreatic cysts | AUC = 0.73 Accuracy = 0.74 Sensitivity = 0.77 Specificity = 0.61 |
|
| Liang et al., 2022 [63] | Preoperative diagnosis | Retrospective | Single centre | Logistic regression | China | CT | n = 193 | Radiological | To differentiate between SCN, MCN, and IPMN | AUC = 0.973 Accuracy = 0.92 Sensitivity = 0.86 Specificity = 1 |
|
| Zhang et al., 2022 [64] | Preoperative diagnosis | Retrospective | Single centre | CNN/GNN | China | CT | n = 263 | Radiological | To differentiate between SCN, MCN, SPN, and IPMN | AUC = 0.856 Accuracy = 0.74 |
|
| Chu et al., 2022 [27] | Preoperative diagnosis | Retrospective | Single centre | Random forest | USA | CT | n = 214 | Radiomics | To classify cysts: IPMNs, MCNs, SCAs, SPNs, and cystic PNETs | AUC = 0.94 Accuracy = 0.94 Sensitivity = 0.94 Specificity = 0.93 |
|
| Tian et al., 2024 [19] | Preoperative diagnosis | Retrospective | Single centre | CNN | China | MRI | n = 314 | Radiomics and clinical | To differentiate between SCN and MCN | AUC = 0.971 Accuracy = 0.92 Specificity = 0.93 |
|
| Chen et al., 2021 [20] | Preoperative diagnosis | Retrospective | Multicentre | Logistic regression | China | CT contrast | n = 128 | Radiological and clinical | To differentiate between SCNs and MCNs | AUC = 0.88 Sensitivity = 0.99 Specificity = 0.84 |
|
| Vilas-Boas et al., 2022 [65] | Preoperative diagnosis | Prospective | Single centre | CNN | Portugal | EUS | n = 5505 | Radiological (EUS image) | To differentiate MCN from non-MCNs | AUC = 1.0 Accuracy = 0.99 Sensitivity = 0.98 Specificity = 0.99 |
|
| Wei et al., 2019 [28] | Preoperative diagnosis | Retrospective | Single centre | SVM | China | CT | n = 214 | Radiomics and clinical | To differentiate SCN from other cystic neoplasms | AUC = 0.77 Sensitivity = 0.69 Specificity = 0.71 |
|
| Yang et al., 2019 [59] | Preoperative diagnosis | Retrospective | Single centre | Random forest | China | MRI | n = 314 | Radiomics | To differentiate SCN and MCN | AUC = 0.75 Accuracy = 0.83 Sensitivity = 0.85 Specificity = 0.83 |
|
| Author | Category | Type | Data Source | AI Model | Country | Imaging Method | Number of Patients | Parameter Used | Endpoint | Performance | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hernandez-Barco et al., 2023 [35] | Preoperative diagnosis | Prospective | Single centre | Linear support vector machine (SVM) | USA | - | n = 575 | Clinical | To classify IPMN | AUC = 0.82 Accuracy = 77 Sensitivity = 83 Specificity = 72 |
|
| Kiritani et al., 2023 [36] | Preoperative diagnosis | Prospective | Multicentre | SVM | Japan, Helsinki | EUS/ERCP | n = 49 | 863 peak intensities obtained from the PESI-MS analysis | To classify IPMN | AUC = 0.924 Sensitivity = 0.88 Specificity = 0.88 Accuracy = 0.88 |
|
| Salanitri et al., 2022 [66] | Preoperative diagnosis | Retrospective | Multicentre | Vision transformers (neural network) | USA, Italy | MRI | n = 139 | Imagistic parameter | To classify IPMN | Accuracy = 0.70 Precision = 0.67 Recall = 0.64 |
|
| Machicado et al., 2021 [37] | Preoperative diagnosis | Prospective | Single centre | CNNs | USA | EUS-nCLE | n = 35 | Histology | To risk stratify IPMN | Accuracy = 0.86 Sensitivity = 0.83 Specificity = 0.88 |
|
| Dominik Schulz et al., 2022 [38] | Preoperative diagnosis | Retrospective/Prospective (7 patients for testing recruited prospectively) | Single centre | CNN | Germany | EUS | n = 70 | EUS images | To classify IPMN | Accuracy = 0.99 Sensitivity = 1 Specificity = 0.97 |
|
| Sijia Cui et al., 2021 [42] | Preoperative diagnosis | Retrospective | Multicentre; China 3 hospitals | Logistic regression (LASSO-based feature selection) | China | MRI and CET images | n = 202 | Radiomics | To classify BD-IPMN | AUC = 0.884 Sensitivity = 0.9 Specificity = 0.79 |
|
| Jae Seung Kang et al., 2020 [39] | Preoperative diagnosis | Retrospective cohort study | Multicentre; international | AutoML package | Korea | CT, MRI, EUS | n = 3708 | Clinical and radiological | Differentiation between benign and malignant IPMNs | AUC = 0.73 |
|
| Takamichi Kuwahara et al., 2019 [29] | Preoperative diagnosis | Retrospective | Single centre | CNN | Japan | EUS | 50 patients | EUS images and clinical | Prediction of malignancy in IPMNs | AUC = 0.98 Sensitivity = 0.95 Specificity = 0.92 Accuracy = 0.94 |
|
| Title | Category | Type | Data Source | AI Model | Country | Imaging Method | Number of Patients | Parameters Used | Endpoint | Performance | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oh et al., 2021 [43] | Preoperative diagnosis | Retrospective | Single centre | CNNs | Korea | EUS | n = 111 | Manual segmentation | Automatic segmentation of pancreatic cyst lesions (PCLs) on endoscopic ultrasonography (EUS) images | Accuracy = 0.972 Specificity = 0.989 Sensitivity = 0.723 |
|
| Park et al., 2022 [30] | Preoperative diagnosis | Retrospective | Multicentre | CNNs | Korea | CT contrast | n = 2044 | Manual segmentation | To identify patients with various solid and cystic pancreatic neoplasms | AUC = 0.87 Sensitivity = 83.3 Specificity = 82.7 Accuracy = 82.9 |
|
| Abi Nader et al., 2023 [67] | Preoperative diagnosis | Retrospective | Europe, USA, and Brazil | CNNs | France | CT | n = 2890 | Radiological | To detect the presence of pancreatic lesions and identify main pancreatic duct dilatation with high accuracy | IPMN AUC = 0.98 Sensitivity = 0.94 Specificity = 0.95 MPD AUC = 0.97 Sensitivity = 0.94 Specificity = 0.90 |
|
| Kooragayala et al., 2022 [68] | Preoperative diagnosis | Retrospective | Single centre | Natural language processing (NLP) | USA | CT | n = 18,769 | Radiological | Identification of potentially concerning pancreatic lesions | Sensitivity = 0.33 Specificity = 0.99 PPV = 0.25 NPV = 0.99 |
|
| Konikoff et al., 2024 [69] | Preoperative Diagnosis | Retrospective | Single centre | CNN | Israel | EUS | n = 1497 | EUS images | Real-time AI-based detection and segmentation of pancreatic lesions on EUS | Accuracy = 0.93 AUC = 0.89 Sensitivity = 0.48 Specificity = 0.98 |
|
| Duh et al., 2023 [31] | Preoperative Diagnosis | Retrospective | Single Centre | CNN | Spain | CT | n = 335 | Manual segmentation | Automated detection of pancreatic cystic lesions (PCLs) on CT scans | Sensitivity = 0.93 Specificity = 0.82 |
|
| Abel et al., 2021 [32] | Preoperative diagnosis | Retrospective | Single centre | CNN | Switzerland | CT | n = 543 | Radiological | Detection of pancreatic cystic lesions using deep learning | Sensitivity = 0.87 |
|
| Title | Category | Type | Data Source | AI Model | Country | Imaging Method | Number of Patients | Parameters Used | Endpoint | Performance | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferres et al., 2024 [23] | Preoperative diagnosis | Retrospective | USA, Europe and Asia | Ensemble | USA | - | n = 850 | Clinical | Stratification into surgery, surveillance, or discharge | Discharge = 0.93 Surveillance = 0.84 Surgery = 0.83 |
|
| Springer et al., 2019 [24] | Preoperative diagnosis | Retrospective | USA, Europe, and Asia | Supervised model | USA | CT, MRI, EUS | n = 862 | Molecular, clinical, and radiological | Management of pancreatic cysts | Sensitivity = 0.9 Specificity = 0.54 |
|
| Aronsson et al., 2021 [25] | Prognosis evaluation | Retrospective | USA | ANN, LASSO | Sweden | - | n = 440 | Clinical | Prediction of 5-year disease-specific survival (DSS) after surgical treatment | ANN model Accuracy = 0.81 Precision = 0.85 Specificity = 0.52 Lasso Accuracy = 0.80 Precision = 0.85 Specificity = 0.52 |
|
References
- Zhang, H.; Qie, Y. Applying Deep Learning to Medical Imaging: A Review. Appl. Sci. 2023, 13, 10521. [Google Scholar] [CrossRef]
- Khosravi, M.; Zare, Z.; Mojtabaeian, S.M.; Izadi, R. Artificial Intelligence and Decision-Making in Healthcare: A Thematic Analysis of a Systematic Review of Reviews. Health Serv. Res. Manag. Epidemiol. 2024, 11, 23333928241234863. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Javaheri, H.; Ghamarnejad, O.; Widyaningsih, R.; Bade, R.; Lukowicz, P.; Karolus, J.; Stavrou, G.A. Enhancing Perioperative Outcomes of Pancreatic Surgery with Wearable Augmented Reality Assistance System: A Matched-Pair Analysis. Ann. Surg. Open 2024, 5, e516. [Google Scholar] [CrossRef]
- Schlanger, D.; Graur, F.; Popa, C.; Mois, E.; Al Hajjar, N. The role of artificial intelligence in pancreatic surgery: A systematic review. Updates Surg. 2022, 74, 417–429. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Kouzahkanan, Z.M.; Radmard, A.R.; Kafieh, R.; Salehnia, A.; Davarpanah, A.H.; Arabalibeik, H.; Ahmadian, A. Segmentation of pancreatic ductal adenocarcinoma (PDAC) and surrounding vessels in CT images using deep convolutional neural networks and texture descriptors. Sci. Rep. 2022, 12, 3092–3099. [Google Scholar] [CrossRef]
- Assawasirisin, C.; Qadan, M.; Aimprasittichai, S.; Kambadakone, A.; Servin-Rojas, M.; Warshaw, A.L.; Lillemoe, K.D.; Fernández-Del Castillo, C. Pancreatic Serous Cystadenoma: A Continuing Diagnostic Challenge. Ann. Surg. 2025, 281, 501–507. [Google Scholar] [CrossRef]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 585–593.e3. [Google Scholar] [CrossRef]
- Gonda, T.A.; Cahen, D.L.; Farrell, J.J. Pancreatic Cysts. N. Engl. J. Med. 2024, 391, 832–843. [Google Scholar] [CrossRef]
- Scholten, L.; van Huijgevoort, N.C.M.; van Hooft, J.E.; Besselink, M.G.; Del Chiaro, M. Pancreatic Cystic Neoplasms: Different Types, Different Management, New Guidelines. Visc. Med. 2018, 34, 173–177. [Google Scholar] [CrossRef]
- Gardner, T.B.; Park, W.G.; Allen, P.J. Diagnosis and Management of Pancreatic Cysts. Gastroenterology 2024, 167, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines, C.; American Gastroenterology, A. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822; quize12-3. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatol. Off. J. Int. Assoc. Pancreatol. (IAP) 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Li, Z.; Miao, H. Accuracy of Fukuoka and American Gastroenterological Association Guidelines for Predicting Advanced Neoplasia in Pancreatic Cyst Neoplasm: A Meta-Analysis. Ann. Surg. Oncol. 2019, 26, 4522–4536. [Google Scholar] [CrossRef]
- Mohapatra, S.; Krishna, S.G.; Pannala, R. Pancreatic Cystic Neoplasms: Translating Guidelines into Clinical Practice. Diagnostics 2023, 13, 749. [Google Scholar] [CrossRef]
- Lekkerkerker, S.J.; Besselink, M.G.; Busch, O.R.; Verheij, J.; Engelbrecht, M.R.; Rauws, E.A.; Fockens, P.; van Hooft, J.E. Comparing 3 guidelines on the management of surgically removed pancreatic cysts with regard to pathological outcome. Gastrointest. Endosc. 2017, 85, 1025–1031. [Google Scholar] [CrossRef]
- Lobo, J.M.; Scheiman, J.M.; Zaydfudim, V.M.; Shami, V.M.; Sauer, B.G. Clinical and Economic Outcomes of Patients Undergoing Guideline-Directed Management of Pancreatic Cysts. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 1689–1697. [Google Scholar] [CrossRef]
- Khaled, Y.S.; Mohsin, M.; Fatania, K.; Yee, A.; Adair, R.; Sheridan, M.; Macutkiewicz, C.; Aldouri, A.; Smith, A.M. Outcome of long interval radiological surveillance of side branch pancreatic duct-involved intraductal papillary mucinous neoplasm in selected patients. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2016, 18, 879–885. [Google Scholar] [CrossRef][Green Version]
- Tian, H.; Zhang, B.; Zhang, Z.; Xu, Z.; Jin, L.; Bian, Y.; Wu, J. DenseNet model incorporating hybrid attention mechanisms and clinical features for pancreatic cystic tumor classification. J. Appl. Clin. Med. Phys. 2024, 25, e14380. [Google Scholar] [CrossRef]
- Chen, H.Y.; Deng, X.Y.; Pan, Y.; Chen, J.Y.; Liu, Y.Y.; Chen, W.J.; Yang, H.; Zheng, Y.; Yang, Y.B.; Liu, C.; et al. Pancreatic Serous Cystic Neoplasms and Mucinous Cystic Neoplasms: Differential Diagnosis by Combining Imaging Features and Enhanced CT Texture Analysis. Front. Media SA 2021, 11, 745001. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lavista Ferres, J.M.; Oviedo, F.; Robinson, C.; Chu, L.; Kawamoto, S.; Afghani, E.; He, J.; Klein, A.P.; Goggins, M.; Wolfgang, C.L.; et al. Performance of explainable artificial intelligence in guiding the management of patients with a pancreatic cyst. Pancreatology 2024, 24, 1182. [Google Scholar] [CrossRef]
- Springer, S.; Masica, D.L.; Dal Molin, M.; Douville, C.; Thoburn, C.J.; Afsari, B.; Li, L.; Cohen, J.D.; Thompson, E.; Allen, P.J.; et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci. Transl. Med. 2019, 11, eaav4772. [Google Scholar] [CrossRef]
- Aronsson, L.; Andersson, R.; Ansari, D. Artificial neural networks versus LASSO regression for the prediction of long-term survival after surgery for invasive IPMN of the pancreas. PLoS ONE 2021, 16, e0249206. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Xue, H.; Qu, T.; Cheng, S.; Li, J.; Li, Y.; Mao, L.; Li, X.; Zhu, L.; et al. A deep learning algorithm to improve readers’ interpretation and speed of pancreatic cystic lesions on dual-phase enhanced CT. Abdom. Radiol. 2022, 47, 2135. [Google Scholar] [CrossRef]
- Chu, L.C.; Park, S.; Soleimani, S.; Fouladi, D.F.; Shayesteh, S.; He, J.; Javed, A.A.; Wolfgang, C.L.; Vogelstein, B.; Kinzler, K.W.; et al. Classification of pancreatic cystic neoplasms using radiomic feature analysis is equivalent to an experienced academic radiologist: A step toward computer-augmented diagnostics for radiologists. Abdom. Radiol. 2022, 47, 4139. [Google Scholar] [CrossRef]
- Wei, R.; Lin, K.; Yan, W.; Guo, Y.; Wang, Y.; Li, J.; Zhu, J. Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol. Cancer Res. Treat. 2019, 18, 1533033818824339. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Okuno, N.; Matsumoto, S.; Obata, M.; Kurita, Y.; Koda, H.; Toriyama, K.; Onishi, S.; et al. Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clin. Transl. Gastroenterol. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, K.; You, M.W.; Kyung, S.G.; Kim, S.Y.; Park, S.H.; Byun, J.H.; Kim, N.; Kim, H.J. Deep Learning–based Detection of Solid and Cystic Pancreatic Neoplasms at Contrast-enhanced CT. Radiol. 2023, 306, 140. [Google Scholar] [CrossRef]
- Duh, M.M.; Torra-Ferrer, N.; Riera-Marín, M.; Cumelles, D.; Rodríguez-Comas, J.; García López, J.; Fernández Planas, M.T. Deep Learning to Detect Pancreatic Cystic Lesions on Abdominal Computed Tomography Scans: Development and Validation Study. JMIR AI 2023, 2, e40702. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.; Wasserth, J.; Weikert, T.; Sauter, A.W.; Nesic, I.; Obradovic, M.; Mehrabi, A.; Kiefer, S.; Attenberger, U.I.; Zhou, S.; et al. Automated Detection of Pancreatic Cystic Lesions on CT Using Deep Learning. Diagnostics 2021, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Liu, J.; Wang, X.; Xie, F.; Wang, S.; Zhang, X.; Mao, L.; Li, X.; Hu, Y.; Jin, Z.; et al. Should All Pancreatic Cystic Lesions with Worrisome or High-Risk Features Be Resected? A Clinical and Radiological Machine Learning Model May Help to Answer. Acad. Radiol. 2024, 31, 1889. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.D.; Lyman, W.B.; Passeri, M.J.; Murphy, K.J.; Sarantou, J.P.; Iannitti, D.A.; Martinie, J.B.; Vrochides, D.; Baker, E.H. Use of Artificial Intelligence Deep Learning to Determine the Malignant Potential of Pancreatic Cystic Neoplasms With Preoperative Computed Tomography Imaging. Am. Surg. 2021, 87, 602–607. [Google Scholar] [CrossRef]
- Hernandez-Barco, Y.G.; Daye, D.; Fernandez-Del Castillo, C.F.; Parker, R.F.; Casey, B.W.; Warshaw, A.L.; Ferrone, C.R.; Lillemoe, K.D.; Qadan, M. IPMN-LEARN: A linear support vector machine learning model for predicting low-grade intraductal papillary mucinous neoplasms. Ann. Hepato-Biliary-Pancreat. Surg. 2023, 27, 195. [Google Scholar] [CrossRef]
- Kiritani, S.; Iwano, T.; Yoshimura, K.; Saito, R.; Nakayama, T.; Yamamoto, D.; Hakoda, H.; Watanabe, G.; Akamatsu, N.; Arita, J.; et al. New Diagnostic Modality Combining Mass Spectrometry and Machine Learning for the Discrimination of Malignant Intraductal Papillary Mucinous Neoplasms. Ann. Surg. Oncol. 2023, 30, 3150. [Google Scholar] [CrossRef]
- Achicado, J.D.; Chao, W.L.; Carlyn, D.E.; Pan, T.Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest. Endosc. 2012, 94, 78–87.e2. [Google Scholar] [CrossRef]
- Schulz, D.; Heilmaier, M.; Phillip, V.; Treiber, M.; Mayr, U.; Lahmer, T.; Mueller, J.; Demir, I.E.; Friess, H.; Reichert, M.; et al. Accurate prediction of histological grading of intraductal papillary mucinous neoplasia using deep learning. Endoscopy 2023, 55, 415–422. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, C.; Song, W.; Choo, W.; Lee, S.; Lee, S.; Han, Y.; Bassi, C.; Salvia, R.; Marchegiani, G.; et al. Risk prediction for malignant intraductal papillary mucinous neoplasm of the pancreas: Logistic regression versus machine learning. Sci. Rep. 2020, 10, 20140. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Tang, T.; Su, Q.; Wang, Y.; Shu, Z.; Yang, W.; Gong, X. Radiomic nomogram based on MRI to predict grade of branching type intraductal papillary mucinous neoplasms of the pancreas: A multicenter study. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2021, 21, 26. [Google Scholar] [CrossRef]
- Oh, S.; Kim, Y.-J.; Park, Y.-T.; Kim, K.-G. Automatic Pancreatic Cyst Lesion Segmentation on EUS Images Using a Deep-Learning Approach. Sensors 2021, 22, 245. [Google Scholar] [CrossRef]
- Ramspek, C.L.; Jager, K.J.; Dekker, F.W.; Zoccali, C.; van Diepen, M. External validation of prognostic models: What, why, how, when and where? Clin. Kidney J. 2020, 14, 49–58. [Google Scholar] [CrossRef]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef]
- van Huijgevoort, N.C.M.; Del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Byun, J.H.; Kim, J.H.; Lee, S.S.; Kim, H.J.; Lee, M.G. Differentiation of Common Pancreatic Cystic Neoplasms Based Upon Multiplicity of CystsNo title. J. Korean Soc. Radiol. 2015, 72, 1–10. [Google Scholar] [CrossRef]
- Lee, L.S. Updates in diagnosis and management of pancreatic cysts. World J. Gastroenterol. 2021, 27, 5700–5714. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Ben, Q.W.; Jin, Z.D.; Du, Y.Q.; Zou, D.W.; Liao, Z.; Li, Z.S. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest. Endosc. 2011, 73, 283–290. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, C.J.; Kolb, J.M.; Benias, P.C.; Shah, H.; Shah, S.; Haluszka, O.; Maranki, J.; Sharzehi, K.; Lam, E.; Gordon, S.R.; et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): Results of a large North American multicenter study. Endosc. Int. Open 2016, 4, 974. [Google Scholar] [CrossRef]
- Gao, R.Y.; Wu, B.H.; Shen, X.Y.; Peng, T.L.; Li, D.F.; Wei, C.; Yu, Z.C.; Luo, M.H.; Xiong, F.; Wang, L.S.; et al. Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition. World J. Gastroenterol. 2020, 26, 6182–6194. [Google Scholar] [CrossRef]
- Woolf, K.M.W.; Liang, H.; Sletten, Z.J.; Russell, D.K.; Bonfiglio, T.A.; Zhou, Z. False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard. Cancer Cytopathol. 2013, 121, 449–458. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- McKinney, S.M.; Sieniek, M.; Godbole, V.; Godwin, J.; Antropova, N.; Ashrafian, H.; Back, T.; Chesus, M.; Corrado, G.S.; Darzi, A.; et al. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Yang, J.; Guo, X.; Ou, X.; Zhang, W.; Ma, X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front. Oncol. 2019, 9, 494. [Google Scholar] [CrossRef]
- Awe, A.M.; Vanden Heuvel, M.M.; Yuan, T.; Rendell, V.R.; Shen, M.; Kampani, A.; Liang, S.; Morgan, D.D.; Winslow, E.R.; Lubner, M.G. Machine learning principles applied to CT radiomics to predict mucinous pancreatic cysts. Abdom. Radiol. 2021, 47, 221. [Google Scholar] [CrossRef]
- De Robertis, R.; Todesco, M.; Autelitano, D.; Spoto, F.; D’Onofrio, M. The role of radiomics in hepato-bilio-pancreatic surgery: A literature review. Artif. Intell. Surg. 2023, 3, 166–179. [Google Scholar] [CrossRef]
- Saraiva, M.M.; González-Haba, M.; Widmer, J.; Mendes, F.; Gonda, T.; Agudo, B.; Ribeiro, T.; Costa, A.; Fazel, Y.; Lera, M.E.; et al. Deep Learning and Automatic Differentiation of Pancreatic Lesions in Endoscopic Ultrasound: A Transatlantic Study. Clin. Transl. Gastroenterol. 2024, 15, e00771. [Google Scholar] [CrossRef]
- Liang, W.; Tian, W.; Wang, Y.; Wang, P.; Wang, Y.; Zhang, H.; Ruan, S.; Shao, J.; Zhang, X.; Huang, D.; et al. Classification prediction of pancreatic cystic neoplasms based on radiomics deep learning models. BMC Cancer 2022, 22, 1237. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, Y.; Li, J.; Li, Y.; Luo, J. A metric learning-based method using graph neural network for pancreatic cystic neoplasm classification from CTs. Med. Phys. 2022, 49, 5523. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, F.; Ribeiro, T.; Afonso, J.; Cardoso, H.; Lopes, S.; Moutinho-Ribeiro, P.; Ferreira, J.; Mascarenhas-Saraiva, M.; Macedo, G. Deep Learning for Automatic Differentiation of Mucinous versus Non-Mucinous Pancreatic Cystic Lesions: A Pilot Study. Diagnostics 2022, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Salanitri, F.P., Bellitto, G., Palazzo, S., Irmakci, I., Wallace, M., Bolan, C., Engels, M., Hoogenboom, S., Aldinucci, M., Bagci, U., Eds.; Neural Transformers for Intraductal Papillary Mucosal Neoplasms (IPMN) Classification in MRI images. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022. [Google Scholar]
- Nader, C.A.; Vetil, R.M.; Wood, L.K.; Rohe, M.-M.; Bône, A.; Karteszi, H.M.; Vullierme, M.-P. Automatic Detection of Pancreatic Lesions and Main Pancreatic Duct Dilatation on Portal Venous CT Scans Using Deep Learning. Investig. Radiol. 2023, 58, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Kooragayala, K.; Crudeli, C.; Kalola, A.; Bhat, V.; Lou, J.; Sensenig, R.; Atabek, U.; Echeverria, K.; Hong, Y. Utilization of Natural Language Processing Software to Identify Worrisome Pancreatic Lesions. Ann. Surg. Oncol. 2022, 29, 8513. [Google Scholar] [CrossRef]
- Konikoff, T.; Loebl, N.; Benson, A.A.; Green, O.; Sandler, H.; Gingold-Belfer, R.; Levi, Z.; Perl, L.; Dotan, I.; Shamah, S. Enhancing detection of various pancreatic lesions on endoscopic ultrasound through artificial intelligence: A basis for computer-aided detection systems. J. Gastroenterol. Hepatol. 2025, 40, 235–240. [Google Scholar] [CrossRef]




| Author (Year) | AI Model | Sample Size | Parameters | Clinical Focus | Performance | Compared to Guidelines/Clinicians | Limitations |
|---|---|---|---|---|---|---|---|
| Wang et al. (2022) [26] | Ensemble | 363 | CT images | Benign vs. malignant PCLs | AUC = 0.91, Acc = 0.84, Sens = 0.96, Spec = 0.68 | Performance similar to senior radiologist, but better than juniors | Retrospective |
| Deng et al. (2024) [33] | LR | 388 | CT images and clinical | Benign vs. malignant PCLs | AUC = 0.95, Acc = 0.90, Sens = 0.96, Spec = 0.83 | Performance better than ACG and European guidelines | Retrospective |
| Watson et al. (2021) [34] | CNN | 27 | CT images | Benign vs. malignant PCLs | Acc = 0.89 | Performance better than Fukuoka guideline, reducing unnecessary surgeries | Small sample size; no AUC data; retrospective |
| Schulz et al. (2022) [38] | CNN | 70 | EUS images | IPMN grading | Acc = 0.99, Sens = 1, Spec = 0.99 | Outperformed existing guidelines | Small prospective cohort (7/70) |
| Cui et al. (2021) [42] | LR (LASSO) | 202 | CT/MRI radiomics | BD-IPMN grading | AUC = 0.88, Sens = 0.90, Spec = 0.79 | Not specified | Retrospective; moderate sample size |
| Oh et al. (2021) [43] | CNN | 111 | EUS images | Segmentation of PCLs | Acc = 0.97, Sens = 0.72, Spec = 0.99 | Comparable to human readers/interpretation | Lower sensitivity; requires manual segmentation |
| Park et al. (2022) [30] | CNN | 2044 | CT (noncontrast) | Cystic vs. solid lesions | AUC = 0.87–0.91, Acc = 0.83–0.86 | Comparable to radiologists if the lesion size is 1.0 cm or higher | Retrospective; performance varies by lesion size |
| Springer et al. (2019) [24] | Supervised | 862 | CT, MRI, EUS images | Management decision support | Acc = 0.69, Sens = 0.91, Spec = 0.54 | Higher accuracy compared to local standard of care accuracy | Retrospective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Jesry, F.; Maliekkal, J.J.; Goulder, L.; Huntly, B.; Smith, A.M.; Khaled, Y.S. Application of Artificial Intelligence in Pancreatic Cyst Management: A Systematic Review. Cancers 2025, 17, 2558. https://doi.org/10.3390/cancers17152558
Lee D, Jesry F, Maliekkal JJ, Goulder L, Huntly B, Smith AM, Khaled YS. Application of Artificial Intelligence in Pancreatic Cyst Management: A Systematic Review. Cancers. 2025; 17(15):2558. https://doi.org/10.3390/cancers17152558
Chicago/Turabian StyleLee, Donghyun, Fadel Jesry, John J. Maliekkal, Lewis Goulder, Benjamin Huntly, Andrew M. Smith, and Yazan S. Khaled. 2025. "Application of Artificial Intelligence in Pancreatic Cyst Management: A Systematic Review" Cancers 17, no. 15: 2558. https://doi.org/10.3390/cancers17152558
APA StyleLee, D., Jesry, F., Maliekkal, J. J., Goulder, L., Huntly, B., Smith, A. M., & Khaled, Y. S. (2025). Application of Artificial Intelligence in Pancreatic Cyst Management: A Systematic Review. Cancers, 17(15), 2558. https://doi.org/10.3390/cancers17152558






