Impact of Preoperative Yttrium-90 Transarterial Radioembolization on Patients Undergoing Right or Extended Right Hepatectomy for Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
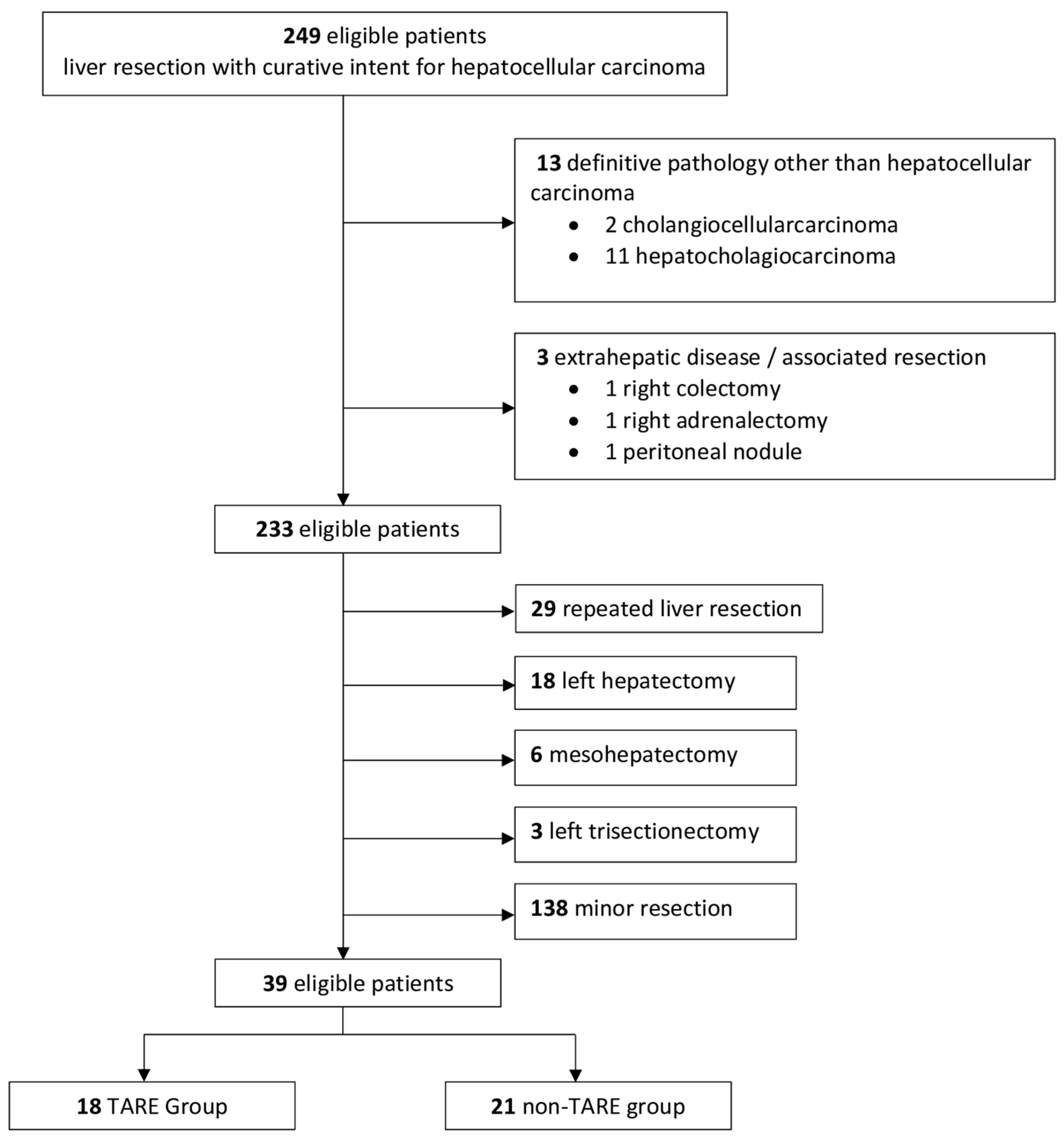
2.1. Patient Selection
2.2. Diagnosis and Preoperative Staging
2.3. TARE Protocol and Technique
2.4. Surgical Technique
2.5. Intra- and Postoperative Outcomes
2.6. Follow-Up
2.7. Definitions of Long-Term Outcomes
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Intraoperative Outcomes
3.3. Postoperative Outcomes and Histological Findings
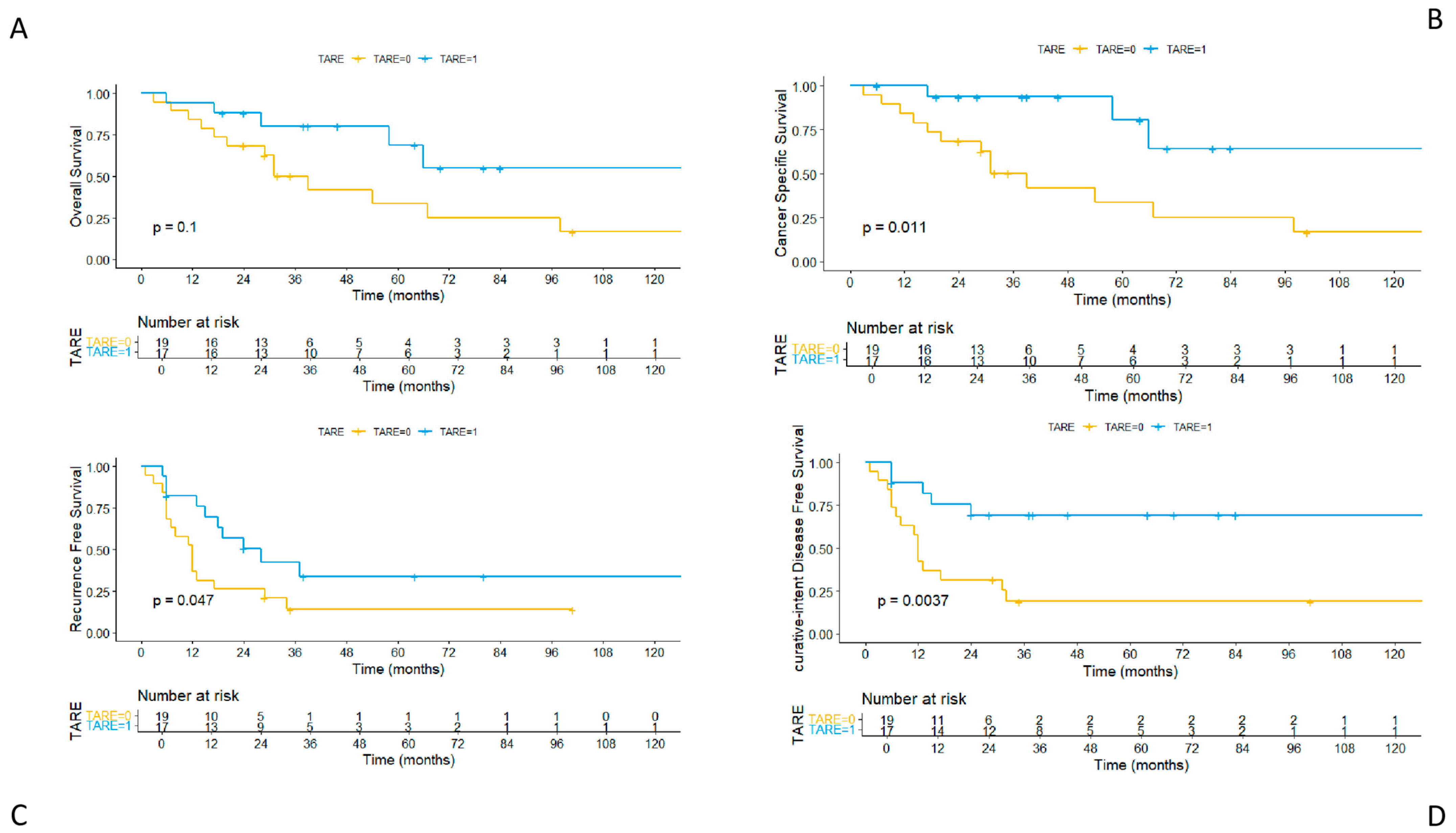
3.4. Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TARE | Yttrium-90 transarterial radioembolization |
| FLR | Future liver remnant |
| PVTT | Portal vein tumor thrombosis |
| OS | Overall survival |
| CSS | Cancer-specific survival |
| RFS | Recurrence-free survival |
| ciDFS | Curative-intent disease-free survival |
| AFP | Alpha-fetoprotein |
| PHLF | Post-hepatectomy liver failure |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the Management of Hepatocellular Carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Taddei, T.H.; Brown, D.B.; Yarchoan, M.; Mendiratta-Lala, M.; Llovet, J.M. Critical Update: AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2025, 82, 272–274. [Google Scholar] [CrossRef]
- Daher, D.; Seif El Dahan, K.; Cano, A.; Gonzales, M.; Ransom, C.; Jaurez, E.; Carranza, O.; Quirk, L.; Morgan, T.; Gopal, P.; et al. Hepatocellular Carcinoma Surveillance Patterns and Outcomes in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2024, 22, 295–304.e2. [Google Scholar] [CrossRef]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Valery, P.C.; Laversanne, M.; Clark, P.J.; Petrick, J.L.; McGlynn, K.A.; Bray, F. Projections of Primary Liver Cancer to 2030 in 30 Countries Worldwide. Hepatology 2018, 67, 600–611. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Rimassa, L.; Chan, S.L.; Sangro, B.; Lau, G.; Kudo, M.; Reig, M.; Breder, V.; Ryu, M.-H.; Ostapenko, Y.; Sukeepaisarnjaroen, W.; et al. Five-Year Overall Survival Update from the HIMALAYA Study of Tremelimumab plus Durvalumab in Unresectable HCC. J. Hepatol. 2025, 82, 1257–1265. [Google Scholar] [CrossRef]
- Vitale, A.; Romano, P.; Cillo, U.; Writing Group for the HE.RC.O.LE.S Collaborative Group; Writing Group for the ITA.LI.CA Collaborative Group; HE.RC.O.LE.S and ITA.LI.CA Collaborative Groups; Lauterio, A.; Sangiovanni, A.; Cabibbo, G.; Missale, G.; et al. Liver Resection vs Nonsurgical Treatments for Patients With Early Multinodular Hepatocellular Carcinoma. JAMA Surg. 2024, 159, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Seyama, Y.; Kokudo, N.; Maema, A.; Sugawara, Y.; Sano, K.; Takayama, T.; Makuuchi, M. One Thousand Fifty-Six Hepatectomies without Mortality in 8 Years. Arch. Surg. 2003, 138, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Kenjo, A.; Miyata, H.; Gotoh, M.; Kitagawa, Y.; Shimada, M.; Baba, H.; Tomita, N.; Kimura, W.; Sugihara, K.; Mori, M. Risk Stratification of 7,732 Hepatectomy Cases in 2011 from the National Clinical Database for Japan. J. Am. Coll. Surg. 2014, 218, 412–422. [Google Scholar] [CrossRef]
- Zaydfudim, V.M.; Kerwin, M.J.; Turrentine, F.E.; Bauer, T.W.; Adams, R.B.; Stukenborg, G.J. The Impact of Chronic Liver Disease on the Risk Assessment of ACS NSQIP Morbidity and Mortality after Hepatic Resection. Surgery 2016, 159, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Cabibbo, G.; Iavarone, M.; Viganò, L.; Pinato, D.J.; Ponziani, F.R.; Lai, Q.; Casadei-Gardini, A.; Celsa, C.; Galati, G.; et al. Personalised Management of Patients with Hepatocellular Carcinoma: A Multiparametric Therapeutic Hierarchy Concept. Lancet Oncol. 2023, 24, e312–e322. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for Hepatocellular Carcinoma Using Yttrium-90 Microspheres: A Comprehensive Report of Long-Term Outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef]
- Garin, E.; Tselikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; de Baere, T.; Assenat, E.; Tacher, V.; Robert, C.; Terroir-Cassou-Mounat, M.; et al. Personalised versus Standard Dosimetry Approach of Selective Internal Radiation Therapy in Patients with Locally Advanced Hepatocellular Carcinoma (DOSISPHERE-01): A Randomised, Multicentre, Open-Label Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 17–29. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Bhoori, S.; Romito, R.; Chiesa, C.; Morosi, C.; Maccauro, M.; Marchianò, A.; Bongini, M.; Lanocita, R.; et al. Yttrium-90 Radioembolization for Intermediate-Advanced Hepatocellular Carcinoma: A Phase 2 Study. Hepatology 2013, 57, 1826–1837. [Google Scholar] [CrossRef]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver Transplantation in Hepatocellular Carcinoma after Tumour Downstaging (XXL): A Randomised, Controlled, Phase 2b/3 Trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Donadon, M.; Torzilli, G. Parenchymal Sparing Anatomical Liver Resections for Hepatocellular Carcinoma. Hepatobiliary Surg. Nutr. 2024, 13, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Fujiyama, Y.; Mishima, K.; Igarashi, K.; Nie, Y.; Berardi, G.; Alomari, M.; Colella, M.; Wakabayashi, G. Laparoscopically Limited Anatomic Liver Resections: A Single-Center Analysis for Oncologic Outcomes of the Conceptual Procedure. Ann. Surg. Oncol. 2024, 31, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Shindoh, J.; Makuuchi, M.; Matsuyama, Y.; Mise, Y.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Complete Removal of the Tumor-Bearing Portal Territory Decreases Local Tumor Recurrence and Improves Disease-Specific Survival of Patients with Hepatocellular Carcinoma. J. Hepatol. 2016, 64, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Makuuchi, M.; Kawasaki, S.; Kakazu, T. Criteria for Safe Hepatic Resection. Am. J. Surg. 1995, 169, 589–594. [Google Scholar] [CrossRef]
- Ikai, I.; Arii, S.; Okazaki, M.; Okita, K.; Omata, M.; Kojiro, M.; Takayasu, K.; Nakanuma, Y.; Makuuchi, M.; Matsuyama, Y.; et al. Report of the 17th Nationwide Follow-up Survey of Primary Liver Cancer in Japan. Hepatol. Res. 2007, 37, 676–691. [Google Scholar] [CrossRef]
- Kokudo, T.; Hasegawa, K.; Matsuyama, Y.; Takayama, T.; Izumi, N.; Kadoya, M.; Kudo, M.; Ku, Y.; Sakamoto, M.; Nakashima, O.; et al. Survival Benefit of Liver Resection for Hepatocellular Carcinoma Associated with Portal Vein Invasion. J. Hepatol. 2016, 65, 938–943. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists. ASA Physical Status Classification System. Last updated 2020. Available online: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system (accessed on 5 April 2025).
- Tabone, M.; Calvo, A.; Russolillo, N.; Langella, S.; Carbonatto, P.; Lo Tesoriere, R.; Richetta, E.; Pellerito, R.; Ferrero, A. Downstaging Unresectable Hepatocellular Carcinoma by Radioembolization Using 90-Yttrium Resin Microspheres: A Single Center Experience. J. Gastrointest. Oncol. 2020, 11, 84–90. [Google Scholar] [CrossRef]
- Martelletti, C.; Ricotti, A.; Gesualdo, M.; Carucci, P.; Gaia, S.; Rolle, E.; Burlone, M.E.; Okolicsanyi, S.; Mattalia, A.; Pirisi, M.; et al. Radioembolization vs Sorafenib in Locally Advanced Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: A Propensity Score and Bayesian Analysis. J. Dig. Dis. 2021, 22, 496–502. [Google Scholar] [CrossRef]
- Capussotti, L.; Ferrero, A.; Russolillo, N.; Langella, S.; Lo Tesoriere, R.; Viganò, L. Routine Anterior Approach during Right Hepatectomy: Results of a Prospective Randomised Controlled Trial. J. Gastrointest. Surg. 2012, 16, 1324–1332. [Google Scholar] [CrossRef]
- Ferrero, A.; Lo Tesoriere, R.; Russolillo, N. Ultrasound Liver Map Technique for Laparoscopic Liver Resections. World J. Surg. 2019, 43, 2607–2611. [Google Scholar] [CrossRef]
- Ferrero, A.; Russolillo, N.; Langella, S.; Forchino, F.; Stasi, M.; Fazio, F.; Lo Tesoriere, R. Ultrasound Liver Map Technique for Laparoscopic Liver Resections: Perioperative Outcomes Are Not Impaired by Technical Complexity. Updates Surg. 2019, 71, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Phillips, C. Use and Dissemination of the Brisbane 2000 Nomenclature of Liver Anatomy and Resections. Ann. Surg. 2013, 257, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Koch, M.; Garden, O.J.; Padbury, R.; Rahbari, N.N.; Adam, R.; Capussotti, L.; Fan, S.T.; Yokoyama, Y.; Crawford, M.; Makuuchi, M.; et al. Bile Leakage after Hepatobiliary and Pancreatic Surgery: A Definition and Grading of Severity by the International Study Group of Liver Surgery. Surgery 2011, 149, 680–688. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Maddern, G.; Koch, M.; Hugh, T.J.; Fan, S.T.; Nimura, Y.; Figueras, J.; Vauthey, J.-N.; et al. Post-Hepatectomy Haemorrhage: A Definition and Grading by the International Study Group of Liver Surgery (ISGLS). HPB 2011, 13, 528–535. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria; 2024. Available online: https://www.R-project.org/ (accessed on 5 April 2025).
- Vouche, M.; Lewandowski, R.J.; Atassi, R.; Memon, K.; Gates, V.L.; Ryu, R.K.; Gaba, R.C.; Mulcahy, M.F.; Baker, T.; Sato, K.; et al. Radiation Lobectomy: Time-Dependent Analysis of Future Liver Remnant Volume in Unresectable Liver Cancer as a Bridge to Resection. J. Hepatol. 2013, 59, 1029–1036. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Donahue, L.; Chokechanachaisakul, A.; Kulik, L.; Mouli, S.; Caicedo, J.; Abecassis, M.; Fryer, J.; Salem, R.; Baker, T. 90Y Radiation Lobectomy: Outcomes Following Surgical Resection in Patients with Hepatic Tumors and Small Future Liver Remnant Volumes. J. Surg. Oncol. 2016, 114, 99–105. [Google Scholar] [CrossRef]
- Gregory, J.; Tselikas, L.; Allimant, C.; de Baere, T.; Bargellini, I.; Bell, J.; Bilbao, J.I.; Bouvier, A.; Chapiro, J.; Chiesa, C.; et al. Defining Textbook Outcome for Selective Internal Radiation Therapy of Hepatocellular Carcinoma: An International Expert Study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 921–928. [Google Scholar] [CrossRef]
- Ahmed, A.; Stauffer, J.A.; LeGout, J.D.; Burns, J.; Croome, K.; Paz-Fumagalli, R.; Frey, G.; Toskich, B. The Use of Neoadjuvant Lobar Radioembolization prior to Major Hepatic Resection for Malignancy Results in a Low Rate of Post Hepatectomy Liver Failure. J. Gastrointest. Oncol. 2021, 12, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Berardi, G.; Guglielmo, N.; Cucchetti, A.; Usai, S.; Colasanti, M.; Meniconi, R.L.; Ferretti, S.; Mariano, G.; Angrisani, M.; Sciuto, R.; et al. Transarterial Radioembolization Can Downstage Intermediate and Advanced Hepatocellular Carcinoma to Liver Transplantation. Transplantation 2025, 109, e54–e63. [Google Scholar] [CrossRef] [PubMed]
- Labgaa, I.; Tabrizian, P.; Titano, J.; Kim, E.; Thung, S.N.; Florman, S.; Schwartz, M.; Melloul, E. Feasibility and Safety of Liver Transplantation or Resection after Transarterial Radioembolization with Yttrium-90 for Unresectable Hepatocellular Carcinoma. HPB 2019, 21, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Meerun, M.A.; Allimant, C.; Rivière, B.; Herrero, A.; Panaro, F.; Assenat, E.; Cassinotto, C.; Mariano-Goulart, D.; Guiu, B. Large, Multifocal or Portal Vein-Invading Hepatocellular Carcinoma (HCC) Downstaged by Y90 Using Personalized Dosimetry: Safety, Pathological Results and Outcomes after Surgery. Hepatobiliary Surg. Nutr. 2023, 12, 351–365. [Google Scholar] [CrossRef]
- Gabr, A.; Abouchaleh, N.; Ali, R.; Baker, T.; Caicedo, J.; Katariya, N.; Abecassis, M.; Riaz, A.; Lewandowski, R.J.; Salem, R. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2018, 29, 1502–1510.e1. [Google Scholar] [CrossRef]
- Son, S.Y.; Geevarghese, R.; Marinelli, B.; Zhao, K.; Covey, A.; Maxwell, A.; Wei, A.C.; Jarnagin, W.; D’Angelica, M.; Yarmohammadi, H. Conversion Therapy to Transplant or Surgical Resection in Patients with Unresectable Hepatocellular Carcinoma Treated with Boosted Dose of Yttrium-90 Radiation Segmentectomy. Cancers 2024, 16, 3024. [Google Scholar] [CrossRef]
- Mangieri, C.W.; Valenzuela, C.D.; Strode, M.A.; Erali, R.A.; Shen, P.; Howerton, R.; Clark, C.J. Effect of Preoperative Liver-Directed Therapy prior to Hepatic Resection. Am. J. Surg. 2023, 225, 703–708. [Google Scholar] [CrossRef]
- Shehta, A.; Lee, J.-M.; Suh, K.-S.; Kim, H.-C.; Hong, S.K.; Cho, J.-H.; Yi, N.-J.; Lee, K.-W. Bridging and Downstaging Role of Trans-Arterial Radio-Embolization for Expected Small Remnant Volume before Liver Resection for Hepatocellular Carcinoma. Ann. Hepatobiliary Pancreat. Surg. 2020, 24, 421–430. [Google Scholar] [CrossRef]
- Rognoni, C.; Ciani, O.; Sommariva, S.; Facciorusso, A.; Tarricone, R.; Bhoori, S.; Mazzaferro, V. Trans-Arterial Radioembolization in Intermediate-Advanced Hepatocellular Carcinoma: Systematic Review and Meta-Analyses. Oncotarget 2016, 7, 72343–72355. [Google Scholar] [CrossRef]
- Torzilli, G.; Belghiti, J.; Kokudo, N.; Takayama, T.; Capussotti, L.; Nuzzo, G.; Vauthey, J.-N.; Choti, M.A.; De Santibanes, E.; Donadon, M.; et al. A Snapshot of the Effective Indications and Results of Surgery for Hepatocellular Carcinoma in Tertiary Referral Centers: Is It Adherent to the EASL/AASLD Recommendations?: An Observational Study of the HCC East-West Study Group. Ann. Surg. 2013, 257, 929–937. [Google Scholar] [CrossRef]
- Famularo, S.; Donadon, M.; Cipriani, F.; Giuliante, F.; Ferri, S.; Celsa, C.; Ferrero, A.; Foschi, F.G.; Baiocchi, G.L.; Biasini, E.; et al. Hepatectomy Versus Sorafenib in Advanced Nonmetastatic Hepatocellular Carcinoma: A Real-Life Multicentric Weighted Comparison. Ann. Surg. 2022, 275, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Juthani, R.; Malalur, P.; Manne, A.; Mittra, A. The Combined Use of Lenvatinib and Locoregional Therapies for the Management of Hepatocellular Carcinoma. Cancers 2025, 17, 1572. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.T.M.; Braat, A.J.A.T.; van Erpecum, K.J.; de Bruijne, J.; Bruijnen, R.C.G.; Sprengers, D.; de Man, R.; Vegt, E.; IJzermans, J.N.M.; Elias, S.G.; et al. Holmium-166 Radioembolisation Dosimetry in HCC. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 993–1003. [Google Scholar] [CrossRef]
- Arar, A.; Heglin, A.; Veluri, S.; Alnablsi, M.W.; Benjamin, J.L.; Choudhary, M.; Pillai, A. Radioembolization of HCC and Secondary Hepatic Tumors: A Comprehensive Review. Q. J. Nucl. Med. Mol. Imaging 2024, 68, 270–287. [Google Scholar] [CrossRef] [PubMed]
| Non-TARE Group n = 21 (53.8%) | TARE Group n = 18 (46.15%) | p-Value | |
|---|---|---|---|
| Age, median [IQR] | 72 [69.0, 77.0] | 74.50 [70.50, 76.0] | 0.489 |
| Male (%) | 16 (76.2) | 17 (94.4) | 0.190 |
| BMI, median [IQR] | 25.93 [21.80, 29.32] | 25.96 [23.99, 28.62] | 0.673 |
| Charlson Comorbidity Index, median [IQR] | 6 [5, 7] | 6 [6, 7] | 0.402 |
| ASA score (%) | |||
| 1 | 1 (4.8) | 0 (0.0) | 0.537 |
| 2 | 6 (28.6) | 3 (16.7) | |
| 3 | 12 (57.1) | 14 (77.8) | |
| 4 | 2 (9.5) | 1 (5.6) | |
| No Virus (%) | 15 (71.4) | 7 (38.9) | 0.033 |
| HBV (%) | 0 (0.0) | 4 (22.2) | |
| HCV (%) | 6 (28.6) | 7 (38.9) | |
| Metabolic Disease (%) | 9 (42.9) | 5 (27.8) | 0.504 |
| Child Pugh Score (%) | |||
| Child Pugh A (%) | 19 (90.5) | 18 (100) | 0.490 |
| Child Pugh B (%) | 2 (9.5) | 0 (0.0) | |
| MELD, median [IQR] | 7.00 [7.00, 8.00] | 7.50 [7.00, 8.75] | 0.588 |
| AFP ng/mL (%) | |||
| ≤100 | 14 (66.7) | 12 (66.7) | 1.000 |
| >100 | 6 (28.6) | 5 (27.8) | |
| Bilirubin preoperative, mg/dl, median [IQR] | 0.71 [0.60, 1.05] | 0.77 [0.61, 0.90] | 0.877 |
| 15′ ICG-RR, median [IQR] | 10.30 [4.30, 12.50] | 14.80 [9.85, 21.73] | 0.033 |
| Esophageal Varices (%) | 1 (4.8) | 2 (11.1) | 0.586 |
| Size of Node (preoperative), mm, median [IQR] | 80.00 [60.00, 150.00] | 70.00 [47.50, 80.00] | 0.171 |
| Single Node (%) | 18 (85.7) | 10 (55.6) | 0.218 |
| Multifocal Disease (%) | 3 (14.3) | 8 (44.4) | |
| Portal Vein Invasion Vp (%) | |||
| Vp0 | 16 (76.2) | 10 (55.6) | 0.043 |
| Vp1 | 0 (0.0) | 1 (5.6) | |
| Vp2 | 4 (19.0) | 1 (5.6) | |
| Vp3 | 1 (4.8) | 6 (33.3) | |
| Hepatic Vein Invasion VV (%) | |||
| VV0 | 10 (47.6) | 12 (66.7) | 0.093 |
| VV1 | 0 (0.0) | 2 (11.1) | |
| VV2 | 10 (47.6) | 4 (22.2) | |
| VV3 | 1 (4.8) | 0 (0.0) | |
| Right Portal Vein Invasion Vp3 (%) | 1 (4.8) | 6 (33.3) | 0.035 |
| Concomitant Portal and Hepatic Vein Invasion (%) | 3 (14.3) | 3 (16.7) | 1.000 |
| BCLC Stage (%) | 0.295 | ||
| A | 9 (42.9) | 5 (27.8) | |
| B | 1 (4.8) | 4 (22.2) | |
| C | 11 (52.4) | 9 (50.0) | |
| FLR/TLV pre-treatment, %, median [IQR] | 40.0 [37.0–47.3] | 39.1 [35.8–50.7] | 0.954 |
| FLR/TLV post-treatment, %, median [IQR] | 51.9 [51.0, 53.9] * | 56.9 [40.0, 62.2] | 0.737 |
| Non-TARE Group n = 21 (53.8%) | TARE Group n = 18 (46.15%) | p-Value | |
|---|---|---|---|
| Operative time, minutes, median [IQR] | 320 [300.00, 420.00] | 330.00 [302.50, 360.00] | 0.899 |
| Operative time (liver transection), minutes, median [IQR] | 90.00 [79.00, 100.00] | 86.50 [67.50, 106.75] | 0.888 |
| Pringle maneuver (%) | 15 (71.4) | 15 (83.3) | 0.464 |
| Duration Pringle maneuver, minutes, median [IQR] | 46.00 [0.00, 68.00] | 53.00 [24.75, 60.50] | 0.734 |
| Blood loss, ml, median [IQR] | 330.00 [190.00, 400.00] | 275.00 [200.00, 570.00] | 0.778 |
| Section plane cm2, median [IQR] | 75.00 [68.00, 101.00] | 64.50 [46.00, 82.00] | 0.143 |
| Type of surgery (%) | |||
| Right hepatectomy (%) | 17 (81.0) | 8 (44.4) | 0.024 |
| Extended right hepatectomy (%) | 4 (19.0) | 10 (55.6) | |
| Minimally invasive resection (%) | 5 (23.8) | 8 (44.4) | 0.196 |
| Intraoperative complication (%) | 3 (14.3) | 1 (5.6) | 0.609 |
| Non-TARE Group n = 21 (53.8%) | TARE Group n = 18 (46.15%) | p-Value | |
|---|---|---|---|
| Postoperative complications (%) | 8 (38.1) | 7 (38.9) | 1.000 |
| Blood transfusions (%) | 3 (14.3) | 1 (5.6) | 0.609 |
| PHLF (%) | 4 (19.0) | 2 (11.1) | 0.667 |
| Grade PHLF sec. ISGLS (%) | |||
| Grade A | 0 (0.0) | 1 (5.6) | 0.609 |
| Grade B | 2 (9.5) | 0 (0.0) | |
| Grade C | 2 (9.5) | 1 (5.6) | |
| Biliary fistula (%) | 3 (14.3) | 2 (11.1) | 1.000 |
| Clavien–Dindo ≥3 (%) | 5 (23.8) | 1 (5.6) | 0.190 |
| CCI ≥ 20.9 (%) | 8 (38.1) | 4 (22.2) | 0.322 |
| Readmission (%) | 2 (9.5) | 1 (5.6) | 1.000 |
| 90-day postoperative mortality (%) | 2 (9.5) | 1 (5.6) | 1.000 |
| Hospital stay, days, median [IQR] | 8.00 [5.00, 11.00] | 6.00 [5.00, 9.00] | 0.318 |
| Histology | |||
| Cirrhosis (%) | 3 (14.3) | 12 (66.7) | 0.001 |
| Multinodular disease (%) | 2 (9.5) | 7 (38.9) | 0.055 |
| Single node disease (%) | 19 (90.5) | 11 (61.1) | |
| Satellitosis (%) | 13 (61.9) | 6 (33.3) | 0.111 |
| Size of node, mm, median [IQR] | 80.00 [60.00, 150.00] | 46.50 [36.25, 77.50] | 0.020 |
| Necrosis percentage, median [IQR] | 10.00 [10.00, 20.00] | 70.00 [50.00, 87.50] | 0.002 |
| Resection margin, mm, median [IQR] | 10.00 [2.00, 15.00] | 2.00 [1.00, 10.00] | 0.032 |
| Positive margin, mm (%) | 1 (4.8) | 2 (11.1) | 0.586 |
| Microvascular invasion (%) | 19 (90.5) | 11 (61.1) | 0.055 |
| Edmonson Grade 3–4 (%) | 18 (85.7) | 10 (55.6) | 0.072 |
| Tumor capsule (%) | 15 (71.4) | 11 (61.1) | 0.520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, A.P.; Russolillo, N.; Maurino, L.; Marengo, A.; Calvo, A.; Ricotti, A.; Langella, S.; Tesoriere, R.L.; Ferrero, A. Impact of Preoperative Yttrium-90 Transarterial Radioembolization on Patients Undergoing Right or Extended Right Hepatectomy for Hepatocellular Carcinoma. Cancers 2025, 17, 2556. https://doi.org/10.3390/cancers17152556
Fontana AP, Russolillo N, Maurino L, Marengo A, Calvo A, Ricotti A, Langella S, Tesoriere RL, Ferrero A. Impact of Preoperative Yttrium-90 Transarterial Radioembolization on Patients Undergoing Right or Extended Right Hepatectomy for Hepatocellular Carcinoma. Cancers. 2025; 17(15):2556. https://doi.org/10.3390/cancers17152556
Chicago/Turabian StyleFontana, Andrea P., Nadia Russolillo, Ludovica Maurino, Andrea Marengo, Amedeo Calvo, Andrea Ricotti, Serena Langella, Roberto Lo Tesoriere, and Alessandro Ferrero. 2025. "Impact of Preoperative Yttrium-90 Transarterial Radioembolization on Patients Undergoing Right or Extended Right Hepatectomy for Hepatocellular Carcinoma" Cancers 17, no. 15: 2556. https://doi.org/10.3390/cancers17152556
APA StyleFontana, A. P., Russolillo, N., Maurino, L., Marengo, A., Calvo, A., Ricotti, A., Langella, S., Tesoriere, R. L., & Ferrero, A. (2025). Impact of Preoperative Yttrium-90 Transarterial Radioembolization on Patients Undergoing Right or Extended Right Hepatectomy for Hepatocellular Carcinoma. Cancers, 17(15), 2556. https://doi.org/10.3390/cancers17152556






