Breaking Barriers with Sound: The Implementation of Histotripsy in Cancer
Simple Summary
Abstract
1. Introduction
2. Technical Background for Clinicians
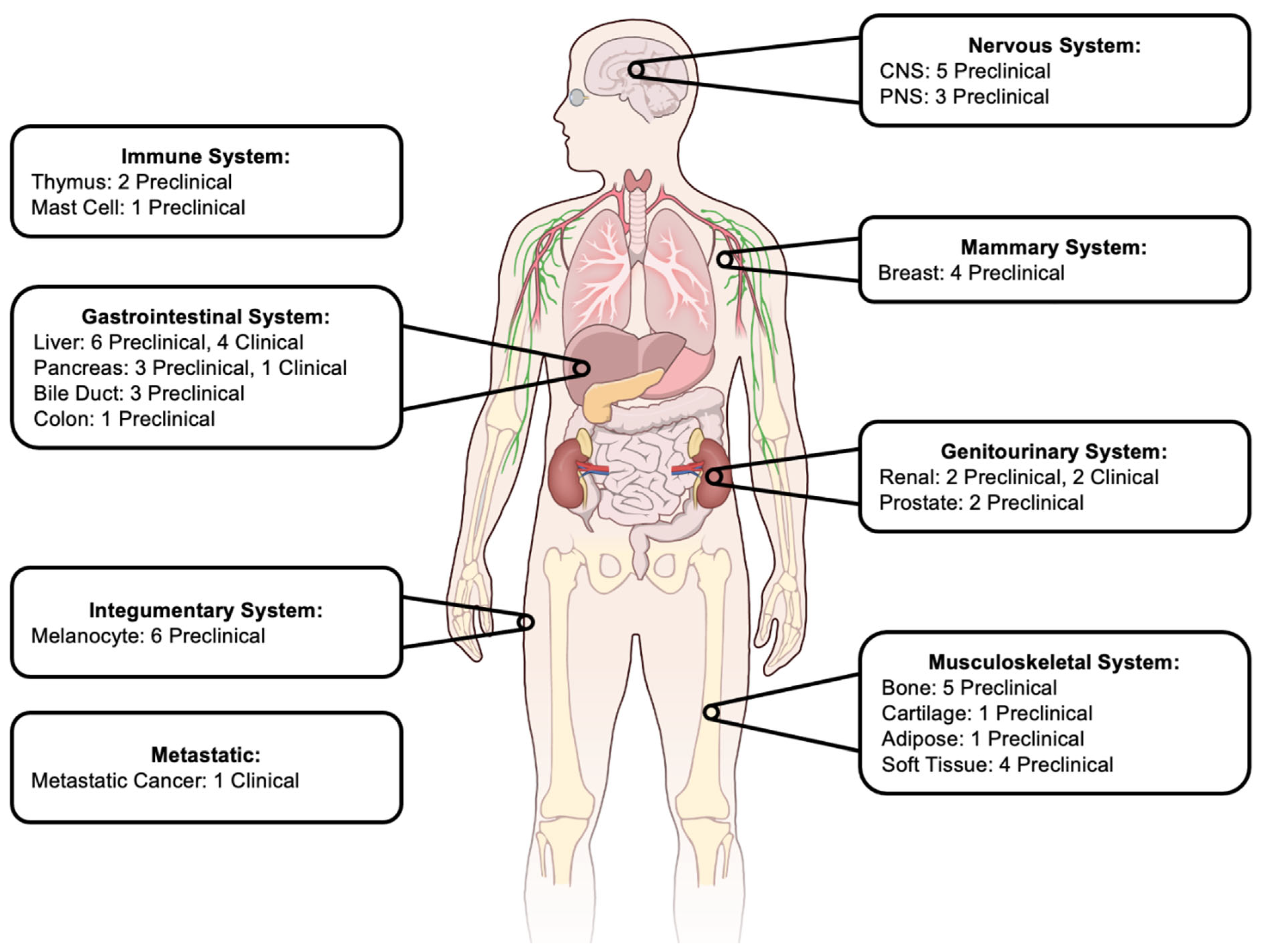
3. Histotripsy in Preclinical Cancer Models
3.1. Nervous System
3.2. Breast
3.3. Gastrointestinal
3.4. Genitourinary
3.5. Musculoskeletal
3.6. Integumentary
3.7. Immune System
4. Clinical Trials
5. Histotripsy in Current Applications
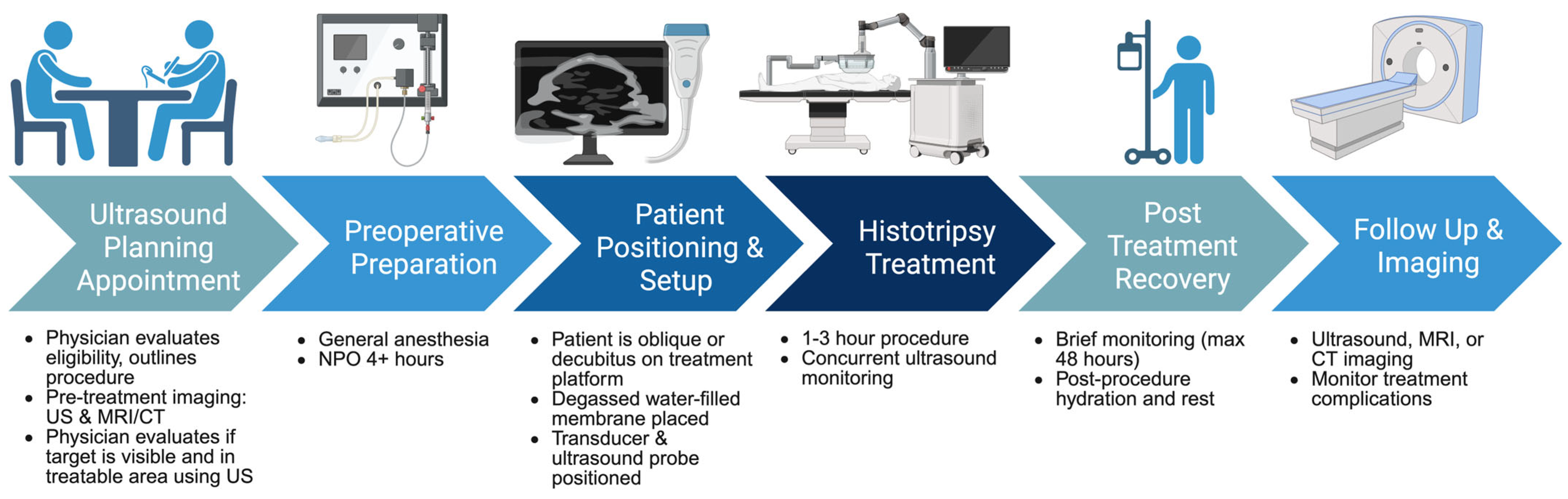
5.1. The Ideal Candidate
5.2. The Typical Procedure
5.3. Peri- and Post-Operative Imaging
5.4. Monitoring and Follow-Up
6. Limitations and Future Work
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Martinez, E.; Landazuri-Navas, S.; Vilchez, E.; Cantu-Hernandez, R.; Mosquera-Moscoso, J.; Encalada, S.; Al Lami, Z.; Zevallos-Delgado, C.; Cinicola, J. Prognostic Scores and Survival Rates by Etiology of Hepatocellular Carcinoma: A Review. J. Clin. Med. Res. 2023, 15, 200–207. [Google Scholar] [CrossRef]
- Vareedayah, A.A.; Alkaade, S.; Taylor, J.R. Pancreatic Adenocarcinoma. Mo. Med. 2018, 115, 230–235. [Google Scholar]
- Janssen, L.M.E.; Ramsay, E.E.; Logsdon, C.D.; Overwijk, W.W. The immune system in cancer metastasis: Friend or foe? J. Immunother. Cancer 2017, 5, 79. [Google Scholar] [CrossRef]
- Kaur, S.; Baine, M.J.; Jain, M.; Sasson, A.R.; Batra, S.K. Early diagnosis of pancreatic cancer: Challenges and new developments. Biomark. Med. 2012, 6, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, G.; Lu, Y.; Zhao, T.; Gao, C.; Liu, W.; Piao, Y.; Chen, Y.; Huang, C.; Chang, A.; et al. Perioperative Serum Scoring Systems Predict Early Recurrence and Poor Prognosis of Resectable Pancreatic Cancer. Front. Oncol. 2022, 12, 841819. [Google Scholar] [CrossRef] [PubMed]
- She, S.; Shi, J.; Zhu, J.; Yang, F.; Yu, J.; Dai, K. Impact of inflammation and the immune system on hepatocellular carcinoma recurrence after hepatectomy. Cancer Med. 2024, 13, e7018. [Google Scholar] [CrossRef]
- Zeng, Z.M.; Mo, N.; Zeng, J.; Ma, F.C.; Jiang, Y.F.; Huang, H.S.; Liao, X.W.; Zhu, G.Z.; Ma, J.; Peng, T. Advances in postoperative adjuvant therapy for primary liver cancer. World J. Gastrointest. Oncol. 2022, 14, 1604–1621. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ye, F.; Kong, Y.; Hu, X.; Deng, X.; Xie, J.; Song, C.; Ou, X.; Wu, S.; Wu, L.; et al. The Single-Cell Landscape of Intratumoral Heterogeneity and The Immunosuppressive Microenvironment in Liver and Brain Metastases of Breast Cancer. Adv. Sci. 2023, 10, e2203699. [Google Scholar] [CrossRef]
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef]
- Breuer, J.A.; Ahmed, K.H.; Al-Khouja, F.; Macherla, A.R.; Muthoka, J.M.; Abi-Jaoudeh, N. Interventional oncology: New techniques and new devices. Br. J. Radiol. 2022, 95, 20211360. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Anders, R.; Are, C.; Brown, D.; Chang, D.T.; et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, L.; Scalise, P.; Bozzi, E.; Candita, G.; Cioni, R. Thermal ablation of hepatocellular carcinoma. J. Med. Imaging Radiat. Oncol. 2023, 67, 817–831. [Google Scholar] [CrossRef]
- Clark, T.W. Complications of hepatic chemoembolization. Semin. Interv. Radiol. 2006, 23, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Solbiati, L.; Meloni, M.F.; Gazelle, G.S.; Halpern, E.F.; Goldberg, S.N. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003, 226, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Barry, A.; Doherty, M.; Fischer, S.; Goldaracena, N.; Rosales, R.; Russo, M.; Beecroft, R.; Ghanekar, A.; Bhat, M.; et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J. Hepatol. 2017, 67, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Slakey, D.P.; Simms, E.; Drew, B.; Yazdi, F.; Roberts, B. Complications of liver resection: Laparoscopic versus open procedures. JSLS 2013, 17, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine 2015, 94, e580. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Xu, Z.; Winterroth, F.; Hall, T.L.; Fowlkes, J.B.; Rothman, E.D.; Roberts, W.W.; Cain, C.A. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy- histotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 995–1005. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Kim, Y.; Owens, G.; Roberts, W.; Cain, C.; Xu, Z. Effects of tissue mechanical properties on susceptibility to histotripsy-induced tissue damage. Phys. Med. Biol. 2014, 59, 253–270. [Google Scholar] [CrossRef]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Imran, K.M.; Ganguly, A.; Paul, T.; Powar, M.; Vlaisavljevich, E.; Cho, C.S.; Allen, I.C. Magic bubbles: Utilizing histotripsy to modulate the tumor microenvironment and improve systemic anti-tumor immune responses. Int. J. Hyperth. 2023, 40, 2244206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, J.B.; Cain, C.A. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta-Lala, M.; Wiggermann, P.; Pech, M.; Serres-Creixams, X.; White, S.B.; Davis, C.; Ahmed, O.; Parikh, N.D.; Planert, M.; Thormann, M.; et al. The #HOPE4LIVER Single-Arm Pivotal Trial for Histotripsy of Primary and Metastatic Liver Tumors. Radiology 2024, 312, e233051. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Burns, K.; Ong, E.; Couillard, A.; Parikh, N.D.; Caoili, E.; Kim, J.; Aucejo, F.; Schlegel, A.; Knott, E.; et al. The first international experience with histotripsy: A safety analysis of 230 cases. J. Gastrointest. Surg. 2025, 29, 102000. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Cain, C.A.; Hall, T.L.; Fowlkes, J.B.; Xu, Z. Probability of cavitation for single ultrasound pulses applied to tissues and tissue-mimicking materials. Ultrasound Med. Biol. 2013, 39, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Mancia, L.; Vlaisavljevich, E.; Yousefi, N.; Rodriguez, M.; Ziemlewicz, T.J.; Lee, F.T.; Henann, D.; Franck, C.; Xu, Z.; Johnsen, E. Modeling tissue-selective cavitation damage. Phys. Med. Biol. 2019, 64, 225001. [Google Scholar] [CrossRef]
- Mancia, L.; Vlaisavljevich, E.; Xu, Z.; Johnsen, E. Predicting Tissue Susceptibility to Mechanical Cavitation Damage in Therapeutic Ultrasound. Ultrasound Med. Biol. 2017, 43, 1421–1440. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Lin, K.W.; Maxwell, A.; Warnez, M.T.; Mancia, L.; Singh, R.; Putnam, A.J.; Fowlkes, B.; Johnsen, E.; Cain, C.; et al. Effects of ultrasound frequency and tissue stiffness on the histotripsy intrinsic threshold for cavitation. Ultrasound Med. Biol. 2015, 41, 1651–1667. [Google Scholar] [CrossRef]
- Maxwell, A.D.; Wang, T.-Y.; Cain, C.A.; Fowlkes, J.B.; Sapozhnikov, O.A.; Bailey, M.R.; Xu, Z. Cavitation clouds created by shock scattering from bubbles during histotripsy. J. Acoust. Soc. Am. 2011, 130, 1888–1898. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X. Effect of pulse duration and pulse repetition frequency of cavitation histotripsy on erosion at the surface of soft material. Ultrasonics 2018, 84, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Winterroth, F.; Xu, Z.; Wang, T.-Y.; Wilkinson, J.E.; Fowlkes, J.B.; Roberts, W.W.; Cain, C.A. Examining and analyzing subcellular morphology of renal tissue treated by histotripsy. Ultrasound Med. Biol. 2011, 37, 78–86. [Google Scholar] [CrossRef][Green Version]
- Khokhlova, T.D.; Canney, M.S.; Khokhlova, V.A.; Sapozhnikov, O.A.; Crum, L.A.; Bailey, M.R. Controlled tissue emulsification produced by high intensity focused ultrasound shock waves and millisecond boiling. J. Acoust. Soc. Am. 2011, 130, 3498–3510. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.D.; Yuldashev, P.V.; Kreider, W.; Khokhlova, T.D.; Schade, G.R.; Hall, T.L.; Sapozhnikov, O.A.; Bailey, M.R.; Khokhlova, V.A. A prototype therapy system for transcutaneous application of boiling histotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Joung, C.; Pahk, K.; Pahk, K.J. Investigation of the long-term healing response of the liver to boiling histotripsy treatment in vivo. Sci. Rep. 2022, 12, 14462. [Google Scholar] [CrossRef]
- Vlaisavljevich, E.; Aydin, O.; Lin, K.W.; Durmaz, Y.Y.; Fowlkes, B.; ElSayed, M.; Xu, Z. The role of positive and negative pressure on cavitation nucleation in nanodroplet-mediated histotripsy. Phys. Med. Biol. 2016, 61, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Vlaisavljevich, E.; Maxwell, A.; Mancia, L.; Johnsen, E.; Cain, C.; Xu, Z. Visualizing the Histotripsy Process: Bubble Cloud-Cancer Cell Interactions in a Tissue-Mimicking Environment. Ultrasound Med. Biol. 2016, 42, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Movahed, P.; Kreider, W.; Maxwell, A.D.; Hutchens, S.B.; Freund, J.B. Cavitation-induced damage of soft materials by focused ultrasound bursts: A fracture-based bubble dynamics model. J. Acoust. Soc. Am. 2016, 140, 1374. [Google Scholar] [CrossRef]
- Mancia, L.; Rodriguez, M.; Sukovich, J.; Xu, Z.; Johnsen, E. Single–bubble dynamics in histotripsy and high–amplitude ultrasound: Modeling and validation. Phys. Med. Biol. 2020, 65, 225014. [Google Scholar] [CrossRef]
- Macoskey, J.J.; Zhang, X.; Hall, T.L.; Shi, J.; Beig, S.A.; Johnsen, E.; Lee, F.T., Jr.; Cain, C.A.; Xu, Z. Bubble-induced color Doppler feedback correlates with histotripsy-induced destruction of structural components in liver tissue. Ultrasound Med. Biol. 2018, 44, 602–612. [Google Scholar] [CrossRef]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-intensity focused ultrasound: A review of mechanisms and clinical applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- Couillard, A.B.; Zlevor, A.M.; Ziemlewicz, T.J.; Kisting, M.A.; Knott, E.; Rossebo, A.E.; White, J.; Lubner, M.G.; Gettle, L.M.; Hinshaw, J.L.; et al. A Comparison of Histotripsy and Percutaneous Cryoablation in a Chronic Healthy Swine Kidney Model. J. Vasc. Interv. Radiol. 2023, 34, 1986–1996. [Google Scholar] [CrossRef]
- Knott, E.A.; Swietlik, J.F.; Longo, K.C.; Watson, R.F.; Green, C.M.; Abel, E.J.; Lubner, M.G.; Hinshaw, J.L.; Smolock, A.R.; Xu, Z.; et al. Robotically-Assisted Sonic Therapy for Renal Ablation in a Live Porcine Model: Initial Preclinical Results. J. Vasc. Interv. Radiol. 2019, 30, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Danev, R.; Yanagisawa, H.; Kikkawa, M. Cryo-electron microscopy methodology: Current aspects and future directions. Trends Biochem. Sci. 2019, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, L.; Liu, S.; Li, J.; Xia, J.; Qin, X.; Li, Y.; Gao, T.; Tang, X. The state-of-the-art and perspectives of laser ablation for tumor treatment. Cyborg Bionic Syst. 2024, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Arnold, L.; Gannon, J.; Simon, A.; Singh, N.; Sheppard, H.; Nagai-Singer, M.A.; Imran, K.M.; Lee, K.; Clark-Deener, S.; et al. Histotripsy Ablation in Preclinical Animal Models of Cancer and Spontaneous Tumors in Veterinary Patients: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 5–26. [Google Scholar] [CrossRef]
- Falk, K.L.; Laeseke, P.F.; Kisting, M.A.; Zlevor, A.M.; Knott, E.A.; Smolock, A.R.; Bradley, C.; Vlaisavljevich, E.; Lee, F.T., Jr.; Ziemlewicz, T.J. Clinical translation of abdominal histotripsy: A review of preclinical studies in large animal models. Int. J. Hyperth. 2023, 40, 2272065. [Google Scholar] [CrossRef]
- Worlikar, T.; Hall, T.; Zhang, M.; Mendiratta-Lala, M.; Green, M.; Cho, C.S.; Xu, Z. Insights from in vivo preclinical cancer studies with histotripsy. Int. J. Hyperth. 2024, 41, 2297650. [Google Scholar] [CrossRef]
- Worlikar, T.; Vlaisavljevich, E.; Gerhardson, T.; Greve, J.; Wan, S.; Kuruvilla, S.; Lundt, J.; Ives, K.; Hall, T.; Welling, T.H.; et al. Histotripsy for Non-Invasive Ablation of Hepatocellular Carcinoma (HCC) Tumor in a Subcutaneous Xenograft Murine Model. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 6064–6067. [Google Scholar] [CrossRef]
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef]
- Worlikar, T.; Mendiratta-Lala, M.; Vlaisavljevich, E.; Hubbard, R.; Shi, J.; Hall, T.L.; Cho, C.S.; Lee, F.T.; Greve, J.; Xu, Z. Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model. BME Front. 2020, 2020, 9830304. [Google Scholar] [CrossRef]
- Worlikar, T.; Zhang, M.; Ganguly, A.; Hall, T.L.; Shi, J.; Zhao, L.; Lee, F.T.; Mendiratta-Lala, M.; Cho, C.S.; Xu, Z. Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model. Cancers 2022, 14, 1612. [Google Scholar] [CrossRef]
- Pepple, A.L.; Guy, J.L.; McGinnis, R.; Felsted, A.E.; Song, B.; Hubbard, R.; Worlikar, T.; Garavaglia, H.; Dib, J.; Chao, H.; et al. Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation. Front. Immunol. 2023, 14, 1012799. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Sereno, J.; Gannon, J.; Zeher, A.; Brock, R.M.; Beitel-White, N.; Simon, A.; Davalos, R.V.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; et al. Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Imran, K.M.; Gannon, J.; Morrison, H.A.; Tupik, J.D.; Tintera, B.; Nagai-Singer, M.A.; Ivester, H.; Madanick, J.M.; Hendricks-Wenger, A.; Uh, K.; et al. Successful In Situ Targeting of Pancreatic Tumors in a Novel Orthotopic Porcine Model Using Histotripsy. Ultrasound Med. Biol. 2023, 49, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Weber, P.; Simon, A.; Saunier, S.; Coutermarsh-Ott, S.; Grider, D.; Vidal-Jove, J.; Allen, I.C.; Luyimbazi, D.; Vlaisavljevich, E. Histotripsy for the Treatment of Cholangiocarcinoma Liver Tumors: In Vivo Feasibility and Ex Vivo Dosimetry Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2953–2964. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Saunier, S.; Simon, A.; Grider, D.; Luyimbazi, D.; Allen, I.C.; Vlaisavljevich, E. Histotripsy for the Treatment of Cholangiocarcinoma in a Patient-Derived Xenograft Mouse Model. Ultrasound Med. Biol. 2022, 48, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.H.; Pahk, K.J.; Jeon, S.; Park, H.J.; Kim, G.B.; Oh, S.J.; Kim, K.; Kim, H.; Yang, Y. Investigation of the Potential Immunological Effects of Boiling Histotripsy for Cancer Treatment. Adv. Ther. 2020, 3, 1900214. [Google Scholar] [CrossRef]
- Tang, S.; McGinnis, R.; Cao, Z.; Baker, J.R., Jr.; Xu, Z.; Wang, S. Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers. Cancers 2025, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Ashar, H.; Singh, A.; Kishore, D.; Neel, T.; More, S.; Liu, C.; Dugat, D.; Ranjan, A. Enabling Chemo-Immunotherapy with HIFU in Canine Cancer Patients. Ann. Biomed. Eng. 2024, 52, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Jing, Y.; Deng, J.; Chang, J.; Sun, W.; Yang, R.; Liu, X.; Zhang, Q.; Wan, M.; Lu, M. Boiling Histotripsy Using Dual-Frequency Protocol on Murine Breast Tumor Model and Promotes Immune Activation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 1773–1785. [Google Scholar] [CrossRef]
- Pieper, A.A.; Stowe, N.A.; Periyasamy, S.; Burkel, B.M.; Tsarovsky, N.W.; Singh, A.P.; Rakhmilevich, A.L.; Sondel, P.M.; Ponik, S.M.; Laeseke, P.F.; et al. Histoplasty Modification of the Tumor Microenvironment in a Murine Preclinical Model of Breast Cancer. J. Vasc. Interv. Radiol. 2024, 35, 900–908 e902. [Google Scholar] [CrossRef]
- Choi, S.W.; Gerhardson, T.I.; Duclos, S.E.; Surowiec, R.K.; Scheven, U.M.; Galban, S.; Lee, F.T.; Greve, J.M.; Balter, J.M.; Hall, T.L.; et al. Stereotactic Transcranial Focused Ultrasound Targeting System for Murine Brain Models. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Duclos, S.; Camelo-Piragua, S.; Chaudhary, N.; Sukovich, J.; Hall, T.; Pandey, A.; Xu, Z. Histotripsy Treatment of Murine Brain and Glioma: Temporal Profile of Magnetic Resonance Imaging and Histological Characteristics Post-treatment. Ultrasound Med. Biol. 2023, 49, 1882–1891. [Google Scholar] [CrossRef]
- Gerhardson, T.; Pal, A.; Sheetz, L.; Sukovich, J.; Lundt, J.; Hall, T.; Chertok, B.; Rehemtulla, A.; Cain, C.; Xu, Z. Histotripsy mediated immunomodulation in a mouse GL261 intracranial glioma model. In Proceedings of the Proc. Int. Symp. Therapeutic Ultrasound, Nashville, TN, USA, 14–17 May 2018. [Google Scholar]
- Duclos, S.; Golin, A.; Fox, A.; Chaudhary, N.; Camelo-Piragua, S.; Pandey, A.; Xu, Z. Transcranial histotripsy parameter study in primary and metastatic murine brain tumor models. Int. J. Hyperth. 2023, 40, 2237218. [Google Scholar] [CrossRef]
- Vezza, C.; Ruger, L.; Langman, M.; Vickers, E.; Prada, F.; Sukovich, J.; Hall, T.; Xu, Z.; Parker, R.L.; Vlaisavljevich, E.; et al. First-In-DOg HISTotripsy for Intracranial Tumors Trial: The FIDOHIST Study. Technol. Cancer Res. Treat. 2024, 23, 15330338241285158. [Google Scholar] [CrossRef]
- Iwanicki, I.; Wu, L.L.; Flores-Guzman, F.; Sundland, R.; Viza-Gomes, P.; Nordgren, R.; Centner, C.S.; Kandel, J.J.; Applebaum, M.A.; Bader, K.B.; et al. Histotripsy induces apoptosis and reduces hypoxia in a neuroblastoma xenograft model. Int. J. Hyperth. 2023, 40, 2222941. [Google Scholar] [CrossRef]
- Eranki, A.; Srinivasan, P.; Ries, M.; Kim, A.; Lazarski, C.A.; Rossi, C.T.; Khokhlova, T.D.; Wilson, E.; Knoblach, S.M.; Sharma, K.V.; et al. High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2020, 26, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, M.; Eikelenboom, D.C.; van den Bijgaart, R.J.E.; Heerschap, A.; Wesseling, P.; den Brok, M.H.; Futterer, J.J.; Adema, G.J. Impact of MR-guided boiling histotripsy in distinct murine tumor models. Ultrason. Sonochem. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Singh, M.P.; Sethuraman, S.N.; Miller, C.; Malayer, J.; Ranjan, A. Boiling histotripsy and in-situ CD40 stimulation improve the checkpoint blockade therapy of poorly immunogenic tumors. Theranostics 2021, 11, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Thim, E.A.; Kitelinger, L.E.; Rivera-Escalera, F.; Mathew, A.S.; Elliott, M.R.; Bullock, T.N.J.; Price, R.J. Focused ultrasound ablation of melanoma with boiling histotripsy yields abscopal tumor control and antigen-dependent dendritic cell activation. Theranostics 2024, 14, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Queen, H.; Ferris, S.F.; McGinnis, R.; Karanam, C.; Gatteno, N.; Buglak, K.; Kim, H.; Xu, J.; Goughenour, K.D.; et al. Histotripsy-focused ultrasound treatment abrogates tumor hypoxia responses and stimulates anti-tumor immune responses in melanoma. Mol. Cancer Ther. 2025, 24, 1088–1098. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Eikelenboom, D.; den Brok, M.H.; Veltien, A.; Wassink, M.; Wesseling, P.; Dumont, E.; Futterer, J.J.; Adema, G.J.; Heerschap, A. In vivo MR guided boiling histotripsy in a mouse tumor model evaluated by MRI and histopathology. NMR Biomed. 2016, 29, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.N.; Hay, A.N.; Vickers, E.R.; Coutermarsh-Ott, S.L.; Gannon, J.M.; Covell, H.S.; Daniel, G.B.; Laeseke, P.F.; Ziemlewicz, T.J.; Kierski, K.R.; et al. Characterizing the Ablative Effects of Histotripsy for Osteosarcoma: In Vivo Study in Dogs. Cancers 2023, 15, 741. [Google Scholar] [CrossRef]
- Ruger, L.; Yang, E.; Gannon, J.; Sheppard, H.; Coutermarsh-Ott, S.; Ziemlewicz, T.J.; Dervisis, N.; Allen, I.C.; Daniel, G.B.; Tuohy, J.; et al. Mechanical High-Intensity Focused Ultrasound (Histotripsy) in Dogs with Spontaneously Occurring Soft Tissue Sarcomas. IEEE Trans. Biomed. Eng. 2023, 70, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.N.; Hay, A.N.; Gannon, J.M.; Sheppard, H.O.; Coutermarsh-Ott, S.L.; Daniel, G.B.; Kierski, K.R.; Ciepluch, B.J.; Vlaisavljevich, E.; Tuohy, J.L. Histotripsy Ablation of Spontaneously Occurring Canine Bone Tumors In Vivo. IEEE Trans. Biomed. Eng. 2022, 70, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ruger, L.; Yang, E.; Coutermarsh-Ott, S.; Vickers, E.; Gannon, J.; Nightengale, M.; Hsueh, A.; Ciepluch, B.; Dervisis, N.; Vlaisavljevich, E.; et al. Histotripsy ablation for the treatment of feline injection site sarcomas: A first-in-cat in vivo feasibility study. Int. J. Hyperth. 2023, 40, 2210272. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Hendricks-Wenger, A.; Coutermarsh-Ott, S.; Gannon, J.; Hay, A.N.; Dervisis, N.; Klahn, S.; Allen, I.C.; Tuohy, J.; Vlaisavljevich, E. Histotripsy Ablation of Bone Tumors: Feasibility Study in Excised Canine Osteosarcoma Tumors. Ultrasound Med. Biol. 2021, 47, 3435–3446. [Google Scholar] [CrossRef]
- Hay, A.N.; Vickers, E.R.; Patwardhan, M.; Gannon, J.; Ruger, L.; Allen, I.C.; Vlaisavljevich, E.; Tuohy, J. Investigating cell death responses associated with histotripsy ablation of canine osteosarcoma. Int. J. Hyperth. 2023, 40, 2279027. [Google Scholar] [CrossRef]
- Schade, G.R.; Keller, J.; Ives, K.; Cheng, X.; Rosol, T.J.; Keller, E.; Roberts, W.W. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J. Urol. 2012, 188, 1957–1964. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Khokhlova, T.D.; Giraldez, M.D.; Schade, G.R.; Starr, F.; Wang, Y.N.; Gallichotte, E.N.; Wang, K.; Hwang, J.H.; Tewari, M. Release of Cell-free MicroRNA Tumor Biomarkers into the Blood Circulation with Pulsed Focused Ultrasound: A Noninvasive, Anatomically Localized, Molecular Liquid Biopsy. Radiology 2017, 283, 158–167. [Google Scholar] [CrossRef]
- Styn, N.R.; Wheat, J.C.; Hall, T.L.; Roberts, W.W. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J. Endourol. 2010, 24, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Schade, G.R.; Wang, Y.N.; D’Andrea, S.; Hwang, J.H.; Liles, W.C.; Khokhlova, T.D. Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response. Ultrasound Med. Biol. 2019, 45, 137–147. [Google Scholar] [CrossRef]
- Verma, Y.; Perera Molligoda Arachchige, A.S. Revolutionizing brain interventions: The multifaceted potential of histotripsy. Neurosurg. Rev. 2024, 47, 124. [Google Scholar] [CrossRef]
- Wagner, M.G.; Minesinger, G.M.; Falk, K.L.; Kutlu, A.Z.; Kisting, M.A.; Speidel, M.A.; Ziemlewicz, T.J.; Hinshaw, J.L.; Swietlik, J.F.; Lee, F.T., Jr.; et al. Evaluation of targeting accuracy of cone beam CT guided histotripsy in an in vivo porcine model. Int. J. Hyperth. 2025, 42, 2455138. [Google Scholar] [CrossRef]
- Hubbard, R.; Choi, D.; Worlikar, T.; Scheven, U.; Kim, H.; Sukovich, J.; Hall, T.L.; Xu, Z. MRI Co-registered Rodent Histotripsy Array for Orthotopic Liver Models. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2025, 72, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Jove, J.; Serres, X.; Vlaisavljevich, E.; Cannata, J.; Duryea, A.; Miller, R.; Merino, X.; Velat, M.; Kam, Y.; Bolduan, R.; et al. First-in-man histotripsy of hepatic tumors: The THERESA trial, a feasibility study. Int. J. Hyperth. 2022, 39, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Wah, T.M.; Pech, M.; Thormann, M.; Serres, X.; Littler, P.; Stenberg, B.; Lenton, J.; Smith, J.; Wiggermann, P.; Planert, M.; et al. A Multi-centre, Single Arm, Non-randomized, Prospective European Trial to Evaluate the Safety and Efficacy of the HistoSonics System in the Treatment of Primary and Metastatic Liver Cancers (#HOPE4LIVER). Cardiovasc. Interv. Radiol. 2023, 46, 259–267. [Google Scholar] [CrossRef]
- HistoSonics. (2024, December 18). World’s First Patients Treated with Novel Edison® Histotripsy System. Available online: https://histosonics.com/news/worlds-first-patients-treated-with-novel-edison-histotripsy-system/ (accessed on 22 February 2025).
- Histotripsy—Clinical Trials Search Results. Available online: https://clinicaltrials.gov/search?intr=Histotripsy (accessed on 21 April 2025).
- Verma, Y.; Arachchige, A. Revolutionizing cardiovascular care: The power of histotripsy. J. Ultrasound 2024, 27, 759–768. [Google Scholar] [CrossRef]
- Schuster, T.G.; Wei, J.T.; Hendlin, K.; Jahnke, R.; Roberts, W.W. Histotripsy Treatment of Benign Prostatic Enlargement Using the Vortx R(x) System: Initial Human Safety and Efficacy Outcomes. Urology 2018, 114, 184–187. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. De Novo Classification Request for Edison System; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023.
- Rogel Cancer Center. Histotripsy FAQ. Available online: https://www.rogelcancercenter.org/liver-cancer/histotripsy-faq (accessed on 10 March 2025).
- Comprehensive Cancer Center. Histotripsy-Liver Cancer Ultrasound. Available online: https://www.uchicagomedicine.org/cancer/types-treatments/histotripsy (accessed on 10 March 2025).
- UW Health. Game-Changing Liver Cancer Treatment. Available online: https://www.uwhealth.org/treatments/histotripsy (accessed on 10 March 2025).
- Histosonics. Patient Education-Histotripsy Procedure in Liver. 2024. Available online: https://myhistotripsy.com/wp-content/uploads/2024/08/PatientInformation.pdf (accessed on 20 February 2025).
- Gastroenterology and GI Surgery. Histotripsy Makes Its Clinical Debut in NYC. Available online: https://physicianfocus.nyulangone.org/histotripsy-makes-its-clinical-debut-in-nyc/ (accessed on 12 March 2025).
- Kumar, Y.N.; Singh, Z.; Wang, Y.N.; Kanabolo, D.; Chen, L.; Bruce, M.; Vlaisavljevich, E.; True, L.; Maxwell, A.D.; Schade, G.R. A comparative study of histotripsy parameters for the treatment of fibrotic ex-vivo human benign prostatic hyperplasia tissue. Sci. Rep. 2024, 14, 20365. [Google Scholar] [CrossRef]
- Kisting, M.A.; Jentink, M.S.; Wagner, M.G.; Xu, Z.; Hinshaw, J.L.; Laeseke, P.F. Imaging for targeting, monitoring, and assessment after histotripsy: A non-invasive, non-thermal therapy for cancer. EMJ Radiol. 2023, 10, 15–21. [Google Scholar] [CrossRef]
- Mouratidis, P.X.E.; Ter Haar, G. Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Medicine. Histotripsy for Liver Tumors. Available online: https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/histotripsy-for-liver-tumors (accessed on 10 March 2025).
- Bader, K.B.; Hendley, S.A.; Anthony, G.J.; Bollen, V. Observation and modulation of the dissolution of histotripsy-induced bubble clouds with high-frame rate plane wave imaging. Phys. Med. Biol. 2019, 64, 115012. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Hall, T.L.; Xu, Z.; Fowlkes, J.B.; Cain, C.A. Imaging feedback of histotripsy treatments using ultrasound shear wave elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, V.V.; Wallach, E.L.; Bader, K.B.; Shekhar, H. Contrast-Enhanced Imaging of Histotripsy Bubble Clouds Using Chirp-Coded Excitation and Volterra Filtering. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 989–998. [Google Scholar] [CrossRef]
- Allen, S.P.; Hernandez-Garcia, L.; Cain, C.A.; Hall, T.L. MR-based detection of individual histotripsy bubble clouds formed in tissues and phantoms. Magn. Reson. Med. 2016, 76, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.P.; Vlaisavljevich, E.; Shi, J.; Hernandez-Garcia, L.; Cain, C.A.; Xu, Z.; Hall, T.L. The response of MRI contrast parameters in in vitro tissues and tissue mimicking phantoms to fractionation by histotripsy. Phys. Med. Biol. 2017, 62, 7167–7180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Kaovasia, T.P.; Komaiha, M.; Nielsen, J.F.; Allen, S.P.; Hall, T.L.; Noll, D.C.; Xu, Z. Transcranial MRI-guided Histotripsy Targeting Using MR-thermometry and MR-ARFI. Ultrasound Med. Biol. 2025, 51, 330–335. [Google Scholar] [CrossRef]
- Longo, K.C.; Knott, E.A.; Watson, R.F.; Swietlik, J.F.; Vlaisavljevich, E.; Smolock, A.R.; Xu, Z.; Cho, C.S.; Mao, L.; Lee, F.T., Jr.; et al. Robotically Assisted Sonic Therapy (RAST) for Noninvasive Hepatic Ablation in a Porcine Model: Mitigation of Body Wall Damage with a Modified Pulse Sequence. Cardiovasc. Interv. Radiol. 2019, 42, 1016–1023. [Google Scholar] [CrossRef]
- Kutlu, A.Z.; Laeseke, P.F.; Zeighami Salimabad, M.; Minesinger, G.M.; Periyasamy, S.; Pieper, A.A.; Hall, T.J.; Wagner, M.G. A Multimodal Phantom for Visualization and Assessment of Histotripsy Treatments on Ultrasound and X-Ray Imaging. Ultrasound Med. Biol. 2023, 49, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Mauch, S.C.; Zlevor, A.M.; Knott, E.A.; Couillard, A.B.; Periyasamy, S.; Williams, E.C.; Swietlik, J.F.; Laeseke, P.F.; Zhang, X.; Xu, Z.; et al. Hepatic and Renal Histotripsy in an Anticoagulated Porcine Model. J. Vasc. Interv. Radiol. 2023, 34, 386–394 e382. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.G.; Periyasamy, S.; Kutlu, A.Z.; Pieper, A.A.; Swietlik, J.F.; Ziemlewicz, T.J.; Hall, T.L.; Xu, Z.; Speidel, M.A.; Lee, F.T., Jr.; et al. An X-Ray C-Arm Guided Automatic Targeting System for Histotripsy. IEEE Trans. Biomed. Eng. 2023, 70, 592–602. [Google Scholar] [CrossRef]
- Serres-Creixams, X.; Vidal-Jove, J.; Ziemlewicz, T.J.; Cannata, J.M.; Escudero-Fernandez, J.M.; Uriarte, I.; Alemany-Botelho, C.; Roson, N.; Escobar, M. Contrast-Enhanced Ultrasound: A Useful Tool to Study and Monitor Hepatic Tumors Treated with Histotripsy—PubMed. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2853–2860. [Google Scholar] [CrossRef]
- Trivedi, V.; Basterrechea, K.; Bader, K.; Shekhar, H. Chirp-Coded Subharmonic Imaging with Volterra Filtering: Histotripsy Bubble Cloud Assessment In Vitro and Ex Vivo–PubMed. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2025, 72, 591–600. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Serres-Creixams, X.; Ziemlewicz, T.J.; Cannata, J.M. Liver Histotripsy Mediated Abscopal Effect-Case Report. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Styn, N.R.; Hall, T.L.; Fowlkes, J.B.; Cain, C.A.; Roberts, W.W. Histotripsy of renal implanted VX-2 tumor in a rabbit model: Investigation of metastases. Urology 2012, 80, 724–729. [Google Scholar] [CrossRef]
- Uysal, M.; Wehrle, C.J.; Coppa, C.; Kamath, S.; Krishnamurthi, S.; Martin, C.; Hag, M.E.; Khalil, M.; Fujiki, M.; Schlegel, A.; et al. Bridging therapy with histotripsy prior to liver transplantation for hepatocellular carcinoma: A first case report. Exp. Hematol. Oncol. 2025, 14, 20. [Google Scholar] [CrossRef]
- Queen, H.; Cho, C.S. How could histotripsy change cancer immunotherapy? Immunotherapy 2025, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mungur, R.; Zheng, J.; Wang, B.; Chen, X.; Zhan, R.; Tong, Y. Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment. Front. Oncol. 2022, 12, 903059. [Google Scholar] [CrossRef] [PubMed]
- Schibber, E.F.; Mittelstein, D.R.; Gharib, M.; Shapiro, M.G.; Lee, P.P.; Ortiz, M. A dynamical model of oncotripsy by mechanical cell fatigue: Selective cancer cell ablation by low-intensity pulsed ultrasound. Proc. Math. Phys. Eng. Sci. 2020, 476, 20190692. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.B.; Kren, N.P.; Durham, P.G.; McRee, A.J.; Dayton, P.A.; Pylayeva-Gupta, Y. Low-Intensity Focused Ultrasound Produces Immune Response in Pancreatic Cancer. Ultrasound Med. Biol. 2022, 48, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
| Ultrasound Parameters | ||||
|---|---|---|---|---|
| Parameter | Intrinsic Threshold Histotripsy | Shock-Scattering Histotripsy | HIFU | Boiling Histotripsy |
| Frequency | 250 kHz–3 MHz | 500 kHz–3 MHz | 1–5 MHz | 1–5 MHz |
| Peak Negative Pressure | 26–30 MPa | 15–20 MPa | 5–10 MPa | 10–20 MPa |
| Peak Positive Pressure | N/A | >50 MPa | <30 MPa | >70 MPa |
| Duty Cycle | <1% | <1% | 10–100% | <2% |
| Pulse Duration | 1–2 cycles | 3–10 cycles | 10–20 ms | High duty cycle |
| Pulse Repetition Frequency | 1 Hz–1 kHz | 1 Hz–1 kHz | – | 1 Hz–2 kHz |
| Mechanism | Mechanical cavitation | Shock-scattering cavitation | Thermal coagulative necrosis | Boiling mechanical disruption |
| Study | Organ System | Cancer Model |
|---|---|---|
| Histotripsy for Non-Invasive Ablation of Hepatocellular Carcinoma (HCC) Tumor in a Subcutaneous Xenograft Murine Model (Worlikar et al., 2018) [49] | Liver: Hepatocellular Carcinoma | Subcutaneous Hep3B tumors in NSG and NOD-SCID mice |
| Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy (Qu et al., 2020) [50] | Liver: Hepatocellular Carcinoma Integumentary: Melanoma | Subcutaneous Hepa1-6 hepatocellular carcinoma in C57BL/6 mice Subcutaneous B16GP33 melanoma in C57BL/6 mice |
| Effects of Histotripsy on Local Tumor Progression in an in vivo Orthotopic Rodent Liver Tumor Model (Worlikar et al., 2020) [51] | Liver: Hepatocellular Carcinoma | Orthotopic N1-S1 hepatic tumors in immunocompetent Sprague Dawley rats |
| Impact of Histotripsy on Development of Intrahepatic Metastases in a Rodent Liver Tumor Model (Worlikar et al., 2022) [52] | Liver: Hepatocellular Carcinoma | Orthotopic McA-RH7777 hepatic tumors in immunocompetent Sprague Dawley rats |
| Spatiotemporal local and abscopal cell death and immune responses to histotripsy focused ultrasound tumor ablation (Pepple et al., 2023) [53] | Liver: Hepatocellular Carcinoma Integumentary: Melanoma | Subcutaneous Hepa1-6 hepatocellular carcinoma in C57BL/6 mice Subcutaneous B16F10 melanoma in C57BL/6 mice |
| Histotripsy Ablation in Preclinical Animal Models of Cancer and Spontaneous Tumors in Veterinary Patients: A Review (Hendricks-Wegner et al., 2021) [46] | Bile Duct: Cholangiocarcinoma Breast: Stage IV Breast Cancer Renal: Renal Cell Carcinoma Pancreas: Epithelioid Carcinoma Liver: Hepatocellular Carcinoma Soft Tissue: Sarcoma Bone: Osteosarcoma | Subcutaneous patient-derived xenograft cholangiocarcinoma in immunocompromised NSG mice Orthotopic 4T1 mammary tumor in BALB/c mice Subcutaneous Renca RCC in BALB/c mice Subcutaneous Panc01 in RAG2/IL2RG deficient pigs Subcutaneous HepG2 in RAG2/IL2RG deficient pigs Spontaneous soft tissue sarcoma in canines Spontaneous hindlimb osteosarcoma in canines |
| Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer (Hendricks-Wenger et al., 2021) [54] | Pancreas: Pancreatic Adenocarcinoma | Subcutaneous Pan02 in C57/Bl6 mice |
| Successful In Situ Targeting of Pancreatic Tumors in a Novel Orthotopic Porcine Model Using Histotripsy (Imran et al., 2023) [55] | Pancreas: Ductal Epithelial Carcinoma | Orthotopic Panc-1 in RAG2/IL2RG double-knockout pigs |
| Histotripsy for the Treatment of Cholangiocarcinoma Liver Tumors: In Vivo Feasibility and Ex Vivo Dosimetry Study (Hendricks-Wenger et al., 2021) [56] | Bile Duct: Cholangiocarcinoma | Subcutaneous patient-derived xenograft cholangiocarcinoma in immunocompromised NSG mice |
| Histotripsy for the Treatment of Cholangiocarcinoma in a Patient-Derived Xenograft Mouse Model (Hendricks-Wenger et al., 2022) [57] | Bile Duct: Cholangiocarcinoma (Adenosquamous Carcinoma) | Subcutaneous patient-derived xenograft of adenosquamous carcinoma (TM01225), a subtype of cholangiocarcinoma, in immunocompromised NSG mice |
| Investigation of the Potential Immunological Effects of Boiling Histotripsy for Cancer Treatment (Nam et al., 2020) [58] | Colon: Colon Carcinoma Breast: Triple-negative Breast Carcinoma | Subcutaneous CT26 colorectal in BALB/c mice Subcutaneous 4T1 triple-negative breast carcinoma in BALB/c mice |
| Ultrasound-Guided Histotripsy Triggers the Release of Tumor-Associated Antigens from Breast Cancers (Tang et al., 2025) [59] | Breast: HER-2+ Breast Cancer | Spontaneous HER2-overexpressing E0771E2 in C57BL/6 HER2 transgenic mice |
| Enabling Chemo-Immunotherapy with HIFU in Canine Cancer Patients (Ashar et al., 2024) [60] | Breast: Mammary Mass Breast: Papillary Adenocarcinoma Musculoskeletal: Soft Tissue Sarcoma (low-grade) Musculoskeleta: Lipoma Immune System: Mass Cell Tumor (low-grade) | Spontaneous tumor in canines |
| Boiling Histotripsy Using Dual-Frequency Protocol on Murine Breast Tumor Model and Promotes Immune Activation (Qi et al., 2023) [61] | Breast: Triple-negative Breast Carcinoma | Subcutaneous 4T1 in BALB/c mice |
| Histoplasty Modification of the Tumor Microenvironment in a Murine Preclinical Model of Breast Cancer (Pieper et al., 2024) [62] | Breast: Triple-negative Breast Carcinoma | Subcutaneous 4T1 in BALB/c mice |
| Stereotactic Transcranial Focused Ultrasound Targeting System for Murine Brain Models (Choi et al., 2020) [63] | Central Nervous System: Glioblastoma | Orthotopic GL261-luciferase in B6 albino mice |
| Histotripsy Treatment of Murine Brain and Glioma: Temporal Profile of Magnetic Resonance Imaging and Histological Characteristics Post-treatment (Choi et al., 2023) [64] | Central Nervous System: Glioma | Orthotopic GL261 in BL6 mice |
| Histotripsy Mediated Immunomodulation in a Mouse GL261 Intracranial Glioma Model (Gerhardson et al., 2018) [65] | Central Nervous System: Glioblastoma | Orthotopic GL261-luc2 in C57 BL/6 albino mice |
| Transcranial histotripsy parameter study in primary and metastatic murine brain tumor models (Duclos et al., 2023) [66] | Central Nervous System: Glioma Central Nervous System: Lung Metastasis | Orthotopic GL261 in C57BL/6 mice Orthotopic LL/2-Luc2 in C57BL/6 mice |
| First-In-DOg HISTotripsy for Intracranial Tumors Trial: The FIDOHIST Study (Vezza et al., 2024) [67] | Central Nervous System: Meningiomas | Spontaneous tumor in canines |
| Histotripsy induces apoptosis and reduces hypoxia in a neuroblastoma xenograft model (Iwanicki et al., 2023) [68] | Peripheral Nervous System: Neuroblastoma | Orthotopic NGP-luciferase in NCR nude mice |
| High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy (Eranki et al., 2020) [69] | Peripheral Nervous System: Neuroblastoma | Subcutaneous Neuro2a in A/J mice |
| Impact of MR-guided boiling histotripsy in distinct murine tumor models (Hoogenboom et al., 2017) [70] | Peripheral Nervous System: Neuroblastoma Integumentary: Melanoma Immune System: Lymphoma | Subcutaneous 9464D in C57Bl/6NCrl mice Subcutaneous B16OVA in C57Bl/6NCrl mice Subcutaneous EL4 in C57Bl/6NCrl mice |
| Boiling histotripsy and in situ CD40 stimulation improve the checkpoint blockade therapy of poorly immunogenic tumors (Singh et al., 2021) [71] | Integumentary: Melanoma | Subcutaneous B16F10 in C57BL/6 mice |
| Focused ultrasound ablation of melanoma with boiling histotripsy yields abscopal tumor control and antigen-dependent dendritic cell activation (Thim et al., 2024) [72] | Integumentary: Melanoma | Subcutaneous B16F10-ZsGreen in C57Bl/6J mice |
| Histotripsy-Focused Ultrasound Treatment Abrogates Tumor Hypoxia Responses and Stimulates Antitumor Immune Responses in Melanoma (Song et al., 2025) [73] | Integumentary: Melanoma | Subctuaneous B16F10 or YUMM1.7 in immunocompetent or CD8-deficient C57BL/6 mice |
| In vivo MR guided boiling histotripsy in a mouse tumor model evaluated by MRI and histopathology (Hoogenboom et al., 2016) [74] | Immune System: Thymoma | Subcutaneous EL4 in C57Bl/6NCrl mice |
| Characterizing the Ablative Effects of Histotripsy for Osteosarcoma: In Vivo Study in Dogs (Ruger et al., 2023) [75] | Musculoskeletal: Bone | Spontaneously arising osteosarcoma and chondrosarcoma in canines |
| Mechanical High-Intensity Focused Ultrasound (Histotripsy) in Dogs with Spontaneously Occurring Soft Tissue Sarcomas (Ruger et al., 2023) [76] | Musculoskeletal: Soft Tissue | Spontaneously arising soft tissue sarcoma in canines |
| Histotripsy Ablation of Spontaneously Occurring Canine Bone Tumors (Ruger at al., 2022) [77] | Musculoskeletal: Bone | Spontaneously arising osteosarcoma and chondrosarcoma in canines |
| Histotripsy ablation for the treatment of feline injection site sarcomas: a first-in-cat in vivo feasibility study (Ruger et al., 2023) [78] | Musculoskeletal: Soft Tissue | Spontaneously arising soft tissue sarcoma in felines |
| Histotripsy Ablation of Bone Tumors: Feasibility Study in Excised Canine Osteosarcoma Tumors (Arnold et al., 2021) [79] | Musculoskeletal: Bone | Spontaneous osteosarcoma tumors in canines in 7.5% gelatin in degassed saline tissue phantom |
| Investigating cell death responses associated with histotripsy ablation of canine osteosarcoma (Hay et al., 2023) [80] | Musculoskeletal: Bone | Spontaneously arising osteosarcoma in canine patients |
| Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model (Schade et al., 2012) [81] | Prostate: Prostate Cancer | Orthotopic ACE-1 prostate tumor in canines |
| Release of Cell-free MicroRNA Tumor Biomarkers into the Blood Circulation with Pulsed Focused Ultrasound: A Noninvasive, Anatomically Localized, Molecular Liquid Biopsy (Chevillet et al., 2016) [82] | Prostate: Prostate Cancer | Subcutaneous MatLyLu cells in Copenhagen rats |
| Histotripsy of VX-2 tumor implanted in a renal rabbit model (Styn et al., 2010) [83] | Kidney: Anaplastic Squamous Cell Carcinoma | Orthotopic VX-2 tumor implanted in New Zealand rabbits |
| Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response (Schade et al. 2019) [84] | Kidney: Renal Cell Carcinoma | Spontaneous renal cell carcinoma in Eker rat model |
| Name of Study | Status | Estimated Completion | Sponsor | Clinical Trials ID | Cancer Type | Enrollment or Estimated | Inclusion Criteria | Exclusion Criteria | Primary Outcome Measures | Secondary Outcome Measures (if Included) |
|---|---|---|---|---|---|---|---|---|---|---|
| Histotripsy (HistoSonics®) for Liver Tumours | Not yet recruiting | 2028-09-01 | The University of Hong Kong | NCT06579833 | primary or secondary liver tumors | 20 | Fit for general anesthesia Liver tumor size < 10 cm Solitary or multifocal Primary liver tumor such as hepatocellular carcinoma or intrahepatic cholangiocarcinoma Secondary liver tumor such as liver metastasis Patients with operable or inoperable liver tumors Liver transplant candidates awaiting for liver graft | Refusal to take part in clinical trial Child C liver cirrhosis Not fit for general anesthesia | Changes in tumor features up to 36 months (size and volume before and after intervention), post procedure adverse events and complication during hospital stay, usually 3 days | |
| The HistoSonics Edison™ System for Treatment of Primary Solid Renal Tumors Using Histotripsy (#HOPE4KIDNEY) (#HOPE4KIDNEY) | Recruiting | 2030-05-01 | HistoSonics, Inc. | NCT05820087 | primary solid renal tumors | 68 | Subject is ≥22 years of age. Subject has signed the Institutional Review Board (IRB) approved trial Informed Consent Form (ICF) prior to any trial related tests/procedures and is willing to comply with trial procedures and required follow-up assessments. Subject is diagnosed with only one (1) non-metastatic solid renal mass ≤ 3 cm confirmed via CT or MRI ≤ 30 days prior to the index procedure date. Subject has had a biopsy to determine the type of tumor, ≥14 days prior to the index procedure. Subject can tolerate general anesthesia. Subject has an Eastern Cooperative Oncology Group Performance Status (ECOG PS) grade 0–2 at baseline screening. Subject meets all the following functional criteria at ≤14 days prior to the planned index procedure date: White Blood Count (WBC) ≥ 3000/mm3 (≥3 × 109/L) Absolute Neutrophil Count (ANC) ≥ 1200/mm3 (≥1.2 × 109/L) Hemoglobin (Hgb) ≥ 9 g/dL Platelet count ≥ 100,000/mm3 (≥100 × 109/L) Subject has an eGFR (Glomerular filtration rate) ≥45 mL/min, ≤14 days prior to the planned index procedure date. The tumor selected for histotripsy treatment must be ≤3 cm in longest diameter. Subject has an adequate acoustic window to visualize targeted tumor using the HistoSonics Edison System. | Subject is pregnant or planning to become pregnant or nursing (lactating) during the trial period. Subject is being actively treated in another pharmaceutical or device trial ≤ 30 days prior to planned index procedure date that may interfere with the primary endpoint(s). Subjects who have active cancers (not in remission for the last two years) other than non-melanomatous skin cancers. In the Investigator’s opinion, the subject has co-morbid disease(s) or condition(s) that would cause undue risk and preclude safe use of the HistoSonics Edison System. Subject is on dialysis, being considered for dialysis or has acute renal failure. Subject has not recovered to Common Terminology Criteria for Adverse Events (CTCAE) grade 2 or better from any adverse effects (except alopecia and neuropathy) related to previous therapy. Subject has an International normalized ratio (INR) > 1.5 or uncorrectable coagulopathy (e.g., known von Willebrand disease, hemophilia, or on anticoagulants), on the planned index procedure date. Subject is taking Aspirin (ASA) or NSAIDS ≤ 7 days prior to the planned index procedure date. Subject has a life expectancy less than one (<1) year. In the investigator’s opinion, histotripsy is not a treatment option for the subject. Subject has a concurrent condition that could jeopardize the safety of the subject or compliance with the protocol. Subject’s targeted tumor has had prior locoregional therapy (e.g., ablation, embolization, radiation). Subject’s targeted tumor is not treatable by the HistoSonics Edison System’s working ranges (refer to User Guide). In the investigator’s opinion, the anticipated risks of intervention outweigh the potential benefits of the intervention. Subject has bilateral kidney tumors or has a single functioning kidney. Subject has a genetic predisposition to kidney cancer such as: Von Hippel Lindau (VHL), Hereditary Papillary Renal Carcinoma (HPRC), Birt-Hogg-Dubé Syndrome (BHD), Tuberous Sclerosis Complex (TSC), Hereditary Leiomyomata’s Renal Cell Carcinoma (HLRCC), Reed’s Syndrome, Succinate Dehydrogenase B Deficiency (SDHB), BRCA 1 associated protein -1 (BAP1) Renal Cell Carcinoma, MITF predisposed Renal Cell Carcinoma The targeted tumor is an angiomyolipoma. Subject has a known sensitivity to contrast media and cannot be adequately pre-medicated. Subject has a urinary tract infection (UTI) ≤7 days prior to the planned index procedure date. The targeted tumor is not clearly visible with ultrasound, MRI or CT. Targeted tumor with adequate margin overlaps the renal pelvis, main renal vessel, ureter, organ or other vital structure. The treatment of the tumor will not allow an adequate margin (as determined by the investigator). | Primary technique efficacy defined as the percentage of targeted tumors that were successfully eliminated after a single histotripsy session as assessed by contrast enhanced MRI or CT at 90 days. Primary Safety Endpoint–Freedom from index procedure related major complications, defined by Clavien–Dindo Classification Grade 3 or higher up to 30 days after the histotripsy procedure. | Technical success demonstrating complete coverage of the targeted tumor as determined post-index procedure (≤36 h) by contrast enhanced MRI or CT in subjects whom treatment was initiated. Secondary Safety Endpoint–Freedom from index procedure related major complications, defined by Clavien–Dindo Classification Grade 3 or higher up to 90 days after the histotripsy procedure. |
| The HistoSonics Investigational System for Treatment of Primary Solid Renal Tumors Using Histotripsy (CAIN) | Active, not recruiting | 2025-06-01 | HistoSonics, Inc. | NCT05432232 | primary solid renal tumors | 20 | Subject is ≥18 years of age. Subject has signed the Ethics Committee (EC) approved trial Informed Consent Form (ICF) prior to any trial related tests/procedures and is willing to comply with trial procedures and required follow-up assessments. Subject is diagnosed with a non-metastatic solid renal mass ≤ 3 cm confirmed via CT or MRI ≤ 30 days prior to the index procedure date. Subject can tolerate general anesthesia. Subject has an Eastern Cooperative Oncology Group Performance Status (ECOG PS) grade 0–2 at baseline screening. Subject meets all the following functional criteria at ≤14 days prior to the planned index procedure date: White Blood Cell (WBC) ≥ 3000/mm3 Absolute Neutrophil Count (ANC) ≥ 1200/mm3 Hemoglobin (Hgb) ≥ 9 g/dL Platelet count ≥ 100,000/mm3 (≥100 × 109/L) White Blood Cell (WBC) ≤ 40 cells/µL via urinalysis Albumin ≤ 300,000 mg/L via urinalysis Subject has an eGFR ≥ 45 mL/min, ≤14 days prior to the planned index procedure date. International Normalized Ratio (INR) score of <1.5 If on anticoagulants, other than aspirin or non-steroidal anti-inflammatory drugs, assessment must be performed on the day of the procedure; OR If only on aspirin or non-steroidal anti-inflammatory drugs, assessment must be performed ≤14 days prior to the planned index procedure date; OR If not on anticoagulants, assessment must be performed ≤14 days prior to the planned index procedure date Biopsy is required to determine the type of tumor and must be performed ≥14 days prior to the planned index procedure date. The tumor selected for histotripsy treatment must be ≤3 cm in longest diameter. Subject has an adequate acoustic window to visualize targeted tumor using the HistoSonics Investigational System. Subject will undergo histotripsy treatment of only one (1) tumor during the index procedure, regardless of how many tumors the subject has. | Subject is pregnant or planning to become pregnant or nursing (lactating) during the trial period. Subject is enrolled and being actively treated in another investigational pharmaceutical or device trial ≤ 30 days prior to planned index procedure date. Subject is undergoing active chemotherapy for any cancer ≤ 14 days prior to planned index procedure date. Subject is undergoing active immunotherapy ≤ 40 days prior to planned index procedure date. In the Investigator’s opinion, the subject has co-morbid disease(s) or condition(s) that would cause undue risk and preclude safe use of the HistoSonics Investigational System. Subject is on dialysis or being considered for dialysis. Subject has not recovered to Common Terminology Criteria for Adverse Events (CTCAE) grade 2 or better from any adverse effects (except alopecia and neuropathy) related to previous anti-cancer therapy. Subject has an uncorrectable coagulopathy other than that induced by aspirin or non-steroidal anti-inflammatory drugs. Subject has a planned cancer treatment (e.g., nephrectomy, chemotherapy, immunotherapy, etc.) prior to completion of the 30-day follow-up visit. Subject has had previous treatments with chemotherapy, radiotherapy, or both that have not been discontinued ≥14 days prior to the planned index procedure date and have not recovered (CTCAE grade 2 or better) from related toxicity (exclusive of alopecia and neuropathy). Subject has previous treatment with immunotherapies that has not been discontinued ≥40 days prior to the planned index procedure date and has not recovered from related toxicity (CTCAE grade 2 or better). Subject has a life expectancy less than one (<1) year. In the investigator’s opinion, histotripsy is not a treatment option for the subject. Subject has a concurrent condition that could jeopardize the safety of the subject or compliance with the protocol. Subjects’ targeted tumor has had prior locoregional therapy (e.g., ablation, embolization, radiation). Subjects’ tumor is not treatable by the HistoSonics Investigational System’s working ranges (refer to User Guide). In the physician’s opinion, the anticipated risk of intervention outweighs the potential benefits of the intervention. Subject has acute renal failure. Subject has a genetic predisposition to kidney cancer such as: Subject has a genetic predisposition to kidney cancer such as: Von Hippel Lindau (VHL), Hereditary Papillary Renal Carcinoma (HPRC), Birt-Hogg-Dubé Syndrome (BHD), Tuberous Sclerosis Complex (TSC), Hereditary Leiomyomata’s Renal Cell Carcinoma (HLRCC), Reed’s Syndrome, Succinate Dehydrogenase B Deficiency (SDHB), BRCA 1 associated protein -1 (BAP1) Renal Cell Carcinoma, MITF predisposed Renal Cell Carcinoma Tumor is an angiomyolipoma. Subject has a known sensitivity to contrast media and cannot be adequately pre-medicated. The targeted tumor is not clearly visible with diagnostic ultrasound and either magnetic resonance imaging (MRI) or computerized tomography (CT). Targeted tumor with adequate margin overlaps the renal pelvis, main renal vessel, ureter, or other vital structure. Targeted tumor with adequate margin overlaps a non-targeted tumor visible via imaging. The treatment of the tumor will not allow for an adequate margin as determined by the investigator. | Technical success, defined as complete coverage of the tumor as determined ≤36 h post-index procedure by magnetic resonance imaging (MRI) or computerized tomography (CT). Primary Safety: Freedom from Index Procedure Related Major Complications. Freedom from index procedure related major complications, defined by Clavien–Dindo Classification Grade 3 or higher up to 30 days after the last histotripsy procedure. | Percentage of targeted tumors successfully eradicated post-index procedure assessed via MRI or CT at 90 days post-index procedure without repeat Histotripsy. Technique Efficacy (Secondary) Percentage of targeted tumors successfully eradicated post-index procedure assessed via MRI or CT at 90 days post-index procedure after repeat Histotripsy |
| Real-world Evaluation of the HistoSonics Edison System for Treatment of Liver Tumors Across Multidisciplinary Users (BOOMBOX: Master Study) | Recruiting | 2031-11-01 | HistoSonics, Inc. | NCT06486454 | primary, metastatic, or benign liver tumors | 5000 | Subject is ≥22 years of age Subject has signed the Ethics Committee (EC), or Institutional Review Board (IRB) approved study Informed Consent Form (ICF) prior to any study related tests/procedures and is willing to comply with study procedures and required follow-up assessments Subject’s liver tumor(s) can be partially or completely treated with histotripsy | Subject is pregnant or planning to become pregnant or nursing (lactating) during the study period Subject is enrolled in an interventional HistoSonics-sponsored trial Subject has a concurrent condition that, in the investigator’s opinion, could jeopardize the safety of the subject or compliance with the protocol | Histotripsy technical success, defined as completion of histotripsy on the target tumor(s) according to the histotripsy treatment plan, assessed by the treating physician on CT or MR imaging at ≤36 h post-histotripsy treatment procedure. The histotripsy treatment plan will include identification of the intended complete or partial treatment of the tumor(s). The histotripsy treatment zone must provide target tumor coverage greater than or equal to the degree of treatment intended. | |
| The HistoSonics System for Treatment of Primary and Metastatic Liver Tumors Using Histotripsy (#HOPE4LIVER US) | Active, not recruiting | 2026-07-01 | HistoSonics, Inc. | NCT04572633 | primary or metastatic liver tumors | 47 | Subject is ≥18 years of age Subject has signed the Ethics Committee (EC) or Institutional Review Board (IRB) approved trial Informed Consent Form (ICF) prior to any trial related tests/procedures and is willing to comply with trial procedures and required follow-up assessments Subject is diagnosed with hepatocellular carcinoma (HCC) or liver metastases (mets) from other primary cancers Subject is able to undergo general anesthesia Subject has a Child-Pugh Score of A or B Subject has an Eastern Cooperative Oncology Group Performance Status (ECOG PS) grade 0–2 at baseline screening Subject meets the following functional criteria, ≤7 days prior to the index-procedure: Liver function: Alanine transaminase (ALT) and Aspartate transaminase (AST) < 2.5× upper limit of normal (ULN) and/or bilirubin < 2.5 ULN, and Renal function: serum creatinine < 2× ULN, and Hematologic function: neutrophil count > 1.0 × 109/L and platelet > 50 × 109/L Subject has an International Normalized Ratio (INR) score of <2.0, ≤7 days prior to the index procedure Subject has not responded to and/or has relapsed and/or is intolerant of other available therapies including locoregional therapies, chemotherapy, immunotherapy and targeted therapies The tumor(s) selected for histotripsy treatment must be ≤3 cm in longest diameter Subject has an adequate acoustic window to visualize targeted tumor(s) using ultrasound imaging Subject has a maximum of three (3) tumors to be treated with histotripsy during the index procedure, regardless of how many tumors the subject has. | Subject is pregnant or planning to become pregnant or nursing (lactating) during the trial period Subject is enrolled in another investigational trial and/or is taking investigational medication and/or has been treated with an investigational device ≤ 30 days prior to planned index procedure date In the Investigator’s opinion, the subject has co-morbid disease(s) or condition(s) that would cause undue risk and preclude safe use of the HistoSonics System Subject has a serum creatinine > 2.0 mg/dL or estimated glomerular filtration rate (EGFR) < 30, unless on dialysis Subject has major surgical procedure or significant traumatic injury ≤ 2 weeks prior to the planned index procedure or not fully recovered (CTCAE grade 1 or better) from side effects/complications of such procedure or trauma Subject has not recovered to common terminology criteria for adverse events (CTCAE) grade 1 or better from any adverse effects (except alopecia, fatigue, nausea, vomiting and peripheral neuropathy) related to previous anti-cancer therapy Subject has a history of, or suspected to have, bleeding disorders that are uncorrectable Subject has coagulopathy that is uncorrectable Subject has a planned cancer treatment (e.g., resection, chemotherapy, etc.) after the planned index-procedure date and prior to completion of the 30-day follow-up visit Subject has previous treatment with bevacizumab that has not been discontinued >40 days prior to the planned index-procedure date Subject has planned bevacizumab treatment prior to completion of the 30-day follow-up visit Subject has previous treatments with chemotherapy and/or radiotherapy that has not been discontinued ≥2 weeks prior to the planned index-procedure date and has not recovered (CTCAE grade 1 or better) from related toxicity (except alopecia and peripheral neuropathy) Subject has previous treatment with immunotherapies that has not been discontinued ≥4 weeks prior to the index-procedure and has not recovered from related toxicity (CTCAE grade 1 or better) Subject has a life expectancy less than six (<6) months In the opinion of the Investigator, histotripsy is not a treatment option for the subject Subject has a concurrent condition that, in the investigator’s opinion, could jeopardize the safety of the subject or compliance with the protocol Subjects’ tumor(s) is not treatable by the System’s working ranges (refer to User Manual) Subject has a known sensitivity to contrast media and cannot be adequately pre-medicated Subjects’ target tumor(s) has/have had prior locoregional therapy (e.g., ablation, embolization, radiation) Subject is eligible for surgical resection Targeted tumor(s) treatment volume overlaps a non-targeted tumor visible via imaging The targeted tumor(s) is not clearly visible with diagnostic ultrasound and computed tomography (CT) or magnetic resonance (MR) imaging The targeted tumor(s) is located in liver segment 1 The Planned Treatment Volume intended to cover the targeted tumor includes or encompasses any portion of the main portal vein, common hepatic duct, common bile duct, gallbladder or stomach/bowel. | Technical success, defined as the treatment volume/treatment dimensions being greater than or equal to the targeted tumor, and with complete tumor coverage, via computed tomography (CT) or magnetic resonance (MR) imaging. [Core Laboratory Adjudicated] Primary efficacy was assessed per tumor with a performance goal of greater than 70%. Primary efficacy was assessed after the first forty (40) consecutive evaluable subjects were enrolled. Evaluable subjects had sufficient CT or MR imaging data to allow the independent core laboratory to evaluate technical success. Procedure-Related Major Complications: Number of index procedure related major complications, including device-related events defined as Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher toxicities observed up to 30 days post index-procedure. Primary safety was assessed per participant with a performance goal of less than 25%. Primary safety was assessed on all subjects enrolled, after the first forty (40) consecutive subjects evaluable for technical success were enrolled. Evaluable subjects had sufficient CT or MR imaging data to allow the independent core laboratory to evaluate technical success. Enrollment of 44 total subjects was required to assess forty (40) subjects evaluable for technical success. | Technical success, defined as the treatment volume/treatment dimensions being greater than or equal to the targeted tumor, and with complete tumor coverage, via computed tomography (CT) or magnetic resonance (MR) imaging. Number of index procedures related to major complications, including device-related events defined as Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher toxicities observed up to 30 days post index-procedure. Technique efficacy, defined as the lack of a nodular or mass-like area of enhancement within or along the edge of the treatment volume assessed via CT or MR imaging at 30 days post-procedure. Number of adverse events (serious and non-serious) reported within 30 days post-index procedure. |
| The HistoSonics System for Treatment of Primary and Metastatic Liver Tumors Using Histotripsy (#HOPE4LIVER EU/UK) (#HOPE4LIVER) | Active, not recruiting | 2026-07-01 | HistoSonics, Inc. | NCT04573881 | primary or metastatic liver tumors | 24 | Subject is ≥18 years of age Subject has signed the Ethics Committee (EC) or Institutional Review Board (IRB) approved trial Informed Consent Form (ICF) prior to any trial related tests/procedures and is willing to comply with trial procedures and required follow-up assessments Subject is diagnosed with hepatocellular carcinoma (HCC) or liver metastases (mets) from other primary cancers Subject is able to undergo general anesthesia Subject has a Child-Pugh Score of A or B (up to B8) Subject has an Eastern Cooperative Oncology Group Performance Status (ECOG PS) grade 0–2 at baseline screening Subject meets the following functional criteria, ≤7 days prior to the index-procedure: Liver function: Alanine transaminase (ALT) and Aspartate transaminase (AST) < 2.5× upper limit of normal (ULN) and bilirubin < 2.5× ULN, and Renal function: serum creatinine < 2× ULN, and Hematologic function: neutrophil count > 1.0 × 109/L and platelet > 50 × 109/L Subject has an International Normalized Ratio (INR) score of <2.0, ≤7 days prior to the index procedure Subject has not responded to and/or has relapsed and/or is intolerant of other available therapies including locoregional therapies, chemotherapy, immunotherapy and targeted therapies. The tumor(s) selected for histotripsy treatment must be ≤3 cm in longest diameter Subject has an adequate acoustic window to visualize targeted tumor(s) using ultrasound imaging Subject has a maximum of three (3) tumors to be treated with histotripsy during the index procedure, regardless of how many tumors the subject has. | Subject is pregnant or planning to become pregnant or nursing (lactating) during the trial period Subject is enrolled in another investigational trial and/or is taking investigational medication or treated with an investigational device ≤ 30 days prior to index procedure In the Investigator’s opinion, the subject has co-morbid disease(s) or condition(s) that would cause undue risk and preclude safe use of the HistoSonics System Subject has a serum creatinine > 2.0 mg/dL or estimated glomerular filtration rate (EGFR) < 30, unless on dialysis Subject has major surgical procedure or significant traumatic injury ≤ 2 weeks prior to the index procedure or not fully recovered from side effects/complications of such procedure or trauma Subject has not recovered to common terminology criteria for adverse events (CTCAE) grade 1 or better from any adverse effects (except alopecia) related to previous anti-cancer therapy Subject has a history of, or suspected to have, bleeding disorders that are uncorrectable Subject has a coagulopathy that is uncorrectable Subject has a planned cancer treatment (e.g., resection, chemotherapy, etc.) from the index-procedure date and prior to completion of the 30 day follow-up visit Subject has previous treatment with bevacizumab that has not been discontinued >40 days prior to the planned index-procedure date Subject has planned bevacizumab treatment prior to completion of the 30 day follow-up visit Subject has previous treatments with chemotherapy and/or radiotherapy that has not been discontinued ≥2 weeks prior to the planned index-procedure date or has not recovered from related toxicity Subject has previous treatment with immunotherapies that has not been discontinued ≥4 weeks prior to the index-procedure or has not recovered from related toxicity Subject has a life expectancy less than six (<6) months In the opinion of the Investigator, histotripsy is not a treatment option for the subject Subject has a concurrent condition that, in the investigator’s opinion, could jeopardize the safety of the subject or compliance with the protocol Subjects’ tumor(s) is not treatable by the System’s working ranges (refer to User Manual) Subject has a known sensitivity to contrast media and cannot be adequately pre-medicated Subjects’ targeted tumor(s) has/have had prior locoregional therapy (e.g., ablation, embolization, radiation) Subject is eligible for surgical resection Targeted tumor(s) treatment volume overlaps a non-targeted tumor visible via imaging The targeted tumor(s) is not clearly visible with diagnostic ultrasound and computed tomography (CT) or magnetic resonance (MR) imaging The targeted tumor(s) is located in liver segment 1 The Planned Treatment Volume intended to cover the targeted tumor includes or encompasses any portion of the main portal vein, common hepatic duct, common bile duct, gallbladder or stomach/bowel | Technical success, defined as the treatment volume/treatment dimensions being greater than or equal to the targeted tumor, and with complete tumor coverage, via computed tomography (CT) or magnetic resonance (MR) imaging. Number of index procedure related major complications, including device-related events defined as Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher toxicities observed up to 30 days post index-procedure. | Technique efficacy, defined as the lack of a nodular or mass-like area of enhancement within or along the edge of the treatment volume assessed via CT or MR imaging at 30 days post-procedure. Number of adverse events (serious and non-serious) reported within 30 days post-index procedure |
| Treatment of Cancer with Immune Checkpoint Inhibition Therapy Boosted by High Intensity Focused Ultrasound Histotripsy | Active, not recruiting | 2030-08-01 | UMC Utrecht | NCT06524570 | metastatic or unresectable cancer | 24 | Histologically confirmed metastatic or unresectable cancer that progressed under standard of care treatment options. Age ≥ 18 years. Has signed and dated written informed consent before performing any study procedure, including screening. Anticipated life expectancy ≥12 weeks by investigator judgment. At least one tumor lesion (primary tumor or metastasis) which is amenable to application of high intensity focused ultrasound histotripsy (determined by a radiologist with HIFU-expertise). The lesion must have a distance of ≤30 mm to the skin. At least part of the lesion must have a distance of ≥10 mm to the skin and other vulnerable structures (e.g., large blood vessels). This part should be sufficient to be able to select at least one HT focus in an area of solid tumor. If the target lesion contains cystic or necrotic regions: the solid component should be ≥10 mm in diameter, sufficient to be able to select at least one HIFU-HT focus in an area of solid tumor with ≥10 mm distance to the skin. Sonication will be performed on tumors that have not previously directly been treated with radiation therapy or surgery unless they showed significant mass regrowth. Measurable disease (at least one lesion besides the HIFU-HT treated lesion) on CT according to RECIST V 1.1 criteria (or on PET-CT according to PERCIST criteria) as assessed by investigator and local radiology review. Performance status of 0 or 1 on the WHO Performance Scale. Screening laboratory values must meet the following criteria: WBC ≥ 2.0 × 109/L, Neutrophils ≥ 1.5 × 109/L Platelets ≥ 100 × 109/L Hemoglobin ≥ 5.5 mmol/L Serum creatinine ≤ 1.5× upper limit of normal (ULN) or calculated creatinine clearance ≥ 60 mL/min (≤Grade 1) Aspartate aminotransferase (AST) ≤ 2.5× ULN; alanine aminotransferase (ALT) ≤ 2.5× ULN; AST/ALT < 5× ULN if liver involvement Serum bilirubin ≤ 1.5× ULN or direct bilirubin ≤ ULN for subjects with total bilirubin levels > 1.5× ULN, except in subjects with Gilbert’s Syndrome Patients must agree to use an adequate method of contraception for the course of the study through 180 days after the last dose of study medication. Patients must be willing to undergo tumor biopsy. | Presence of known central nervous system, meningeal, or epidural metastatic disease. However, subjects with known brain metastases are allowed if the brain metastases are stable for ≥4 weeks before the first dose of study treatment. Stable is defined as neurological symptoms not present or resolved to baseline, no radiologic evidence of progression, and steroid requirement of prednisone ≤10 mg/day or equivalent. Patients currently participating and receiving study therapy or patients who participated in a study of an investigational agent and received study therapy or used an investigational device within 4 weeks prior to the first dose of the study treatment. Prior chemotherapy, targeted small molecule therapy or monoclonal antibodies within 4 weeks prior to the first dose of the study treatment. Prior radiotherapy within 8 weeks prior to the first dose of the study treatment. The patient will be excluded from the study if the only targetable lesion has directly been treated with radiation therapy in the past with an exception for lesions that showed massive regrowth. Prior surgery or ablative therapy within 4 weeks prior to the first dose of the study treatment. The patient will be excluded from the study if the only targetable lesion has directly been treated with ablative therapy in the past. Ongoing adverse events > Grade 1 due to a previously administered therapy. Subjects with ≤Grade 2 neuropathy, vitiligo, thyroid disorders, hypocortisolism or alopecia of any grade are an exception to this criterion and may qualify for the study. History of other malignancies, except adequately treated and a cancer-related life-expectancy of more than 5 years. Concurrent medical condition requiring the use of immunosuppressive medications, or immunosuppressive doses of systemic or absorbable topical corticosteroids; exceeding prednisolone 10 mg or equivalent. Active autoimmune disease that has required systemic treatment in the past 2 years (i.e., with use of disease modifying agents, high-dose corticosteroids or immunosuppressive drugs). Replacement therapy (e.g., thyroxine, insulin, or physiologic corticosteroid replacement therapy for adrenal or pituitary insufficiency, etc.) is not considered a form of systemic treatment. Active infection requiring systemic therapy. History of (non-infectious) pneumonitis that required steroids or current pneumonitis. Known history of active Tuberculosis. Receipt of a live vaccine within 4 weeks prior to the first dose of the study treatment. Hypersensitivity to any of the study drugs or their excipients. Contra-indications to MR imaging (e.g., certain pacemakers or severe claustrophobia). Contra-indications to gadolinium-based contrast agents are not an exclusion criterion, as a different brand of gadolinium can be used or if necessary the MRI can be performed without contrast. Pregnancy or lactation. Any other medical or social condition that, in the opinion of the Principal Investigator, might put the subject at risk of harm during the study or might adversely affect the interpretation of the study data. | Number and severity of adverse events until 100 days after the last study treatment Discontinuation rate due to adverse events at every visit until 2 years post treatment Patient reported tolerability by HIFU-HT-tolerability questionnaire: The HIFU-HT tolerability questionnaire is a self-reported, customized questionnaire that describes the burden/complaints a respondent experienced following HIFU-histotripsy treatment. The questionnaire comprises questions about pain, use of pain medication, complaints other than pain, burden of MRI scan, burden of peri-procedural analgesia, time burden of treatment. Respondents are asked to grade the experienced complaints or burden on a scale of 5 options, ranging from no complaints/no burden to severe complaints/severe burden. If respondents report pain, they are asked to grade their pain on a scale ranging from 0 to 10 (0 reflecting no pain, 10 reflecting worst possible pain) and respondents are asked for how many days the pain was present (ranging from 0 to 7 days). This will be performed at days 8 and 15. Patient reported tolerability by EQ-5D: The EuroQol Group EQ-5D questionnaire (Dutch version) is a self-reported questionnaire that reflects a respondent’s health. The EQ-5D comprises questions on 5 domains (mobility, self care, daily activities, pain/complaints, mood), for each of these domains respondents state whether they have no problems, some problems or severe problems. Respondents are also asked to grade their general health status on a scale of 0–100 (0 reflecting the worst possible health status, 100 reflecting the best possible health status). This will be performed at baseline, days 1, 8, 15, 22, 43, 64, 91; thereafter every 4 to 8 weeks until 2 years after start of therapy Patient reported tolerability by USD-I: The Utrecht symptom diary immunotherapy (USD-I) is a self-reported questionnaire that was developed and validated in the UMC Utrecht to score symptoms patients might experience during/after treatment with checkpoint inhibition therapy. The questionnaire comprise questions on 19 possible symptoms (apetite, stool pattern, diarrhea, abdominal pain, coughing, eye complaints, skin rash, pruritus, headache, myalgia, arthralgia, paresthesias, pain, sleeping problems). Respondents are asked to grade these symptoms on a scale of 0–10 (0 reflecting no problems, 10 reflecting worst possible problem). This will be performed at baseline, days 1, 8, 15, 22, 43, 64, 91; thereafter every 4 weeks until 2 years after start of therapy Feasibility: Number of technically effective HIFU-HT procedures on day 8, percentage of screening failures at baseline, and time burden of the study procedures through study completion up to two years after start of study treatment | Radiological response: MRI- Local response of HIFU-HT treated tumor as assessed by MRI directly and 12 weeks after HIFU-HT Radiological response: CT—Best overall systemic response using RECIST 1.1 as assessed by CT-scan every 12 weeks (or using PERCIST as assessed by PET-CT if not RECIST measurable) Immunologic response: Analysis of immunological parameters in peripheral blood. Analysis of immune infiltrates in tumor biopsies taken at baseline and 7 days after HIFU-HT-Baseline and days 1, 8, 9, 15, 22, 64 Overall survival: Explorative analysis to assess overall survival in months while taking into consideration the heterogeneous patient population in this basket design. Performed every 12 weeks until 2 years. Progression free survival: Explorative analysis to assess progression-free survival in months while taking into consideration the heterogeneous patient population in this basket design. Done every 12 weeks until 2 years. |
| The HistoSonics Edison™ System for Treatment of Pancreatic Adenocarcinoma Using Histotripsy | Recruiting | 2026-01-01 | HistoSonics, Inc. | NCT06282809 | pancreatic adenocarcinoma | 50 | Subject is ≥18 years of age. Subject has signed the Ethics Committee (EC) approved trial Informed Consent Form (ICF) prior to any trial related tests/procedures and is willing to comply with trial procedures and required follow-up assessments. Subject is diagnosed with unresectable pancreatic adenocarcinoma, locally advanced (Stage 3) or oligometastatic disease (Stage 4) confirmed via CT or MR imaging ≤30 days prior to the index procedure date. NOTE: If Stage 4 disease, there must be ≤5 metastatic tumors and the tumors are located only in the liver and/or lung. Subject is not a surgical candidate and has received chemotherapy ≥8 weeks. Subject can tolerate general anesthesia. Subject has an Eastern Cooperative Oncology Group Performance Status (ECOG PS) grade 0–1 at baseline. Subject meets the following criteria ≤14 days prior to the planned index procedure date: Hemoglobin ≥ 9 g/dL, Neutrophil count > 1.0 × 109/L, Platelet > 50 × 109/L, Total bilirubin ≤ 2.5× Institutional Upper Limit of Normal (IULN), Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) ≤2.5× IULN, International Normalized Ratio (INR) value <1.5, Serum creatinine < 2.0 mg/dL or an estimated glomerular filtration rate (eGFR) ≥45 mL/min. The targeted pancreatic tumor is ≥2 cm in longest diameter. The planned histotripsy treatment volume is ≥1.0 cm from any portion of the duodenum, small intestine, stomach, or colon as visualized on ultrasound, and CT, or MR imaging. Subject has an adequate acoustic window to visualize targeted tumor using the HistoSonics Edison System. Subject will undergo histotripsy treatment of only one (1) tumor during the index procedure, regardless of how many tumors are present in the pancreas. | Subject is pregnant or planning to become pregnant or nursing (lactating) during the trial period. Subject has had prior pancreatic, bilioenteric, or gastric surgery. Subject is being actively treated in another pharmaceutical or device trial that has not completed its primary endpoint prior to the index procedure or may interfere with the primary outcome measure of this trial. Subject has an uncorrectable coagulopathy. Subject has a life expectancy of less than six (6) months. Subject has a biliary or pancreatic stent and/or percutaneous biliary tube that encompasses the planned histotripsy treatment volume. Subject has metastases to organs other than the liver and/or lung (e.g., bone, brain, peritoneum). Subject has a known sensitivity to contrast media and cannot be adequately pre-medicated. Subject has an active duodenal or gastric ulcer requiring medical management. Subject is undergoing active chemotherapy for any cancer ≤ 14 days prior to planned index procedure date. Subject is undergoing active immunotherapy ≤ 30 days prior to planned index procedure date. Subject’s targeted tumor has had prior locoregional therapy (e.g., ablation, embolization, or radiation). Subject has a planned cancer treatment (e.g., pancreatic surgery, chemotherapy, immunotherapy, etc.) prior to completion of the 30 day follow-up visit. Subject has not recovered (CTCAE grade 2 or better) from chemotherapy or immunotherapy related toxicities (exclusive of alopecia, neuropathy, and exocrine insufficiency). In the investigator’s opinion, histotripsy is not a treatment option for the subject. Subject has a concurrent condition that could jeopardize the safety of the subject or compliance with the protocol. Subject’s tumor is not treatable by the System’s working ranges (refer to User Guide). | Evaluate the safety of the HistoSonics Edison System for the destruction of pancreatic adenocarcinomas using histotripsy: Index procedure-related complications ≤ 30 days post index procedure, graded using Clavien–Dindo Classification and Common Terminology Criteria for Adverse Events (CTCAE). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, A.P.; Kotlarz, P.L.; Giff, A.E.; Goundry, K.A.; Laeseke, P.; Koepsel, E.M.K.; Alhamami, M.; Daye, D. Breaking Barriers with Sound: The Implementation of Histotripsy in Cancer. Cancers 2025, 17, 2548. https://doi.org/10.3390/cancers17152548
Raman AP, Kotlarz PL, Giff AE, Goundry KA, Laeseke P, Koepsel EMK, Alhamami M, Daye D. Breaking Barriers with Sound: The Implementation of Histotripsy in Cancer. Cancers. 2025; 17(15):2548. https://doi.org/10.3390/cancers17152548
Chicago/Turabian StyleRaman, Ashutosh P., Parker L. Kotlarz, Alexis E. Giff, Katherine A. Goundry, Paul Laeseke, Erica M. Knavel Koepsel, Mosa Alhamami, and Dania Daye. 2025. "Breaking Barriers with Sound: The Implementation of Histotripsy in Cancer" Cancers 17, no. 15: 2548. https://doi.org/10.3390/cancers17152548
APA StyleRaman, A. P., Kotlarz, P. L., Giff, A. E., Goundry, K. A., Laeseke, P., Koepsel, E. M. K., Alhamami, M., & Daye, D. (2025). Breaking Barriers with Sound: The Implementation of Histotripsy in Cancer. Cancers, 17(15), 2548. https://doi.org/10.3390/cancers17152548







