Minimally Invasive Total Versus Partial Thymectomy for Early-Stage Thymoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
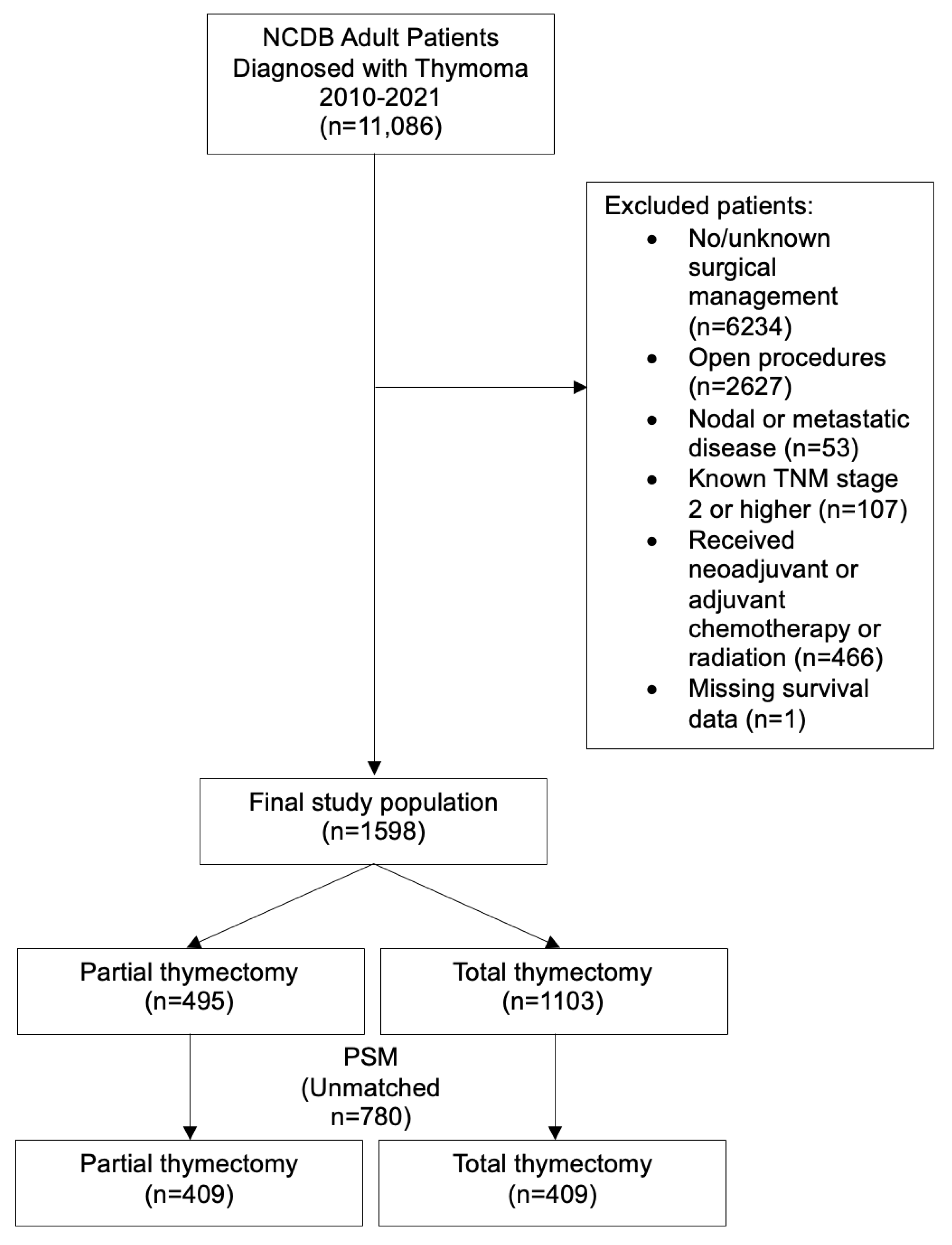
2.2. Patient Population
2.3. Main Exposure and Independent Variables
2.4. Outcome Variables
2.5. Statistical Analysis
3. Results
3.1. Unmatched Analysis
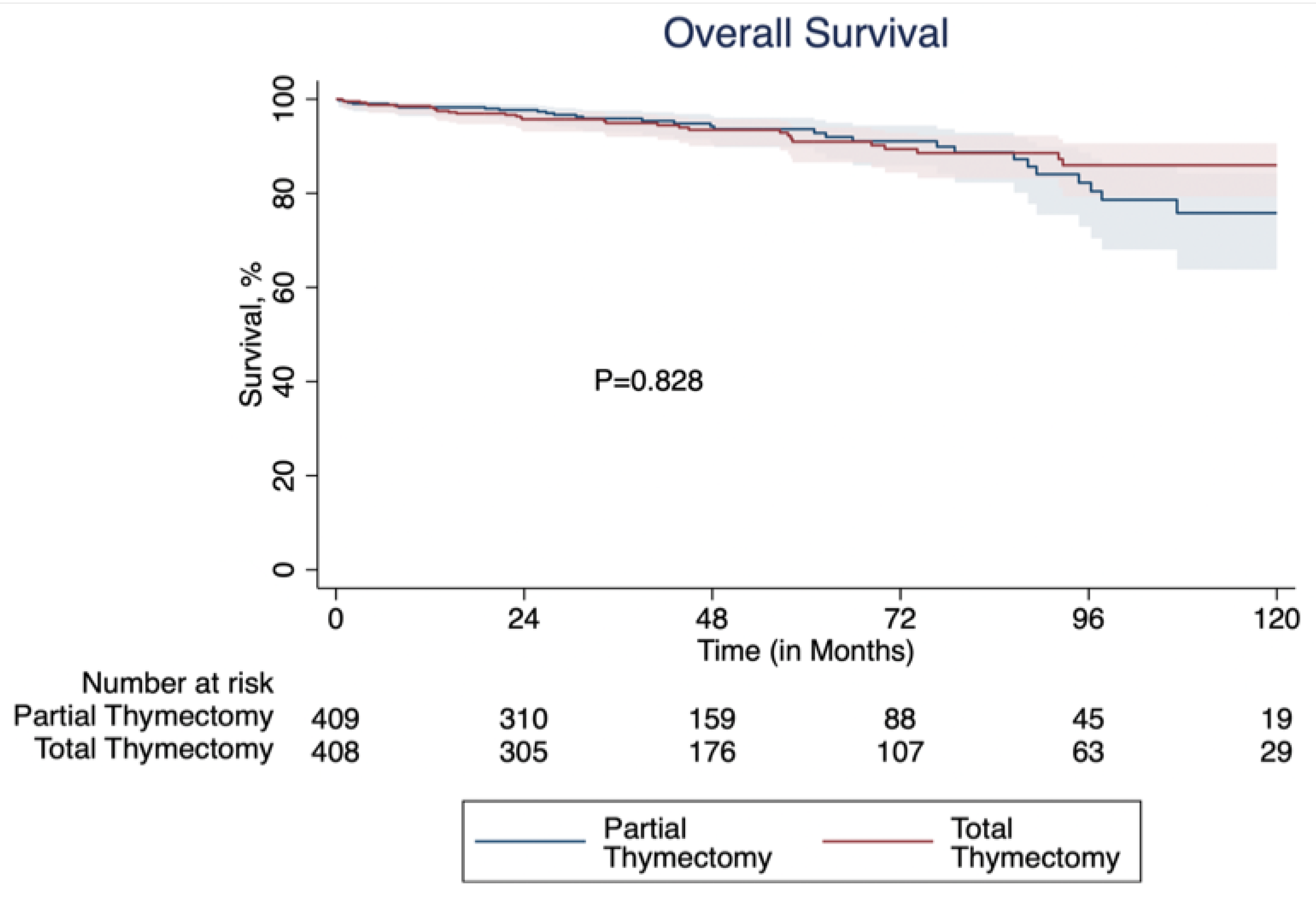
3.2. Propensity Score Matched Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| NEJM | New England Journal of Medicine |
| NCDB | National Cancer Database |
| TNM | Tumor node metastasis |
| AJCC | American Joint Committee on Cancer |
| aHR | Adjusted hazard ratio |
References
- Kooshesh, K.A.; Foy, B.H.; Sykes, D.B.; Gustafsson, K.; Scadden, D.T. Health Consequences of Thymus Removal in Adults. N. Engl. J. Med. 2023, 389, 406–417. [Google Scholar] [CrossRef]
- Liou, D.Z.; Berry, M.F.; Brown, L.M.; Demmy, T.L.; Huang, J.; Khullar, O.V.; Padda, S.K.; Shah, R.D.; Taylor, M.D.; Toker, S.A.; et al. The Society of Thoracic Surgeons Expert Consensus Document on the Surgical Management of Thymomas. Ann. Thorac. Surg. 2024, 118, 975–1004. [Google Scholar] [CrossRef]
- Zielinski, M.; Kuzdzal, J.; Szlubowski, A.; Soja, J. Comparison of late results of basic transsternal and extended transsternal thymectomies in the treatment of myasthenia gravis. Ann. Thorac. Surg. 2004, 78, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, F.; Falcoz, P.E.; Moser, B.; van Raemdonck, D.; Bille, A.; Toker, A.; Spaggiari, L.; Ampollini, L.; Filippini, C.; Thomas, P.A.; et al. Thymomectomy plus total thymectomy versus simple thymomectomy for early-stage thymoma without myasthenia gravis: A European Society of Thoracic Surgeons Thymic Working Group Study. Eur. J. Cardio-Thorac. Surg. 2021, 60, 881–887. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Shen, Y.; Wei, Y.; Tan, L.; Zhang, P.; Han, Y.; Chen, C.; Zhang, R.; Li, Y.; et al. Thymectomy versus tumor resection for early-stage thymic malignancies: A Chinese Alliance for Research in Thymomas retrospective database analysis. J. Thorac. Dis. 2016, 8, 680–686. [Google Scholar] [CrossRef]
- Narm, K.S.; Lee, C.Y.; Do, Y.W.; Jung, H.S.; Byun, G.E.; Lee, J.G.; Kim, D.J.; Hwang, Y.; Park, I.K.; Kang, C.H.; et al. Limited thymectomy as a potential alternative treatment option for early-stage thymoma: A multi-institutional propensity-matched study. Lung Cancer 2016, 101, 22–27. [Google Scholar] [CrossRef]
- Voulaz, E.; Perroni, G.; Russo, A.; Patirelis, A.; Mangiameli, G.; Alloisio, M.; Ambrogi, V. Thymomectomy versus complete thymectomy in early-stage non-myasthenic thymomas: A multicentric propensity score-matched study. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac167. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yokoi, K.; Nakajima, J.; Tanaka, F.; Maniwa, Y.; Suzuki, M.; Nagayasu, T.; Asamura, H. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann. Thorac. Surg. 2016, 101, 520–526. [Google Scholar] [CrossRef]
- Kaminski, H.J.; Kusner, L.L.; Cutter, G.R.; Le Panse, R.; Wright, C.D.; Perry, Y.; Wolfe, G.I. Does Surgical Removal of the Thymus Have Deleterious Consequences? Neurology 2024, 102, e209482. [Google Scholar] [CrossRef]
- The American College of Surgeons. National Cancer Database. 2021. Available online: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/ (accessed on 3 December 2024).
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Lunt, M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am. J. Epidemiol. 2014, 179, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.; Javidfar, J.; Newmark, A.; Lavelle, M.; Bacchetta, M.; Gorenstein, L.; D’Ovidio, F.; Ginsburg, M.E.; Sonett, J.R. Minimally invasive thymectomy and open thymectomy: Outcome analysis of 263 patients. Ann. Thorac. Surg. 2012, 94, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Nguyen, D.; Groth, S.S.; Palivela, N.; Ripley, R.T.; Makris, K.I.; Farjah, F.; Cornwell, L.; Massarweh, N.N. Utilization of Minimally Invasive Thymectomy and Margin-Negative Resection for Early-Stage Thymoma. Ann. Thorac. Surg. 2019, 108, 405–411. [Google Scholar] [CrossRef]
- Strobel, P.; Bauer, A.; Puppe, B.; Kraushaar, T.; Krein, A.; Toyka, K.; Gold, R.; Semik, M.; Kiefer, R.; Nix, W.; et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: A retrospective analysis. J. Clin. Oncol. 2004, 22, 1501–1509. [Google Scholar] [CrossRef]
- Safieddine, N.; Liu, G.; Cuningham, K.; Ming, T.; Hwang, D.; Brade, A.; Bezjak, A.; Fischer, S.; Xu, W.; Azad, S.; et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J. Thorac. Oncol. 2014, 9, 1018–1022. [Google Scholar] [CrossRef]
- Burt, B.M.; Yao, X.; Shrager, J.; Antonicelli, A.; Padda, S.; Reiss, J.; Wakelee, H.; Su, S.; Huang, J.; Scott, W. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J. Thorac. Oncol. 2017, 12, 129–136. [Google Scholar] [CrossRef]
- Papadimas, E.; Tan, Y.K.; Luo, H.; Choong, A.; Tam, J.K.C.; Kofidis, T.; Mithiran, H. Partial Versus Complete Thymectomy in Non-Myasthenic Patients With Thymoma: A Systematic Review and Meta-Analysis of Clinical Outcomes. Heart Lung Circ. 2022, 31, 59–68. [Google Scholar] [CrossRef]
- Pulle, M.V.; Asaf, B.B.; Puri, H.V.; Bishnoi, S.; Kumar, A. Meta-Analysis of Limited Thymectomy versus Total Thymectomy for Masaoka Stage I and II Thymoma. J. Chest Surg. 2021, 54, 127–136. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Kaminski, H.J.; Aban, I.B.; Minisman, G.; Kuo, H.C.; Marx, A.; Strobel, P.; Mazia, C.; Oger, J.; Cea, J.G.; et al. Randomized trial of thymectomy in myasthenia gravis. N. Engl. J. Med. 2016, 375, 511–522. [Google Scholar] [CrossRef]
- Lucchi, M.; Ricciardi, R.; Melfi, F.; Duranti, L.; Basolo, F.; Palmiero, G.; Murri, L.; Mussi, A. Association of thymoma and myasthenia gravis: Oncological and neurological results of the surgical treatment. Eur. J. Cardiothorac. Surg. 2009, 35, 812–816. [Google Scholar] [CrossRef]
- Hess, N.R.; Sarkaria, I.S.; Pennathur, A.; Levy, R.M.; Christie, N.A.; Luketich, J.D. Minimally invasive versus open thymectomy: A systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann. Cardiothorac. Surg. 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.K.; Lee, S.K.; Kim, H.Y.; Park, S.Y.; Park, I.K.; Kim, D.J.; Chung, K.Y. Recurrence after thymoma resection according to the extent of the resection. J. Cardiothorac. Surg. 2014, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Q.; Li, J.; Qiu, H.; Zhu, K.; Chen, D.; Jin, Z.; Zhang, J.; Zhang, B.; Witharana, P.; et al. Prognosis and surgical outcomes of the total thymectomy versus thymomectomy in non-myasthenic patients with early-stage thymoma: A systematic review and meta-analysis. Asian J. Surg. 2023, 46, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
| Unmatched | Matched | |||||||
|---|---|---|---|---|---|---|---|---|
| Partial Thymectomy (N = 495) | Total Thymectomy (N = 1103) | p-Value | Standardized Bias|%| | Partial Thymectomy (N = 409) | Total Thymectomy (N = 409) | p-Value | Standardized Bias|%| | |
| Sex, female | 266(53.7%) | 589(53.4%) | 0.914 | 2.9% | 226(55.3%) | 224(54.8%) | 0.944 | 0.1% |
| Age, mean (SD) | 63.7(12.2) | 61.1(13.3) | <0.001 | 17.6% | 63.4(12.2) | 63.4(12.1) | 0.970 | 0.2% |
| Race | 0.921 | 4.6% | 0.990 | 2.6% | ||||
| White | 369(74.5%) | 816(74.0%) | 306(74.8%) | 308(75.3%) | ||||
| Black | 62(12.5%) | 151(13.7%) | 52(12.7%) | 53(13.0%) | ||||
| Asian | 48(9.7%) | 103(9.3%) | 39(9.5%) | 36(8.8%) | ||||
| Other | 16(3.2%) | 33(3%) | 12(2.9%) | 12(2.9%) | ||||
| Ethnicity, Hispanic | 36(7.3%) | 83(7.5%) | 0.918 | 4.2% | 28(6.9%) | 26(6.4%) | 0.888 | 2.0% |
| Charlson-Deyo Index | 0.742 | 6.0% | 0.953 | 2.4% | ||||
| 0 | 381(77.0%) | 833(75.5%) | 313(76.5%) | 317(77.5%) | ||||
| 1 | 77(15.6%) | 189(17.1%) | 63(15.4%) | 60(14.7%) | ||||
| 2+ | 37(7.5%) | 81(7.3%) | 33(8.1%) | 32(7.8%) | ||||
| Tumor Size, mm (SD) | 48.7(26.4) | 50.4(28.0) | 0.455 | 7.0% | 48.4(26.7) | 49.6(26.6) | 0.512 | 4.3% |
| Histology | 0.587 | 9.4% | >0.999 | 2.5% | ||||
| Type A | 71(14.3%) | 151(13.7%) | 62(15.2%) | 59(14.4%) | ||||
| Type AB | 182(36.8%) | 380(34.5%) | 148(36.2%) | 148(36.2%) | ||||
| Type B1 | 71(14.3%) | 185(16.8%) | 57(13.9%) | 57(13.9%) | ||||
| Type B2 | 80(16.2%) | 193(17.5%) | 69(16.9%) | 71(17.4%) | ||||
| Type B3 | 32(6.5%) | 55(5%) | 23(5.6%) | 24(5.9%) | ||||
| Thymoma, NOS | 59(11.9%) | 139(12.6%) | 50(12.2%) | 50(12.2%) | ||||
| Primary Payor | 0.063 | 18.4% | 0.986 | 5.8% | ||||
| Private Insurance | 221(44.6%) | 530(48.1%) | 190(46.5%) | 187(45.7%) | ||||
| Medicare | 239(48.3%) | 458(41.5%) | 191(46.7%) | 189(46.2%) | ||||
| Medicaid/Government | 26(5.2%) | 89(8.1%) | 17(4.2%) | 20(4.9%) | ||||
| Not Insured | 5(1%) | 20(1.8%) | 3(0.7%) | 3(0.7%) | ||||
| Unknown | 4(0.8%) | 6(0.5%) | 4(1.0%) | 4(1%) | ||||
| Median Income Quartiles | 0.330 | 11.1% | 0.707 | 3.1% | ||||
| <$30,000 | 26(6.1%) | 81(8.9%) | 25(6.1%) | 26(6.4%) | ||||
| $30,000–$34,999 | 59(13.9%) | 112(12.3%) | 57(13.9%) | 54(13.2%) | ||||
| $35,000–$45,999 | 103(24.3%) | 219(24.1%) | 101(24.7%) | 98(24.0%) | ||||
| ≥$46,000 | 235(55.6%) | 496(54.6%) | 226(55.3%) | 231(56.5%) | ||||
| Center Type | 0.058 | 13.1% | 0.622 | 3.9% | ||||
| Academic | 264(53.3%) | 531(48.1%) | 224(54.8%) | 232(56.7%) | ||||
| Non-academic | 231(46.7%) | 572(51.9%) | 185(45.2%) | 177(43.3%) | ||||
| Approach | 0.135 | 0.937 | ||||||
| Robotic | 333(67.3%) | 783(71.0%) | 277(67.7%) | 280(68.5%) | ||||
| VATS | 162(32.7%) | 320(29.0%) | 132(32.3%) | 129(31.5%) | ||||
| Unmatched Cohort | Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Partial Thymectomy | Total Thymectomy | p Value | Partial Thymectomy | Total Thymectomy | p Value | |
| 30-Day Mortality | 4 (0.8%) | 7 (0.6%) | 0.747 | 4 (1.0%) | 4 (1.0%) | >0.999 |
| 90-Day Mortality | 4 (0.8%) | 9 (0.8%) | >0.999 | 4 (1.0%) | 4 (1.0%) | >0.999 |
| Resection Margins | 0.386 | 0.905 | ||||
| Positive | 47 (9.9%) | 90 (8.5%) | 38 (9.7%) | 40 (10.1%) | ||
| Negative | 428 (90.1%) | 963 (91.5%) | 356 (90.3%) | 356 (89.9%) | ||
| Length of Stay, median days (IQR) | 2 (1–3) | 2 (1–3) | 0.943 | 2 (1–3) | 2 (1–3) | 0.315 |
| Readmission within 30 days | 8 (1.6%) | 24 (2.2%) | 0.564 | 7 (1.7%) | 7 (1.7%) | >0.999 |
| Variable | aHR | p-Value | 95% CI |
|---|---|---|---|
| Partial Thymectomy | 1.00 | 0.976 | (0.68–1.48) |
| Negative Margins(R0) | 0.91 | 0.761 | (0.54–1.58) |
| Tumor Size(mm) | 1.01 | 0.004 | (1.00–1.01) |
| Age | 1.06 | <0.001 | (1.04–1.08) |
| Sex (Female) | 0.76 | 0.146 | (0.53–1.10) |
| Histology | |||
| A | Reference | ||
| AB | 0.93 | 0.773 | (0.55–1.57) |
| B1 | 0.74 | 0.392 | (0.38–1.46) |
| B2 | 0.96 | 0.899 | (0.51–1.82) |
| B3 | 1.53 | 0.243 | (0.75–3.12) |
| NOS | 0.91 | 0.790 | (0.46–1.81) |
| Race | |||
| White | Reference | ||
| Black | 1.07 | 0.810 | (0.59–1.96) |
| Asian | 0.91 | 0.775 | (0.47–1.77) |
| Other | 0.98 | 0.981 | (0.24–4.06) |
| Hispanic | 2.20 | 0.022 | (1.12–4.31) |
| Academic Center | 0.66 | 0.030 | (0.46–0.96) |
| Charlson-Deyo Index | |||
| 0 | Reference | ||
| 1 | 0.93 | 0.788 | (0.58–1.51) |
| 2+ | 2.91 | <0.001 | (1.73–4.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohlman, A.; Odeh, B.; Helenowski, I.; Coughlin, J.M.; Raad, W.; Lubawski, J.; Abdelsattar, Z.M. Minimally Invasive Total Versus Partial Thymectomy for Early-Stage Thymoma. Cancers 2025, 17, 2518. https://doi.org/10.3390/cancers17152518
Pohlman A, Odeh B, Helenowski I, Coughlin JM, Raad W, Lubawski J, Abdelsattar ZM. Minimally Invasive Total Versus Partial Thymectomy for Early-Stage Thymoma. Cancers. 2025; 17(15):2518. https://doi.org/10.3390/cancers17152518
Chicago/Turabian StylePohlman, Alexander, Bilal Odeh, Irene Helenowski, Julia M. Coughlin, Wissam Raad, James Lubawski, and Zaid M. Abdelsattar. 2025. "Minimally Invasive Total Versus Partial Thymectomy for Early-Stage Thymoma" Cancers 17, no. 15: 2518. https://doi.org/10.3390/cancers17152518
APA StylePohlman, A., Odeh, B., Helenowski, I., Coughlin, J. M., Raad, W., Lubawski, J., & Abdelsattar, Z. M. (2025). Minimally Invasive Total Versus Partial Thymectomy for Early-Stage Thymoma. Cancers, 17(15), 2518. https://doi.org/10.3390/cancers17152518







