Targeted Therapies and Immunotherapies for Diffuse Large B-Cell Lymphoma
Simple Summary
Abstract
1. Introduction
1.1. DLBCL Epidemiology and Subtypes
1.2. Limitations of Standard Therapy and the Need for Novel Approaches in DLBCL
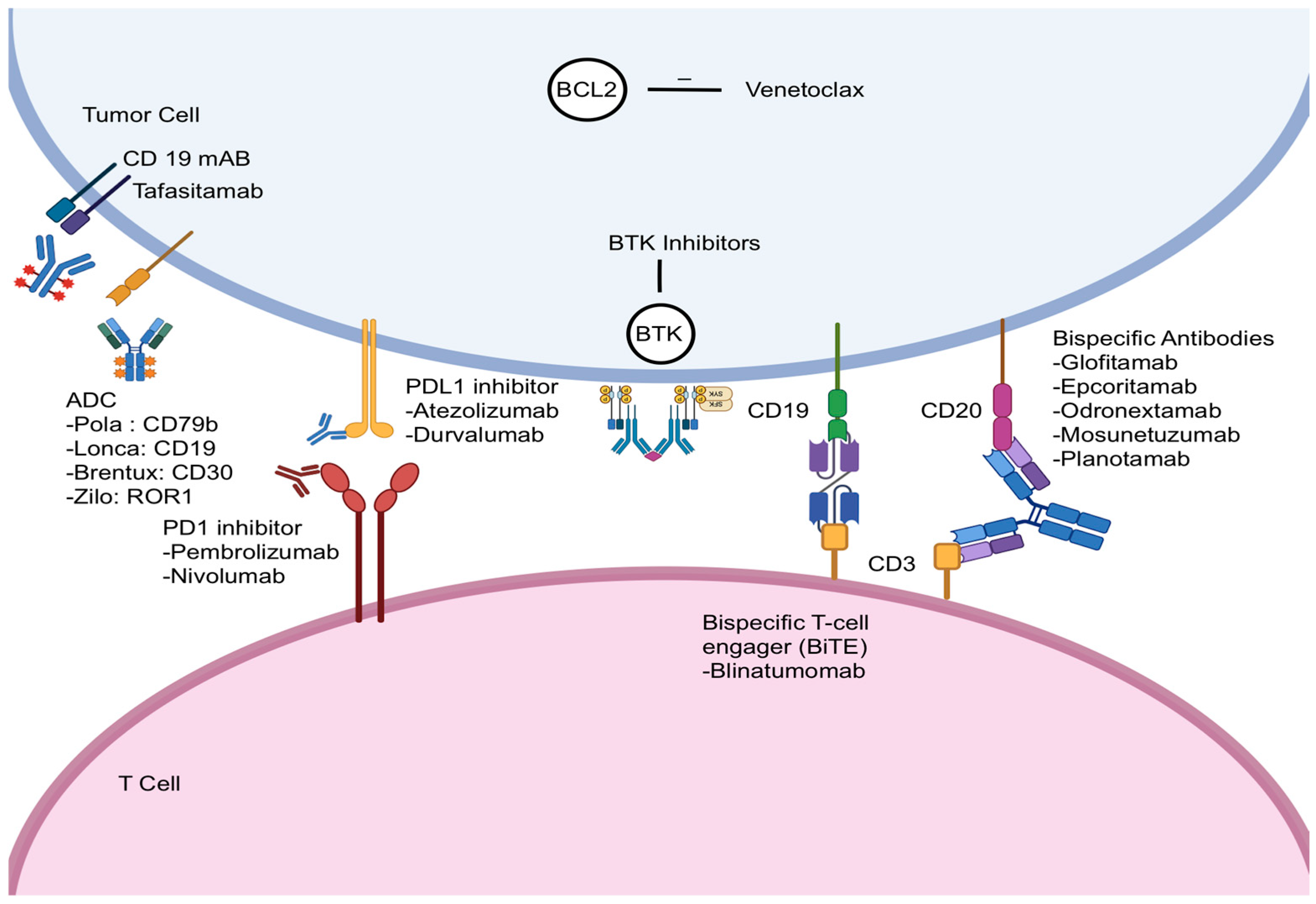
2. Bispecific Antibodies
2.1. Epcoritamab
2.2. Glofitamab
2.3. Odronextamab
2.4. Mosunetuzumab
2.5. Planotamab
2.6. Blinatumomab
3. Checkpoint Inhibitors
3.1. Atezolizumab
3.2. Durvalumab
3.3. Pembrolizumab
3.4. Nivolumab
4. Antibody–Drug Conjugates
4.1. Polatuzumab Vedotin
4.2. Loncastuximab Tesirine
4.3. Brentuximab Vedotin
4.4. Zilovertamab Vedotin
4.5. Naratuximab Emtansine
| Trial/Regimen | Indication/Line | N (Active Arm) | ORR (%) | CR (%) | Median PFS (Months) | Median OS (Months) | CRS (Any Grade, %) | CRS (Grade 3+, %) | ICANS (Any Grade, %) | ICANS (Grade 3+, %) |
|---|---|---|---|---|---|---|---|---|---|---|
| Epcoritamab Monotherapy (EPCORE NHL-1) [11] | 3L+ R/R DLBCL/LBCL | 157 | 63.1 | 39.5 | 4.2 (overall); 37.3 (CR pts) | 18.5 (overall); NR (CR pts) | 51 | 2.5–3 | 6–6.4 | 0.6 (1 fatal event) |
| Epcoritamab + GemOx (EPCORE NHL-2) [15] | 2L+ ASCT-ineligible R/R DLBCL | 103 | 85 | 61 | 11.2 (overall); 26.7 (CR pts) | 21.6 (overall); NR (CR pts) | 52 | 1 | 2.9 | 1 (G3) |
| Glofitamab Monotherapy (NP30179) [24,25,26] | 3L+ R/R DLBCL/LBCL | 155 | 59 | 38 | 1-yr PFS in CR: 71% PFS—CR at EOT 80% | NR (18-mo OS: 41%; 1-yr OS in CR: 92%) | 64 | 4 | 8 | 3 |
| Glofitamab + GemOx (STARGLO) [23] | 2L+ R/R DLBCL (ASCT-ineligible) | 183 (Glofit-GemOx arm) | 68.3 | 58.5 | 13.8 | 25·5 (18·3–NE) | 44.8 | 2.3 | 2.3 | 0.6 |
| Mosunetuzumab + Polatuzumab Vedotin (Phase 1b/2, NCT03671018) [43,44] | 2L+ R/R LBCL | 98 (Phase 2 expansion) | 59.2 | 45.9 | 11.4 | 23.3 | 16.7 | 3.1 | 1 (G2 seizure) | Not reported |
| Mosunetuzumab + Polatuzumab Vedotin (SUNMO) [45,46] | 2L+ R/R aNHL (DLBCL, HGBCL, trFL, FL3B) | 138 (NCT05171647) | 70.3 | 51.4 | 11.5 months | 23.2 months | 25.9 | 0.7 | 0 | 0 |
| Polatuzumab Vedotin + Bendamustine + Rituximab (Pola-BR, GO29365) [85] | 3L+ R/R DLBCL (ASCT-ineligible) | 40 | 62.5 | 52.5 | 9.2 month | 12.4 | N/A | N/A | N/A | N/A |
| Polatuzumab Vedotin + R-GemOx (POLARGO) [87] | R/R DLBCL (ASCT-ineligible) | Not specified | 52.7 | 40.3 | 7.4 | 19.5 | N/A | N/A | N/A | N/A |
| Loncastuximab Tesirine Monotherapy (LOTIS-2) [101] | 3L+ R/R DLBCL/LBCL | 145 | 48.3 | 24.8 | 4.9(1-yr EFS 54%) | 9.5 (1-yr OS 74%) | N/A | N/A | N/A | N/A |
| Brentuximab Vedotin + Lenalidomide + Rituximab (ECHELON-3) [107] | 3L+ R/R DLBCL (ASCT/CAR-T ineligible) | 155 | 70.9 | 45.6 | 5.8 | 15.9 | N/A | N/A | N/A | N/A |
| ViPOR (Venetoclax + Ibrutinib + Prednisone + Obinutuzumab + Lenalidomide) [124] | R/R DLBCL | 50 | 54 | 38 | Not reported (2-yr PFS 34%) | Not reported (2-yr OS 36%) | N/A | N/A | N/A | N/A |
5. Monoclonal Antibody
Tafasitamab
6. BTK Inhibitors
7. BCL2 Inhibitors
8. Tazemetostat and Tulmimetostat
9. Selixenor
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Modi, D.; Potugari, B.; Uberti, J. Immunotherapy for Diffuse Large B-Cell Lymphoma: Current Landscape and Future Directions. Cancers 2021, 13, 5827. [Google Scholar] [CrossRef]
- SEER. Diffuse Large B-Cell Lymphoma—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/dlbcl.html (accessed on 9 June 2025).
- Trabolsi, A.; Arumov, A.; Schatz, J.H. Bispecific antibodies and CAR-T cells: Dueling immunotherapies for large B-cell lymphomas. Blood Cancer J. 2024, 14, 27. [Google Scholar] [CrossRef]
- Elsawy, M.; Chavez, J.C.; Avivi, I.; Larouche, J.-F.; Wannesson, L.; Cwynarski, K.; Osman, K.; Davison, K.; Rudzki, J.D.; Dahiya, S.; et al. Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma. Blood 2022, 140, 2248–2260. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Jacobsen, E.D.; Miklos, D.B.; Lekakis, L.J.; Braunschweig, I.; Oluwole, O.O.; Lin, Y.; Siddiqi, T.; et al. Durability of response in ZUMA-1, the pivotal phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients (Pts) with refractory large B-cell lymphoma. J. Clin. Oncol. 2018, 36, 3003. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Jacobson, C.A.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2023, 141, 2307–2315. [Google Scholar] [PubMed]
- Kamdar, M.K.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glaß, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.J.; et al. Lisocabtagene maraleucel (liso-cel) vs standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (pt) with R/R large B-cell lymphoma (LBCL): 3-year follow-up (FU) from the randomized, phase 3 TRANSFORM study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 7013. [Google Scholar]
- Gajra, A.; Zalenski, A.; Sannareddy, A.; Jeune-Smith, Y.; Kapinos, K.; Kansagra, A. Barriers to Chimeric Antigen Receptor T-Cell (CAR-T) Therapies in Clinical Practice. Pharm. Med. 2022, 36, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Sehn, L.H. CAR T cells as a second-line therapy for large B-cell lymphoma: A paradigm shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef]
- Falchi, L.; Vardhana, S.A.; Salles, G.A. Bispecific antibodies for the treatment of B-cell lymphoma: Promises, unknowns, and opportunities. Blood 2023, 141, 467–480. [Google Scholar] [CrossRef]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell–Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Karimi, Y.; Abrisqueta, P.; de Vos, S.; Nijland, M.; Offner, F.; Osei-Bonsu, K.; Rana, A.; Archer, K.G.; Song, Y.; Cordoba, R.; et al. Epcoritamab + R-DHAX/C in transplant-eligible patients (pts) with high-risk relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2024, 42, 7032. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Falchi, L.; Nijland, M.; de Vos, S.; Offner, F.; Rana, A.; Archer, K.; Song, Y.; Osei-Bonsu, K.; Karimi, Y.; et al. ABCL-272 Epcoritamab + R-DHAX/C Induces Deep, Durable Responses in Transplant-Eligible Patients With Relapsed or Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL), Including High-Risk Disease. Clin. Lymphoma Myeloma Leuk. 2024, 24, S468. [Google Scholar] [CrossRef]
- Brody, J.D.; Jørgensen, J.; Belada, D.; Costello, R.; Trněný, M.; Vitolo, U.; Lewis, D.J.; Karimi, Y.H.; Sureda, A.; André, M.; et al. Epcoritamab plus GemOx in transplant-ineligible relapsed/refractory DLBCL: Results from the EPCORE NHL-2 trial. Blood 2025, 145, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.; Joergensen, J.M.; Belada, D.; Costello, R.T.; Trněný, M.; Vitolo, U.; Lewis, D.; Karimi, Y.H.; Balari, A.M.S.; André, M.; et al. Epcoritamab SC + GemOx Leads to High Complete Metabolic Response Rates in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma Ineligible for Autologous Stem Cell Transplant: Updated Results from Epcore NHL-2. Blood 2023, 142, 3092. [Google Scholar] [CrossRef]
- Leslie, L.A.; Cheah, C.Y.; Morschhauser, F.; Darrah, J.M.; Brody, J.; Belada, D.; Duras, J.; Vermaat, J.S.; Musuraca, G.; Osei-Bonsu, K.; et al. Fixed-Duration Epcoritamab + R-Mini-CHOP in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma Ineligible for Full-Dose R-CHOP: Updated Results from Arm 8 of the Epcore NHL-2 Trial. Blood 2024, 144, 3106. [Google Scholar] [CrossRef]
- Cordoba, R. First Disclosure of Epcoritamab + R-Ice in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL) Eligible for Autologous Stem Cell Transplantation (ASCT): Epcore NHL-2. Available online: https://library.ehaweb.org/eha/2025/eha2025-congress/4159322/raul.cordoba.first.disclosure.of.epcoritamab.2B.r-ice.in.patients.with.relapsed.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D2882%2Aot_id%3D31553%2Amarker%3D5843%2Afeatured%3D19595 (accessed on 15 June 2025).
- Gurion, R.; Mazza, I.A.; Thieblemont, C.; Kim, W.S.; Masszi, A.; García-Sancho, A.M.; Nagy, Z.; Tessoulin, B.; Ubieto, A.J.; Ko, P.-S.; et al. Fixed-Duration Epcoritamab Plus Lenalidomide in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma (DLBCL): Updated Results from Arm 1 of the Epcore NHL-5 Trial. Blood 2024, 144, 3110. [Google Scholar] [CrossRef]
- Kerr, D.A.; Lavie, D.; Avigdor, A.; Avivi, I.; Patel, K.; Thieblemont, C.; Abrisqueta, P.; Izutsu, K.; Seliem, M.; Siddani, S.R.; et al. ABCL-417 First Data From Subcutaneous Epcoritamab + Polatuzumab Vedotin Rituximab, Cyclophosphamide Doxorubicin, and Prednisone (Pola-RCHP) for First-Line Diffuse Large B-Cell Lymphoma (DLBCL): EPCORE NHL-5. Clin. Lymphoma Myeloma Leuk. 2024, 24, S478–S479. [Google Scholar] [CrossRef]
- Morschhauser, F.; Belada, D.; Duell, J.; Jurczak, W.; Kim, T.M.; Kim, W.S.; Kumode, T.; Jiménez, J.L.; Meert, C.; Alcaide, S.O.; et al. Epcore DLBCL-3 First Disclosure: Fixed-Duration Epcoritamab Monotherapy in Older (≥75 y), Anthracycline-Ineligible Patients with Previously Untreated Large B-Cell Lymphoma. Blood 2024, 144 (Suppl. S1), 867. [Google Scholar] [CrossRef]
- Belada, D.; Kwiatek, M.; Burgues, J.M.B.; Morschhauser, F.; Duell, J.; Jurczak, W.; Kim, T.M.; Kim, W.S.; Kumode, T.; Jiménez, J.L.; et al. Epcore Dlbcl-3: Fixed-Duration Epcoritamab Monotherapy in Older (≥75Y), Anthracycline-Ineligible Patients with Previously Untreated Large B-Cell Lymphoma (lbcl). Hematol. Oncol. 2025, 43 (Suppl. S3), e158_70093. [Google Scholar] [CrossRef]
- Thieblemont, C.; Clausen, M.R.; Balari, A.S.; Zinzani, P.L.; Fox, C.; Kim, S.Y.; Vindeloev, S.D.; Lugtenburg, P. Phase 3 trial (GCT3013-05) of epcoritamab versus standard of care in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS7569. [Google Scholar] [CrossRef]
- Abramson, J.S.; Ku, M.; Hertzberg, M.; Huang, H.-Q.; Fox, C.P.; Zhang, H.; Yoon, D.H.; Kim, W.-S.; Abdulhaq, H.; Townsend, W.; et al. Glofitamab plus gemcitabine and oxaliplatin (GemOx) versus rituximab-GemOx for relapsed or refractory diffuse large B-cell lymphoma (STARGLO): A global phase 3, randomised, open-label trial. Lancet 2024, 404, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Carlo-Stella, C.; Morschhauser, F.; Hutchings, M.; Bachy, E.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Glofitamab monotherapy in pts with relapsed/refractory (R/R) large B-cell lymphoma (LBCL): Extended follow-up and landmark analyses from a pivotal phase II study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 7550. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Cartron, G.; Corradini, P.; Bartlett, N.L.; Iacoboni, G.; Khan, C.; Hertzberg, M.S.; et al. Fixed-Duration Glofitamab Monotherapy Continues to Demonstrate Durable Responses in Patients with Relapsed or Refractory Large B-Cell Lymphoma: 3-Year Follow-up from a Pivotal Phase II Study. Blood 2024, 144, 865. [Google Scholar] [CrossRef]
- Cartron, G.; Houot, R.; Al Tabaa, Y.; Le Bras, F.; Ysebaert, L.; Choquet, S.; Jardin, F.; Bay, J.O.; Gros, F.X.; Morschhauser, F.; et al. Glofitamab in refractory or relapsed diffuse large B cell lymphoma after failing CAR-T cell therapy: A phase 2 LYSA study. Nat. Cancer 2025, 6, 1173–1183. [Google Scholar] [CrossRef]
- Hutchings, M.; Balari, A.S.; Bosch, F.; Larsen, T.S.; Corradini, P.; Avigdor, A.; Terol, M.J.; Dominguez, A.R.; Pinto, A.; Skarbnik, A.P.; et al. Glofitamab in Combination with Polatuzumab Vedotin Maintains Durable Responses and a Manageable Safety Profile in Patients with Heavily Pre-Treated Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL) Including High-Grade B-Cell Lymphoma (HGBCL): Extended Follow-up of a Phase Ib/II Study. Blood 2024, 144, 988. [Google Scholar] [CrossRef]
- Dickinson, M.; Viardot, A.; Marks, R.; Topp, M.S.; Morschhauser, F.; Jacobs, B.; Tani, M.; Bosch, F.; Esteban, D.; Cordoba, R.; et al. Glofitamab + Pola-R-CHP in patients with previously untreated diffuse large B-cell lymphoma (DLBCL): Results from a phase Ib study. J. Clin. Oncol. 2023, 41, 7549. [Google Scholar] [CrossRef]
- Advani, R.H.; Dickinson, M.J.; Fox, C.P.; Kahl, B.; Herrera, A.F.; Lenz, G.; Song, Y.; Tao, R.; Cai, Q.; Kim, T.M.; et al. SKYGLO: A Global Phase III Randomized Study Evaluating Glofitamab Plus Polatuzumab Vedotin + Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (Pola-R-CHP) Versus Pola-R-CHP in Previously Untreated Patients with Large B-Cell Lymphoma (LBCL). Blood 2024, 144, 1718-1. [Google Scholar] [CrossRef]
- Falchi, L.; Jardin, F.; Haioun, C.; Wrobel, T.; Joergensen, J.M.; Bastos-Oreiro, M.; Mou, E.; Martinez-Lopez, J.; Budde, L.E.; Bartlett, N.L.; et al. Glofitamab (Glofit) Plus R-CHOP Has a Favorable Safety Profile and Induces High Response Rates in Patients with Previously Untreated (1L) Large B-Cell Lymphoma (LBCL) Defined As High Risk By Circulating Tumor DNA (ctDNA) Dynamics: Preliminary Safety and Efficacy Results. Blood 2023, 142, 858. [Google Scholar] [CrossRef]
- Diefenbach, C.S.; Caimi, P.F.; Saba, N.S.; Madueno, F.V.; Hamadani, M.; Fayad, L.E.; Riedell, P.A.; Gillis-Smith, A.; Simko, S.; Orellana-Noia, V.; et al. Glofitamab in Combination with Rituximab Plus Ifosfamide, Carboplatin, and Etoposide Shows Favorable Efficacy and Manageable Safety in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma, Eligible for Stem Cell Transplant or Chimeric Antigen Receptor T-Cell Therapy: Results from a Phase Ib Study. Blood 2024, 144, 987. [Google Scholar] [CrossRef]
- Hutchings, M.; Dickinson, M.J.; Gritti, G.; Carlo-Stella, C.; Townsend, W.; Bosch, F.; Bartlett, N.L.; Cartron, G.; Ghesquieres, H.; Houot, R.; et al. Englumafusp Alfa (CD19-4-1BBL) Combined with Glofitamab Is Safe and Efficacious in Patients with r/r B-NHL: Extended Follow up Analysis of the Dose-Escalation Part of Phase 1 Trial BP41072. Blood 2024, 144, 990. [Google Scholar] [CrossRef]
- Minson, A.; Hamad, N.; Yannakou, C.K.; Wong, S.M.; Butler, J.P.; Seymour, J.F.; Dickinson, M. Trial in Progress: A Multicentre, Parallel Arm, Open-Label Trial of Frontline R-CHOP/Polatuzumab Vedotin-RCHP and Glofitamab in Younger Patients with Higher Risk Diffuse Large B Cell Lymphoma (COALITION). Blood 2021, 138, 3571. [Google Scholar] [CrossRef]
- Minson, A.; Verner, E.; Giri, P.; Butler, J.; Janowski, W.; Cheah, C.Y.; Ratnasingam, S.; Wong, S.M.; Ku, M.; Hertzberg, M.S.; et al. A Randomized Phase 2, Investigator-Led Trial of Glofitamab-R-CHOP or Glofitamab-Polatuzumab Vedotin-R-CHP (COALITION) in Younger Patients with High Burden, High-Risk Large B-Cell Lymphoma Demonstrates Safety, Uncompromised Chemotherapy Intensity, a High Rate of Durable Remissions, and Unique FDG-PET Response Characteristics. Blood 2024, 144, 582. [Google Scholar] [CrossRef]
- Topp, M.S.; Matasar, M.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; Arnason, J.E.; Michot, J.-M.; Goldschmidt, N.; O’bRien, S.M.; Abadi, U.; et al. Odronextamab monotherapy in R/R DLBCL after progression with CAR T-cell therapy: Primary analysis of the ELM-1 study. Blood 2025, 145, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, T.M.; Cho, S.-G.; Jarque, I.; Iskierka-Jażdżewska, E.; Poon, L.M.; Prince, H.M.; Zhang, H.; Cao, J.; Zhang, M.; et al. Odronextamab monotherapy in patients with relapsed/refractory diffuse large B cell lymphoma: Primary efficacy and safety analysis in phase 2 ELM-2 trial. Nat. Cancer 2025, 6, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.; Turgut, B.; Tessoulin, B.; Guidez, S.; Altuntas, F.; Iqbal, N.; Namuduri, M.; Cao, A.; Sophos, N.; Uppala, A.; et al. Phase 3 trial evaluating efficacy and safety of odronextamab plus CHOP vs rituximab plus CHOP in previously untreated diffuse large B-cell lymphoma (DLBCL.; OLYMPIA-3). J. Clin. Oncol. 2024, 42, TPS7086. [Google Scholar] [CrossRef]
- Hawkes, E.A.; Kim, T.M.; Martín, E.M.D.; Núñez-García, A.; Chong, G.; Namuduri, M.; Risal, A.; Cheng, Y.; Flink, D.M.; Zhu, M.; et al. Phase 3 trial evaluating the efficacy and safety of odronextamab versus standard-of-care (SOC) therapy in relapsed/refractory (R/R) aggressive B-cell non-Hodgkin lymphoma (B-NHL.; OLYMPIA-4). J. Clin. Oncol. 2024, 42, TPS7093. [Google Scholar] [CrossRef]
- Bartlett, N.L.; Assouline, S.; Giri, P.; Schuster, S.J.; Cheah, C.Y.; Matasar, M.; Gregory, G.P.; Yoon, D.H.; Shadman, M.; Fay, K.; et al. Mosunetuzumab monotherapy is active and tolerable in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2023, 7, 4926–4935. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Phillips, T.J.; Hoffmann, M.S.; Armand, P.; Kim, T.M.; Yoon, D.H.; Mehta, A.; Greil, R.; Westin, J.; Lossos, I.S.; et al. Mosunetuzumab in combination with CHOP in previously untreated DLBCL: Safety and efficacy results from a phase 2 study. Blood Adv. 2023, 7, 6055–6065. [Google Scholar] [CrossRef]
- Olszewski, A.; Avigdor, A.; Babu, S.; Levi, I.; Eradat, H.; Abadi, U.; Holmes, H.; McKinney, M.; Woszczyk, D.; Giannopoulos, K.; et al. ABCL-366: Mosunetuzumab (Mosun) Monotherapy for Elderly/Unfit Patients with First-Line Diffuse Large B-Cell Lymphoma (DLBCL) Continues to Show Promising Safety and Efficacy with Durable Complete Responses. Clin. Lymphoma Myeloma. Leuk. 2021, 21, S395–S396. [Google Scholar] [CrossRef]
- Budde, L.E.; Olszewski, A.J.; Assouline, S.; Lossos, I.S.; Diefenbach, C.; Kamdar, M.; Ghosh, N.; Modi, D.; Sabry, W.; Naik, S.; et al. Mosunetuzumab with polatuzumab vedotin in relapsed or refractory aggressive large B cell lymphoma: A phase 1b/2 trial. Nat. Med. 2023, 30, 229–239. [Google Scholar] [CrossRef]
- Chavez, J.C.; Olszewski, A.J.; Bastos-Oreiro, M.; Assouline, S.E.; Lossos, I.S.; Diefenbach, C.; Ghosh, N.; Modi, D.; Sabry, W.; Naik, S.; et al. A Randomized Phase II Study of Mosunetuzumab SC Plus Polatuzumab Vedotin Demonstrates Improved Outcomes Versus Rituximab Plus Polatuzumab Vedotin in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2024, 144, 989. [Google Scholar] [CrossRef]
- Westin, J.; Olszewski, A.J.; Fogliatto, L.; Kim, W.S.; Shin, H.-J.; Jeon, Y.-W.; Norasetthada, L.; Pavlovsky, A.; Rego, E.; Wu, H.; et al. SUNMO: A phase III trial evaluating the efficacy and safety of mosunetuzumab in combination with polatuzumab vedotin vs rituximab plus gemcitabine and oxaliplatin in patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS7586. [Google Scholar] [CrossRef]
- Westin, J.; Zhang, H.; Kim, W.; Fogliatto, L.; Maruyama, D.; Farias, D.; Norasetthada, L.; Hong, H.; Ozcan, M.; Pavlovsky, A.; et al. Mosunetuzumab Plus Polatuzumab Vedotin is Superior to R-GemOx in Transplant-Ineligible Patients with R/R Lbcl: Primary Results of The Phase III Sunmo Trial. Hematol. Oncol. 2025, 43, 1–6. [Google Scholar] [CrossRef]
- Kambhampati, S.; Kallam, A.; Borogovac, A.; Chen, L.; Puverel, S.; Johnson, J.; Melgar, I.; Baird, J.H.; Danilov, A.; Godfrey, J.; et al. A Phase 2 Study of Loncastuximab Tesirine Plus Mosunetuzumab in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2024, 144 (Suppl. S1), 1742-1. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). A Phase 1 Study of Mosunetuzumab with Polatuzumab Vedotin and Lenalidomide (M+Pola+Len) in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Report No.: NCT06015880. 2025. Available online: https://clinicaltrials.gov/study/NCT06015880 (accessed on 8 June 2025).
- Patel, K.; Riedell, P.A.; Tilly, H.; Ahmed, S.; Michot, J.M.; Ghesquieres, H.; de Colella, J.-M.S.; Khan, A.C.; Bouabdallah, K.; Tessoulin, B.; et al. A Phase 1 Study of Plamotamab, an Anti-CD20 x Anti-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Non-Hodgkin’s Lymphoma: Recommended Dose Safety/Efficacy Update and Escalation Exposure-Response Analysis. Blood 2022, 140 (Suppl. S1), 9470–9472. [Google Scholar] [CrossRef]
- Riedell, P.A.; Patel, K.; Dunleavy, V.; Karimi, Y.H.; Shah, N.N.; Ribrag, V.; Ysebaert, L.; Noel, R.; Brisou, G.; Kline, J.; et al. Plamotamab: First Presentation of Subcutaneous Administration in a Phase 1 Dose Escalation Study in Heavily Pretreated R/R NHL Patients Who Had Prior CAR-T Cell Therapy. Blood 2024, 144, 4497. [Google Scholar] [CrossRef]
- Patel, K.; Koh, Y.; Ayyappan, S.; Karimi, Y.; Lossos, I.S.; Merchant, A.; Lee, P.; Jin, J.; Clynes, R.; Kanodia, J.; et al. Phase 2 Randomized, Open-Label, Multicenter Study to Evaluate the Efficacy and Safety of Plamotamab Combined with Tafasitamab (Tafa) + Lenalidomide (Len) Vs Tafa+Len in Relapsed or Refractory DLBCL. Blood 2022, 140, 12066–12067. [Google Scholar] [CrossRef]
- Viardot, A.; Goebeler, M.-E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef]
- Giri, P.; Patil, S.; Ratnasingam, S.; Prince, H.M.; Milliken, S.; Meijide, J.B.; Coyle, L.; Van Der Poel, M.; Mulroney, C.M.; Farooqui, M.Z.H.; et al. Results from a phase 1b study of blinatumomab-pembrolizumab combination in adults with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2022, 40, e19584. [Google Scholar] [CrossRef]
- Ghobadi, A.; Foley, N.C.; Cohen, J.; Rettig, M.P.; Cashen, A.F.; Gehrs, L.; Christ, S.; Street, E.; Wallace, N.; Ritchey, J.; et al. Blinatumomab consolidation post–autologous stem cell transplantation in patients with diffuse large B-cell lymphoma. Blood Adv. 2024, 8, 513–522. [Google Scholar] [CrossRef]
- Katz, D.A.; Morris, J.D.; Chu, M.P.; David, K.A.; Thieblemont, C.; Morley, N.J.; Khan, S.S.; Viardot, A.; García-Sancho, A.M.; Rodríguez-García, G.; et al. Open-label, phase 2 study of blinatumomab after frontline R-chemotherapy in adults with newly diagnosed, high-risk DLBCL. Leuk. Lymphoma 2022, 63, 2063–2073. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). A Phase I Trial of the Combination of Lenalidomide and Blinatumomab in Patients With Relapsed or Refractory Non-Hodgkins Lymphoma (NHL).Report No.: NCT02568553. 2025. Available online: https://clinicaltrials.gov/study/NCT02568553 (accessed on 5 June 2025).
- Nijland, M.; Issa, D.E.; Bult, J.A.A.; Deeren, D.; Velders, G.A.; Nijziel, M.R.; Sandberg, Y.; Vergote, V.; Oosterveld, M.; Fijnheer, R.; et al. Atezolizumab consolidation in patients with high-risk diffuse large B-cell lymphoma in complete remission after R-CHOP. Blood Adv. 2025, 9, 3530–3539. [Google Scholar] [CrossRef]
- Palomba, M.L.; Till, B.G.; Park, S.I.; Morschhauser, F.; Cartron, G.; Marks, R.; Shivhare, M.; Hong, W.-J.; Raval, A.; Chang, A.C.; et al. Combination of Atezolizumab and Obinutuzumab in Patients with Relapsed/Refractory Follicular Lymphoma and Diffuse Large B-Cell Lymphoma: Results from a Phase 1b Study. Clin. Lymphoma Myeloma Leuk. 2022, 22, e443–e451. [Google Scholar] [CrossRef]
- Palomba, M.L.; Cartron, G.; Popplewell, L.; Ribrag, V.; Westin, J.; Huw, L.-Y.; Agarwal, S.; Shivhare, M.; Hong, W.-J.; Raval, A.; et al. Combination of Atezolizumab and Tazemetostat in Patients With Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Results From a Phase Ib Study. Clin. Lymphoma Myeloma Leuk. 2022, 22, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Burke, J.M.; Cheson, B.D.; Diefenbach, C.S.; Ferrari, S.; Hahn, U.H.; Hawkes, E.A.; Khan, C.; Lossos, I.S.; Musuraca, G.; et al. Safety and efficacy of atezolizumab with rituximab and CHOP in previously untreated diffuse large B-cell lymphoma. Blood Adv. 2023, 7, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Othman, T.; Frankel, P.; Allen, P.; Popplewell, L.L.; Shouse, G.; Siddiqi, T.; Danilov, A.V.; Ruel, N.; Daniels, S.; Peters, L.; et al. Atezolizumab combined with immunogenic salvage chemoimmunotherapy in patients with transformed diffuse large B-cell lymphoma. Haematologica 2024, 110, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Frustaci, A.M.; Condoluci, A.; Coscia, M.; Chiarle, R.; Zinzani, P.L.; Motta, M.; Gaidano, G.; Quaresmini, G.; Scarfò, L.; et al. Atezolizumab, venetoclax, and obinutuzumab combination in Richter transformation diffuse large B-cell lymphoma (MOLTO): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 1298–1309. [Google Scholar] [CrossRef]
- Frustaci, A.M.; Montillo, M.; Rossi, D.; Zinzani, P.L.; Motta, M.; Gaidano, G.; Quaresmini, G.; Scarfo, L.; Pietrasanta, D.; Coscia, M.; et al. Results of MOLTO, a multicenter, open label, phase II clinical trial evaluating venetoclax, atezolizumab and obinutuzumab combination in Richter syndrome. J. Clin. Oncol. 2023, 41 (Suppl. S16), 7502. [Google Scholar] [CrossRef]
- Herbaux, C.; Bachy, E.; Bouabdallah, R.; Guidez, S.; Casasnovas, O.; Feugier, P.; Damaj, G.; Tilly, H.; Ysebaert, L.; Le Gouill, S.; et al. Atezolizumab, obinutuzumab and venetoclax for the treatment of patients with relapsed/refractory B non-Hodgkin lymphoma: Final analysis of a phase II trial from the LYSA group. Br. J. Haematol. 2025, 207, 110–122. [Google Scholar] [CrossRef]
- Herbaux, C.; Casasnovas, O.; Feugier, P.; Damaj, G.; Bouabdallah, R.; Guidez, S.; Ysebaert, L.; Tilly, H.; Le Gouill, S.; Fornecker, L.; et al. Atezolizumab + obinutuzumab + venetoclax in patients with relapsed or refractory diffuse large B-cell Lymphomas (R/R DLBCL): Primary analysis of a phase II trial from LYSA. J. Clin. Oncol. 2020, 38, 8053. [Google Scholar] [CrossRef]
- Jaeger, U.; Kalakonda, N.; Everaus, H.; Fustier, P.; Jaeger, J.; Manzke, O.; Nowakowski, G.S. Phase II Study of Durvalumab (anti–Pd-L1) Combined with Either R-Chop or Lenalidomide and R-Chop in Previously Untreated, High-Risk Diffuse Large B-Cell Lymphoma. Hematol. Oncol. 2017, 35 (Suppl. S2), 419–420. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Bastos-Oreiro, M.; Ungar, D.; Dautaj, I.; Kalac, M. Safety and Anti-Tumor Activity Study of Loncastuximab Tesirine and Durvalumab in Diffuse Large B-Cell, Mantle Cell, or Follicular Lymphoma. Blood 2019, 134, 2807. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Thieblemont, C.; Melnichenko, V.; Bouabdallah, K.; Walewski, J.; Majlis, A.; Fogliatto, L.M.; Garcia-Sancho, A.M.; Christian, B.; Gulbas, Z.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma: Final Analysis of KEYNOTE-170. Blood 2023, 142, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; LaPlant, B.R.; Parikh, S.A.; Leis, J.F.; Call, T.G.; Shanafelt, T.D.; He, R.; Micallef, I.N.; Nowakowski, G.S.; Habermann, T.M.; et al. Efficacy of pembrolizumab monotherapy and in combination with BCR inhibitors for Richter transformation of chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2024, 42, 7050. [Google Scholar] [CrossRef]
- Jaeger, U.; Worel, N.; McGuirk, J.P.; Riedell, P.A.; Fleury, I.; Du, Y.; Han, X.; Pearson, D.; Redondo, S.; Waller, E.K. Safety and efficacy of tisagenlecleucel plus pembrolizumab in patients with r/r DLBCL: Phase 1b PORTIA study results. Blood Adv. 2023, 7, 2283–2286. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Armand, P.; Hamadani, M.; Kline, J.; Moskowitz, C.H.; Avigan, D.; Brody, J.D.; Ribrag, V.; Herrera, A.F.; Morschhauser, F.; et al. Pembrolizumab for patients with non-Hodgkin lymphoma: Phase 1b KEYNOTE-013 study. Leuk. Lymphoma 2022, 64, 130–139. [Google Scholar] [CrossRef]
- Gregory, G.P.; Kumar, S.K.; Wang, D.; Mahadevan, D.; Walker, P.; Wagner-Johnston, N.D.; Escobar, C.; Bannerji, R.; Bhutani, D.; Chang, J.; et al. Pembrolizumab plus dinaciclib in patients with hematologic malignancies: The phase 1b KEYNOTE-155 study. Blood Adv. 2022, 6, 1232–1242. [Google Scholar] [CrossRef]
- Godfrey, J.; Mei, M.; Chen, L.; Song, J.Y.; Bedell, V.; Budde, E.; Armenian, S.; Puverel, S.; Nikolaenko, L.; Chen, R.; et al. Results from a phase I trial of pembrolizumab plus vorinostat in relapsed/refractory B-cell non-Hodgkin lymphoma. Haematologica 2023, 109, 533–542. [Google Scholar] [CrossRef]
- Witzig, T.E.; Maddocks, K.J.; De Vos, S.; Lyons, R.M.; Edenfield, W.J.; Sharman, J.P.; Vose, J.; Yimer, H.A.; Wei, H.; Chan, E.M.; et al. Phase 1/2 trial of acalabrutinib plus pembrolizumab (Pem) in relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2019, 37, 7519. [Google Scholar] [CrossRef]
- Berinstein, N. Clinical Effectiveness of Combination Immunotherapy DPX-Survivac, Low Dose Cyclophosphamide, and Pembrolizumab in Recurrent/Refractory DLBCL: The Spirel Study. In Proceedings of the 62nd ASH Annual Meeting and Exposition, San Diego, CA, USA, 5–8 December 2020; Available online: https://ash.confex.com/ash/2020/webprogram/Paper136751.html (accessed on 7 June 2025).
- Ho, C.; Fromm, J.R.; Fang, M.; Till, B.G.; Shadman, M.; Cowan, A.; Lynch, R.C.; Wu, V.; Voutsinas, J.M.; Rasmussen, H.A.; et al. Long-term efficacy and safety of pembrolizumab with R-CHOP as first-line therapy for DLBCL. J. Clin. Oncol. 2023, 41 (Suppl. S16), 7561. [Google Scholar] [CrossRef]
- Chong, E.A.; Alanio, C.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Lacey, S.F.; Ruella, M.; Bhattacharyya, S.; Wherry, E.J.; Schuster, S.J. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood 2022, 139, 1026–1038. [Google Scholar] [CrossRef]
- Osborne, W.; Marzolini, M.; Tholouli, E.; Ramakrishnan, A.; Bachier, C.R.; McSweeney, P.A.; Irvine, D.; Zhang, M.; Al-Hajj, M.A.; Pule, M.; et al. Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR T cell therapy, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. J. Clin. Oncol. 2020, 38 (Suppl. S15), 8001. [Google Scholar] [CrossRef]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.; Reddy, N.; et al. Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar] [CrossRef]
- Smykova, O.G.; Markelov, V.V.; Lepik, K.V.; Kondakova, E.V.; Stelmakh, L.V.; Moiseev, I.S.; Mikhailova, N.B.; Kulagin, A.D. LY-07. Immunochemotherapy with nivolumab, bendamustine, gemcitabine and rituximab (BeGeRN) in relapsed or refractory B-cell Non-Hodgkin Lymphoma: NCT03259529. Cell. Ther. Transplant. 2022, 11, 1–132. [Google Scholar] [CrossRef]
- de Jonge, A.V.; van Werkhoven, E.D.; Nijland, M.; de Heer, K.; Van Der Poel, M.W.; Sandberg, Y.; Klerk, C.; Drees, E.E.; Roemer, M.G.; de Jong, D.; et al. High Grade B Cell Lymphoma with MYC and BCL2 and/or BCL6 Rearrangements Treated with DA-EPOCH-R Induction and Nivolumab Consolidation Treatment: Interim Results of the HOVON-152 Phase II Trial. Blood 2021, 138, 1414. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N.; et al. Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study. J. Clin. Oncol. 2019, 37, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Morschhauser, F.; Sehn, L.H.; Herrera, A.F.; Friedberg, J.W.; Trněný, M.; Lenz, G.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; et al. Five-Year Analysis of the POLARIX Study: Prolonged Follow-up Confirms Positive Impact of Polatuzumab Vedotin Plus Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (Pola-R-CHP) on Outcomes. Blood 2024, 144, 469. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Hertzberg, M.; Opat, S.; Herrera, A.F.; Assouline, S.; Flowers, C.R.; Kim, T.M.; McMillan, A.K.; Özcan, M.; Safar, V.; et al. Polatuzumab Vedotin Plus Bendamustine and Rituximab in Relapsed/Refractory Diffuse Large B-Cell Lymphoma (R/R DLBCL): Final Results of a Phase Ib/II Randomized Study and Single-Arm Extension (Ext) Study. Blood 2022, 140 (Suppl. S1), 9464–9467. [Google Scholar] [CrossRef]
- Melani, C.; Lakhotia, R.; Pittaluga, S.; Phelan, J.D.; Muppidi, J.R.; Gordon, M.J.; Yang, Y.; Xu, W.; Davies-Hill, T.; Huang, D.W.; et al. Phase 1/2 Study of Polatuzumab in Combination with Venetoclax, Ibrutinib, Prednisone, Obinutuzumab, and Lenalidomide (ViPOR-P) in Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Preliminary Safety, Efficacy, and Molecular Analysis. Blood 2024, 144 (Suppl. S1), 1722. [Google Scholar] [CrossRef]
- Matasar, M.J.; Haioun, C.; Sancho, J.-M.; Viardot, A.; Hirata, J.; Perretti, T.; Musick, L.; McMillan, A.K. POLARGO: Randomized Phase III Study of Polatuzumab Vedotin Plus Rituximab, Gemcitabine, and Oxaliplatin in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2021, 138 (Suppl. S1), 3568. [Google Scholar] [CrossRef]
- Jerkeman, M.; Leppä, S.; Hamfjord, J.; Brown, P.; Ekberg, S.; Ferreri, A.J.M. S227: Initial Safety Data from The Phase 3 Polar Bear Trial in Elderly or Frail Patients with Diffuse Large Cell Lymphoma, Comparing R-Pola-Mini-Chp and R-Mini-Chop. HemaSphere 2023, 7, e91359ec. [Google Scholar] [CrossRef]
- Lynch, R.C.; Poh, C.; Ujjani, C.S.; Warren, E.H.; Smith, S.D.; Shadman, M.; Morris, K.; Rasmussen, H.; Ottemiller, S.; Shelby, M.; et al. Polatuzumab vedotin with dose-adjusted etoposide, cyclophosphamide, doxorubicin, and rituximab (Pola-DA-EPCH-R) for upfront treatment of aggressive B-cell non-Hodgkin lymphomas. J. Clin. Oncol. 2022, 40, 7546. [Google Scholar] [CrossRef]
- Reagan, P. Phase 2 Study of Polatuzumab Vedotin, Rituximab and Dose Attenuated CHP in Older Patients with DLBCL. Report No.: NCT04594798. 2024. Available online: https://clinicaltrials.gov/study/NCT04594798 (accessed on 8 June 2025).
- City of Hope Medical Center. A Phase 2 Study of Polatuzumab Vedotin with Rituximab, Ifosfamide, Carboplatin, and Etoposide (PolaR-ICE) as Initial Salvage Therapy for Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Report No.: NCT04665765. 2025. Available online: https://clinicaltrials.gov/study/NCT04665765 (accessed on 8 June 2025).
- GWT-TUD GmbH. Open-Label, Prospective Phase III Clinical Study to Compare Polatuzumab Vedotin Plus Rituximab, Ifosfamide, Carboplatin and Etoposide (Pola-R-ICE) with Rituximab, Ifosfamide, Carboplatin and Etoposide (R-ICE) Alone as Salvage Therapy in Patients with Primary Refractory or Relapsed Diffuse Large B-Cell Lymphoma (DLBCL). Report No.: NCT04833114. 2025. Available online: https://clinicaltrials.gov/study/NCT04833114 (accessed on 8 June 2025).
- Modi, D.; Kim, S.; Pregja, S.; Houde, C.; Reichel, K.A.; Ramchandren, R. A Phase II Study Evaluating Safety and Efficacy of Polatuzumab Vedotin in Combination with Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone in Patients with Previously Untreated Double and Triple Hit Lymphoma. Blood 2021, 138 (Suppl. S1), 3561. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Li, Q.; Yin, H.; Xiao, X.; Li, J.; Huang, W.; Jing, H.; Zhang, Y. Polatuzumab Vedotin, Rituximab and Lenalidomide (Pola-R2) As First-Line Therapy for Unfit and Frail Elderly Diffuse Large B-Cell Lymphoma Patients: Preliminary Result of a Prospective, Phase II, Multi-Center Study. Blood 2024, 144 (Suppl. S1), 1728. [Google Scholar] [CrossRef]
- Liu, Y. A Prospective, Multicenter, Phase II Study of POLA-R-CHP in the First-Line Treatment of Transformed DLBCL. Report No.: NCT06743945. 2025. Available online: https://clinicaltrials.gov/study/NCT06743945 (accessed on 8 June 2025).
- Shi, W. A Prospective, Single-arm, Multicenter Clinical Study of Polatuzumab, Rituximab and Orelabrutinib Combination Regimen (PRO) in the Treatment of Elderly Patients with Frail Treatment-naive Non-germinal Center Subtype Diffuse Large B-Cell Lymphoma. Report No.: NCT06530511. 2025. Available online: https://clinicaltrials.gov/study/NCT06530511 (accessed on 8 June 2025).
- The First Affiliated Hospital of Soochow University. Polatuzumab Vedotin and Zanubrutinib Plus R-CHP for Patients in Treatment of Newly Diagnosed Untreated Non-GCB DLBCL with Extranodal Involvement. Report No.: NCT06468943. 2024. Available online: https://clinicaltrials.gov/study/NCT06468943 (accessed on 8 June 2025).
- Tao, R. Evaluation of the Efficacy and Safety of Polatuzumab Vedotin Combined with Rituximab, Gemcitabine, and Oxaliplatin (Pola-R-GemOx) as Salvage Therapy for Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) Patients Ineligible for Autologous Transplantation: A Prospective, Multicenter, Single-Arm Clinical Study. Report No.: NCT07001540. 2025. Available online: https://clinicaltrials.gov/study/NCT07001540 (accessed on 8 June 2025).
- Qian, L. Polatuzumab Vedotin, Zanubrutinib, Rituximab, Lenalidomide and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. Report No.: NCT06664411. 2024. Available online: https://clinicaltrials.gov/study/NCT06664411 (accessed on 8 June 2025).
- Gritti, G.; Marlton, P.; Phillips, T.J.; Arthur, C.; Bannerji, R.; Corradini, P.; Johnston, A.; Seymour, J.F.; Yuen, S.; Hirata, J.; et al. Polatuzumab Vedotin Plus Venetoclax with Rituximab in Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Primary Efficacy Analysis of a Phase Ib/II Study. Blood 2020, 136, 45–47. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.Z.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. P1132: Long-Term Responses with Loncastuximab Tesirine: Updated Results from Lotis-2, The Pivotal Phase 2 Study in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma. HemaSphere 2023, 7, e402511c. [Google Scholar] [CrossRef]
- Carlo-Stella, C.; Zinzani, P.L.L.; Janakiram, M.; Dia, V.; He, X.; Ervin-Haynes, A.; Depaus, J. Planned Interim Analysis of a Phase 2 Study of Loncastuximab Tesirine Plus Ibrutinib in Patients with Advanced Diffuse Large B-Cell Lymphoma (LOTIS-3). Blood 2021, 138, 54. [Google Scholar] [CrossRef]
- Kwiatek, M.T.; Carlo-Stella, C.; Urban, A.; Niewiarowski, A.; Wang, L.; Laughlin, M.; Hamadani, M. ABCL-351 LOTIS-5, an Ongoing, Phase 3 Randomized Study of Loncastuximab Tesirine With Rituximab (Lonca-R) Versus Immunochemotherapy in Patients With R/R DLBCL. Clin. Lymphoma Myeloma Leuk. 2024, 24, S473–S474. [Google Scholar] [CrossRef]
- Alderuccio, J.P. Initial Results from Lotis-7: A Phase 1B Study of Loncastuximab Tesirine Plus Glofitamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Available online: https://library.ehaweb.org/eha/2025/eha2025-congress/4160985/juan.pablo.alderuccio.initial.results.from.lotis-7.a.phase.1b.study.of.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1 (accessed on 8 June 2025).
- Istituto Clinico Humanitas. Use of LOncastuximab Tesirine in Patients With RElapsed/Refractory Diffuse LargeB-Cell LYmphoma (DLBCL) or High Grade B-Cell Lymphoma (HGBCL) Who Have Progressive Disease After CAR T-Cell Treatment. Report No.: NCT06918912. 2025. Available online: https://clinicaltrials.gov/study/NCT06918912 (accessed on 8 June 2025).
- Jacobsen, E.D.; Sharman, J.P.; Oki, Y.; Advani, R.H.; Winter, J.N.; Bello, C.M.; Spitzer, G.; Palanca-Wessels, M.C.; Kennedy, D.A.; Levine, P.; et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood 2015, 125, 1394–1402. [Google Scholar] [CrossRef]
- Kim, J.A.; Hahn, U.; Kim, W.-S.; Fleury, I.; Laribi, K.; Burgues, J.M.B.; Bouabdallah, K.; Forward, N.; Bijou, F.; MacDonald, D.; et al. Brentuximab vedotin in combination with lenalidomide and rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma: Results from the phase 3 ECHELON-3 study. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA7005. [Google Scholar] [CrossRef]
- Reagan, P. Phase II Pilot Study of Brentuximab Vedotin, Rituximab and Dose Attenuated CHP in Elderly Patients with DLBCL. Report No.: NCT02734771. 2024. Available online: https://clinicaltrials.gov/study/NCT02734771 (accessed on 8 June 2025).
- Svoboda, J.; Bair, S.M.; Landsburg, D.J.; Nasta, S.D.; Nagle, S.J.; Barta, S.K.; Khan, N.; Filicko-O’Hara, J.; Gaballa, S.; Strelec, L.; et al. Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas. Haematologica 2020, 106, 1705–1713. [Google Scholar] [CrossRef]
- Merck Sharp; Dohme LLC. A Randomized, Open-label, Multicenter, Phase 2 Study Evaluating the Efficacy and Safety of Zilovertamab Vedotin (MK-2140) Plus R-CHP Versus Polatuzumab Vedotin Plus R-CHP in Treatment-naïve Participants with GCB Subtype of Diffuse Large B-Cell Lymphoma (DLBCL). Report No.: NCT06890884. 2025. Available online: https://clinicaltrials.gov/study/NCT06890884 (accessed on 8 June 2025).
- Ozcan, M.; Barca, E.G.; Kim, T.M.; Paszkiewicz-Kozik, E.; Zaucha, J.M.; Hohaus, S.; Ladetto, M.; Patti, C.; Giebel, S.; Robak, T.; et al. Waveline-007: Dose Escalation and Confirmation, and Efficacy Expansion Trial of Zilovertamab Vedotin in Combination with Cyclophosphamide, Doxorubicin, and Prednisone Plus Rituximab in Patients with Diffuse Large B Cell Lymphoma. Blood 2024, 144, 578. [Google Scholar] [CrossRef]
- Gollard, R.P.T.; Flynn, J.; Reddy, N.; Yusuf, R.Z.; Al-Janadi, A. waveLINE-010: Zilovertamab vedotin plus R-CHP versus R-CHOP in untreated diffuse large B-cell lymphoma. J. Clin. Oncol. 2025, 43, 16. [Google Scholar] [CrossRef]
- Armand, P.; Lee, S.T.; Jurczak, W.; Stevens, D.A.; Choquet, S.; Ghesquieres, H.; Norasetthada, L.; Pinto, A.; Saydam, G.; Zhou, H.; et al. WaveLINE-003: Phase 2/3 trial of zilovertamab vedotin plus standard of care in relapsed/refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2025, 43, 7005. [Google Scholar] [CrossRef]
- Norasetthada, L.; Diaz, J.; Lv, F.; Song, Y.; Lee, S.T.; Paszkiewicz-Kozik, E.; Modi, D.; Santoro, A.; Pathiraja, K.; Reddy, N.; et al. Updated Results from the Phase 2 Waveline-004 Study of Zilovertamab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Blood 2024, 144, 1738. [Google Scholar] [CrossRef]
- Levy, M.Y.; Jagadeesh, D.; Grudeva-Popova, Z.; Trněný, M.; Jurczak, W.; Pylypenko, H.; André, M.; Nasta, S.D.; Rechavi-Robinson, D.; Toffanin, S.; et al. Safety and Efficacy of CD37-Targeting Naratuximab Emtansine PLUS Rituximab in Diffuse Large B-Cell Lymphoma and Other NON-Hodgkin’S B-Cell Lymphomas—A Phase 2 Study. Blood 2021, 138, 526. [Google Scholar] [CrossRef]
- Abramson, J.S.; Ku, M.; Hertzberg, M.; Fox, C.P.; Herbaux, C.; Huang, H.; Yoon, D.H.; Kim, W.S.; Zhang, H.; Abdulhaq, H.; et al. Glofitamab Plus Gemcitabine And Oxaliplatin (Glofit-Gemox) In Patients With Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (Dlbcl): 2-Year Follow-Up Of Starglo. Hematol. Oncol. 2025, 43, e76_70093. [Google Scholar] [CrossRef]
- Melchardt, T. Frontline Phase II Rituximab-Polatuzumab-Glofitmab (R-POLA-GLO) Trial Demonstrates a Manageable Safety Profile and High Response Rates in Elderly and Medical Unfit Patients with Aggressive Lymphoma. Available online: https://library.ehaweb.org/eha/2025/eha2025-congress/4159325/thomas.melchardt.frontline.phase.ii.rituximab-polatuzumab-glofitmab.html?f=menu%3D6%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Ace_id%3D2882%2Aot_id%3D31553%2Amarker%3D5843%2Afeatured%3D19595 (accessed on 5 June 2025).
- Study Details. Rituximab in Combination with Glofitamab and Polatuzumab Vedotin in Patients with Previously Untreated Aggressive B-Cell Lymphoma Ineligible for R-CHOP. Available online: https://www.clinicaltrials.gov/study/NCT05798156 (accessed on 5 June 2025).
- Mohan, M.; Szabo, A.; Cheruvalath, H.; Clennon, A.; Bhatlapenumarthi, V.; Patwari, A.; Balev, M.; Bhutani, D.; Shrestha, A.; Thanendrarajan, S.; et al. Effect of Intravenous Immunoglobulin (IVIG) Supplementation on infection-free survival in recipients of BCMA-directed bispecific antibody therapy for multiple myeloma. Blood Cancer J. 2025, 15, 74. [Google Scholar] [CrossRef]
- Crochet, G.; Iacoboni, G.; Couturier, A.; Bachy, E.; Iraola-Truchuelo, J.; Gastinne, T.; Cartron, G.; Fradon, T.; Lesne, B.; Kwon, M.; et al. Efficacy of CAR T-cell therapy is not impaired by previous bispecific antibody treatment in large B-cell lymphoma. Blood 2024, 144, 334–338. [Google Scholar] [CrossRef]
- Brooks, T.R.; Zabor, E.C.; Bedelu, Y.; Yang, X.; Karimi, Y.H.; Nedved, A.N.; Wang, Y.; Dave, N.K.; Landsburg, D.J.; Baron, K.; et al. Real-world outcomes of patients with aggressive B-cell lymphoma treated with epcoritamab or glofitamab. Blood 2025, in press. [Google Scholar] [CrossRef]
- Calabretta, E.; Lu, K.; Zhao, X.; Stewart, C.; Palazzo, M.; Crombie, J.L.; Murakami, M.A.; Herrera, A.F.; Lenz, G.; Morschhauser, F.; et al. The Benefit OF Pola-R-Chp in Dlbclass-Defined Molecular Subsets of Newly Diagnosed Dlbcl in The Polarix Trial. Hematol. Oncol. 2025, 43 (Suppl. S3), eLBA1_70110. [Google Scholar] [CrossRef]
- Alderuccio, J.P.; Okada, C.; Crochet, G.; Ayers, E.C.; Hu, M.; Ferrari, S.; Caimi, P.F.; Hamadani, M.; Depaus, J.; Derenzini, E.; et al. Initial Results From Lotis-7: A Phase 1b Study Of Loncastuximab Tesirine Plus Glofitamab In Patients With Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (dlbcl). Hematol. Oncol. 2025, 43, e78_70093. [Google Scholar] [CrossRef]
- Melani, C.; Lakhotia, R.; Pittaluga, S.; Phelan, J.D.; Huang, D.W.; Wright, G.; Simard, J.; Muppidi, J.; Thomas, C.J.; Ceribelli, M.; et al. Combination Targeted Therapy in Relapsed Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2024, 390, 2143–2155. [Google Scholar] [CrossRef]
- Pirosa, M.C.; Stathis, A.; Zucca, E. Tafasitamab for the treatment of patients with diffuse large B-cell lymphoma. Hum. Vaccines Immunother. 2024, 20, 2309701. [Google Scholar] [CrossRef]
- Düll, J.; Maddocks, K.J.; Gonzalez-Barca, E.; Jurczak, W.; Liberati, A.M.; Obr, A.; Gaidano, G.; Abrisqueta, P.; André, M.; Dreyling, M.H.; et al. Long-term analyses from L-MIND, a phase II study of tafasitamab (MOR208) combined with lenalidomide (LEN) in patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). J. Clin. Oncol. 2021, 39, 7513. [Google Scholar] [CrossRef]
- Larsen, T.S.; Manzke, O.; Leibovitz, D.; Arbushites, M. A Phase 3 Study of Tafasitamab Plus Lenalidomide in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma (firmMIND). Blood 2022, 140 (Suppl. S1), 12077–12078. [Google Scholar] [CrossRef]
- Vitolo, U.; Nowakowski, G.S.; Burke, J.M.; Fox, C.P.; Trneny, M.; Chiappella, A.; Waldron-Lynch, M.; Hadar, N.; Pachori, A.; Lenz, G. frontMIND: A phase III, randomized, double-blind study of tafasitamab + lenalidomide + R-CHOP versus R-CHOP alone for newly diagnosed high-intermediate and high-risk diffuse large B-cell lymphoma. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS7590. [Google Scholar] [CrossRef]
- Johnson, P.W.M.; Balasubramanian, S.; Hodkinson, B.; Shreeve, S.M.; Sun, S.; Srinivasan, S.; Steele, A.J.; Vermeulen, J.; Sehn, L.H.; Wilson, W.H. Clinical impact of ibrutinib plus R-CHOP in untreated DLBCL coexpressing BCL2 and MYC in the phase 3 PHOENIX trial. Blood Adv. 2023, 7, 2008–2017. [Google Scholar] [CrossRef]
- Wilson, W.H.; Wright, G.W.; Huang, D.W.; Hodkinson, B.; Balasubramanian, S.; Fan, Y.; Vermeulen, J.; Shreeve, M.; Staudt, L.M. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021, 39, 1643–1653.e3. [Google Scholar] [CrossRef]
- Dyer, M.J.; De Vos, S.; Ruan, J.; Flowers, C.; Maddocks, K.J.; Rule, S.; Hamdy, A.M.; Izumi, R.; Slatter, J.G.; Cheung, J.; et al. Acalabrutinib monotherapy in patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2018, 36, 7547. [Google Scholar] [CrossRef]
- Christofyllakis, K.; Poeschel, V.; Altmann, B.; Maurer, S.; Lesan, V.; Bittenbring, J.; Kos, I.A.; Kaddu-Mulindwa, D.; Abdi, Z.; Fleser, O.; et al. A Randomized, Open-Label, Phase 3 Study of Acalabrutinib in Combination with Rituximab and Reduced Dose CHOP (R-miniCHOP) in Older Adults with Untreated Diffuse Large B-Cell Lymphoma (ARCHED/GLA 2022-1). Blood 2023, 142, 6248. [Google Scholar] [CrossRef]
- Sehn, L.H.; Kahl, B.S.; Matasar, M.J.; Lenz, G.; Izutsu, K.; Zhao, W.; Tao, L.; Calvo, R.; Zinzani, P.L. ESCALADE: A phase 3 study of acalabrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for patients ≤65y with untreated non-germinal center B-cell–like (non-GCB) diffuse large B-cell lymphoma (DLBCL). J. Clin. Oncol. 2021, 39 (Suppl. S15), TPS7572. [Google Scholar] [CrossRef]
- Yin, X.; He, Q.; Liu, D.; Xie, L.; Wang, H.; Chen, C.; Zhao, C.; Shan, N.; Shi, S.; Wei, H.; et al. Zanubrutinib plus R-CHOP for the treatment of newly diagnosed double-expressor lymphoma: A phase 2 clinical study. Cancer 2025, 131, e35697. [Google Scholar] [CrossRef]
- Westin, J.; Davis, R.E.; Feng, L.; Hagemeister, F.; Steiner, R.; Lee, H.J.; Fayad, L.; Nastoupil, L.; Ahmed, S.; Rodriguez, A.; et al. Smart Start: Rituximab, Lenalidomide, and Ibrutinib in Patients With Newly Diagnosed Large B-Cell Lymphoma. J. Clin. Oncol. 2023, 41, 745–755. [Google Scholar] [CrossRef]
- Westin, J.; E Steiner, R.; Chihara, D.; Ahmed, S.; Jain, P.; Malpica, L.; Iyer, S.P.; Fayad, L.; Nair, R.; Nastoupil, L.J.; et al. Smart Stop: Lenalidomide, Tafasitamab, Rituximab, and Acalabrutinib Alone and with Combination Chemotherapy for the Treatment of Newly Diagnosed Diffuse Large B-Cell Lymphoma. Blood 2023, 142 (Suppl. S1), 856. [Google Scholar] [CrossRef]
- Morschhauser, F.; Feugier, P.; Flinn, I.W.; Gasiorowski, R.; Greil, R.; Illés, Á.; Johnson, N.A.; Larouche, J.-F.; Lugtenburg, P.J.; Patti, C.; et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021, 137, 600–609. [Google Scholar] [CrossRef]
- Vitolo, U.; Novo, M. Bcl-2 inhibition in DLBCL: “the times they are a-changing”? Blood 2021, 137, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, U.; Trněný, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2017, 35, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V.; Morschhauser, F.; McKay, P.; Salles, G.A.; Batlevi, C.L.; Schmitt, A.; Tilly, H.; Cartron, G.; Thieblemont, C.; Fruchart, C.; et al. Interim Results from an Ongoing Phase 2 Multicenter Study of Tazemetostat, an EZH2 Inhibitor, in Patients with Relapsed or Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2018, 132 (Suppl. S1), 4196. [Google Scholar] [CrossRef]
- Sarkozy, C.; Molina, T.J.; Houot, R.; Dubois, S.; Pica, G.M.; Ruminy, P.; Herbaux, C.; Gastinne, T.; Haioun, C.; Guidez, S.; et al. Results of the Phase II of Epirchop Study, Evaluating the Efficacy of Tazemetostat in Combination with R-CHOP in Elderly Newly Diagnosed Diffuse Large B Cell Lymphoma (DLBCL): A Lysa Study. Blood 2023, 142, 853. [Google Scholar] [CrossRef]
- Kalakonda, N.; Maerevoet, M.; Cavallo, F.; Follows, G.; Goy, A.; Vermaat, J.S.P.; Casasnovas, O.; Hamad, N.; Zijlstra, J.M.; Bakhshi, S.; et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020, 7, e511–e522. [Google Scholar] [CrossRef]
| Trial Name | Drug(s) | Population | Phase | OR (CR) |
|---|---|---|---|---|
| Epcoritamab | ||||
| EPCORE NHL-1; GCT3013-01 [11] | Subcutaneous Epcoritamab | CD20+ mature B-cell neoplasm (diffuse large B-cell lymphoma (DLBCL) or other aggressive non-Hodgkin lymphomas, including primary mediastinal LBCL, high-grade B-cell lymphoma, or follicular lymphoma grade 3B) | I/II | 63 (39) |
| EPCORE NHL-2 (NCT04663347) [12,13,14,15,16,17] | Epcoritamab plus GemOx [15] | ASCT-ineligible relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | IB/II | 91 (59) |
| Epcoritamab + R-DHAX/C [13] | Transplant-eligible patients (pts) with high-risk re lapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | IB/II | 76 (69) | |
| Epcoritamab SC + R-mini-CHOP [16] | 1L DLBCL who were not considered candidates for full-dose R-CHOP | IB/II | 89 (82) | |
| Epcoritamab + RICE [17] | R/R DLBCL eligible for ASCT | IB/II | 87 (65) | |
| EPCORE NHL-5 (NCT05283720) | Epcoritamab Plus Lenalidomide [18] | Relapsed or refractory (R/R) DLBCL | IB/II | 67 (51) |
| Epcoritamab + pola-R-CHP [19] | Newly diagnosed CD20+ DLBCL | IB/II | 100 (97) | |
| EPCORE DLBCL-3 [20,21] | Epcoritamab monotherapy | Elderly pts with 1L CD20+ LBCL who were ineligible for anthracycline-based tx due to age ≥ 80 y or age ≥ 75 y with underlying comorbidities | II | 78 (70) |
| EPCORE DLBCL-1 NCT04628494 [22] | Epcoritamab vs. investigator’s choice of chemotherapy | Relapsed, refractory DLBCL who have failed or are ineligible for high-dose chemotherapy and autologous stem cell transplant (HDT-ASCT) | III | Ongoing |
| Glotifamab | ||||
| STARGLO (NCT0353283) [23] | Glofitamab plus gemcitabine–oxaliplatin (Glofit-GemOx) versus rituximab (R)-GemOx | Transplant-ineligible patients (aged ≥ 18 years) with histologically confirmed relapsed or refractory diffuse large B-cell lymphoma | III | 25.5 vs.12.9 |
| NCT03075696 [24,25,26] | Glofitamab + obinutuzumab | Relapsed or refractory DLBCL who had received at least two lines of therapy previously | II | 52 (39) |
| LYSA NCT04703686 [27] | Glofitamab | Refractory or relapsed diffuse large B-cell lymphoma after failing CAR-T cell therapy | II | 76 (45) |
| NCT03533283 [28] | Glofitamab + Polatuzumab Vedotin | Heavily pretreated relapsed/refractory (R/R) large B-cell lymphoma (LBCL), including high-grade B-cell lymphoma (HGBCL) | IB/II | 80 (62) |
| NCT03467373 [29] | Glofitamab + Pola-R-CHP | Previously untreated diffuse large B-cell lymphoma (DLBCL) | IB | 100 (76) |
| SKYGLO NCT06047080 [30] | Glofitamab Plus Polatuzumab Vedotin + Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (Pola-R-CHP) versus Pola-R-CHP | Previously untreated CD20-positive LBCL | III | Ongoing |
| NCT04980222 [31] | Glofitamab Plus R-CHOP | Previously untreated (1L) large B-cell lymphoma (LBCL) defined as high risk by circulating tumor DNA (ctDNA) dynamics | II | 93 (80) |
| NCT05364424 [32] | Glofitamab in Combination with Rituximab Plus Ifosfamide, Carboplatin, and Etoposide | Relapsed or refractory diffuse large B-cell lymphoma, eligible for stem cell transplant or chimeric antigen receptor T-cell therapy | IB | 78 (68) |
| NCT04077723 [33] | Englumafusp Alfa + Glofitamab | Relapsed or refractory B-NHL after at least one prior treatment | IB | (aNHL/iNHL) ORR 67.5%/91.7% CR 56.6%/79.2% |
| COALITION NCT04914741 [34,35] | Glofitamab-R-CHOP or Glofitamab-Polatuzumab Vedotin-R-CHP | Younger patients with high-burden, high-risk large B-cell lymphoma | IB/II | 100 (92) |
| NCT05798156 [34,35] | R-Pola-Glo | Elderly/frail and medically unfit pts with aggressive lymphoma | II | 90 (82) |
| Odronextamab | ||||
| ELM-1 study NCT02290951 [36] | Odronextamab | Relapsed/Refractory (R/R) B-cell non-Hodgkin lymphoma after ≥2 lines of therapy—post CAR-T DLBCL cohort | I | 48 (31) |
| ELM-2 study NCT03888105 [37] | Odronextamab | Relapsed/Refractory (R/R) B-cell non-Hodgkin lymphoma after ≥2 lines of therapy—DLBCL cohort | II | 52 (31.5) |
| OLYMPIA-3 (NCT06091865) [38] | Odronextamab plus CHOP vs. rituximab plus CHOP | Previously untreated diffuse large B-cell lymphoma | III | Ongoing |
| OLYMPIA-4 (EudraCT 2022-502783-21-00) [39] | Odronextamab versus Standard of care | Previously treated aggressive B-NHL | III | Ongoing |
| Mosunetuzumab | ||||
| NCT02500407 [40] | Mosunetuzumab | Relapsed/refractory diffuse large B-cell lymphoma | I/II | 42 (24) |
| NCT03677141 [41] | Mosunetuzumab with CHOP | Previously untreated DLBCL | II | 87.5 (85) |
| NCT03677154 [42] | Mosunetuzumab | Elderly/unfit patients with 1L DLBCL | I/II | 67.7 (42) |
| NCT03671018 [43,44] | Mosunetuzumab + polatuzumab vedotin [43] | Relapsed/refractory aggressive large B-cell lymphoma (LBCL) | Ib/II | 59.2 (45.9) |
| M (SC administration)-Pola vs. rituximab (R)-Pola [44] | II | OR 78 vs. 50; CR 58 vs. 35 | ||
| SUNMO (NCT05171647) [45,46] | Mosun + Pola (M-Pola) vs. IV rituximab + gemcitabine and oxaliplatin (R-GemOx) | CD20-positive R/R aNHL, ≥1 prior systemic therapy (if only one prior line of therapy, pts must be ineligible for ASCT) | III | 70.3 (51.4) |
| NCT05672251 [47] | Loncastuximab tesirine (Lonca) + Mosunetuzumab | R/RL DLBCL after ≥2 prior lines of therapy | II | Ongoing |
| NCT06015880 [48] | Mosunetuzumab, Pola, Lenalidomide | Relapsed/refractory diffuse large B-cell lymphoma | I | Ongoing |
| Plamotamab | ||||
| NCT02924402 [49,50] | Plamotamab IV [49] | Relapsed/refractory (R/R) non-Hodgkin’s lymphoma (NHL) | I | 47 (26) |
| Plamotamab SC [50] | Heavily pretreated R/R NHL patients who had prior CAR-T cell therapy | I | 53 (24) | |
| XmAb13676-03 NCT05328102 [51] | Plamotamab + TAFA + LEN versus TAFA + LEN | DLBCL who have relapsed or are refractory to ≥1 prior line of therapy and are ineligible for or refuse ASCT | II | Ongoing |
| Blinatumomab | ||||
| NCT01741792 [52] | Blinatumomab | Relapsed/refractory DLBCL | II | 43 (19) |
| NCT03340766 [53] | Blinatumomab + Pembrolizumab | Relapsed/refractory DLBCL | IB | 30, Terminated due to lack of efficacy |
| NCT03072771 [54] | Blinatumomab consolidation after auto-SCT | Relapsed diffuse large B-cell lymphoma (DLBCL), who undergo autologous stem cell transplant | I | 1 yr—50% CR |
| NCT03023878 [55] | Blinatumomab following an induction with R- chemotherapy | Adults with newly diagnosed, high-risk DLBCL | II | - |
| NCT02568553 [56] | Lenalidomide + Blinatumomab | Relapsed or refractory non-Hodgkin’s lymphoma (NHL) | I | Ongoing |
| Atezolizumab | ||||
| HOVON 151 trial NCT03463057 [57] | Atezolizumab | DLBCL patients with an international prognostic index (IPI) score of ≥3 and CMR after R-CHOP | II | 2-year: DFS 87.9% OS 96.3% |
| NCT02220842 | Atezolizumab + Obinutuzumab [58] | Relapsed or refractory DLBCL or FL | Ib | 17 (4) |
| Atezolizumab + Tazemetostat [59] | Relapsed or refractory DLBCL | Ib | 16 (7) | |
| NCT02596971 [60] | Atezolizumab + RCHOP | Previously untreated advanced DLBCL | Ib/II | 87.5 (77.5) |
| NCT03321643 [61] | Atezolizumab + rituximab + GemOx (R-GemOx + Atezo) | R/R transformed DLBCL, including Richter transformation | I/II | 59 (33) |
| NCT04082897 MOLTO [62,63] | Atezolizumab + venetoclax + obinutuzumab | Richter transformation diffuse large B-cell lymphoma | II | 67.9 (28.6) |
| NCT03276468 LYSA [64,65] | Atezolizumab + venetoclax + obinutuzumab | Relapsed/refractory B non-Hodgkin lymphoma | II | 23.6 (18) |
| Durvalumab | ||||
| NCT03003520 [66] | Durvalumab in combination with R-CHOP or R2-CHOP | Previously untreated, high-risk DLBCL | II | Ongoing; 54% Arm A; 67% Arm B |
| NCT03685344 [67] | Loncastuximab Tesirine + Durvalumab | R/R DLBCL, MCL, or FL | I/II | Ongoing |
| Pembrolizumab | ||||
| KEYNOTE-170 NCT02576990 [68] | Pembrolizumab | Relapsed/refractory (R/R) primary mediastinal B-cell lymphoma (PMBCL) whose disease progressed after or who were ineligible for autologous stem cell transplantation | II | 41 (20) |
| NCT02332980 [69] | Pembrolizumab Alone or With Idelalisib or Ibrutinib | CLL patients with biopsy-proven Richter transformation to diffuse large B-cell lymphoma | II | 23 (7.7) |
| PORTIA trial NCT03630159 [70] | Tisagenlecleucel + pembrolizumab | Relapsed or refractory DLBCL | Ib | 50 (33.3) |
| KEYNOTE-013 study NCT01953692 [71] | pembrolizumab (cohort 4) or pembrolizumab plus lenalidomide (cohort5) | Relapsed or refractory NHL who were ineligible for or failed hematopoietic cell transplantation (HCT) | Ib | 22 (12)/39 (22) |
| NCT02684617 KEYNOTE-155 [72] | Pembrolizumab + Dinaciclib | Relapsed or refractory DLBCL | Ib | 21 (10.5) |
| NCT03150329 [73] | Pembrolizumab + vorinostat | Relapsed/refractory B-cell NHL—DLBCL cohort | I | 55 (45) |
| KEYNOTE145 NCT02362035 [74] | Acalabrutinib + pembrolizumab | Relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL) | I/II | 26 (7) |
| SPiReL trial NCT03349450 [75] | Pembrolizumab, low-dose cyclophosphamide, +DPX-Survivac | Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) | II | 63 (27) |
| NCT02541565 [76] | Pembrolizumab with R-CHOP | Untreated DLBCL or grade 3b follicular lymphoma | 90 (77) | |
| NCT02650999 [77] | Pembrolizumab | Relapsed/refractory B-cell lymphomas after CD19-directed CAR T cells | Ia/II | 25 |
| Alexander NCT03287817 [78] | Pembrolizumab + AUTO3, a CAR T targeting CD19/22 | r/r DLBCL (NOS) or transformed (tDLBCL) | I | 64 (55) |
| Nivolumab | ||||
| CheckMate 139 NCT02038933 [79] | Nivolumab | Relapsed/refractory DLBCL who were ineligible for autologous hematopoietic cell transplantation (auto-HCT) or who had experienced failure with auto-HCT | II | auto-HCT–failed: 10 (3) auto-HCT–ineligible: 3 (0) |
| NCT03259529 [80] | Nivolumab, bendamustine, gemcitabine + rituximab (BeGeRN) | Relapsed or refractory B-cell non-Hodgkin lymphoma | I/II | 45.5 (18) |
| HOVON-152 NCT03620578 [81] | DA-EPOCH-R Induction and Nivolumab Consolidation Treatment | Newly diagnosed High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements | II | 61% CMR |
| CheckMate 436 NCT02581631 [82] | Nivolumab + Brentuximab Vedotin | Relapsed/refractory primary mediastinal large B-cell lymphoma | II | 70 (43) |
| Polatuzumab Vedotin | ||||
| POLARIX NCT03274492 [83,84] | Polatuzumab Vedotin + Rituximab, Cyclophosphamide, Doxorubicin, Prednisone (Pola-R-CHP) vs. R-CHOP | Previously untreated intermediate-risk or high-risk DLBCL | III | 2 year PFS: 76.7% (Pola-R-CHP) vs. 70.2% (R-CHOP) |
| NCT02257567 [85] | Pola + bendamustine + rituximab (Pola + BR) vs. BR alone | Relapsed/refractory diffuse large B-cell lymphoma | Ib/II | BoR:62.5% vs. 25.0% BCR:52.5% vs. 22.5% PFS:9.2 vs. 3.7 mo |
| NCT04739813 [86] | Polatuzumab, Venetoclax, Ibrutinib, Prednisone, Obinutuzumab, Lenalidomide (ViPOR-P) | Relapsed/refractory diffuse large B-cell lymphoma | I/II | 75 (50) |
| POLARGO NCT04182204 [87] | Pola-R-GemOx vs. R-GemOx | Relapsed/refractory diffuse large B-cell lymphoma | III | 52.7 (40.3) |
| POLAR BEAR NCT04332822 [88] | Pola-R-miniCHP vs. R-miniCHOP | Newly diagnosed DLBCL, >80 years, or 75–80 years and frail | III | Ongoing |
| NCT04231877 PERCH [89] | Pola, etoposide, cyclophosphamide, doxorubicin, rituximab (Pola-DA-EPCH-R) | Aggressive large B-cell lymphomas | I | 93 (71) |
| NCT04594798 [90] | Pola, Rituximab and Dose Attenuated CHP | Newly diagnosed DLBCL patients 75 years and older | II | Ongoing |
| NCT04665765 [91] | Pola, rituximab, ifosfamide, carboplatin, etoposide (PolaR-ICE) | First salvage therapy for relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | II | Ongoing |
| NCT04833114 [92] | Pola, rituximab, ifosfamide, carboplatin, etoposide (Pola-R-ICE) vs. rituximab, ifosfamide, carboplatin and etoposide (R-ICE) | Salvage therapy in patients with primary refractory or relapsed diffuse large B-cell lymphoma (DLBCL) | III | Ongoing |
| NCT04479267 [93] | Pola, Rituximab, Cyclophosphamide, Doxorubicin, Prednisone | Patients aged > 18 years with previously untreated double- or triple-hit lymphoma | II | Ongoing |
| NCT06176729 [94] | Pola, Rituximab Lenalidomide (Pola-R2) | Newly diagnosed DLBCL patients aged over 70 years old and unfit or frail | II | EOT CRR 100% |
| NCT06743945 [95] | POLA-R-CHP | Patients with transformed DLBCL | II | Ongoing |
| NCT06530511 [96] | Polatuzumab, Rituximab, Orelabrutinib (PRO) | Elderly patients with frail, treatment-naïve, non-germinal center subtype diffuse large B-cell lymphoma | II | Ongoing |
| NCT06468943 [97] | Pola, Zanubrutinib, R-CHP | Newly diagnosed untreated non-GCB DLBCL patients with extranodal involvement | II | Ongoing |
| NCT06015880 [48] | Mosunetuzumab, Pola, Lenalidomide | Relapsed/refractory diffuse large B-cell lymphoma | I | Ongoing |
| NCT07001540 [98] | Pola-R-GemOx | Salvage therapy for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) patients ineligible for autologous transplantation | II | Ongoing |
| NCT06664411 [99] | Pola, Zanubrutinib, Rituximab, Lenalidomide Prednisone Pola-ZR2P | Previously untreated DLBCL | II | Ongoing |
| NCT02611323 [100] | Pola, Venetoclax, Rituximab Pola-Ven-R | Relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) | Ib/II | 65 (31) |
| Loncastuximab tesirine | ||||
| LOTIS 2 NCT03589469 [101] | Loncastuximab | Relapsed/refractory diffuse large B-cell lymphoma | II | 48 (25) |
| LOTIS-3 NCT03684694 [102] | Loncastuximab + ibrutinib | Relapsed/refractory diffuse large B-cell lymphoma | I/II | 57 (34) |
| LOTIS-5 NCT04384484 [103] | Lonca-R vs. R + gemcitabine + oxaliplatin (R-GemOx) | Relapsed/refractory diffuse large B-cell lymphoma | III | Ongoing Prelim: 80 (50) |
| LOTIS-7 NCT04970901 [104] | Lonca + Pola (arm C), glofitamab (arm E), or mosunetuzumab (arm F) | Relapsed/refractory diffuse large B-cell lymphoma | Ib | Lonca + Glofit 95 (90.9) |
| LORELY NCT06918912 [105] | Loncastuximab | Relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma (HGBCL) following CAR-T therapy failure | II | Ongoing |
| Brentuximab vedotin | ||||
| NCT01421667 [106] | Brentuximab | Relapsed/refractory diffuse large B-cell lymphoma | II | 44 (17) |
| ECHELON-3 NCT04404283 [107] | Brentuximab vedotin, lenalidomide, rituximab vs. R2 | Relapsed/refractory diffuse large B-cell lymphoma | III | 70.9 (45.6) |
| NCT02734771 [108] | Brentuximab Vedotin, Rituximab, Dose Attenuated CHP | Elderly patients with newly diagnosed DLBCL | II | Ongoing |
| NCT01994850 [109] | Brentuximab vedotin (BV) rituximab, cyclophosphamide, doxorubicin, prednisone (R-CHP) | CD30-positive (+) B-cell lymphomas | I/II | 100 (86) |
| Zilovertamab Vedotin | ||||
| waveLINE-011 NCT06890884 [110] | Zilovertamab Vedotin +R-CHP vs. Pola + R-CHP | Treatment-naïve DLBCL, GCB subtype | II | Ongoing |
| waveLINE-007 NCT05406401 [111] | Zilovertamab vedotin + R-CHP | Previously untreated DLBCL | II | 100 (100) |
| waveLINE-010 NCT06717347 [112] | Zilovertamab vedotin + R-CHP vs. R-CHOP | Previously untreated DLBCL | III | Ongoing |
| waveLINE-003 NCT05139017 [113] | Zilovertamab vedotin, rituximab, gemcitabine-oxaliplatin (R-GemOx) | Relapsed/refractory DLBCL | II/III | 56 |
| waveLINE-004 NCT05144841 [114] | Zilovertamab vedotin | Relapsed/refractory DLBCL | II | 29 (13) |
| Naratuximab Emtansine | ||||
| NCT02564744 [115] | Naratuximab Emtansine, rituximab | Relapsed and/or refractory (R/R) B-NHL | II | 44 (31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhari, J.; Shah, N.N. Targeted Therapies and Immunotherapies for Diffuse Large B-Cell Lymphoma. Cancers 2025, 17, 2517. https://doi.org/10.3390/cancers17152517
Chaudhari J, Shah NN. Targeted Therapies and Immunotherapies for Diffuse Large B-Cell Lymphoma. Cancers. 2025; 17(15):2517. https://doi.org/10.3390/cancers17152517
Chicago/Turabian StyleChaudhari, Jahnavi, and Nikesh N. Shah. 2025. "Targeted Therapies and Immunotherapies for Diffuse Large B-Cell Lymphoma" Cancers 17, no. 15: 2517. https://doi.org/10.3390/cancers17152517
APA StyleChaudhari, J., & Shah, N. N. (2025). Targeted Therapies and Immunotherapies for Diffuse Large B-Cell Lymphoma. Cancers, 17(15), 2517. https://doi.org/10.3390/cancers17152517






