Comprehensive Receptor Repertoire and Functional Analysis of Peripheral NK Cells in Soft Tissue Sarcoma Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. NK Cell Degranulation and Intracellular Staining of IFNγ
2.3. Flow Cytometry Analysis
2.4. Statistical Analysis
3. Results
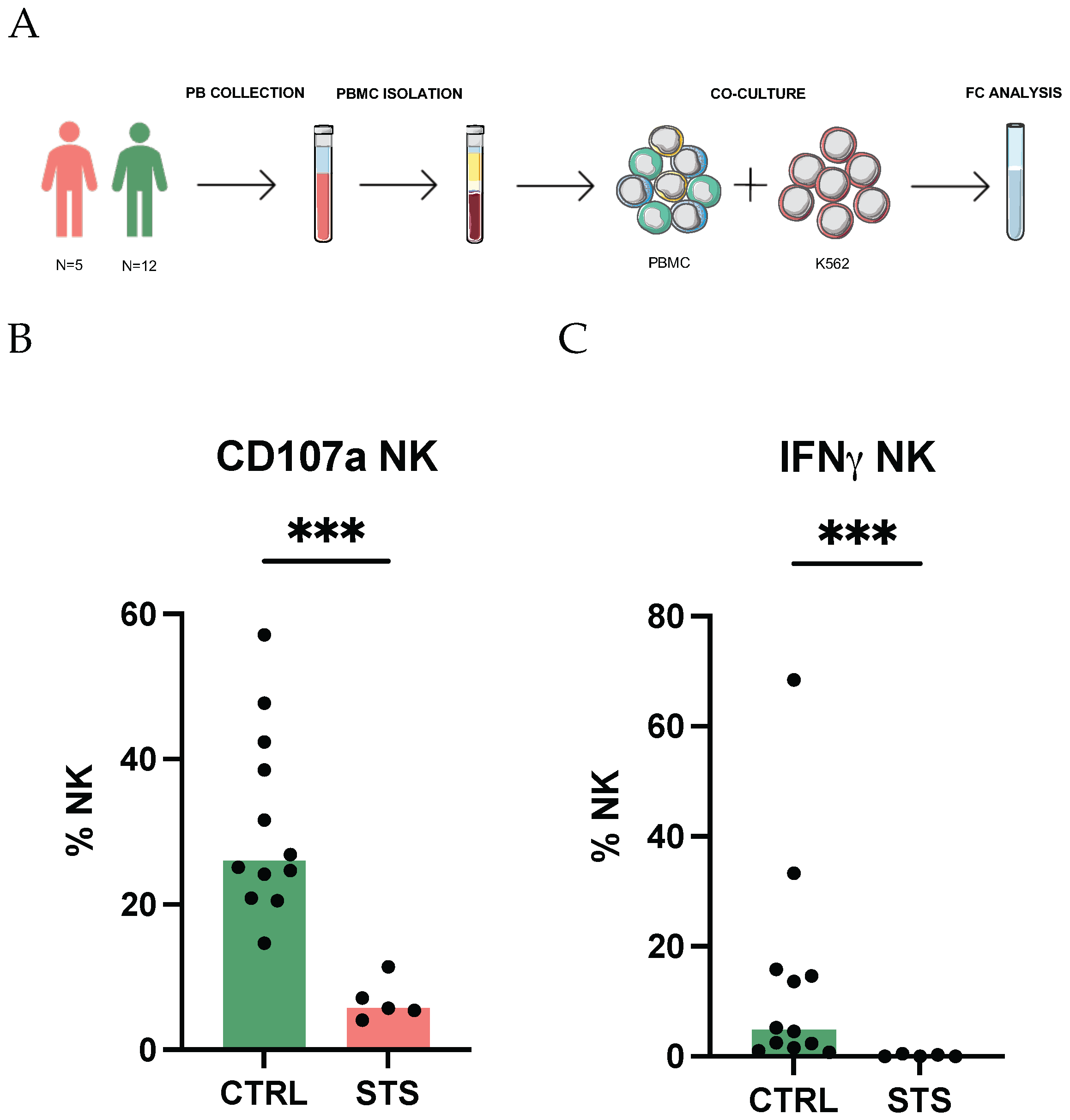
3.1. Impaired Degranulation and IFNγ Production Highlight NK Cell Dysfunction in STS Patients
3.2. Comprehensive Phenotypic Profiling Reveals Altered NK Cell Receptor Repertoire in STS Patients
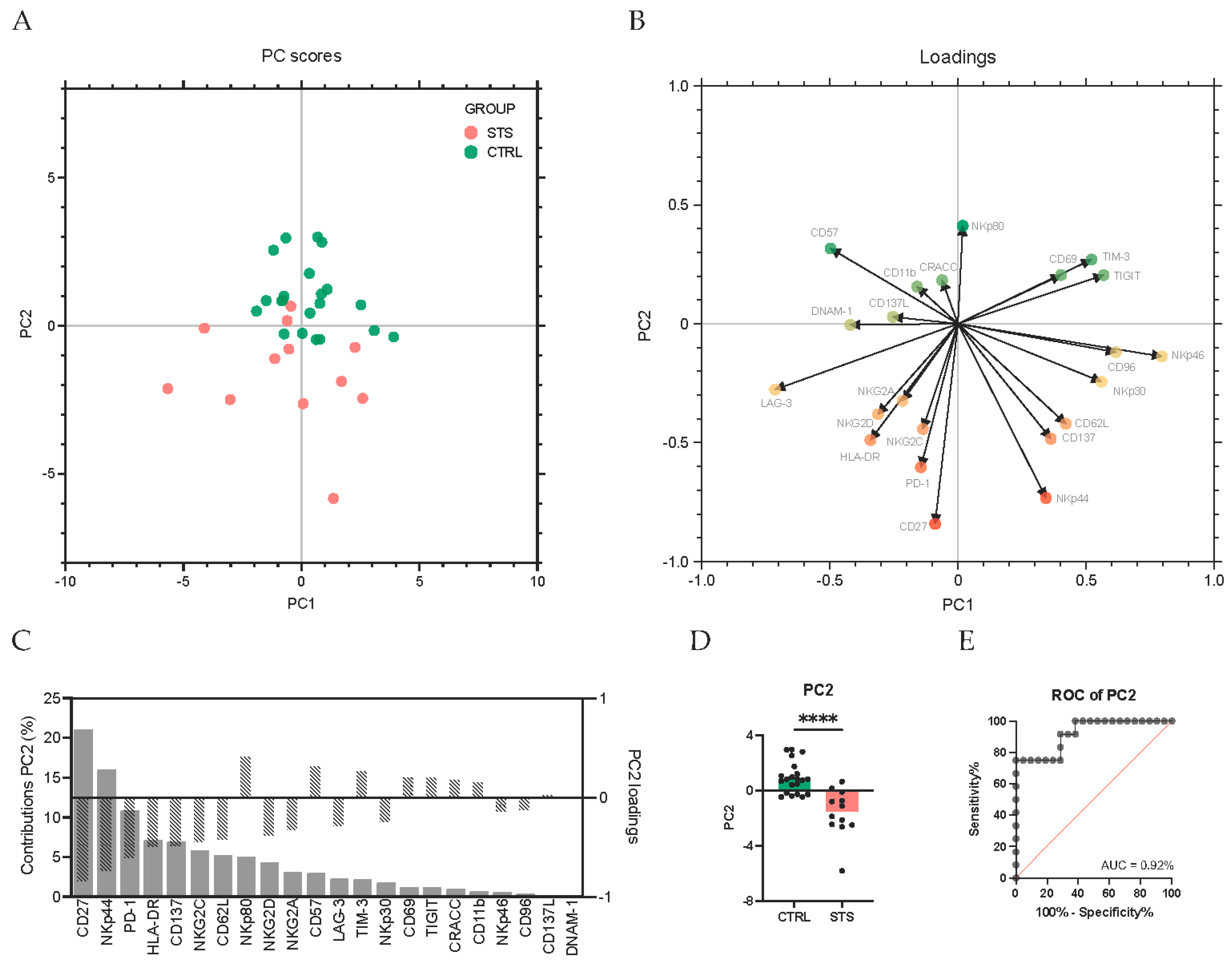
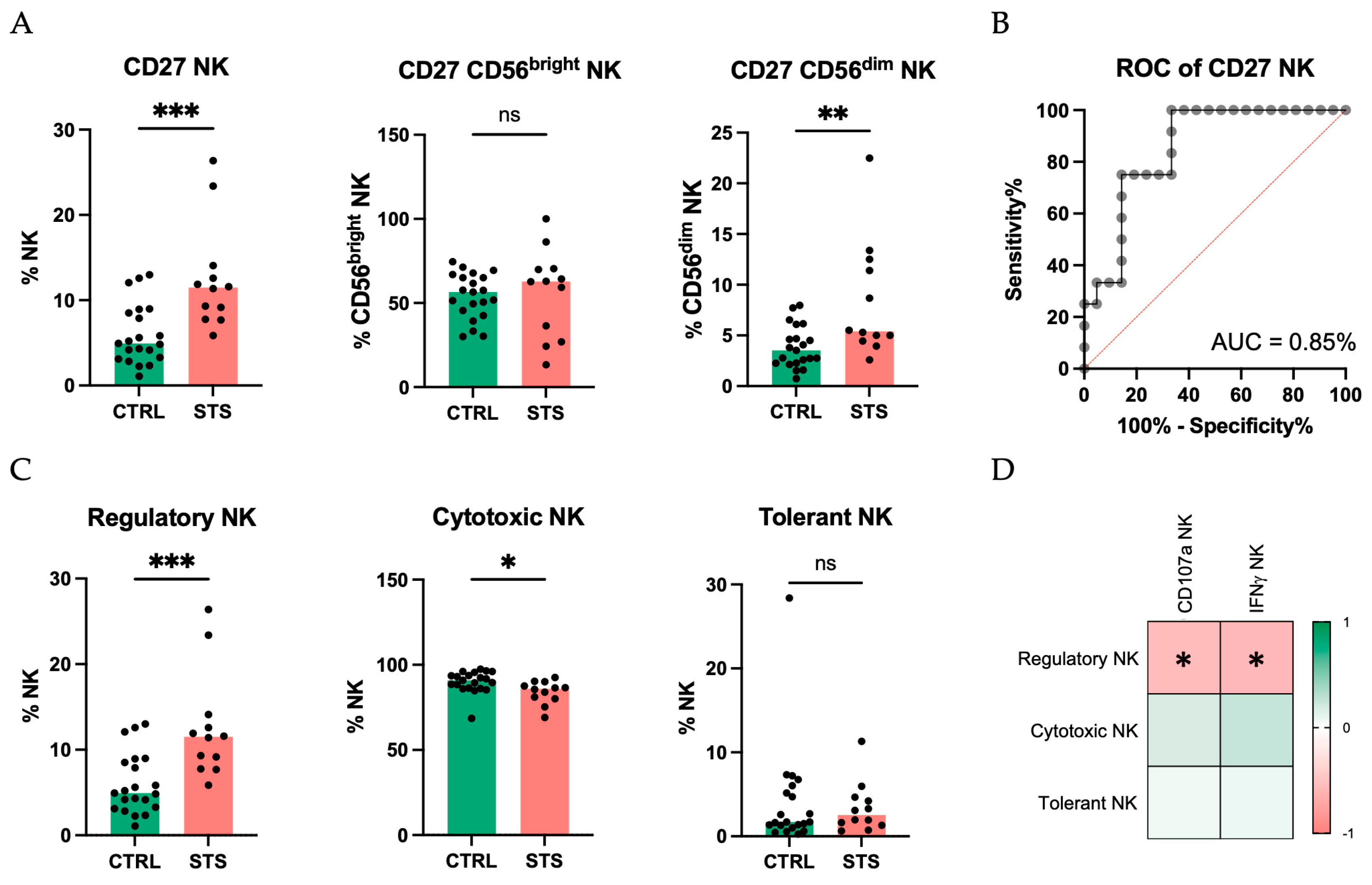
3.3. Principal Component Analysis Identifies CD27 and NKp44 as Key Discriminators of NK Cell Alterations in STS Patients
4. Discussion
4.1. Impaired Degranulation and IFNγ Production Highlight NK Cell Dysfunction in STS Patients
4.2. Comprehensive Phenotypic Profiling Reveals Altered NK Cell Receptor Repertoire in STS Patients
4.3. Principal Component Analysis Identifies CD27 and NKp44 as Key Discriminators of NK Cell Alterations in STS Patients
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CTRL | Control group |
| IPST | Portuguese Institute for Blood and Transplantation |
| IQR | Inter-quartile range |
| NCR | Natural cytotoxicity receptor |
| NK | Natural killer |
| PBMC | Peripheral blood mononuclear cell |
| PBS | Phosphate-buffered saline |
| PC2 | Second principal component |
| PCA | Principal component analysis |
| ROC | Receiver operating characteristic |
| STS | Soft tissue sarcoma |
| SVD | Single value decomposition |
| TIL | Tumor-infiltrating lymphocyte |
| ULSC | Coimbra Local Health Unit |
References
- Mohindra, N.; Agulnik, M. Targeted Therapy and Promising Novel Agents for the Treatment of Advanced Soft Tissue Sarcomas. Expert Opin. Investig. Drugs 2015, 24, 1409–1418. [Google Scholar] [CrossRef]
- Doyle, L.A. Sarcoma Classification: An Update Based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 2014, 120, 1763–1774. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3. [Google Scholar]
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus Erysipelas and the Bacillus Prodigiosus). Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef]
- Antonescu, C.R. The Role of Genetic Testing in Soft Tissue Sarcoma. Histopathology 2006, 48, 13–21. [Google Scholar] [CrossRef]
- Taylor, B.S.; Barretina, J.; Maki, R.G.; Antonescu, C.R.; Singer, S.; Ladanyi, M. Advances in Sarcoma Genomics and New Therapeutic Targets. Nat. Rev. Cancer 2011, 11, 541–557. [Google Scholar] [CrossRef]
- Banks, L.B.; D’Angelo, S.P. The Role of Immunotherapy in the Management of Soft Tissue Sarcomas: Current Landscape and Future Outlook. J. Natl. Compr. Cancer Netw. 2022, 20, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Tazzari, M.; Bergamaschi, L.; De Vita, A.; Collini, P.; Barisella, M.; Bertolotti, A.; Ibrahim, T.; Pasquali, S.; Castelli, C.; Vallacchi, V. Molecular Determinants of Soft Tissue Sarcoma Immunity: Targets for Immune Intervention. Int. J. Mol. Sci. 2021, 22, 7518. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, V.; Villamayor, M.L.; Pozas, J.; Chamorro, J.; Rosero, D.I.; San Román, M.; Guerrero, P.; Pérez de Aguado, P.; Calvo, J.C.; García de Quevedo, C.; et al. Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers 2023, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.M.; Hiam, K.J.; Burnett, C.E.; Venida, A.; DeBarge, R.; Tenvooren, I.; Marquez, D.M.; Cho, N.W.; Carmi, Y.; Spitzer, M.H. Systemic Dysfunction and Plasticity of the Immune Macroenvironment in Cancer Models. Nat. Med. 2020, 26, 1125–1134. [Google Scholar] [CrossRef]
- Almeida, J.S.; Sousa, L.M.; Couceiro, P.; Andrade, T.F.; Alves, V.; Martinho, A.; Rodrigues, J.; Fonseca, R.; Freitas-Tavares, P.; Santos-Rosa, M.; et al. Peripheral Immune Profiling of Soft Tissue Sarcoma: Perspectives for Disease Monitoring. Front. Immunol. 2024, 15, 1391840. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Herrero, S.; López-Soto, A.; Sordo-Bahamonde, C.; Gonzalez-Rodriguez, A.; Vitale, M.; Gonzalez, S. NK Cell-Based Immunotherapy in Cancer Metastasis. Cancers 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Fortes-Andrade, T.; Almeida, J.S.; Sousa, L.M.; Santos-Rosa, M.; Freitas-Tavares, P.; Casanova, J.M.; Rodrigues-Santos, P. The Role of Natural Killer Cells in Soft Tissue Sarcoma: Prospects for Immunotherapy. Cancers 2021, 13, 3865. [Google Scholar] [CrossRef]
- Greppi, M.; De Franco, F.; Obino, V.; Rebaudi, F.; Goda, R.; Frumento, D.; Vita, G.; Baronti, C.; Melaiu, O.; Bozzo, M.; et al. NK Cell Receptors in Anti-Tumor and Healthy Tissue Protection: Mechanisms and Therapeutic Advances. Immunol. Lett. 2024, 270, 106932. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.S. Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells. Front. Immunol. 2018, 9, 2041. [Google Scholar] [CrossRef]
- Sousa, L.M.; Almeida, J.S.; Fortes-Andrade, T.; Santos-Rosa, M.; Freitas-Tavares, P.; Casanova, J.M.; Rodrigues-Santos, P. Tumor and Peripheral Immune Status in Soft Tissue Sarcoma: Implications for Immunotherapy. Cancers 2021, 13, 3885. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B Cells Are Associated with Survival and Immunotherapy Response in Sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Cho, D.; Shook, D.R.; Shimasaki, N.; Chang, Y.-H.; Fujisaki, H.; Campana, D. Cytotoxicity of Activated Natural Killer Cells against Pediatric Solid Tumors. Clin. Cancer Res. 2010, 16, 3901–3909. [Google Scholar] [CrossRef]
- Stahl, D.; Gentles, A.J.; Thiele, R.; Gütgemann, I. Prognostic Profiling of the Immune Cell Microenvironment in Ewing´s Sarcoma Family of Tumors. Oncoimmunology 2019, 8, e1674113. [Google Scholar] [CrossRef]
- Krzewski, K.; Coligan, J.E. Human NK Cell Lytic Granules and Regulation of Their Exocytosis. Front. Immunol. 2012, 3, 335. [Google Scholar] [CrossRef] [PubMed]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a Expression and Cytotoxic Activity. Cell Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a Functional Marker for the Identification of Natural Killer Cell Activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Fregni, G.; Messaoudene, M.; Fourmentraux-Neves, E.; Mazouz-Dorval, S.; Chanal, J.; Maubec, E.; Marinho, E.; Scheer-Senyarich, I.; Cremer, I.; Avril, M.-F.; et al. Phenotypic and Functional Characteristics of Blood Natural Killer Cells from Melanoma Patients at Different Clinical Stages. PLoS ONE 2013, 8, e76928. [Google Scholar] [CrossRef] [PubMed]
- Stringaris, K.; Sekine, T.; Khoder, A.; Alsuliman, A.; Razzaghi, B.; Sargeant, R.; Pavlu, J.; Brisley, G.; de Lavallade, H.; Sarvaria, A.; et al. Leukemia-Induced Phenotypic and Functional Defects in Natural Killer Cells Predict Failure to Achieve Remission in Acute Myeloid Leukemia. Haematologica 2014, 99, 836–847. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.H.; Ryu, J.H.; Bae, H.J.; Choi, A.; Lee, H.; Lim, J.; Han, K.; Park, C.H.; Jung, E.S.; et al. Natural Killer Cell Activity for IFN-Gamma Production as a Supportive Diagnostic Marker for Gastric Cancer. Oncotarget 2017, 8, 70431–70440. [Google Scholar] [CrossRef]
- Borrego, F.; Masilamani, M.; Marusina, A.I.; Tang, X.; Coligan, J.E. The CD94/NKG2 Family of Receptors: From Molecules and Cells to Clinical Relevance. Immunol. Res. 2006, 35, 263–278. [Google Scholar] [CrossRef]
- Erokhina, S.A.; Streltsova, M.A.; Kanevskiy, L.M.; Telford, W.G.; Sapozhnikov, A.M.; Kovalenko, E.I. HLA-DR+ NK Cells Are Mostly Characterized by Less Mature Phenotype and High Functional Activity. Immunol. Cell Biol. 2018, 96, 212–228. [Google Scholar] [CrossRef]
- Burt, B.M.; Plitas, G.; Nguyen, H.M.; Stableford, J.A.; Bamboat, Z.M.; DeMatteo, R.P. Circulating HLA-DR+ Natural Killer Cells Have Potent Lytic Ability and Weak Antigen-Presenting Cell Function. Hum. Immunol. 2008, 69, 469–474. [Google Scholar] [CrossRef]
- Lichtfuss, G.F.; Cheng, W.-J.; Farsakoglu, Y.; Paukovics, G.; Rajasuriar, R.; Velayudham, P.; Kramski, M.; Hearps, A.C.; Cameron, P.U.; Lewin, S.R.; et al. Virologically Suppressed HIV Patients Show Activation of NK Cells and Persistent Innate Immune Activation. J. Immunol. 2012, 189, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Fogli, M.; Costa, P.; Murdaca, G.; Setti, M.; Mingari, M.C.; Moretta, L.; Moretta, A.; De Maria, A. Significant NK Cell Activation Associated with Decreased Cytolytic Function in Peripheral Blood of HIV-1-Infected Patients. Eur. J. Immunol. 2004, 34, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Yano, N.; Endoh, M.; Nomoto, Y.; Sakai, H.; Rifai, A. Increase of HLA-DR-Positive Natural Killer Cells in Peripheral Blood from Patients with IgA Nephropathy. Hum. Immunol. 1996, 49, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef]
- van Hall, T.; André, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; van der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the Novel Immune Checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef]
- Borst, L.; van der Burg, S.H.; van Hall, T. The NKG2A–HLA-E Axis as a Novel Checkpoint in the Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 5549–5556. [Google Scholar] [CrossRef]
- Peng, Y.-P.; Zhu, Y.; Zhang, J.-J.; Xu, Z.-K.; Qian, Z.-Y.; Dai, C.-C.; Jiang, K.-R.; Wu, J.-L.; Gao, W.-T.; Li, Q.; et al. Comprehensive Analysis of the Percentage of Surface Receptors and Cytotoxic Granules Positive Natural Killer Cells in Patients with Pancreatic Cancer, Gastric Cancer, and Colorectal Cancer. J. Transl. Med. 2013, 11, 262. [Google Scholar] [CrossRef]
- Rocca, Y.S.; Roberti, M.P.; Arriaga, J.M.; Amat, M.; Bruno, L.; Pampena, M.B.; Huertas, E.; Loria, F.S.; Pairola, A.; Bianchini, M.; et al. Altered Phenotype in Peripheral Blood and Tumor-Associated NK Cells from Colorectal Cancer Patients. Innate Immun. 2013, 19, 76–85. [Google Scholar] [CrossRef]
- Krijgsman, D.; de Vries, N.L.; Skovbo, A.; Andersen, M.N.; Swets, M.; Bastiaannet, E.; Vahrmeijer, A.L.; van de Velde, C.J.H.; Heemskerk, M.H.M.; Hokland, M.; et al. Characterization of Circulating T-, NK-, and NKT Cell Subsets in Patients with Colorectal Cancer: The Peripheral Blood Immune Cell Profile. Cancer Immunol. Immunother. 2019, 68, 1011–1024. [Google Scholar] [CrossRef]
- Vitale, M.; Falco, M.; Castriconi, R.; Parolini, S.; Zambello, R.; Semenzato, G.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Identification of NKp80, a Novel Triggering Molecule Expressed by Human NK Cells. Eur. J. Immunol. 2001, 31, 233–242. [Google Scholar] [CrossRef]
- Freud, A.G.; Keller, K.A.; Scoville, S.D.; Mundy-Bosse, B.L.; Cheng, S.; Youssef, Y.; Hughes, T.; Zhang, X.; Mo, X.; Porcu, P.; et al. NKp80 Defines a Critical Step during Human Natural Killer Cell Development. Cell Rep. 2016, 16, 379–391. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Farkas, A.M.; Audenet, F.; Anastos, H.; Oh, W.K.; Galsky, M.D.; Sfakianos, J.P.; Bhardwaj, N. Tim-3 and TIGIT Mark NK and T Cells Susceptible to Effector Dysfunction in Human Bladder Cancer. J Immunol. 2018, 200, 124.14. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 Expression in Peripheral NK Cells Predicts a Poorer Prognosis and Tim-3 Blockade Improves NK Cell-Mediated Cytotoxicity in Human Lung Adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, J.; Gu, H.; Yuan, Y.; Zhang, B.; Zhu, D.; Zhou, J.; Zhu, Y.; Chen, W. The Clinical Significance of Abnormal Tim-3 Expression on NK Cells from Patients with Gastric Cancer. Immunol. Investig. 2015, 44, 578–589. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.P.; Gallois, A.; Jimenez-Baranda, S.; Khan, S.; Anderson, A.C.; Kuchroo, V.K.; Osman, I.; Bhardwaj, N. Reversal of NK-Cell Exhaustion in Advanced Melanoma by Tim-3 Blockade. Cancer Immunol. Res. 2014, 2, 410–422. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Kučan Brlić, P.; Lenac Roviš, T.; Cinamon, G.; Tsukerman, P.; Mandelboim, O.; Jonjić, S. Targeting PVR (CD155) and Its Receptors in Anti-Tumor Therapy. Cell Mol. Immunol. 2019, 16, 40–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New Checkpoint Receptor Targets for Cancer Immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef]
- Chan, C.J.; Martinet, L.; Gilfillan, S.; Souza-Fonseca-Guimaraes, F.; Chow, M.T.; Town, L.; Ritchie, D.S.; Colonna, M.; Andrews, D.M.; Smyth, M.J. The Receptors CD96 and CD226 Oppose Each Other in the Regulation of Natural Killer Cell Functions. Nat. Immunol. 2014, 15, 431–438. [Google Scholar] [CrossRef]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavilla, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural Killer Cells Infiltrating Human Nonsmall-cell Lung Cancer Are Enriched in CD56 bright CD16− Cells and Display an Impaired Capability to Kill Tumor Cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; André, P.; Dieu-Nosjean, M.-C.; Alifano, M.; Régnard, J.-F.; et al. Profound Coordinated Alterations of Intratumoral NK Cell Phenotype and Function in Lung Carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 Marks Human Natural Killer Cell Maturation and Suppresses Cell-Mediated Cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef]
- Golden-Mason, L.; Waasdorp Hurtado, C.E.; Cheng, L.; Rosen, H.R. Hepatitis C Viral Infection Is Associated with Activated Cytolytic Natural Killer Cells Expressing High Levels of T Cell Immunoglobulin- and Mucin-Domain-Containing Molecule-3. Clin. Immunol. 2015, 158, 114–125. [Google Scholar] [CrossRef]
- Wakiyama, H.; Masuda, T.; Motomura, Y.; Hu, Q.; Tobo, T.; Eguchi, H.; Sakamoto, K.; Hirakawa, M.; Honda, H.; Mimori, K. Cytolytic Activity (CYT) Score Is a Prognostic Biomarker Reflecting Host Immune Status in Hepatocellular Carcinoma (HCC). Anticancer Res. 2018, 38, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Tian, Z.; Wei, H. Subsets of Human Natural Killer Cells and Their Regulatory Effects. Immunology 2014, 141, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Smyth, M.J. CD27 Dissects Mature NK Cells into Two Subsets with Distinct Responsiveness and Migratory Capacity. J. Immunol. 2006, 176, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Vossen, M.T.M.; Matmati, M.; Hertoghs, K.M.L.; Baars, P.A.; Gent, M.-R.; Leclercq, G.; Hamann, J.; Kuijpers, T.W.; van Lier, R.A.W. CD27 Defines Phenotypically and Functionally Different Human NK Cell Subsets. J. Immunol. 2008, 180, 3739–3745. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Huntington, N.D.; Nutt, S.L.; Smyth, M.J. Functional Subsets of Mouse Natural Killer Cells. Immunol. Rev. 2006, 214, 47–55. [Google Scholar] [CrossRef]
- Chiossone, L.; Chaix, J.; Fuseri, N.; Roth, C.; Vivier, E.; Walzer, T. Maturation of Mouse NK Cells Is a 4-Stage Developmental Program. Blood 2009, 113, 5488–5496. [Google Scholar] [CrossRef]
- Darvishvand, R.; Rezaeifard, S.; Kiani, R.; Tahmasebi, S.; Faghih, Z.; Erfani, N. Natural Killer Cell Subsets and Their Functional Molecules in Peripheral Blood of the Patients with Breast Cancer. Immun. Inflamm. Dis. 2024, 12, e1255. [Google Scholar] [CrossRef]
- Sivori, S.; Parolini, S.; Marcenaro, E.; Castriconi, R.; Pende, D.; Millo, R.; Moretta, A. Involvement of Natural Cytotoxicity Receptors in Human Natural Killer Cell-Mediated Lysis of Neuroblastoma and Glioblastoma Cell Lines. J. Neuroimmunol. 2000, 107, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.; Hoffmann, S.C.; Jarahian, M.; Momburg, F.; Watzl, C. Expression Analysis of the Ligands for the Natural Killer Cell Receptors NKp30 and NKp44. PLoS ONE 2007, 2, e1339. [Google Scholar] [CrossRef] [PubMed]
- Horton, N.C.; Mathew, S.O.; Mathew, P.A. Novel Interaction between Proliferating Cell Nuclear Antigen and HLA I on the Surface of Tumor Cells Inhibits NK Cell Function through NKp44. PLoS ONE 2013, 8, e59552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nieto-Velázquez, N.G.; Torres-Ramos, Y.D.; Muñoz-Sánchez, J.L.; Espinosa-Godoy, L.; Gómez-Cortés, S.; Moreno, J.; Moreno-Eutimio, M.A. Altered Expression of Natural Cytotoxicity Receptors and NKG2D on Peripheral Blood NK Cell Subsets in Breast Cancer Patients. Transl. Oncol. 2016, 9, 384–391. [Google Scholar] [CrossRef]
- Essa, E.S.; Tawfeek, G.A.-E.; El Hassanin, S.A.; Emara, K.G.M. Modulation the Expression of Natural Killer Cell Activating Receptor (NKp44) in the Peripheral Blood of Diffuse Large B-Cell Lymphoma Patients and the Correlation with Clinic Pathological Features. Clin. Immunol. 2018, 188, 38–44. [Google Scholar] [CrossRef]


| Clinicopathological Characteristic | Value | Frequency (%) |
|---|---|---|
| N | 12 | |
| Median age (range), years | 57 (19–78) | |
| Sex | ||
| Female | 5 | 42 |
| Male | 7 | 58 |
| Soft tissue sarcoma histology | ||
| Leiomyosarcoma | 5 | 42 |
| Liposarcoma | 2 | 17 |
| Synovial sarcoma | 2 | 17 |
| Haemangiosarcoma | 1 | 8 |
| Malignant fibrous histiocytoma | 1 | 8 |
| Clear cell sarcoma | 1 | 8 |
| Localization | ||
| Connective and soft tissue of limb | 3 | 25 |
| Retroperitoneum | 2 | 17 |
| Connective and soft tissue of head | 1 | 8 |
| Connective and soft tissue of abdomen | 1 | 8 |
| Connective and soft tissue of trunk | 1 | 8 |
| Connective and soft tissue of pelvis | 1 | 8 |
| Jejunum | 1 | 8 |
| Myometrium | 1 | 8 |
| Adrenal gland | 1 | 8 |
| Tumor type | ||
| Primary | 2 | 17 |
| Recurrent | 2 | 17 |
| Metastatic | 4 | 33 |
| Recurrent/Metastatic | 4 | 33 |
| Therapy | ||
| Anthracycline-based therapy | 1 | 8 |
| Trabectedin-based therapy | 2 | 17 |
| Anthracycline- and trabectedin-based therapy | 6 | 50 |
| Anthracycline- and trabectedin-based therapy and others | 3 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.M.; Almeida, J.-S.; Fortes-Andrade, T.; Couceiro, P.; Rodrigues, J.; Fonseca, R.; Santos-Rosa, M.; Freitas-Tavares, P.; Casanova, J.M.; Rodrigues-Santos, P. Comprehensive Receptor Repertoire and Functional Analysis of Peripheral NK Cells in Soft Tissue Sarcoma Patients. Cancers 2025, 17, 2508. https://doi.org/10.3390/cancers17152508
Sousa LM, Almeida J-S, Fortes-Andrade T, Couceiro P, Rodrigues J, Fonseca R, Santos-Rosa M, Freitas-Tavares P, Casanova JM, Rodrigues-Santos P. Comprehensive Receptor Repertoire and Functional Analysis of Peripheral NK Cells in Soft Tissue Sarcoma Patients. Cancers. 2025; 17(15):2508. https://doi.org/10.3390/cancers17152508
Chicago/Turabian StyleSousa, Luana Madalena, Jani-Sofia Almeida, Tânia Fortes-Andrade, Patrícia Couceiro, Joana Rodrigues, Rúben Fonseca, Manuel Santos-Rosa, Paulo Freitas-Tavares, José Manuel Casanova, and Paulo Rodrigues-Santos. 2025. "Comprehensive Receptor Repertoire and Functional Analysis of Peripheral NK Cells in Soft Tissue Sarcoma Patients" Cancers 17, no. 15: 2508. https://doi.org/10.3390/cancers17152508
APA StyleSousa, L. M., Almeida, J.-S., Fortes-Andrade, T., Couceiro, P., Rodrigues, J., Fonseca, R., Santos-Rosa, M., Freitas-Tavares, P., Casanova, J. M., & Rodrigues-Santos, P. (2025). Comprehensive Receptor Repertoire and Functional Analysis of Peripheral NK Cells in Soft Tissue Sarcoma Patients. Cancers, 17(15), 2508. https://doi.org/10.3390/cancers17152508








