Simple Summary
CEBPAbZIP-inf mutations are associated with favorable outcomes in acute myeloid leukemia (AML). However, data on the comprehensive integration of clinical data, genetic characteristics, and measurable residual disease in those patients are limited. We found that IKZF1 mutations/deletions and FLT3-ITD mutations predicted poor outcomes in AML patients with CEBPAbZIP-inf mutations. And we identified a potential high-risk population with adverse prognostic factors in CEBPAbZIP-inf AML patients for which transplantation should be considered.
Abstract
Background: CCAAT/enhancer-binding protein alpha–basic leucine zipper in-frame (CEBPAbZIP-inf) mutations are associated with favorable outcomes in acute myeloid leukemia (AML). So far, there are limited data on integrating clinical and genomic features impacting the outcomes. Methods: Clinical and genomic data from consecutive patients with CEBPAbZIP-inf were reviewed. A Cox proportional hazards regression was used to identify the variables associated with event-free survival (EFS), relapse-free survival (RFS) and survival. Results: 224 CEBPAbZIP-inf patients were included in this study. In the 201 patients, except for the 19 receiving the transplant in the first complete remission with no events (the transplant cohort), multivariate analyses showed that IKZF1 mutations/deletions were significantly associated with poor EFS (p = 0.001) and RFS (p < 0.001); FLT3-ITD mutations, poor RFS (p = 0.048). In addition, increasing WBC count, lower hemoglobin concentration, non-intensive induction, and MRD positivity after first consolidation predicted poor outcomes. On the basis of the number of adverse prognostic covariates for RFS, the 201 patients were classified into low-, intermediate- or high-risk subgroups, and there were significant differences in the 3-year EFS, RFS and survival rates (all p < 0.001); however, except for survival in the low-risk group, these metrics were lower than those in the transplant cohort. Conclusions: We identified a potential high-risk population with adverse prognostic factors in CEBPAbZIP-inf AML patients for which transplantation should be considered.
1. Introduction
CCAAT/enhancer-binding protein α (CEBPA) is detected in approximately 10% of patients with acute myeloid leukemia (AML). Recent studies revealed that mutations in the basic leucine zipper region of CEBPA (CEBPAbZIP-inf) were associated with favorable outcomes regardless of the mono or biallelic status [1,2], which is a favorable risk group per the 2022 European LeukemiaNet (ELN) and International Consensus Classification (ICC) [3,4]. However, certain patients with CEBPAbZIP-inf mutations fail to achieve a complete remission (CR), experience relapse, or even die, suggesting clinical and biological heterogeneity.
Several studies have reported that increasing age, higher white blood cell (WBC) count, and non-intensive induction are associated with poor outcomes and that CSF3R, WT1, NRAS, KIT, TET2, DNMT3A, and FLT3-ITD mutations predict a high likelihood of relapse; however, most of these mutations were identified by univariate analysis [1,5,6,7]. Even so, the data are limited, and precise risk stratification to identify high-risk subgroups and optimal treatment strategies for patients with CEBPAbZIP-inf-mutated AML are needed. Therefore, we integrated data from 224 consecutive AML patients with CEBPAbZIP-inf mutations to explore the clinical covariates and genetic abnormalities associated with specified outcomes.
2. Patients and Methods
2.1. Patients
The data of consecutive AML patients with CEBPAbZIP-inf mutations from January 2017 to August 2024 who were diagnosed and treated at Peking University People’s Hospital were reviewed. Diagnosis and classification were performed according to the 2022 ELN recommendations for AML [3]. The last follow-up date was January 2025. The study was approved by the Ethics Committee of Peking University People’s Hospital (2021PHB136-001), and written informed consent was obtained from all patients in compliance with the Declaration of Helsinki.
2.2. Immune, Cytogenetic, and Molecular Analyses and Next-Generation Sequencing (NGS)
Immunophenotyping was performed using diagnostic bone marrow (BM) aspirate samples, which were analyzed by flow cytometry, as previously described [8]. The karyotype was determined by the G-banding method following the 2013 International System for Human Cytogenetic Nomenclature [9]. Bone marrow aspirates were collected for measurable residual disease (MRD) monitoring by multiparameter flow cytometry (MPFC) after each chemotherapy cycle, and the MRD test sensitivity was 0.01%, as previously reported [8]. NGS was used to detect gene mutations in patients, as previously described [10]. The hematologic tumor panel was shown in Supplemental Table S1. The algorithm independently developed by the Guangzhou Jinyu Company was used to detect variations. A variant allele frequency (VAF) cutoff of 1.0% was used for SNVs and indels.
2.3. Treatment
Induction treatment included intensive therapy, such as the “3 + 7” regimen and homoharringtonine combined with the cytarabine and aclarubicin (HAA) regimen, or non-intensive therapy, such as the low-dose cytarabine or anthracycline-based regimen and venetoclax plus azacytidine regimen. The details of the treatment were described in the Supplementary Materials.
2.4. Definitions
The response to the first induction therapy was evaluated on days 21–28. For patients whose blood cell counts have not fully recovered, we reevaluated the peripheral blood and BM conditions two weeks later to determine the remission status. CR, CRi, and relapse were defined according to the 2022 ELN recommendations for AML [3]. MRD relapse was defined as the conversion from MRD negativity to MRD positivity (>0.01%) in patients with CR/CRi by MPFC. Events were defined as failure to achieve CR/CRi after 2 cycles of induction therapy, hematologic relapse, MRD relapse, or death from any cause. Event-free survival (EFS) was measured from the time of diagnosis to the date of the first event or the last follow-up. Relapse-free survival (RFS) was calculated as the time from achieving CR/CRi to hematologic relapse, death, or the last follow-up. Survival was calculated from the time of diagnosis of AML to death or the last follow-up.
2.5. Statistical Analysis
Baseline characteristics were summarized using descriptive statistical methods. Categorical variables were expressed as frequencies (percentages), whereas continuous measures were summarized using medians with interquartile ranges (IQR). Comparative analyses employed the following appropriate statistical tests: χ2 tests or Fisher’s exact tests for categorical variables and Student’s t-tests for normally distributed continuous variables. X-tile plots were constructed to determine the optimal cutoff values for continuous covariates for predicting outcomes [11]. Continuous variables without significant prognostic significance are presented as medians. Variables, including clinical and genetic alterations (frequency ≥ 5%), first underwent univariate screening for association with outcomes. Variables demonstrating marginal significance (p < 0.20) were subsequently entered into multivariable Cox proportional hazards regression models employing backward elimination. Final models identified independent prognostic variables, with effect estimates expressed as hazard ratios (HRs) accompanied by 95% confidence intervals (CIs). Significant covariates from the multivariate analysis of RFS were used to develop risk stratification. Weighted scores were assigned to these covariates according to the regression coefficient [12]. The risk stratification model underwent internal validation through a bootstrap resampling procedure with 1000 iterations [13,14]. Model discrimination was evaluated by calculating the area under the receiver operating characteristic curve (AU-ROC) [15]. A two-tailed p-value < 0.05 was established as the threshold for statistical significance. All statistical analyses were performed using SPSS 26.0 (IBM Corp., Armonk, NY, USA), R statistical environment version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA), with the latter also employed for data visualization.
3. Results
3.1. Patient Characteristics
The data of 224 consecutive CEBPAbZIP-inf mutated AML patients were reviewed. A total of 124 (55%) patients were male. Median age was 42 years (IQR, 32–54 years). A total of 58 (26%) patients had cytogenetic abnormalities, including 15 with adverse cytogenetic risk. A total of 194 patients received intensive induction chemotherapy, and 30 received non-intensive induction chemotherapy (Table 1). Among the 14 patients with FLT3-ITD mutations, 9 received chemotherapy combined with sorafenib from induction therapy, and 1 received sorafenib after relapse.

Table 1.
Patients’ characteristics.
3.2. Mutation Topography
By targeted sequencing, additional mutations were identified in 178 of 224 (79%) patients with CEBPAbZIP-inf mutations. The most frequently mutated genes were WT1 (n = 63, 28%), followed by GATA2 (n = 54, 24%), NRAS (n = 37, 17%), TET2 (n = 24, 11%), KIT (n = 16, 7%), FLT3-ITD (n = 14, 6%), CSF3R (n = 14, 6%), IKZF1 mutations (n = 10) and deletions (n = 3, 6%), and DNMT3A (n = 12, 5%) (Figure 1A). Except for that in CEBPA, the median mutation number per patient was two (IQR, 1–3; range, 0–11). When the co-occurrence and mutual exclusivity patterns of mutations with frequencies > 5% were explored, we found co-mutations in CSF3R and KIT and in DNMT3A and TET2. GATA2 mutations and WT1 or TET2 mutations were mutually exclusive, as were those in IKZF1 and NRAS (Figure 1B).
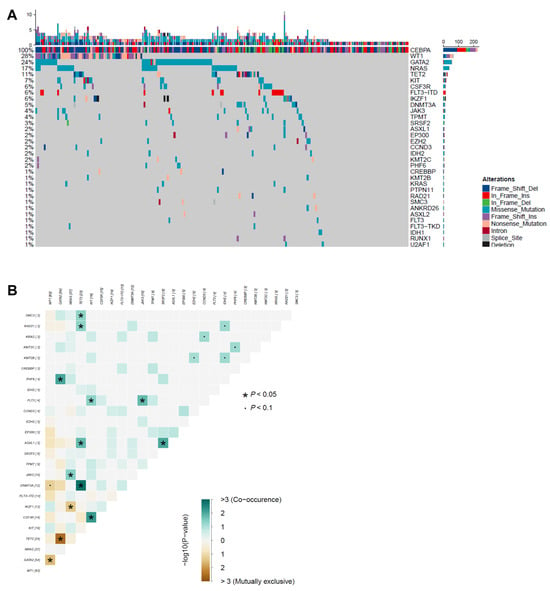
Figure 1.
Mutation topography in AML with CEBPAbZIP-inf mutation. (A) Co-mutations. Each column indicates the data of one sample; each row indicates a gene. The top bar indicates the mutation frequency (mutation/Mb DNA), and the right bar indicates the frequency of different mutated genes. Sorted by frequency of mutations in the entire CEBPAbZIP-inf AML patients. (B) Pairwise associations between gene mutations. Gene interaction patterns were assessed through pairwise co-occurrence analysis. Fisher’s exact tests quantified association strength between gene pairs, with odds ratios (OR) and corresponding p-values computed under the null hypothesis of independent distribution. Associations reaching significance threshold (* p < 0.05, ∙ p < 0.01) underwent visual encoding using a two-dimensional matrix. Forest green hues signified synergistic co-occurrence (OR > 1), whereas amber tones indicated mutual exclusivity (OR < 1).
3.3. Outcomes
Among the 224 AML patients, 204 (91%) achieved CR/CRi after the first induction therapy, and 221 (99%) ultimately achieved CR/CRi; 69 (31%) showed MRD positivity after induction therapy, and 27 (12%) showed MRD positivity after the first consolidation therapy. With a median follow-up of 24 (IQR, 13–42) months for all patients and 25 (IQR, 12–43) months for survivors, 29 (13%) and 80 (36%) experienced MRD and hematologic relapse, respectively; 74 (33%) underwent a transplant, including 19 in CR1 with MRD negativity based on their preference (the transplant cohort), 18 in CR1 with MRD positivity, and 37 in CR2. A total of 30 (13%) patients died of no response (n = 1), hematologic relapse (n = 25), severe infection (n = 2), or transplantation-related mortality (n = 2). The baseline characteristics of the transplant cohort are presented in Table 1. There were some differences in the patients’ characteristics at diagnosis between the transplant and nontransplant cohorts, including younger age (p < 0.001), higher WBC counts (p = 0.015), higher BM blast proportions (p = 0.012), and more KIT (p = 0.003) and FLT3-ITD (p = 0.033) mutations were observed in the transplant cohort compared to the nontransplant cohort. Notably, no events occurred in the transplant cohort during the follow-up period. The 3-year probabilities of EFS (100% vs. 38% [95% CI [31, 47%], p < 0.001), RFS (100% vs. 48% [41, 57%], p < 0.001), and survival (100% vs. 80% [72, 87%], p = 0.024) were higher in the transplant cohort than in the nontransplant cohort.
3.4. Impact of Co-Mutations and Cytogenetic Abnormalities on Outcomes
Because no events occurred in the transplant cohort during the follow-up period, we explored the effects of clinical covariates and genetic abnormalities (frequencies ≥ 5%) on outcomes in the nontransplant cohort for which MRD data were available. Univariate analyses revealed that IKZF1 mutations/deletions were associated with poor EFS (p = 0.007) and RFS (p < 0.001); FLT3-ITD mutations and DNMT3A mutations had a tendency toward adverse RFS (p = 0.063 and p = 0.074) (Figure 2). Other common co-mutations had no prognostic significance. Notably, myelodysplasia-related gene mutations and cytogenetic abnormalities had no impact on outcomes (Supplemental Table S2).
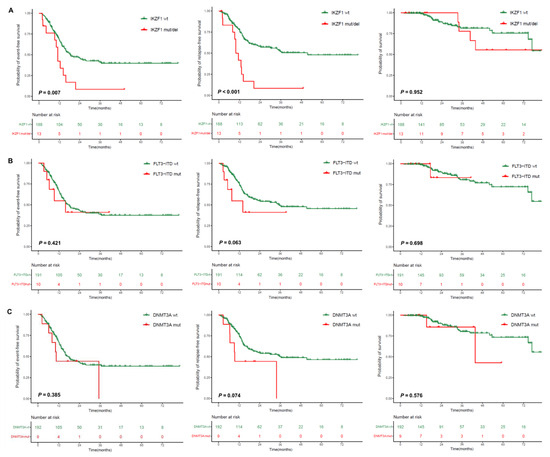
Figure 2.
Impact of co-mutations on outcomes. (A) IKZF1 mutations/deletions; (B) FLT3-ITD mutations; (C) DNMT3A mutations.
The frequency of IKZF1 mutations (n = 10) or deletions (n = 3) was 6%, with a median VAF of 24% (range: 2–45%) observed in the mutated patients. Missense mutations were the predominant alterations (n = 4), followed by frameshift deletions (n = 3), in-frame deletions (n = 1), nonsense mutations (n = 1), and frameshift insertions (n = 1) (Supplemental Figure S1). In addition, three patients had IKZF1 exon 4–8 deletion.
3.5. Identifying Covariates Associated with Outcomes
In the 201 patients (the nontransplant cohort), clinical variables that may be related to outcomes, including baseline characteristics (age, sex, complete blood count, BM blasts, cytogenetic abnormalities, and genomic abnormalities [frequencies ≥ 5%]), induction chemotherapy, and MRD after induction and first consolidation, were explored. The univariate analysis results are displayed in Supplemental Table S2. In the multivariate analyses, IKZF1 mutations/deletions were significantly associated with poor EFS (HR = 3.0 [95% CI 1.6, 5.8], p = 0.001) and RFS (HR = 3.8 [1.9, 7.3]); FLT3-ITD mutations, poor RFS (HR = 2.5 [1.0, 6.5], p = 0.048). In addition, increasing WBC count was significantly associated with poor EFS (HR = 1.8 [1.2, 2.7], p = 0.003), RFS (HR = 2.0 [1.3, 3.0], p = 0.002), and survival (HR = 1.7 [0.8, 3.5], p = 0.038); non-intensive induction, poor EFS (HR = 3.2 [1.8, 5.6], p < 0.001) and RFS (HR = 3.9 [2.2, 6.9], p < 0.001); MRD positivity after first consolidation, worse RFS (HR = 2.4 [1.3, 4.2], p = 0.005) and survival (HR = 4.9 [2.2, 11.2], p < 0.001); and increasing HGB concentration, favorable RFS (HR = 0.1 [0.01, 0.3], p = 0.001) (Table 2). Using X-tile, we determined that the optimal cutoff values for the WBC count were 32, 69, and 45 × 109/L for EFS, RFS, and survival, respectively, and the HGB concentration was 72 g/L for RFS. In multivariate analyses, these categorical covariates impacting outcomes were still significantly associated with outcomes (Supplemental Table S3).

Table 2.
Multivariate analyses of outcomes.
Next, we analyzed patients according to the induction regimen. In 172 patients receiving intensive induction therapy, the results were similar to those of all patients except for an increased WBC count, which had no impact on survival (Supplemental Table S4). Analysis was not performed for those receiving non-intensive induction therapy because of the small number of patients.
3.6. Risk Stratification
Patients in our cohort had a higher relapse rate, so we selected adverse variables affecting RFS to develop the risk stratification. In the 201 patients (the nontransplant cohort), we used the number of adverse prognostic covariates (including WBC count ≥ 69 × 109/L, HGB ≤ 72 g/L, non-intensive induction, MRD positivity after the first consolidation, FLT3-ITD mutations, and IKZF1 mutations/deletions; points were assigned to these covariates according to the regression coefficient, each scored as 1 point) for RFS to divide the patients into low- (score 0; n = 108; 54%), intermediate- (score 1; n = 73; 36%), and high-risk (score ≥ 2; n = 20; 10%) subgroups. Combining the transplant cohort, there were significant differences in EFS, RFS, and survival among the four subgroups (all p values for trends <0.001). The transplant cohort had the best outcomes. Differences in EFS and RFS were detected between the subgroups. There was no difference in survival between the intermediate- and high-risk subgroups, and there was no difference between the low-risk subgroup and the transplant cohort, which was significantly greater than that in the intermediate- and high-risk subgroups (Figure 3).
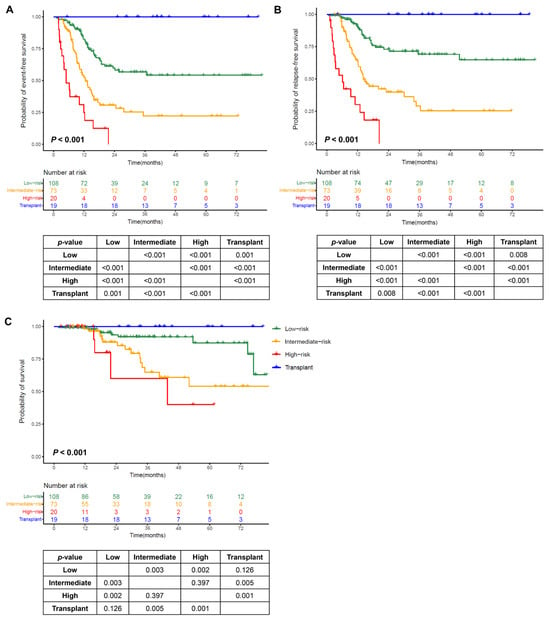
Figure 3.
Outcomes by the number of adverse prognostic covariates. (A) Event-free survival (EFS); (B) relapse-free survival (RFS); (C) survival.
To evaluate the risk stratification performance for predicting outcomes, we plotted ROC curves for the probabilities of RFS at 1, 2, and 3 years (Supplemental Figure S2). The risk stratification showed good sensitivity and specificity with an AUROC value of 0.74 (0.65, 0.81) for 1-year RFS, 0.73 (0.64, 0.80) for 2-year RFS, and 0.74 (0.64, 0.82) for 3-year RFS.
In the transplant cohort, 19 patients were divided into intermediate-risk (n = 6) and high-risk (n = 13) subgroups on the basis of risk classification. The 3-year probabilities of EFS (p values < 0.001–0.003), RFS (p values < 0.001–0.006), and survival (p values = 0.009) in the transplant cohort were higher than those in the nontransplant cohort, except for a tendency toward poor survival (p = 0.095) in the intermediate-risk subgroup. (Figure 4A–C).
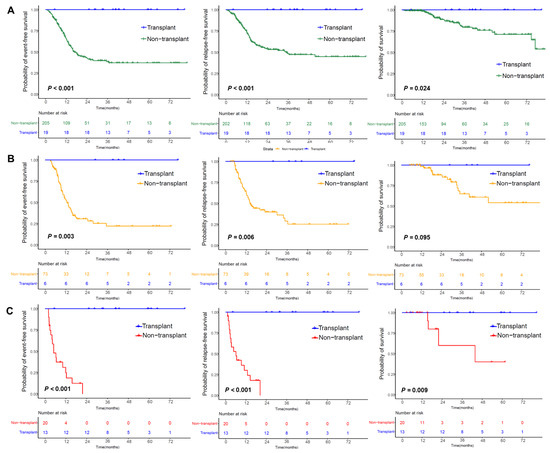
Figure 4.
Outcomes in patients with CEBPAbZIP-inf mutations. (A) Outcomes in the nontransplant cohort and transplant cohort. (B) Outcomes in the intermediate-risk subgroup of the nontransplant cohort and transplant cohort. (C) Outcomes in the high-risk subgroup of the nontransplant cohort and transplant cohort.
4. Discussion
In this study, we identified IKZF1 mutations/deletions (6%) as an independent risk factor for worse EFS and RFS in AML patients with CEBPAbZIP-inf mutations, which has rarely been reported. IKZF1 alterations are relatively common (30–50%) in adult acute lymphoblastic leukemia (ALL) patients [16,17], and the most common pattern of alteration is heterozygous deletion of either the whole gene or specific exons with subsequent loss of function [18]. In numerous studies, IKZF1 mutation was reported to be an independent marker of adverse risk in ALL patients [18,19,20]. However, studies on IKZF1 alterations in AML patients are limited, with frequencies of 2–5%, which are much lower than those reported in ALL patients, although these frequencies are also associated with poor outcomes [21,22,23].
Consistent with previous studies, FLT3-ITD mutations predicted a high likelihood of relapse and worse survival through univariate analysis in AML patients (did not receive FLT3 inhibitors) with CEBPAbZIP-inf mutations or with biallelic CEBPA mutations [6,24]. In our study, multivariate analysis revealed that the FLT3-ITD mutation was significantly associated with poor RFS. AML with FLT3-ITD (without adverse-risk genetic lesions) is classified as intermediate risk [25], but there is limited evidence to support the reclassification of AML with CEBPAbZIP-inf- FLT3-ITD mutations. Although FLT3 inhibitors significantly improve outcomes in AML patients with FLT3 mutations [26], whether they can improve the prognosis of patients with AML CEBPAbZIP-inf with FLT3 mutations needs further investigation.
Consistent with previous studies [6,7,27], higher WBC count and MRD positivity after the first consolidation were associated with poor outcomes in our study, and patients may have benefited from intensive induction therapy. Moreover, myelodysplasia-related gene mutations or cytogenetic abnormalities had no prognostic significance in AML patients with CEBPAbZIP-inf mutations.
On the basis of the adverse covariates of RFS identified in this study, all 19 patients in the transplant cohort classified in the intermediate- or high-risk subgroup had no events during the follow-up period, whereas those receiving consistent chemotherapy had a high likelihood of relapse and death, suggesting that transplantation might improve the outcomes in AML patients with CEBPAbZIP-inf mutations. Although transplantation is not recommended as an essential treatment strategy for patients with CEBPAbZIP-inf-mutated AML, our findings suggest that intermediate- and high-risk AML patients with CEBPAbZIP-inf mutations should consider transplantation.
Our study has several limitations. First, it was a retrospective study. Second, the chemotherapy regimens used were not uniform, and not all patients with FLT3-ITD mutations were treated with FLT3 inhibitors. Third, the number of patients in our study, especially the transplant cohort, was relatively small, and there might be selection bias, which might influence the results and a prospective study is needed to validate the conclusions.
5. Conclusions
IKZF1 mutations/deletions and FLT3-ITD mutations predicted poor outcomes in AML patients with CEBPAbZIP-inf mutations in addition to a high WBC count, lower hemoglobin concentration, non-intensive induction therapy, and MRD positivity after the first consolidation. We identified a potential high-risk population for transplantation that should be considered. Our findings need to be verified in future studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17152494/s1: Supplemental Table S1: List of 290 genes targeted by next generation sequencing panel; Supplemental Table S2: Univariable analyses of outcomes; Supplemental Table S3: Multivariate analyses of outcomes; Supplemental Table S4: Multivariate analyses of outcomes in patients receiving intensive induction therapy; Supplemental Figure S1: Lollipop plot illustrating IKZF1 mutations; Supplemental Figure S2: ROC curves of the risk stratification for the 1-, 2- and 3-year probabilities of RFS.
Author Contributions
Q.J.: conceptualization; methodology; investigation; validation; funding acquisition; writing—original draft; writing—review and editing; supervision; project administration; resources; data curation; and formal analysis. Q.J., S.Y., and L.H.: data curation; formal analysis and writing—original draft. Y.Q., G.R., Y.W. (Yazhe Wang), Y.L., and H.S.: Resources. H.J., F.T., T.Z., J.J., J.W., Q.F., X.Z., L.X., Y.W. (Yu Wang), Y.S., and X.H.: data curation and investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970140 and No. 82370161) and Peking University People’s Hospital Research and Development Funds (RDL2023-11).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Peking University People’s Hospital (approval number 2021PHB136-001) on 24 May 2021.
Informed Consent Statement
Informed consent was obtained from all individual participants or their guardians included in the study.
Data Availability Statement
Data are available on reasonable request from the corresponding authors and consistent with the laws of China.
Acknowledgments
We gratefully acknowledge the next-generation sequencing technical help provided by Chengcheng Yan and Jinghua Feng (Guangzhou Jinyu Company, Guangzhou, China).
Conflicts of Interest
The authors declare no competing interests.
References
- Taube, F.; Georgi, J.A.; Kramer, M.; Stasik, S.; Middeke, J.M.; Röllig, C.; Krug, U.; Krämer, A.; Scholl, S.; Hochhaus, A.; et al. CEBPA mutations in 4708 patients with acute myeloid leukemia: Differential impact of bZIP and TAD mutations on outcome. Blood 2022, 139, 87–103. [Google Scholar] [CrossRef]
- Wakita, S.; Sakaguchi, M.; Oh, I.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; Mori, S.; et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022, 6, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Tien, F.M.; Yao, C.Y.; Tsai, X.C.; Lo, M.Y.; Chen, C.Y.; Lee, W.H.; Lin, C.C.; Kuo, Y.Y.; Peng, Y.L.; Tseng, M.H.; et al. Dysregulated immune and metabolic pathways are associated with poor survival in adult acute myeloid leukemia with CEBPA bZIP in-frame mutations. Blood Cancer J. 2024, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Georgi, J.A.; Stasik, S.; Kramer, M.; Meggendorfer, M.; Röllig, C.; Haferlach, T.; Valk, P.; Linch, D.; Herold, T.; Duployez, N.; et al. Prognostic impact of CEBPA mutational subgroups in adult AML. Leukemia 2024, 38, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, Z.; Xie, J.; Zhang, J.; Wu, Q.; Jiang, R.; Zhao, X.; Yang, X.; Chen, S. CEBPA bZIP in-frame mutations in acute myeloid leukemia: Prognostic and therapeutic implications. Blood Cancer J. 2024, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.; Ruan, G.; Liu, Y.; Wang, Y.; Zhang, X.; Xu, L.; Huang, X.; Chang, Y. Impact of pre-transplantation minimal residual disease determined by multiparameter flow cytometry on the outcome of AML patients with FLT3-ITD after allogeneic stem cell transplantation. Ann. Hematol. 2018, 97, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.G.; McGowan-Jordan, J.; Schmid, M. ISCN 2013: An International System for Human Cytogenetic Nomenclature (2013); Karger Medical and Scientific Publishers: Basel, Switzerland, 2013. [Google Scholar]
- Yu, S.; Yang, S.; Hu, L.; Duan, W.; Zhao, T.; Qin, Y.; Wang, Y.; Lai, Y.; Shi, H.; Tang, F.; et al. Genetic abnormalities predict outcomes in patients with core binding factor acute myeloid leukemia. Ann. Hematol. 2025, 104, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.B.; Mehta, V.; Girman, C.J.; Adhikari, D.; Johnson, M.L. Regression coefficient-based scoring system should be used to assign weights to the risk index. J. Clin. Epidemiol. 2016, 79, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Iba, K.; Shinozaki, T.; Maruo, K.; Noma, H. Re-evaluation of the comparative effectiveness of bootstrap-based optimism correction methods in the development of multivariable clinical prediction models. BMC Med. Res. Methodol. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Harrell, F.E., Jr.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, A.N.; Cox, T.; Kolamunnage-Dona, R. Time-dependent ROC curve analysis in medical research: Current methods and applications. BMC Med. Res. Methodol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Yamaguchi, S.; Iwanaga, E.; Nanri, T.; Shimomura, T.; Suzushima, H.; Mitsuya, H.; Asou, N. High frequency of IKZF1 genetic alterations in adult patients with B-cell acute lymphoblastic leukemia. Eur. J. Haematol. 2013, 91, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Goorha, S.; Radtke, I.; Miller, C.B.; Coustan-Smith, E.; Dalton, J.D.; Girtman, K.; Mathew, S.; Ma, J.; Pounds, S.B.; et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007, 446, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Vairy, S.; Tran, T.H. IKZF1 alterations in acute lymphoblastic leukemia: The good, the bad and the ugly. Blood Rev. 2020, 44, 100677. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Lhermitte, L.; Dourthe, M.E.; Lengliné, E.; Graux, C.; Grardel, N.; Cayuela, J.M.; Arnoux, I.; Gandemer, V.; Ifrah, N.; et al. IKZF1 alterations predict poor prognosis in adult and pediatric T-ALL. Blood 2021, 137, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, A.; Liu, L.; Qin, J.; Wang, C.; Yang, M.; Lou, Y.; Wang, L.; Ni, X.; Hu, X.; et al. The clinical impact of IKZF1 mutation in acute myeloid leukemia. Exp. Hematol. Oncol. 2023, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.N.; Stasik, S.; Röllig, C.; Petzold, A.; Sauer, T.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. Mutated IKZF1 is an independent marker of adverse risk in acute myeloid leukemia. Leukemia 2023, 37, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Li, X.; Lv, Y.; Zhu, Y.; Wang, J.; Jin, J.; Yu, W. The specific distribution pattern of IKZF1 mutation in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Green, C.L.; Koo, K.K.; Hills, R.K.; Burnett, A.K.; Linch, D.C.; Gale, R.E. Prognostic significance of CEBPA mutations in a large cohort of younger adult patients with acute myeloid leukemia: Impact of double CEBPA mutations and the interaction with FLT3 and NPM1 mutations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Long, N.; Saultz, J.; Gandhi, A.; Newell, L.F.; Hayes-Lattin, B.; Maziarz, R.T.; Leonard, J.; Bottomly, D.; McWeeney, S.; et al. Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia. Blood Adv. 2023, 7, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Mrózek, K.; Kohlschmidt, J.; Blachly, J.S.; Nicolet, D.; Carroll, A.J.; Archer, K.J.; Mims, A.S.; Larkin, K.T.; Orwick, S.; Oakes, C.C.; et al. Outcome prediction by the 2022 European LeukemiaNet genetic-risk classification for adults with acute myeloid leukemia: An Alliance study. Leukemia 2023, 37, 788–798. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).