The Role of Prophylactic HIPEC in High-Risk Gastric Cancer Patients: Where Do We Stand?
Simple Summary
Abstract
1. Introduction
2. High-Risk Gastric Cancer and HIPEC
3. Prophylactic HIPEC in High-Risk Gastric Cancer
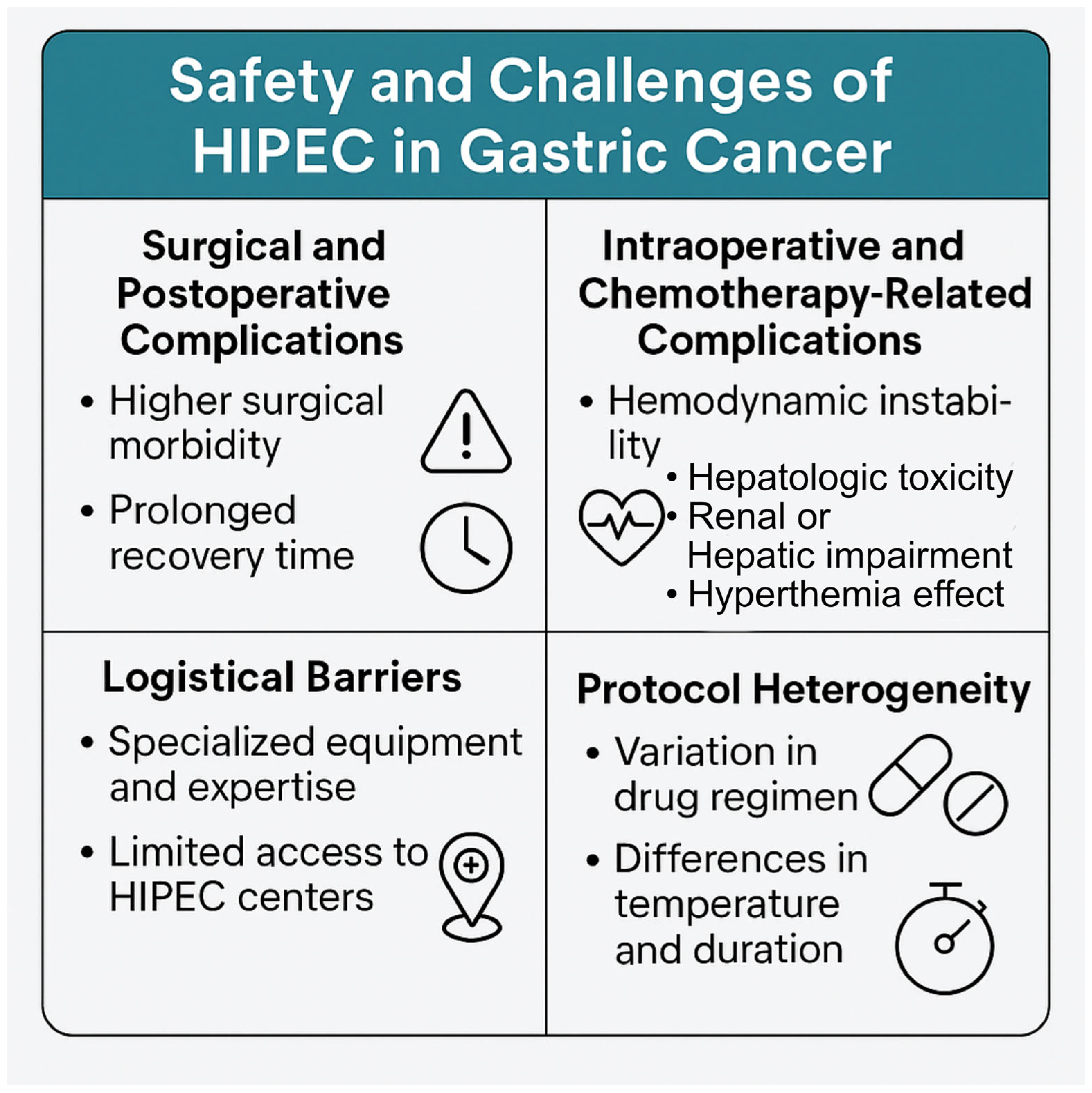
4. Challenges and Considerations
5. Quality of Life Following HIPEC
6. Future Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sampson, J.A. Implantation Peritoneal Carcinomatosis of Ovarian Origin. Am. J. Pathol. 1931, 7, 423. [Google Scholar]
- Flanagan, M.; Solon, J.; Chang, K.H.; Deady, S.; Moran, B.; Cahill, R.; Shields, C.; Mulsow, J. Peritoneal metastases from extra-abdominal cancer—A population-based study. Eur. J. Surg. Oncol. 2018, 44, 1811–1817. [Google Scholar] [CrossRef]
- Solon, J.G.; O’Neill, M.; Chang, K.H.; Deady, S.; Cahill, R.; Moran, B.; Shields, C.; Mulsow, J. An 18 year population-based study on site of origin and outcome of patients with peritoneal malignancy in Ireland. Eur. J. Surg. Oncol. 2017, 43, 1924–1931. [Google Scholar] [CrossRef]
- Kusamura, S.; Baratti, D.; Zaffaroni, N.; Villa, R.; Laterza, B.; Balestra, M.R.; Deraco, M. Pathophysiology and biology of peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2010, 2, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Surg. Oncol. Clin. N. Am. 2003, 12, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Evolution of cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis: Are there treatment alternatives? Am. J. Surg. 2011, 201, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Surgical responsibilities in the management of peritoneal carcinomatosis. J. Surg. Oncol. 2010, 101, 713–724. [Google Scholar] [CrossRef]
- Moyer, H.R.; Delman, K.A. The role of hyperthermia in optimizing tumor response to regional therapy. Int. J. Hyperth. 2008, 24, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- de Bree, E.; Tsiftsis, D.D. Principles of perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. Recent Results Cancer Res. 2007, 169, 39–51. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest. Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Byrne, R.M.; Gilbert, E.W.; Dewey, E.N.; Herzig, D.O.; Lu, K.C.; Billingsley, K.G.; Deveney, K.E.; Tsikitis, V.L. Who Undergoes Cytoreductive Surgery and Perioperative Intraperitoneal Chemotherapy for Appendiceal Cancer? An Analysis of the National Cancer Database. J. Surg. Res. 2019, 238, 198–206. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Deraco, M.; Kusamura, S.; Virzì, S.; Puccio, F.; Macrì, A.; Famulari, C.; Solazzo, M.; Bonomi, S.; Iusco, D.R.; Baratti, D. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: Multi-institutional phase-II trial. Gynecol. Oncol. 2011, 122, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef]
- Elias, D.; David, A.; Sourrouille, I.; Honoré, C.; Goéré, D.; Dumont, F.; Stoclin, A.; Baudin, E. Neuroendocrine carcinomas: Optimal surgery of peritoneal metastases (and associated intra-abdominal metastases). Surgery 2014, 155, 5–12. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Gerardi, C.; Abatini, C.; Melotti, G.; Bonavina, L.; Torri, V.; Santullo, F.; Garattini, S.; De Luca, M.; Rulli, E.; et al. Prophylactic surgery plus hyperthermic intraperitoneal chemotherapy (HIPEC CO2) versus standard surgery for gastric carcinoma at high risk of peritoneal carcinomatosis: Short and long-term outcomes (GOETH STUDY)—A collaborative randomized controlled trial by ACOI, FONDAZIONE AIOM, SIC, SICE, and SICO. Trials 2022, 23, 969. [Google Scholar]
- Zhang, J.F.; Lv, L.; Zhao, S.; Zhou, Q.; Jiang, C.G. Hyperthermic intraperitoneal chemotherapy (HIPEC) combined with surgery: A 12-year meta-analysis of this promising treatment strategy for advanced gastric cancer at different stages. Ann. Surg. Oncol. 2022, 29, 3170–3186. [Google Scholar] [CrossRef]
- Götze, T.O.; Piso, P.; Lorenzen, S.; Bankstahl, U.S.; Pauligk, C.; Elshafei, M.; Amato, G.; Reim, D.; Bechstein, W.O.; Königsrainer, A.; et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma–the phase III “PREVENT”-(FLOT9) trial of the AIO/CAOGI/ACO. BMC Cancer 2021, 21, 1158. [Google Scholar] [CrossRef]
- Coccolini, F.; Celotti, A.; Ceresoli, M.; Montori, G.; Marini, M.; Catena, F.; Ansaloni, L. Hyperthermic intraperitoneal chemotherapy (HIPEC) and neoadjuvant chemotherapy as prophylaxis of peritoneal carcinosis from advanced gastric cancer—Effects on overall and disease free survival. J. Gastrointest. Oncol. 2016, 7, 523. [Google Scholar] [CrossRef]
- Beeharry, M.K.; Zhu, Z.L.; Liu, W.T.; Yao, X.X.; Yan, M.; Zhu, Z.G. Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: Personal experience from a randomized case control study. BMC Cancer 2019, 19, 932. [Google Scholar]
- Beeharry, M.K.; Ni, Z.T.; Yang, Z.Y.; Zheng, Y.N.; Feng, R.H.; Liu, W.T.; Yan, C.; Yao, X.X.; Li, C.; Yan, M.; et al. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): Dragon II trial. BMC Cancer 2020, 20, 224. [Google Scholar] [CrossRef]
- Jain, A.J.; Badgwell, B.D. Current evidence for the use of HIPEC and cytoreductive surgery in gastric cancer metastatic to the peritoneum. J. Clin. Med. 2023, 12, 6527. [Google Scholar] [CrossRef]
- Kunte, A.R.; Parray, A.M.; Bhandare, M.S.; Solanki, S.L. Role of prophylactic HIPEC in non-metastatic, serosa-invasive gastric cancer: A literature review. Pleura Peritoneum 2022, 7, 103–115. [Google Scholar] [CrossRef]
- Zhuang, X.; He, Y.; Ma, W. Prophylactic hyperthermic intraperitoneal chemotherapy may benefit the long-term survival of patients after radical gastric cancer surgery. Sci. Rep. 2022, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Hamazoe, R.; Maeta, M.; Shimizu, N.; Murakami, A.; Wakatsuki, T. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 1988, 61, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kaibara, N.; Hamazoe, R.; Iitsuka, Y.; Maeta, M.; Koga, S. Hyperthermic peritoneal perfusion combined with anticancer chemotherapy as prophylactic treatment of peritoneal recurrence of gastric cancer. Hepatogastroenterology 1989, 36, 75–78. [Google Scholar]
- Hamazoe, R.; Maeta, M.; Kaibara, N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994, 73, 2048–2052. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Kondou, A.; Oka, A.; Tsujitani, S.; Maeta, M.; Kaibara, N. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur. J. Surg. 1995, 161, 581–586. [Google Scholar]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999, 85, 529–534. [Google Scholar] [CrossRef]
- Yonemura, Y.; de Aretxabala, X.; Fujimura, T.; Fushida, S.; Katayama, K.; Bandou, E.; Sugiyama, K.; Kawamura, T.; Kinoshita, K.; Endou, Y.; et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepatogastroenterology 2001, 48, 1776–1782. [Google Scholar]
- Kuramoto, M.; Shimada, S.; Ikeshima, S.; Matsuo, A.; Yagi, Y.; Matsuda, M.; Yonemura, Y.; Baba, H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann. Surg. 2009, 250, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.J.; Wei, Z.G.; Zhen, L.; Li, G.X.; Uang, X.C.; Qing, S.H. Clinical application of perioperative continuous hyperthermic peritoneal perfusion chemotherapy for gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao 2009, 29, 295–297. [Google Scholar] [PubMed]
- Zhang, T.; Feng, J.; Cai, C.; Zhang, X. Synthesis and field test of three candidates for soybean pod borer’s sex pheromone. Nat. Prod. Commun. 2011, 6, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.B.; Ge, H.E.; Bai, X.Y.; Zhang, W.; Zhang, Y.Y.; Wang, J.; Li, X.; Xing, L.P.; Guo, S.H.; Wang, Z.Y. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp. Ther. Med. 2014, 7, 1083–1088. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Reutovich, M.Y.; Krasko, O.V.; Sukonko, O.G. Hyperthermic intraperitoneal chemotherapy in serosa-invasive gastric cancer patients. Eur. J. Surg. Oncol. 2019, 45, 2405–2411. [Google Scholar] [CrossRef]
- Fan, B.; Bu, Z.; Zhang, J.; Zong, X.; Ji, X.; Fu, T.; Jia, Z.; Zhang, Y.; Wu, X. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer 2021, 21, 216. [Google Scholar] [CrossRef]
- Kunisaki, C.; Shimada, H.; Nomura, M.; Akiyama, H.; Takahashi, M.; Matsuda, G. Lack of efficacy of prophylactic continuous hyperthermic peritoneal perfusion on subsequent peritoneal recurrence and survival in patients with advanced gastric cancer. Surgery 2002, 131, 521–528. [Google Scholar] [CrossRef]
- Li, C.; Yan, M.; Chen, J.; Xiang, M.; Zhu, Z.G.; Yin, H.R.; Lin, Y.Z. Surgical resection with hyperthermic intraperitoneal chemotherapy for gastric cancer patients with peritoneal dissemination. J. Surg. Oncol. 2010, 102, 361–365. [Google Scholar] [CrossRef]
- Hultman, B.; Lind, P.; Glimelius, B.; Sundbom, M.; Nygren, P.; Haglund, U.; Mahteme, H. Phase II study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy. Acta Oncol. 2013, 52, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.Y.; Mok, K.T.; Liu, S.I.; Tsai, C.C.; Wang, B.W.; Chen, I.S.; Chen, Y.C.; Chang, B.M.; Chou, N.H. Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion. J. Chin. Med. Assoc. 2013, 76, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Yarema, R.R.; Ohorchak, M.A.; Zubarev, G.P.; Mylyan, Y.P.; Oliynyk, Y.Y.; Zubarev, M.G.; Gyrya, P.I.; Kovalchuk, Y.J.; Safiyan, V.I.; Fetsych, T.G. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperth. 2014, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Chow, O.; Parikh, K.; Blank, S.; Jibara, G.; Kadri, H.; Labow, D.M.; Hiotis, S.P. Peritoneal carcinomatosis in patients with gastric cancer, and the role for surgical resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy. Am. J. Surg. 2014, 207, 78–83. [Google Scholar] [CrossRef]
- Boerner, T.; Graichen, A.; Jeiter, T.; Zemann, F.; Renner, P.; März, L.; Soeder, Y.; Schlitt, H.J.; Piso, P.; Dahlke, M.H. CRS-HIPEC Prolongs Survival but is Not Curative for Patients with Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 3972–3977. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtröder, C.; Schüle, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer. 2020, 23, 11–22. [Google Scholar] [CrossRef]
- Xie, T.Y.; Wu, D.; Li, S.; Qiu, Z.Y.; Song, Q.Y.; Guan, D.; Wang, L.P.; Li, X.G.; Duan, F.; Wang, X.X. Role of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer. World J. Gastrointest. Oncol. 2020, 12, 782–790. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Z.; Wu, Y.; Liu, P.; Qian, J.; Yu, S.; Xia, B.; Lai, J.; Ma, S.; Wu, Z. Prophylactic chemotherapeutic hyperthermic intraperitoneal perfusion reduces peritoneal metastasis in gastric cancer: A retrospective clinical study. BMC Cancer 2020, 20, 827. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, J.; Bai, X.; Sun, Y.; Liu, H.; Ma, S.; Li, Y.; Kang, W.; Ma, F.; Li, W.; et al. Lobaplatin in Prophylactic Hyperthermic Intraperitoneal Chemotherapy for Advanced Gastric Cancer: Safety and Efficacy Profiles. Cancer Manag. Res. 2020, 12, 5141–5146. [Google Scholar] [CrossRef]
- Diniz, T.P.; da Costa, W.L., Jr.; de Jesus, V.H.F.; Ribeiro, H.S.C.; Diniz, A.L.; de Godoy, A.L.; de Farias, I.C.; Torres, S.M.; Felismino, T.C.; Coimbra, F.J.F. Does hipec improve outcomes in gastric cancer patients treated with perioperative chemotherapy and radical surgery? A propensity-score matched analysis. J. Surg. Oncol. 2020, 121, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Quero, G.; Fiorillo, C.; Cina, C.; Alfieri, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases: Analysis of short- and long-term outcomes. Langenbecks Arch. Surg. 2021, 406, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Pocard, M. Randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: A systematic review. Pleura Peritoneum 2016, 1, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.L., Jr.; Coimbra, F.J.; Ribeiro, H.S.; Diniz, A.L.; de Godoy, A.L.; Begnami, M.; Silva, M.J.; Fanelli, M.F.; Mello, C.A. Safety and preliminary results of perioperative chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC) for high-risk gastric cancer patients. World J. Surg. Oncol. 2012, 10, 195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Ma, S.; Zhang, S.; Aizezi, Y.; Wang, Q. Optimal hyperthermic intraperitoneal chemotherapy regimen for advanced and peritoneal metastatic gastric cancer: A systematic review and Bayesian network meta-analysis. Front. Oncol. 2024, 14, 1466473. [Google Scholar] [CrossRef]
- Wajekar, A.S.; Solanki, S.L.; Patil, V.P. Postoperative complications and critical care management after cytoreduction surgery and hyperthermic intraperitoneal chemotherapy: A systematic review of the literature. World J. Crit. Care Med. 2022, 11, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Shirsalimi, N.; Sedighi, E. Challenges following CRS and HIPEC surgery in cancer patients with peritoneal metastasis: A comprehensive review of clinical outcomes. Front. Surg. 2024, 11, 1498529. [Google Scholar] [CrossRef]
- Finlay, B.; Price, T.; Hewett, P. Neutropenia and thrombocytopenia after cytoreductive surgery and heated intraperitoneal chemotherapy. Pleura Peritoneum 2017, 2, 137–141. [Google Scholar] [CrossRef]
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114. [Google Scholar] [CrossRef]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Boussios, S.; Moschetta, M.; Karathanasi, A.; Tsiouris, A.K.; Kanellos, F.S.; Tatsi, K.; Katsanos, K.H.; Christodoulou, D.K. Malignant peritoneal mesothelioma: Clinical aspects, and therapeutic perspectives. Ann. Gastroenterol. 2018, 31, 659–669. [Google Scholar] [CrossRef]
- Grimmig, T.; Moll, E.M.; Kloos, K.; Thumm, R.; Moench, R.; Callies, S.; Kreckel, J.; Vetterlein, M.; Pelz, J.; Polat, B.; et al. Upregulated heat shock proteins after hyperthermic chemotherapy point to induced cell survival mechanisms in affected tumor cells from peritoneal carcinomatosis. Cancer Growth Metastasis 2017, 10, 1179064417730559. [Google Scholar] [CrossRef]
- Samara, A.A.; Lafioniatis, A.; Ioannou, M.; Tsiapakidou, S.; Gerede, A.; Anastasakis, E.; Daponte, A.; Sotiriou, S. The role of heat shock proteins in placental ischemic disease: A narrative review of the current literature. Int. J. Gynaecol. Obstet. 2025, 169, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Nogueiro, J.; Fathi, N.Q.; Guaglio, M.; Baratti, D.; Kusamura, S.; Deraco, M. Risk factors for gastrointestinal perforation and anastomotic leak in patients submitted to cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur. J. Surg. Oncol. 2023, 49, 107020. [Google Scholar] [CrossRef] [PubMed]
- Samara, A.A.; Diamantis, A.; Magouliotis, D.; Tolia, M.; Tsavalas, V.; Tzovaras, G.; Tepetes, K. Assessing Preoperative (EORTC) QLQ-C30 Score in Elderly Patients with Colorectal Cancer: Results from a Prospective Cohort Study. J. Clin. Med. 2024, 13, 6193. [Google Scholar] [CrossRef] [PubMed]
- Leimkühler, M.; Hentzen, J.E.K.R.; Hemmer, P.H.J.; Been, L.B.; van Ginkel, R.J.; Kruijff, S.; van Leeuwen, B.L.; de Bock, G.H. Systematic Review of Factors Affecting Quality of Life After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2020, 27, 3973–3983. [Google Scholar] [CrossRef]
- Shan, L.L.; Saxena, A.; Shan, B.L.; Morris, D.L. Quality of life after cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy for peritoneal carcinomatosis: A systematic review and meta-analysis. Surg. Oncol. 2014, 23, 199–210. [Google Scholar] [CrossRef]
- Seretis, C.; Youssef, H. Quality of life after cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: A systematic review. Eur. J. Surg. Oncol. 2014, 40, 1605–1613. [Google Scholar] [CrossRef]
- Passot, G.; Bakrin, N.; Roux, A.S.; Vaudoyer, D.; Gilly, F.N.; Glehen, O.; Cotte, E. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: A prospective study of 216 patients. Eur. J. Surg. Oncol. 2014, 40, 529–535. [Google Scholar] [CrossRef]
- Morgan, R.B.; Tun, S.; Eng, O.S. Quality of life after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A narrative review. Dig. Med. Res. 2020, 3, 53. [Google Scholar] [CrossRef]
- Duckworth, K.E.; McQuellon, R.P.; Russell, G.B.; Cashwell, C.S.; Shen, P.; Stewart, J.H., 4th; Levine, E.A. Patient rated outcomes and survivorship following cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CS + HIPEC). J. Surg. Oncol. 2012, 106, 376–380. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Bockelmann, C.; Raue, W.; Menenakos, C.; Perez, S.; Rau, B.; Hartmann, J. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Is it worth the risk? Ann. Surg. Oncol. 2013, 20, 226–232. [Google Scholar] [CrossRef]
- Lv, C.B.; Tong, L.Y.; Zeng, W.M.; Chen, Q.X.; Fang, S.Y.; Sun, Y.Q.; Cai, L.S. Efficacy of neoadjuvant chemotherapy combined with prophylactic intraperitoneal hyperthermic chemotherapy for patients diagnosed with clinical T4 gastric cancer who underwent laparoscopic radical gastrectomy: A retrospective cohort study based on propensity score matching. World J. Surg. 2024, 22, 244. [Google Scholar]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Van der Speeten, K.; Kusamura, S.; Villeneuve, L.; Piso, P.; Verwaal, V.J.; González-Moreno, S.; Glehen, O. The 2022 PSOGI International Consensus on HIPEC Regimens for Peritoneal Malignancies: HIPEC Technologies. Ann. Surg. Oncol. 2024, 31, 7090–7110. [Google Scholar] [CrossRef]

| Intervention | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | HIPEC | Control Group | NAC | AC | HIPEC Duration (min) | Drug | Temp (°C) | HIPEC | Control | HIPEC | Control |
| Koga et al. [27] | HIPEC + RS | RS | ND | ND | 50–60 | MMC | 44–45 | 3-year: 83.0% | 67.30% | ||
| Kaibara et al. [28] | HIPEC + RS | RS | ND | ND | 50–60 | MMC | 44–45 | 5-year: 71.5% | 59.70% | ||
| Hamazoe et al. [29] | HIPEC + RS | RS | ND | ND | 50–60 | MMC | 40–45 | 5-year: 64% | 52% | ||
| Ikeguchi et al. [30] | HIPEC + RS | Systemic chemotherapy | No | Yes | MMC | 44–45 | 30 months | 23 months | |||
| Fujimoto et al. [31] | HIPEC + RS | RS | ND | Yes | 120 | MMC | 43–44 | 4-year: 76% | 58% | ||
| Yonemura et al. [32] | HIPEC + RS | RS | ND | Yes | 60 | MMC/CIS | 42–43 | 5-year: 61% | 42% | ||
| Kuramoto et al. [33] | HIPEC + RS | RS | ND | Yes | 60 | CIS | 5-year: 43.8% | 0% | |||
| Deng et al. [34] | HIPEC + RS | RS | ND | Yes | 60–90 | MMC/5-FU | 42–43 | 3-year: 59.09% | 34.15% | ||
| Zhang et al. [35] | HIPEC + CRS | CRS | NR | NR | 60–90 | MMC/CIS | 42.5–43.5 | 32-month survival: 14.7% | 2.9% | ||
| Cui et al. [36] | HIPEC + RS | RS | YES | YES | 90 | CIS/5-FU | 41–43 | 3-year: 75% | 35.41% | ||
| Rudloff et al. [37] | HIPEC + CRS | Systemic chemotherapy | NR | NR | 30 | Oxaliplatin | 41 | 11.3 months median survival | All dead within 12 months | ||
| Reutovich et al. [38] | HIPEC + RS | RS | ND | ND | 60 | CIS/doxorubicin | 42 | 3-year progression-free survival: 47% (95% CI 36–61) | 3-year progression-free survival: 27% (95% CI 17–43) | p = 0.0024. | |
| Beeharry et al. [21] | HIPEC + RS | RS | ND | Yes | 60 | CIS | 42 | 3-year: 93% | 65% | ||
| Fan et al. [39] | HIPEC + RS | RS | ND | Yes | 30 | CIS | 42.5–43 | 3-year: 87.9% | 100% | ||
| Intervention | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | HIPEC | Control Group | NAC | AC | HIPEC Duration (min) | Drug | Temp (°C) | HIPEC | Control | HIPEC | Control |
| Kunisaki [40] | HIPEC + RS | RS | yes | 40 | MMC/CIS/etoposide | 42–43 | 5-year: 49% | 56% | |||
| Li [41] | HIPEC + CRS | CRS | 60 | MMC/CIS | 40–45 | ||||||
| Hultman [42] | HIPEC + CRS | Systemic chemotherapy | Yes | 90 | CIS/doxorubicin | 42–44 | 17.4 months mean survival | 11.1 months | |||
| Kang [43] | HIPEC + RS | RS | ND | Yes | 60 | MMC/CIS/etoposide | 41–4 | 3-year: 66.03% | 28.87% | 5-year: 43% | 10% |
| Yarema [44] | HIPEC + RS | RS | ND | ND | 90 | MMC | 41–43.5 | 1-year: 100%/ | 12 months/52.6% 1 year | ||
| HIPEC + CRS | Systemic chemotherapy | Yes | 90 | MMC | 41–43.5 | 1-year: 68.8% | 25% | ||||
| Kim [45] | HIPEC + CRS | CRS | 90 | MMC | 41 | ||||||
| Coccolini [20] | HIPEC + RS | RS | Yes | ND | 90 | CIS/paclitaxel | 40–41 | 34.5 months | 21.6–27.7 | 36.6 months mean survival | 27.1–28.2 |
| Boerner [46] | HIPEC + CRS | Systemic chemotherapy | NR | NR | 60 | CIS/doxorubicin | 42–43 | 17.2 median survival | 11.0 median survival | ||
| Bonnot [47] | HIPEC + CRS | CRS | Yes | Yes | 30–90 | MMC/CIS/oxaliplatin | 42–44 | 5-year: 10.82% | 5-year: 6.43% | ||
| Rau [48] | HIPEC + CRS | Systemic chemotherapy | Yes | Yes | 60 | MMC/CIS | 41 | 3-year: 17.5% | 0% | ||
| Xie [49] | HIPEC + RS | RS | ND | Yes | 60 | CIS | 42–43 | 3-year: 63% | 60% | 3-year: 68% | 66% |
| Zhu [50] | HIPEC + RS | RS | ND | Yes | 60 | CIS | 41.5–42.5 | 36.5 months | 24.5 | NR | 33 months |
| Zhong [51] | HIPEC + RS | RS | ND | Yes | 60 | Lobaplatin | 43 | 3-year: 89.4% | 73.90% | 3-year: 89.4% | 84.30% |
| Diniz [52] | HIPEC + RS | RS | Yes | Yes | 90 | MMC | 41–42 | 5-year: 49.5% | 65.80% | 5-year: 59.5% | 68.70% |
| Rosa [53] | HIPEC +CRS | RS | ND | ND | 90 | MMC | 41–42 | 5-year: 30% | 9% | 5-year: 33% | 9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantis, A.; Samara, A.A.; Lafioniatis, A.; Janho, M.B.; Floros, T.; Tepetes, K. The Role of Prophylactic HIPEC in High-Risk Gastric Cancer Patients: Where Do We Stand? Cancers 2025, 17, 2492. https://doi.org/10.3390/cancers17152492
Diamantis A, Samara AA, Lafioniatis A, Janho MB, Floros T, Tepetes K. The Role of Prophylactic HIPEC in High-Risk Gastric Cancer Patients: Where Do We Stand? Cancers. 2025; 17(15):2492. https://doi.org/10.3390/cancers17152492
Chicago/Turabian StyleDiamantis, Alexandros, Athina A. Samara, Anastasios Lafioniatis, Michel B. Janho, Theodoros Floros, and Konstantinos Tepetes. 2025. "The Role of Prophylactic HIPEC in High-Risk Gastric Cancer Patients: Where Do We Stand?" Cancers 17, no. 15: 2492. https://doi.org/10.3390/cancers17152492
APA StyleDiamantis, A., Samara, A. A., Lafioniatis, A., Janho, M. B., Floros, T., & Tepetes, K. (2025). The Role of Prophylactic HIPEC in High-Risk Gastric Cancer Patients: Where Do We Stand? Cancers, 17(15), 2492. https://doi.org/10.3390/cancers17152492







