Impact of Anastomotic Leak on Long-Term Survival After Gastrectomy: Results from an Individual Patient Data Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Extraction
2.3. Outcomes of Interest and Definitions
2.4. Quality Assessment and Assessment of Certainty of Evidence
2.5. Statistical Analysis
3. Results
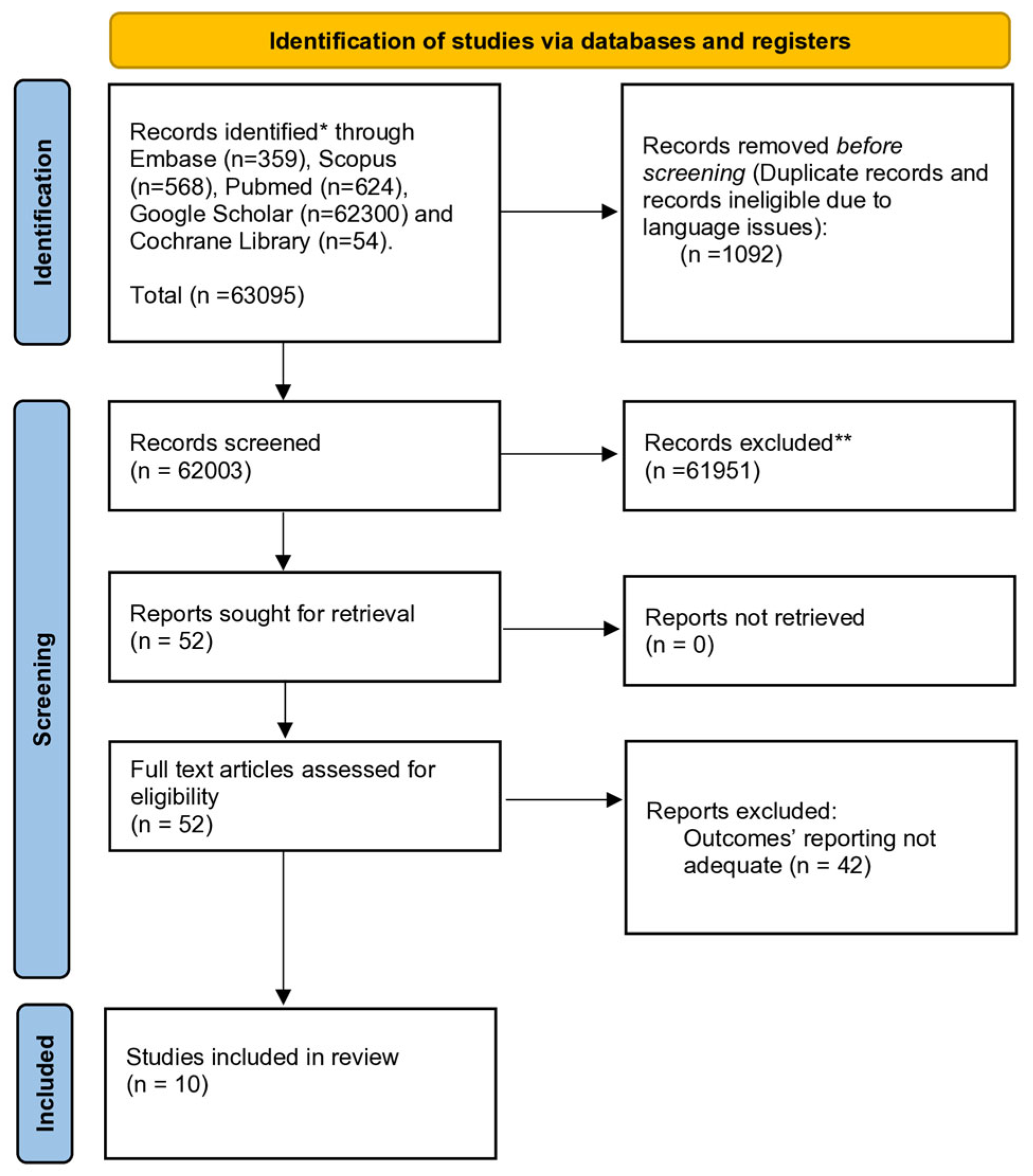
3.1. Systematic Review
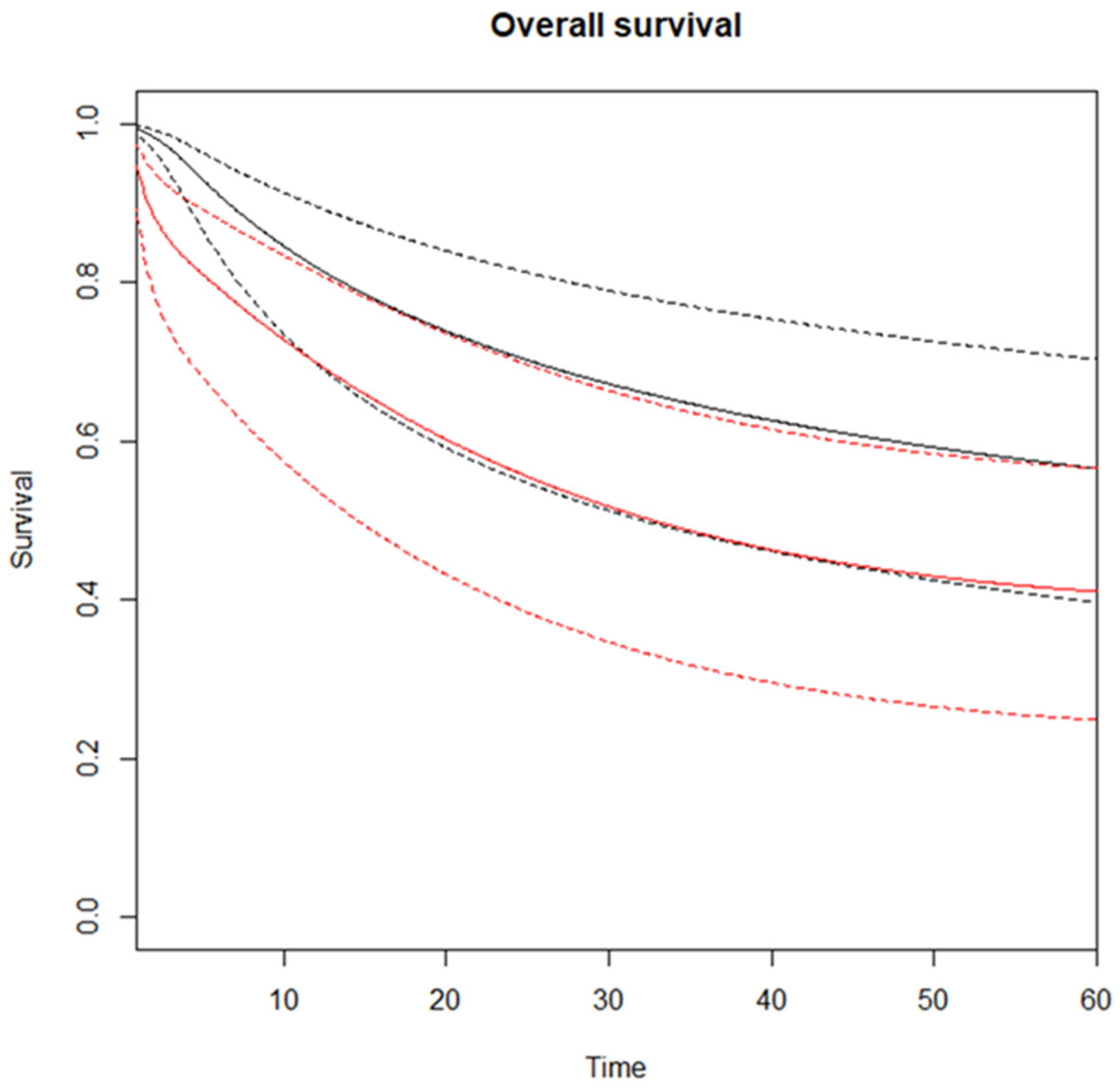
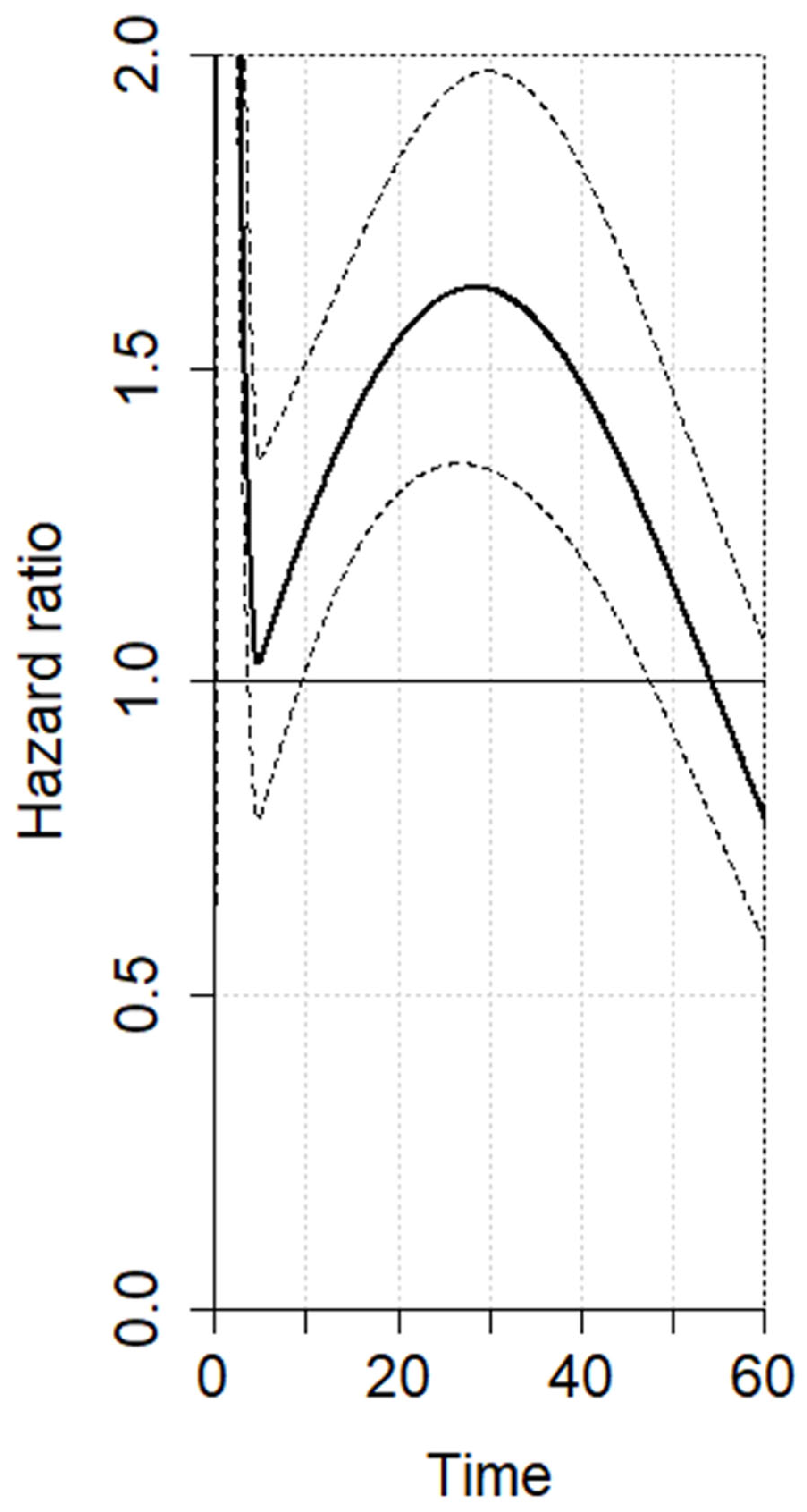
3.2. Meta-Analysis—Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Search Strategy
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://Gco.Iarc.Who.Int/Today (accessed on 4 May 2025).
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on Global Epidemiology, Risk and Prognostic Factors of Gastric Cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef]
- Schneider, M.A.; Kim, J.; Berlth, F.; Sugita, Y.; Grimminger, P.P.; Sano, T.; Rosati, R.; Baiocchi, G.L.; Bencivenga, M.; De Manzoni, G.; et al. Defining Benchmarks for Total and Distal Gastrectomy: Global Multicentre Analysis. Br. J. Surg. 2024, 111, znad379. [Google Scholar] [CrossRef]
- Van Hootegem, S.J.M.; Van Der Linde, M.; Schneider, M.A.; Kim, J.; Berlth, F.; Sugita, Y.; Grimminger, P.P.; Baiocchi, G.L.; De Manzoni, G.; Bencivenga, M.; et al. Impact of Postoperative Complications on Clinical Outcomes after Gastrectomy for Cancer: Multicentre Study. Br. J. Surg. 2025, 112, znaf043. [Google Scholar] [CrossRef]
- Van Der Werf, L.R.; Busweiler, L.A.D.; Van Sandick, J.W.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.L. Reporting National Outcomes after Esophagectomy and Gastrectomy According to the Esophageal Complications Consensus Group (ECCG). Ann. Surg. 2020, 271, 1095–1101. [Google Scholar] [CrossRef]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; Van Berge Henegouwen, M.I. Outcomes of Esophagogastric Cancer Surgery During Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Giacopuzzi, S.; Marrelli, D.; Reim, D.; Piessen, G.; Matos da Costa, P.; Reynolds, J.V.; Meyer, H.J.; Morgagni, P.; Gockel, I.; et al. International Consensus on a Complications List after Gastrectomy for Cancer. Gastric Cancer 2019, 22, 172–189. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Giacopuzzi, S.; Reim, D.; Piessen, G.; Da Costa, P.M.; Reynolds, J.V.; Meyer, H.J.; Morgagni, P.; Gockel, I.; Santos, L.L.; et al. Incidence and Grading of Complications after Gastrectomy for Cancer Using the GASTRODATA Registry a European Retrospective Observational Study. Ann. Surg. 2020, 272, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Giacopuzzi, S.; Vittimberga, G.; De Pascale, S.; Pastorelli, E.; Gelmini, R.; Viganò, J.; Graziosi, L.; Vagliasindi, A.; Rosa, F.; et al. Clinical Outcomes of Patients with Complicated Post-Operative Course after Gastrectomy for Cancer: A GIRCG Study Using the GASTRODATA Registry. Updates Surg. 2023, 75, 419–427. [Google Scholar] [CrossRef]
- Baum, P.; Diers, J.; Lichthardt, S.; Kastner, C.; Schlegel, N.; Germer, C.T.; Wiegering, A. Mortality and Complications Following Visceral Surgery. Dtsch. Ärzteblatt Int. 2019, 116, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, B.; Huang, Y. Impact of Anastomotic Leakage on Survival after Surgery for Gastric Carcinoma: A PRISMA Systematic Review and Meta-Analysis. Medicine 2023, 102, e35417. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, H.; Zhou, L.; Li, Q.; Wang, L.; Zhang, D.; Xu, H.; Xu, Z. Risk Factors and Conservative Therapy Outcomes of Anastomotic Leakage after Gastrectomy: Experience of 3926 Patients from a Single Gastric Surgical Unit. Front. Oncol. 2023, 13, 1163463. [Google Scholar] [CrossRef]
- Ren, L.-F.; Xu, Y.-H.; Long, J.-G. Prognostic Value of Postoperative Complication for Gastric Cancer. J. Laparoendosc. Adv. Surg. Tech. 2024, 34, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Navidi, M.; Griffin, S.M.; Phillips, A.W. Impact of Anastomotic Leak on Long-Term Survival in Patients Undergoing Gastrectomy for Gastric Cancer. Br. J. Surg. 2020, 107, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Sierzega, M.; Kolodziejczyk, P.; Kulig, J. Impact of Anastomotic Leakage on Long-Term Survival after Total Gastrectomy for Carcinoma of the Stomach. Br. J. Surg. 2010, 97, 1035–1042. [Google Scholar] [CrossRef]
- Saunders, J.H.; Yanni, F.; Dorrington, M.S.; Bowman, C.R.; Vohra, R.S.; Parsons, S.L. Impact of Postoperative Complications on Disease Recurrence and Long-Term Survival Following Oesophagogastric Cancer Resection. Br. J. Surg. 2020, 107, 103–112. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Walpita, Y.N.; Nurmatov, U. ROBINS-I: A Novel and Promising Tool for Risk of Bias Assessment of Nonrandomized Studies of Interventions. J. Coll. Community Physicians Sri Lanka 2020, 26, 183. [Google Scholar] [CrossRef]
- Royston, P.; Parmar, M.K. Restricted Mean Survival Time: An Alternative to the Hazard Ratio for the Design and Analysis of Randomized Trials with a Time-to-Event Outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Charvat, H.; Belot, A. Mexhaz: An R Package for Fitting Flexible Hazard-Based Regression Models for Overall and Excess Mortality with a Random Effect. J. Stat. Softw. 2021, 98, 1–36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://Www.R-Project.Org/ (accessed on 30 April 2025).
- Yoo, H.M.; Lee, H.H.; Shim, J.H.; Jeon, H.M.; Park, C.H.; Song, K.Y. Negative Impact of Leakage on Survival of Patients Undergoing Curative Resection for Advanced Gastric Cancer. J. Surg. Oncol. 2011, 104, 734–740. [Google Scholar] [CrossRef]
- Nagasako, Y.; Satoh, S.; Isogaki, J.; Inaba, K.; Taniguchi, K.; Uyama, I. Impact of Anastomotic Complications on Outcome after Laparoscopic Gastrectomy for Early Gastric Cancer. Br. J. Surg. 2012, 99, 849–854. [Google Scholar] [CrossRef]
- Kim, S.H.; Son, S.Y.; Park, Y.S.; Ahn, S.H.; Park, D.J.; Kim, H.H. Risk Factors for Anastomotic Leakage: A Retrospective Cohort Study in a Single Gastric Surgical Unit. J. Gastric Cancer 2015, 15, 167–175. [Google Scholar] [CrossRef]
- Barchi, L.C.; Ramos, M.F.K.P.; Pereira, M.A.; Dias, A.R.; Ribeiro-Júnior, U.; Zilberstein, B.; Cecconello, I. Esophagojejunal Anastomotic Fistula: A Major Issue after Radical Total Gastrectomy. Updates Surg. 2019, 71, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Adachi, Y.; Taniguchi, A.; Kimura, Y.; Iitaka, D.; Iwata, G.; Yamaoka, N. Prognostic Impacts of Categorized Postoperative Complications in Surgery for Gastric Cancer. Asian J. Surg. 2023, 46, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mittelstädt, A.; Reitberger, H.; Fleischmann, J.; Elshafei, M.; Brunner, M.; Anthuber, A.; Krautz, C.; Lucio, M.; Merkel, S.; Grützmann, R.; et al. Effect of Circular Stapler Diameter on Anastomotic Leakage Rate and Stenosis After Open Total Gastrectomy With Esophagojejunostomy. Ann. Surg. Open 2022, 3, e195. [Google Scholar] [CrossRef]
- D’Souza, J.; McCombie, A.; Roberts, R. The Influence of Short-Term Postoperative Outcomes on Overall Survival after Gastric Cancer Surgery. ANZ J. Surg. 2023, 93, 2875–2884. [Google Scholar] [CrossRef]
- Ishida, R.; Komatsu, S.; Takashima, Y.; Nishibeppu, K.; Ohashi, T.; Kosuga, T.; Konishi, H.; Shiozaki, A.; Kubota, T.; Fujiwara, H.; et al. Pancreatic Fistula as a Pivotal Prognostic Factor among Postoperative Complications in Gastric Cancer. Am. J. Cancer Res. 2023, 13, 6063. [Google Scholar]
- Calì, M.; Bona, D.; Kim, Y.M.; Hyung, W.; Cammarata, F.; Bonitta, G.; Bonavina, L.; Aiolfi, A. Effect of Minimally Invasive versus Open Distal Gastrectomy on Long-Term Survival in Patients with Gastric Cancer: Individual Patient Data Meta-Analysis. Ann. Surg. Oncol. 2024, 32, 2161–2171. [Google Scholar] [CrossRef]
- Aiolfi, A.; Sozzi, A.; Bonitta, G.; Lombardo, F.; Cavalli, M.; Campanelli, G.; Bonavina, L.; Bona, D. Short-Term Outcomes of Different Esophagojejunal Anastomotic Techniques during Laparoscopic Total Gastrectomy: A Network Meta-Analysis. Surg. Endosc. 2023, 37, 5777–5790. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Lombardo, F.; Matsushima, K.; Sozzi, A.; Cavalli, M.; Panizzo, V.; Bonitta, G.; Bona, D. Systematic Review and Updated Network Meta-Analysis of Randomized Controlled Trials Comparing Open, Laparoscopic-Assisted, and Robotic Distal Gastrectomy for Early and Locally Advanced Gastric Cancer. Surgery 2021, 170, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Calì, M.; Aiolfi, A.; Sato, S.; Hwang, J.; Bonitta, G.; Albanesi, F.; Bonavina, G.; Cavalli, M.; Campanelli, G.; Biondi, A.; et al. Effect of Indocyanine Green-Guided Lymphadenectomy During Gastrectomy on Survival: Individual Patient Data Meta-Analysis. Cancers 2025, 17, 980. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Bona, D.; Bonitta, G.; Lombardo, F.; Manara, M.; Sozzi, A.; Schlanger, D.; Popa, C.; Cavalli, M.; Campanelli, G.; et al. Long-Term Impact of D2 Lymphadenectomy during Gastrectomy for Cancer: Individual Patient Data Meta-Analysis and Restricted Mean Survival Time Estimation. Cancers 2024, 16, 424. [Google Scholar] [CrossRef]
- Seicean, R.; Puscasu, D.; Gheorghiu, A.; Pojoga, C.; Seicean, A.; Dindelegan, G. Anastomotic Leakage after Gastrectomy for Gastric Cancer. J. Gastrointest. Liver Dis. 2023, 32, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, E.C.; Goense, L.; Brenkman, H.J.F.; van Hillegersberg, R.; Ruurda, J.P. Identification of the Clinically Most Relevant Postoperative Complications after Gastrectomy: A Population-Based Cohort Study. Gastric Cancer 2020, 23, 339–348. [Google Scholar] [CrossRef]
- Andreou, A.; Biebl, M.; Dadras, M.; Struecker, B.; Sauer, I.M.; Thuss-Patience, P.C.; Chopra, S.; Fikatas, P.; Bahra, M.; Seehofer, D.; et al. Anastomotic Leak Predicts Diminished Long-Term Survival after Resection for Gastric and Esophageal Cancer. Surgery 2016, 160, 191–203. [Google Scholar] [CrossRef]
- Climent, M.; Hidalgo, N.; Vidal; Puig, S.; Iglesias, M.; Cuatrecasas, M.; Ramón, J.M.; García-Albéniz, X.; Grande, L.; Pera, M. Postoperative Complications Do Not Impact on Recurrence and Survival after Curative Resection of Gastric Cancer. Eur. J. Surg. Oncol. 2016, 42, 132–139. [Google Scholar] [CrossRef][Green Version]
- Aiolfi, A.; Griffiths, E.A.; Sozzi, A.; Manara, M.; Bonitta, G.; Bonavina, L.; Bona, D. Effect of Anastomotic Leak on Long-Term Survival After Esophagectomy: Multivariate Meta-Analysis and Restricted Mean Survival Times Examination. Ann. Surg. Oncol. 2023, 30, 5564–5572. [Google Scholar] [CrossRef]
- Koedam, T.W.A.; Bootsma, B.T.; Deijen, C.L.; Van De Brug, T.; Kazemier, G.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Haglind, E.; Tuynman, J.B.; et al. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann. Surg. 2022, 275, E420–E427. [Google Scholar] [CrossRef]
- Spooner, C.E.; Markowitz, N.P.; Saravolatz, L. The Role of Tumor Necrosis Factor in Sepsis. Clin. Immunol. Immunopathol. 1992, 62, S11–S17. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Brenkman, H.J.F.; Claassen, L.; Hannink, G.; Van Der Werf, L.R.; Ruurda, J.P.H.; Nieuwenhuizen, G.A.P.; Luyer, M.D.P.; Kouwenhoven, E.A.; Van Det, M.J.; Van Berge Henegouwen, M.I.; et al. Learning Curve of Laparoscopic Gastrectomy: A Multicenter Study. Ann. Surg. 2023, 277, E808–E816. [Google Scholar] [CrossRef]
- Semenov, N.; Dalgatov, K.; Izrailov, R. FLOT compared to FOLFOX/XELOX as a neoadjuvant chemotherapy in locally advanced gastric cancer: Experience of two clinics. J. Clin. Oncol. 2025, 42, 3. [Google Scholar] [CrossRef]
- Cho, H.; Nakamura, J.; Asaumi, Y.; Yabusaki, H.; Sakon, M.; Takasu, N.; Kobayashi, T.; Aoki, T.; Shiraishi, O.; Kishimoto, H.; et al. Long-term Survival Outcomes of Advanced Gastric Cancer Patients Who Achieved a Pathological Complete Response with Neoadjuvant Chemotherapy: A Systematic Review of the Literature. Ann. Surg. Oncol. 2015, 22, 787–792. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Coccolini, F.; Nardi, M.; Montori, G.; Ceresoli, M.; Celotti, A.; Cascinu, S.; Fugazzola, P.; Tomasoni, M.; Glehen, O.; Catena, F.; et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int. J. Surg. 2018, 51, 120–127. [Google Scholar] [CrossRef]
- Putila, E.; Helminen, O.; Helmiö, M.; Huhta, H.; Jalkanen, A.; Kallio, R.; Koivukangas, V.; Kokkola, A.; Laine, S.; Lietzen, E.; et al. Postoperative Complications After Neoadjuvant Chemotherapy Versus Upfront Surgery in Gastric Adenocarcinoma: A Population-Based Nationwide Study in Finland. Ann. Surg. Oncol. 2024, 31, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Lowy, A.M.; Mansfield, P.F.; Leach, S.D.; Pazdur, R.; Dumas, P.; Ajani, J.A. Response to Neoadjuvant Chemotherapy Best Predicts Survival After Curative Resection of Gastric Cancer. Ann. Surg. 1999, 151, 303–308. [Google Scholar] [CrossRef]
- Eto, K.; Hiki, N.; Kumagai, K.; Shoji, Y.; Tsuda, Y.; Kano, Y.; Yasufuku, I.; Okumura, Y.; Tsujiura, M.; Ida, S.; et al. Prophylactic effect of neoadjuvant chemotherapy in gastric cancer patients with postoperative complications. Gastric Cancer 2018, 21, 703–709. [Google Scholar] [CrossRef] [PubMed]
| Author, Country, Year | Period | No. Patients | Age | M/F | BMI | Tumor Location | NA/A | Histology | pS 0-I | pS IIa | pS IIb | pSIII | pSIV | N0 | N1 | N2 | N3 | ASA | Surgical Procedure (DG-TG) | Lymphadenectomy (D1-D1+-D2) | Approach (Op-H- TMI) | Operating Time (In Min) | AL | HLOS | 30d Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sierzega, Poland, 2010 [15] | 1999–2004 | 690 | 63.7 (26–84) | 458/232 | nr | 290U/103M 148L/149WS | 0–196 | nr | nr | nr | nr | nr | nr | 202 | 185 | 128 | 175 | nr | 0/690 | 123/332/235 | 690/0/0 | 300 < 240 m; 390 ≥ 240 m | 41 | 13/31 | 27 |
| Yoo, South Korea, 2011 [23] | 2000–2005 | 478 | 58 ± 10 | 326/152 | nr | 250UM/228L | Nr–363 | 172D/306UN | nr | nr | nr | nr | nr | 177 | 173 | 70 | 58 | nr | 295/183 | 37/426/15 | 478/0/0 | nr | 32 | nr | nr |
| Nagasako, Japan, 2012 [24] | 1997–2008 | 400 | 63.4 (32–92) | 278/122 | 22.2 (17.1–30.8) | 48U/216M/107L/29ML | 0–0 | 252D/148UN | nr | nr | nr | nr | nr | 368 | nr | nr | nr | nr | 296/33 (44P/27PP | 166/168/66 (D1/D1+/D2) | 0/400/0 | 303.3 (152–865) | 14 | nr | nr |
| Kim, South Korea, 2015 [25] | 2003–2012 | 3827 | 1722 < 60/ 2105 ≥ 60 | 2602/1225 | 2683 < 25 1045 ≥ 25 3 | 733U/1013M/ 1935L/81WS | nr | nr | 2249 | 579 | 875 | 113 | 2298 | 482 | 369 | 671 | 3476 < 3 351 ≥ 3 | 2913/752 (162P) | nr | 1460/2367/0 | 3616 ≤ 300 m; 211 > 300 m | 72 | 15.8WL | 0 | |
| Barchi, Brazil, 2019 [26] | 2009–2017 | 258 | 62.3 (25–94) | 180/78 | 23.8 | 170ML | 44–123 | 107D/151UN | 113 | 145 | 91 | 167 | 202 < 3/56 ≥ 3 | 0/208 (50CG) | 90/168 (D0-D1/D2) | 244/0/14 | nr | 15 | 11.6/36.7 | 11 | |||||
| Nagata, Japan, 2022 [27] | 2012–2018 | 197 | 73.2 ± 10.2 | 135/62 | nr | 29U/82M/86L | 0–nr | 9D/75 SRC | nr | nr | nr | nr | nr | 103 | 34 | 31 | 29 | nr | 136/61 | nr | 105/92/0 | 356.5 | 9 | nr | 0 |
| Mittelstadt, Germany, 2022 [28] | 2000–2018 | 356 | 65 ± 12 | 254/102 | 26.0 ± 4.3 | nr | 183–13 | nr | 88 | 75 | 92 | 80 | nr | nr | nr | nr | 15/211/125/5 | 0/100 | nr | 356/0/0 | nr | 22 | nr | nr | |
| D’Souza, New Zeland, 2023 [29] | 2014–2022 | 77 | 65 (54–76) | 47/30 | nr | 39ML/25 U | 43–382 | 4 DF/2 I/4CR | 31 | 23 | 22 | 1 | 47 | 30 | 8/29/36/4 | 25/52 | 22/42/0 | 70/7/0 | nr | 6 | 8 | 1% | |||
| He, China, 2023 [12] | 2014–2021 | 3926 | 1394 < 60/ 2532 ≥ 60 | 2845/1081 | 789 <25/1031 ≥25 AND <30/106 ≥3 | 1146U/1127M 1456L/197O | 184–nr | nr | 1450 | 897 | 1532 | 47 | 1859 | 552 | 557 | 958 | 3335 < 3 591 ≥ 3 | 1967/1818 (141P) | nr | 1493/2433/0 | 2095 < 180 m; 1831 ≥ 180 m | 80 | nr | nr | |
| Ishida, Japan, 2023 [30] | 1997–2018 | 1653 | 1288 < 75/ 365 ≥ 75 | 1113/540 | nr | nr | nr | nr | nr | nr | nr | nr | nr | 1191 | 461 | nr | nr | nr | 1069/571/13 | nr | 47 | nr | nr | ||
| Time Horizon | No. Trials | RMSTD (mos) | SE | 95% CI | p-Value |
|---|---|---|---|---|---|
| 12 months | 10 | −1.1 | 0.3 | −1.6, −0.5 | 0.00008 |
| 24 months | 10 | −3.1 | 0.8 | −4.7, −1.5 | 0.0002 |
| 36 months | 9 | −5.2 | 1.3 | −7.8, −2.6 | 0.00008 |
| 48 months | 9 | −8.1 | 1.9 | −11.8, −4.4 | 0.00001 |
| 60 months | 8 | −10.6 | 2.5 | −15.4, −5.7 | 0.00001 |
| Time Horizon | HR (95% CI) |
|---|---|
| 12 months | 1.32 (1.11–1.58) |
| 24 months | 1.61 (1.34–1.92) |
| 36 months | 1.55 (1.27–1.91) |
| 48 months | 1.22 (1.02–1.53) |
| 60 months | 0.79 (0.59–1.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calì, M.; Bona, D.; De Bernardi, S.; Kim, Y.M.; Li, P.; Aljohani, E.; Bonavina, G.; Bonitta, G.; Wang, Q.; Biondi, A.; et al. Impact of Anastomotic Leak on Long-Term Survival After Gastrectomy: Results from an Individual Patient Data Meta-Analysis. Cancers 2025, 17, 2471. https://doi.org/10.3390/cancers17152471
Calì M, Bona D, De Bernardi S, Kim YM, Li P, Aljohani E, Bonavina G, Bonitta G, Wang Q, Biondi A, et al. Impact of Anastomotic Leak on Long-Term Survival After Gastrectomy: Results from an Individual Patient Data Meta-Analysis. Cancers. 2025; 17(15):2471. https://doi.org/10.3390/cancers17152471
Chicago/Turabian StyleCalì, Matteo, Davide Bona, Sara De Bernardi, Yoo Min Kim, Ping Li, Emad Aljohani, Giulia Bonavina, Gianluca Bonitta, Quan Wang, Antonio Biondi, and et al. 2025. "Impact of Anastomotic Leak on Long-Term Survival After Gastrectomy: Results from an Individual Patient Data Meta-Analysis" Cancers 17, no. 15: 2471. https://doi.org/10.3390/cancers17152471
APA StyleCalì, M., Bona, D., De Bernardi, S., Kim, Y. M., Li, P., Aljohani, E., Bonavina, G., Bonitta, G., Wang, Q., Biondi, A., Bonavina, L., & Aiolfi, A. (2025). Impact of Anastomotic Leak on Long-Term Survival After Gastrectomy: Results from an Individual Patient Data Meta-Analysis. Cancers, 17(15), 2471. https://doi.org/10.3390/cancers17152471










