Neo-Adjuvant Chemotherapy in Gastric Adenocarcinoma: Impact on Surgical and Oncological Outcomes in a Western Referral Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Outcomes
2.2. Statistical Analysis
3. Results
3.1. Comparative Analysis of Clinico-Demographic and Perioperative Outcomes Between Upfront Surgery and NACT Patients
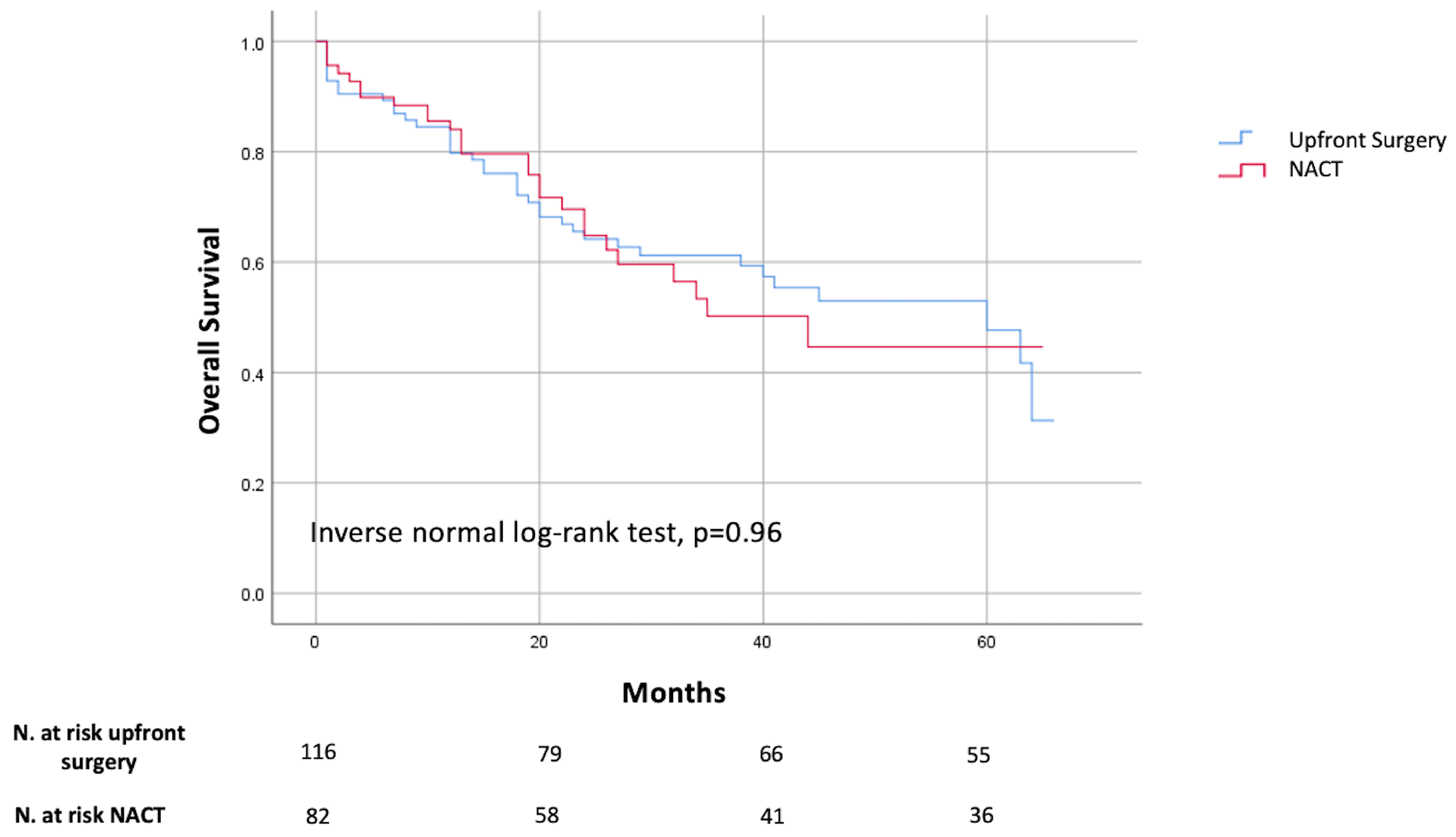
3.2. Long Terms Outcomes
3.3. Comparison Between FLOT Group and Other Regimes in Patients Who Underwent NACT
3.4. Multivariate Analysis for Perioperative Mortality, Severe Complications, and Re-Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | Gastric Cancer |
| NACT | Neo-adjuvant chemotherapy |
| ASA | American Society of Anesthesiologists Physical Status Classification System |
| OS | Overall survival |
| TG | Total gastrectomy |
| STG | Subtotal gastrectomy |
| DS | Disease-free survival |
| QR | Quartile rank |
| OR | Odds Ratio |
| C.I. | Confidence Interval |
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020-40: A Population-Based Modelling Study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Iwu-Jaja, C.J. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene 2023, 3, 256–268. [Google Scholar] [CrossRef]
- Rosa, F.; Alfieri, S.; Tortorelli, A.; Fiorillo, C.; Costamagna, G.; Doglietto, G. Trends in Clinical Features, Postoperative Outcomes, and Long-Term Survival for Gastric Cancer: A Western Experience with 1,278 Patients over 30 Years. World J. Surg. Oncol. 2014, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Gastric Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Nicole McMillian, N.R.; MaryElizabeth Stein, M.; Ajani, J.A.; D’Amico, T.A.; Chair, V.; Bentrem, D.J.; Gibson, M.K.; Grierson, P.; Gupta, G.; Hofstetter, W.L.; et al. NCCN Guidelines Version 2.2025 Gastric Cancer Continue NCCN Guidelines Panel Disclosures; NCCN: Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Guida, L. Neoplasie Dello Stomaco E Della Giunzione Esofago-Gastrica Edizione. AIOM 2021, 51–58. Available online: https://www.iss.it/documents/20126/8403839/LG-177_Stomaco_AIOM_agg2021 (accessed on 20 July 2025).
- De Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) Guidelines for Gastric Cancer Staging and Treatment: 2015. Gastric Cancer 2017, 20, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Nardi, M.; Montori, G.; Ceresoli, M.; Celotti, A.; Cascinu, S.; Fugazzola, P.; Tomasoni, M.; Glehen, O.; Catena, F.; et al. Neoadjuvant Chemotherapy in Advanced Gastric and Esophago-Gastric Cancer. Meta-Analysis of Randomized Trials. Int. J. Surg. 2018, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Laterza, V.; Schena, C.A.; Tondolo, V.; Strippoli, A.; Covino, M.; Pacini, G.; Quero, G.; Fiorillo, C.; De Sio, D.; et al. Surgery for Locally Advanced Gastric Cancer in the Era of Neoadjuvant Therapies: Something New? Minerva Surg. 2023, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.L.K.; Ying, A.H.S.; Wen, S.N.H.; Yeo, S.C.; Tay, K.V. Systematic Review and Meta-Analysis of the Outcomes Following Neoadjuvant Therapy in Upfront Resectable Gastric Cancers Compared to Surgery Alone in Phase III Randomised Controlled Trials. J. Gastrointest. Surg. 2023, 27, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Wang, Z.-Z.; Fan, Y.-C.; Liu, M.-X.; Xu, K.; Zhang, N.; Yao, Z.-D.; Yang, H.; Zhang, C.-H.; Xing, J.-D.; et al. Comparison of Neoadjuvant Chemotherapy Followed by Surgery vs. Surgery Alone for Locally Advanced Gastric Cancer: A Meta-Analysis. Chin. Med. J. 2021, 134, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Sivacoumarane, S.; Dutta, S.; Dubashi, B.; Adithan, S.; Toi, P.C.; Nelamangala Ramakrishnaiah, V.P. Role of Neoadjuvant Chemotherapy in Locally Advanced Carcinoma Stomach: An Analysis of the Short-Term Outcomes. Cureus 2022, 14, e23936. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hosoda, K.; Niihara, M.; Hiki, N. History and Emerging Trends in Chemotherapy for Gastric Cancer. Ann. Gastroenterol. Surg. 2021, 5, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.; Sano, T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer Using the National Cancer Database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Quero, G.; Laterza, V.; Mascagni, P.; Longo, F.; Menghi, R.; Razionale, F.; Rosa, F.; Mezza, T.; Boskoski, I.; et al. Postoperative Hyperglycemia Affects Survival after Gastrectomy for Cancer: A Single-Center Analysis Using Propensity Score Matching. Surgery 2020, 167, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Quero, G.; Fiorillo, C.; Longo, F.; Laterza, V.; Rosa, F.; Cina, C.; Menghi, R.; Tortorelli, A.P.; Barbaro, F.; Pecere, S.; et al. Propensity Score-Matched Comparison of Short- and Long-Term Outcomes between Surgery and Endoscopic Submucosal Dissection (ESD) for Intestinal Type Early Gastric Cancer (EGC) of the Middle and Lower Third of the Stomach: A European Tertiary Referral Center Experience. Surg. Endosc. 2021, 35, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Lirosi, M.C.; D’Ugo, D.; Fico, V.; Ricci, R.; Santullo, F.; Rizzuto, A.; Cananzi, F.C.; Persiani, R. Neo-Adjuvant Chemo(Radio)Therapy in Gastric Cancer: Current Status and Future Perspectives. World J. Gastrointest. Oncol. 2015, 7, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Schena, C.A.; Laterza, V.; Quero, G.; Fiorillo, C.; Strippoli, A.; Pozzo, C.; Papa, V.; Alfieri, S. The Role of Surgery in the Management of Gastric Cancer: State of the Art. Cancers 2022, 14, 5542. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. New Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological Regression after Neoadjuvant Docetaxel, Oxaliplatin, Fluorouracil, and Leucovorin versus Epirubicin, Cisplatin, and Fluorouracil or Capecitabine in Patients with Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4-AIO): Results from the Phase 2 Part of a Multicentre, Open-Label, Randomised Phase 2/3 Trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of Comorbidity on Chemotherapy Use and Outcomes in Solid Tumors: A Systematic Review. J. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; López-Fernánde, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klei, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on Cardio-Oncology Developed in Collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting Chemotherapy Toxicity in Older Adults with Cancer: A Prospective Multicenter Study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.B.; Straker, R.J.; Keele, L.; Fraker, D.L.; Roses, R.E.; Miura, J.T.; Karakousis, G.C. Lymph Node Evaluation after Neoadjuvant Chemotherapy for Patients with Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Van Den Ende, T.; Ter Veer, E.; Machiels, M.; Mali, R.M.A.; Abe Nijenhuis, F.A.; De Waal, L.; Laarman, M.; Gisbertz, S.S.; Hulshof, M.C.C.M.; Van Oijen, M.G.H.; et al. The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-Analysis. Cancers 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Putila, E.; Helminen, O.; Helmiö, M.; Huhta, H.; Jalkanen, A.; Kallio, R.; Koivukangas, V.; Kokkola, A.; Laine, S.; Lietzen, E.; et al. Postoperative Complications After Neoadjuvant Chemotherapy Versus Upfront Surgery in Gastric Adenocarcinoma: A Population-Based Nationwide Study in Finland. Ann. Surg. Oncol. 2024, 31, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ge, L.; Qin, Y.; Huang, M.; Chen, J.; Yang, Y.; Zhong, J. Postoperative Morbidity and Mortality after Neoadjuvant Chemotherapy versus Upfront Surgery for Locally Advanced Gastric Cancer: A Propensity Score Matching Analysis. Cancer Manag. Res. 2019, 11, 6011–6018. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Pauligk, C.; Homann, N.; Schmalenberg, H.; Jäger, E.; Al-Batran, S.-E. Feasibility of Perioperative Chemotherapy with Infusional 5-FU, Leucovorin, and Oxaliplatin with (FLOT) or without (FLO) Docetaxel in Elderly Patients with Locally Advanced Esophagogastric Cancer. Br. J. Cancer 2013, 108, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Shah, M.M.; Shah, A.R. Fluoropyrimidine-Associated Cardiotoxicity: Revisited. Expert. Opin. Drug Saf. 2009, 8, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.J. Fluorouracil Cardiotoxicity. Ann. Pharmacother. 1994, 28, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, I.G.; Walker, G.R.; Ferry, K.; Franceschi, D.; Livingstone, A.S. Neoadjuvant Treatment for Resectable Cancer of the Esophagus and the Gastroesophageal Junction: A Meta-Analysis of Randomized Clinical Trials. Ann. Surg. Oncol. 2003, 10, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Neoadjuvant Chemotherapy for Advanced Gastric Cancer: A Meta-Analysis. World J. Gastroenterol. 2010, 16, 5621. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 254) | Upfront Surgery (n = 144) | NACT (n = 110) | p | |
|---|---|---|---|---|
| Clinico-demographic characteristics | ||||
| Sex, n (%) | ||||
| Male | 162 (63.8) | 84 (58.3) | 78 (70.9) | 0.04 |
| Female | 92 (36.2) | 60 (41.7) | 32 (29.1) | |
| Age, years, median (QR) | 69 (61–78) | 72 (65–81) | 65 (57–74) | 0.001 |
| Diabetes, n (%) | 38 (15) | 23 (16) | 15 (13.6) | 0.60 |
| Cardiovascular diseases, n (%) | 51 (20.1) | 37 (25.7) | 14 (12.7) | 0.01 |
| Respiratory diseases, n (%) | 21 (8.3) | 11 (7.6) | 10 (9.1) | 0.67 |
| Nefropathy, n (%) | 3 (1.2) | 3 (7.6) | 0 | 0.13 |
| Smoker, n (%) | ||||
| Current | 27 (10.6) | 13 (9) | 14 (12.7) | 0.05 |
| Ex | 67 (26.2) | 31 (21.5) | 36 (32.7) | |
| ASA ≥ 3, n (%) | 71 (28) | 56 (38.9) | 15 (13.6) | 0.001 |
| Charlson > 3, n (%) | 197 (77.6) | 125 (86.8) | 72 (65.5) | 0.001 |
| Tumor location, n (%) | ||||
| Distal | 101 (39.8) | 69 (47.9) | 32 (29.1) | 0.001 |
| Middle | 62 (24.4) | 38 (26.4) | 24 (21.8) | |
| Proximal | 91 (35.8) | 37 (25.7) | 54 (49.1) | |
| Perioperative outcomes | ||||
| Surgical procedure, n (%) | ||||
| Subtotal gastrectomy | 156 (61.4) | 100 (69.4) | 56 (50.9) | 0.003 |
| Total gastrectomy | 98 (38.6) | 44 (30.6) | 54 (49.1) | |
| HIPEC, n (%) | 26 (10.3) | 9 (6.3) | 17 (15.5) | 0.02 |
| Associated organs resection, n (%) | 42 (16.5) | 25 (17.4) | 17 (15.5) | 0.68 |
| Clavien–Dindo ≥ 3 complications, n (%) | 53 (20.9) | 30 (20.8) | 23 (20.9) | 0.99 |
| Anastomotic leakage, n (%) | 27 (10.6) | 13 (9) | 14 (12.7) | 0.34 |
| Reoperation, n (%) | 36 (14.2) | 24 (16.7) | 12 (10.9) | 0.19 |
| Post-operative mortality, n (%) | 16 (6.3) | 11 (7.6) | 5 (4.5) | 0.32 |
| LOS, days, median (QR) | 9 (7–15) | 9 (7–16) | 10 (8–22) | 0.26 |
| Study Population (n = 254) | Upfront Surgery (n = 144) | NACT (n = 110) | p | |
|---|---|---|---|---|
| T stage, n (%) | ||||
| T0 | 6 (2.4) | 3 (2.1) | 3 (2.8) | 0.07 |
| T1–2 | 77 (30.3) | 52 (36.1) | 25 (22.7) | |
| T3–4 | 171 (67.3) | 89 (61.8) | 82 (74.5) | |
| N stage, n (%) | ||||
| N0 | 88 (34.6) | 60 (42) | 28 (25.9) | 0.007 |
| N+ | 166 (65.4) | 84 (58) | 82 (74.1) | |
| Harvested lymph nodes, median (QR) | 25 (14–40) | 26 (10–43) | 24 (14–45) | 0.91 |
| Positive lymph nodes, median (QR) | 2 (0–10) | 1 (0–10) | 3 (0–12) | 0.01 |
| Histotype *, n(%) | ||||
| Intestinal | 91 (35.9) | 70 (56.9) | 21 (35.6) | 0.03 |
| Diffuse | 66 (25.9) | 39 (31.7) | 27 (45.8) | |
| Mixed | 25 (9.9) | 14 (11.4) | 11 (18.6) | |
| R status, n(%) | ||||
| 0 | 213 (83.9) | 123 (85.4) | 90 (81.8) | 0.63 |
| 1 | 29 (11.4) | 14 (9.7) | 15 (13.7) | |
| 2 | 12 (4.7) | 7 (4.9) | 5 (4.5) |
| Study Population (n = 110) | Non-FLOT (n = 36) | FLOT (n = 74) | p | |
|---|---|---|---|---|
| Clinico-demographic characteristics | ||||
| Sex, n (%) | ||||
| Male | 78 (70.9) | 26 (78.8) | 52 (68.4) | 0.39 |
| Female | 32 (29.1) | 8 (23.5) | 24 (31.6) | |
| Age, years, median (QR) | 65 (62–69) | 69 (65–73) | 62 (60–67) | 0.003 |
| Diabetes, n (%) | 15 (13.6) | 4 (11.8) | 11 (14.5) | 0.70 |
| Cardiovascular diseases, n (%) | 14 (12.7) | 7 (20.6) | 7 (9.2) | 0.1 |
| Respiratory diseases, n (%) | 10 (9.1) | 5 (14.7) | 58 (6.6) | 0.17 |
| Nefropathy, n (%) | 0 | |||
| Smoker, n (%) | ||||
| Current | 14 (12.7) | 3 (8.8) | 11 (14.5) | 0.22 |
| Ex | 36 (32.7) | 15 (44.1) | 21 (27.6) | |
| ASA ≥ 3, n (%) | 15 (13.6) | 9 (26.5) | 6 (7.9) | <0.01 |
| Charlson > 3, n (%) | 72 (65.5) | 26 (72.2) | 46 (62.2) | 0.29 |
| Tumor location, n (%) | ||||
| Distal | 32 (29.1) | 11 (30.6) | 21 (28.4) | 0.66 |
| Middle | 24 (21.8) | 6 (16.7) | 18 (24.3) | |
| Proximal | 54 (49.1) | 19 (52.8) | 35 (47.3) | |
| Perioperative outcomes | ||||
| Surgical procedure, n (%) | ||||
| Subtotal gastrectomy | 56 (50.9) | 23 (63.9) | 33 (44.6) | 0.003 |
| Total gastrectomy | 54 (49.1) | 13 (36.1) | 41 (55.4) | |
| HIPEC, n (%) | 17 (15.5) | 5 (11.8) | 12 (17.1) | 0.47 |
| Associated organs resection, n (%) | 17 (15.5) | 9 (25) | 8 (10.9) | 0.05 |
| Clavien–Dindo ≥ 3 complications, n (%) | 23 (20.9) | 11 (30.5) | 12 (16.2) | 0.05 |
| Anastomotic leakage, n (%) | 14 (12.7) | 7 (19.4) | 7 (9.6) | 0.14 |
| Reoperation, n (%) | 12 (10.9) | 8 (22.2) | 4 (5.4) | 0.008 |
| Post-operative mortality, n (%) | 5 (4.5) | 3 (8.3) | 2 (2.7) | 0.12 |
| LOS, days, median (QR) | 10 (8–22) | 12 (7–30) | 9 (7–17) | 0.18 |
| Study Population (n = 110) | Non-FLOT (n = 36) | FLOT (n = 74) | p | |
|---|---|---|---|---|
| T stage, n (%) | ||||
| T0 | 3 (2.7) | 2 (5.6) | 1 (1.4) | 0.40 |
| T1–2 | 25 (22.7) | 7 (19.4) | 18 (24.3) | |
| T3–4 | 82 (74.6) | 27 (75) | 55 (74.3) | |
| N stage, n (%) | ||||
| N0 | 28 (25.4) | 11 (30.6) | 17 (23) | 0.39 |
| N+ | 82 (74.6) | 25 (69.4) | 57 (77) | |
| Harvested lymph nodes, median (QR) | 24 (14–45) | 20 (9–53) | 27 (15–44) | 0.10 |
| Positive lymph nodes, median (QR) | 3 (0–12) | 1 (0–12) | 4 (0–13) | 0.10 |
| Histotype *, n(%) | ||||
| Intestinal | 21 (19.1) | 6 (40) | 15 (34.1) | 0.48 |
| Diffuse | 27 (24.5) | 5 (33.3) | 22 (50) | |
| Mixed | 11 (10) | 4 (26.7) | 7 (15.9) | |
| R status, n(%) | ||||
| 0 | 90 (81.8) | 28 (77.8) | 62 (83.8) | 0.41 |
| 1 | 15 (13.6) | 5 (13.9) | 10 (13.5) | |
| 2 | 5 (4.6) | 3 (8.3) | 2 (2.7) |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Study Population | Clavien < 3 | Clavien ≥ 3 | p | |
| Age ≥ 65 | 56 | 43 (76.8) | 13 (23.2) | 0.55 |
| Cardiovascular diseases, n (%) | 14 | 9 (64.3) | 5 (35.7) | 0.14 |
| ASA ≥ 3, n (%) | 15 | 9 (60) | 6 (40) | 0.05 |
| Charlson > 3, n (%) | 72 | 56 (77.8) | 16 (22.2) | 0.64 |
| Proximal tumor | 54 | 36 (66.7) | 18 (33.3) | 0.002 |
| Surgical procedure, n (%) | ||||
| Subtotal gastrectomy | 56 | 45 (80.4) | 11 (19.6) | 0.74 |
| Total gastrectomy | 54 | 42 (77.8) | 12 (22.2) | |
| HIPEC, n (%) | 17 | 16 (94.1) | 1 (5.9) | 0.09 |
| FLOT | 74 | 62 (83.8) | 12 (16.2) | 0.08 |
| Multivariate analysis | ||||
| Variables | OR | 95% C.I. | p | |
| FLOT | 0.5 | 0.18–1.39 | 0.18 | |
| ASA ≥ 3 | 1.80 | 0.50–6.44 | 0.36 | |
| Upper third location | 4.7 | 1.56–14.18 | 0.006 | |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Study Population | No-Reoperation | Reoperation | p | |
| Age ≥ 65 | 56 | 52 (92.8) | 4 (7.4) | 0.25 |
| Cardiovascular diseases, n (%) | 14 | 11 (78.6) | 3 (21.4) | 0.18 |
| ASA ≥ 3, n (%) | 15 | 11 (73.3) | 4 (26.7) | 0.03 |
| Charlson > 3, n (%) | 72 | 64 (88.9) | 8 (11.1) | 0.92 |
| Proximal tumor | 54 | 44 (81.5) | 10 (18.5) | 0.01 |
| Surgical procedure, n (%) | ||||
| Subtotal gastrectomy | 56 | 51 (91.1) | 5 (8.9) | 0.49 |
| Total gastrectomy | 54 | 47 (87) | 7 (13) | |
| HIPEC, n (%) | 17 | 16 (94.1) | 1 (5.9) | 0.47 |
| FLOT | 74 | 70 (94.6) | 4 (5.4) | 0.008 |
| Multivariate analysis | ||||
| Variables | O.R. | 95% C.I. | p | |
| FLOT | 0.22 | 0.06–0.86 | 0.003 | |
| ASA ≥ 3 | 1.85 | 0.41–8.35 | 0.43 | |
| Upper third location | 5.59 | 1.1–28.45 | 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, C.; Biffoni, B.; Di Cesare, L.; Rosa, F.; Alfieri, S.; Langellotti, L.; Menghi, R.; Tondolo, V.; Quero, G. Neo-Adjuvant Chemotherapy in Gastric Adenocarcinoma: Impact on Surgical and Oncological Outcomes in a Western Referral Center. Cancers 2025, 17, 2465. https://doi.org/10.3390/cancers17152465
Fiorillo C, Biffoni B, Di Cesare L, Rosa F, Alfieri S, Langellotti L, Menghi R, Tondolo V, Quero G. Neo-Adjuvant Chemotherapy in Gastric Adenocarcinoma: Impact on Surgical and Oncological Outcomes in a Western Referral Center. Cancers. 2025; 17(15):2465. https://doi.org/10.3390/cancers17152465
Chicago/Turabian StyleFiorillo, Claudio, Beatrice Biffoni, Ludovica Di Cesare, Fausto Rosa, Sergio Alfieri, Lodovica Langellotti, Roberta Menghi, Vincenzo Tondolo, and Giuseppe Quero. 2025. "Neo-Adjuvant Chemotherapy in Gastric Adenocarcinoma: Impact on Surgical and Oncological Outcomes in a Western Referral Center" Cancers 17, no. 15: 2465. https://doi.org/10.3390/cancers17152465
APA StyleFiorillo, C., Biffoni, B., Di Cesare, L., Rosa, F., Alfieri, S., Langellotti, L., Menghi, R., Tondolo, V., & Quero, G. (2025). Neo-Adjuvant Chemotherapy in Gastric Adenocarcinoma: Impact on Surgical and Oncological Outcomes in a Western Referral Center. Cancers, 17(15), 2465. https://doi.org/10.3390/cancers17152465







