Bioinformatics Screening of Tumor-Derived Neuropeptides Mediating Neuroimmune Axis of Head and Neck Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Acquisition of Neuropeptide Dataset
2.2. Differential Gene Expression Analysis
2.3. Gene Pair Correlation and Immune Cell Infiltration Analysis
2.4. Immunotherapy Outcome
2.5. Survival Analysis
3. Results
3.1. More Than Half of Neuropeptide Genes Expressed in HNSCC
3.2. PTHLH, NMB, GAST, LYNX1, and AGT Neuropeptides Linked to Immune Cell Infiltration in HNSC
3.3. PTHLH, SCG5, APLN, and UCN2 Correlated with Immune Checkpoint Genes
3.4. Differentially Expressed Neuropeptides Correlated with Key Neurotrophic Factors (ARTN, TGFB1, SEMA4F) in HNSC
3.5. LYNX1, UCN2, AGT, SCG5, and GAST Correlated with HNSC Survival
3.6. Stage Plot Analysis Revealed Consistent Expression of Neuropeptide Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Zhang, Y.; Gibbons, D.L.; Deneen, B.; Kwiatkowski, D.J.; Ittmann, M.; Creighton, C.J. Pan-Cancer Molecular Classes Transcending Tumor Lineage Across 32 Cancer Types, Multiple Data Platforms, and over 10,000 Cases. Clin. Cancer Res. 2018, 24, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Schmitd, L.B.; Scanlon, C.S.; D’Silva, N.J. Perineural Invasion in Head and Neck Cancer. J. Dent. Res. 2018, 97, 742–750. [Google Scholar] [CrossRef]
- Lee, T.L.; Chiu, P.H.; Li, W.Y.; Yang, M.H.; Wei, P.Y.; Chu, P.Y.; Wang, Y.F.; Tai, S.K. Nerve-Tumour Interaction Enhances the Aggressiveness of Oral Squamous Cell Carcinoma. Clin. Otolaryngol. 2019, 44, 1087–1095. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the Central Nervous System Drive Neurogenesis in Cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of P53 Drives Neuron Reprogramming in Head and Neck Cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Bhandage, A.K.; Jin, Z.; Korol, S.V.; Shen, Q.; Pei, Y.; Deng, Q.; Espes, D.; Carlsson, P.O.; Kamali-Moghaddam, M.; Birnir, B. GABA Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and CD4(+) T Cells and Is Immunosuppressive in Type 1 Diabetes. eBioMedicine 2018, 30, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Case, A.J.; Zimmerman, M.C. Sympathetic-Mediated Activation Versus Suppression of the Immune System: Consequences for Hypertension. J. Physiol. 2016, 594, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Cutz, E.; Chan, W.; Track, N.S.; Goth, A.; Said, S.I. Release of Vasoactive Intestinal Polypeptide in Mast Cells by Histamine Liberators. Nature 1978, 275, 661–662. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Regulation of Immune Tolerance by Anti-Inflammatory Neuropeptides. Nat. Rev. Immunol. 2007, 7, 52–63. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Delgado, M. Anti-inflammatory neuropeptide receptors: New therapeutic targets for immune disorders? Trends Pharmacol. Sci. 2007, 28, 482–491. [Google Scholar] [CrossRef]
- Rozengurt, E. Autocrine Loops, Signal Transduction, and Cell Cycle Abnormalities in the Molecular Biology of Lung Cancer. Curr. Opin. Oncol. 1999, 11, 116–122. [Google Scholar] [CrossRef]
- Wu, Y.; Berisha, A.; Borniger, J.C. Neuropeptides in Cancer: Friend and Foe? Adv. Biol. 2022, 6, e2200111. [Google Scholar] [CrossRef]
- Souza-Moreira, L.; Campos-Salinas, J.; Caro, M.; Gonzalez-Rey, E. Neuropeptides as Pleiotropic Modulators of the Immune Response. Neuroendocrinology 2011, 94, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.P.; Arleth, A.J.; Feuerstein, G.Z. Neuropeptide Y Upregulates the Adhesiveness of Human Endothelial Cells for Leukocytes. Circ. Res. 1991, 68, 314–318. [Google Scholar] [CrossRef]
- Chen, W.C.; Liu, Y.B.; Liu, W.F.; Zhou, Y.Y.; He, H.F.; Lin, S. Neuropeptide Y Is an Immunomodulatory Factor: Direct and Indirect. Front. Immunol. 2020, 11, 580378. [Google Scholar] [CrossRef]
- Bull, H.A.; Hothersall, J.; Chowdhury, N.; Cohen, J.; Dowd, P.M. Neuropeptides Induce Release of Nitric Oxide from Human Dermal Microvascular Endothelial Cells. J. Investig. Dermatol. 1996, 106, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Bakos, J.; Zatkova, M.; Bacova, Z.; Ostatnikova, D. The Role of Hypothalamic Neuropeptides in Neurogenesis and Neuritogenesis. Neural Plast. 2016, 2016, 3276383. [Google Scholar] [CrossRef] [PubMed]
- Zaben, M.J.; Gray, W.P. Neuropeptides and Hippocampal Neurogenesis. Neuropeptides 2013, 47, 431–438. [Google Scholar] [CrossRef]
- Kim, Y.; Bark, S.; Hook, V.; Bandeira, N. Neuropedia: Neuropeptide Database and Spectral Library. Bioinformatics 2011, 27, 2772–2773. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Yin, S.; Jang, R.; Wang, J.; Xue, Z.; Xu, T. Neuropep: A Comprehensive Resource of Neuropeptides. Database 2015, 2015, bav038. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Miao, Y.R.; Xie, G.Y.; Luo, M.; Hu, H.; Kwok, H.F.; Feng, J.; Guo, A.Y. ICBatlas: A Comprehensive Resource for Depicting Immune Checkpoint Blockade Therapy Characteristics from Transcriptome Profiles. Cancer Immunol. Res. 2022, 10, 1398–1406. [Google Scholar] [CrossRef]
- Kim, J.; He, M.J.; Widmann, A.K.; Lee, F.S. The Role of Neurotrophic Factors in Novel, Rapid Psychiatric Treatments. Neuropsychopharmacology 2024, 49, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Tilan, J.; Kitlinska, J. Neuropeptide Y (NPY) in Tumor Growth and Progression: Lessons Learned From Pediatric Oncology. Neuropeptides 2016, 55, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Katzmann Rider, G.; Gajra, S.; Narra, V.; Ramavajla, V.; Chang, Y.J.; Tao, Y.; Soteropoulos, P.; Husain, S.; Khan, J.; et al. Differential Gene Expression Changes in the Dorsal Root Versus Trigeminal Ganglia Following Peripheral Nerve Injury Rats. Eur. J. Pain 2020, 24, 967–982. [Google Scholar] [CrossRef]
- Zuo, S.; Wei, M.; Wang, S.; Dong, J.; Wei, J. Pan-Cancer Analysis of Immune Cell Infiltration Identifies a Prognostic Immune-Cell Characteristic Score (ICCS) in Lung Adenocarcinoma. Front. Immunol. 2020, 11, 1218. [Google Scholar] [CrossRef]
- Yuen, G.J.; Demissie, E.; Pillai, S.B. Lymphocytes And Cancer: A Love-Hate Relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells Across Human Cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Kuske, M.; Haist, M.; Jung, T.; Grabbe, S.; Bros, M. Immunomodulatory Properties of Immune Checkpoint Inhibitors—More than Boosting T-Cell Responses? Cancers 2022, 14, 1710. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; England, C.G.; McNeel, D.G.; Cai, W. Molecular Imaging of Immunotherapy Targets in Cancer. J. Nucl. Med. 2016, 57, 1487–1492. [Google Scholar] [CrossRef]
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic Targets and Biomarkers of Tumor Immunotherapy: Response Versus Non-Response. Signal Transduct Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef]
- Yang, W.F.; Wong, M.C.M.; Thomson, P.J.; Li, K.Y.; Su, Y.X. The Prognostic Role of PD-L1 Expression for Survival in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef]
- Zhou, W.T.; Jin, W.L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Hoover, G.; Gilbert, S.; Curley, O.; Obellianne, C.; Lin, M.T.; Hixson, W.; Pierce, T.W.; Andrews, J.F.; Alexeyev, M.F.; Ding, Y.; et al. Nerve-To-Cancer Transfer of Mitochondria During Cancer Metastasis. Nature 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baloh, R.H.; Tansey, M.G.; Lampe, P.A.; Fahrner, T.J.; Enomoto, H.; Simburger, K.S.; Leitner, M.L.; Araki, T.; Johnson, E.M., Jr.; Milbrandt, J. Artemin, a Novel Member of the GDNF Ligand Family, Supports Peripheral and Central Neurons and Signals Through the Gfralpha3-RET Receptor Complex. Neuron 1998, 21, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.A.; Kessler, J.A. TGF-beta Family Signaling in Neural and Neuronal Differentiation, Development, and Function. Cold Spring Harb. Perspect. Biol. 2017, 17, 9. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-Related Axonogenesis And Neurogenesis In Prostate Cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar] [CrossRef]
- Szpunar, M.J.; Belcher, E.K.; Dawes, R.P.; Madden, K.S. Sympathetic Innervation, Norepinephrine Content, and Norepinephrine Turnover in Orthotopic and Spontaneous Models of Breast Cancer. Brain Behav. Immun. 2016, 53, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-M.; Lin, Y.-F.; Su, C.-Y.; Peng, H.-Y.; Chang, Y.-C.; Hsiao, J.-R.; Chen, C.-L.; Chang, J.-Y.; Shieh, Y.-S.; Hsiao, M. Parathyroid Hormone-Like Hormone Is a Poor Prognosis Marker of Head and Neck Cancer and Promotes Cell Growth via RUNX2 Regulation. Sci. Rep. 2017, 7, 41131. [Google Scholar] [CrossRef]
- Almadori, G.; Coli, A.; De Corso, E.; Mele, D.A.; Settimi, S.; Di Cintio, G.; Brigato, F.; Scannone, D.; Lauriola, L.; Ranelletti, F.O. Parathyroid Hormone-Related Peptide and Parathyroid Hormone-Related Peptide Receptor Type 1 in Locally Advanced Laryngeal Cancer as Prognostic Indicators of Relapse And Survival. BMC Cancer 2022, 22, 704. [Google Scholar] [CrossRef]
- Pitarresi, J.R.; Norgard, R.J.; Chiarella, A.M.; Suzuki, K.; Bakir, B.; Sahu, V.; Li, J.; Zhao, J.; Marchand, B.; Wengyn, M.D. Pthrp Drives Pancreatic Cancer Growth and Metastasis and Reveals a New Therapeutic Vulnerability. Cancer Discov. 2021, 11, 1774–1791. [Google Scholar] [CrossRef]
- Chilug, L.E.; Niculae, D.; Leonte, R.A.; Nan, A.; Turcu, R.; Mustaciosu, C.; Serban, R.M.; Lavric, V.; Manda, G. Preclinical Evaluation of NHS-Activated Gold Nanoparticles Functionalized with Bombesin or Neurotensin-like Peptides for Targeting Colon and Prostate Tumours. Molecules 2020, 25, 3363. [Google Scholar] [CrossRef]
- Duan, S.; Rico, K.; Merchant, J.L. Gastrin: From Physiology to Gastrointestinal Malignancies. Function 2022, 3, zqab062. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Perrault, E.N.; Birbrair, A.; Mattson, B.J.; Gutmann, D.H.; Mabbott, D.J.; Cukierman, E.; Repasky, E.A.; Sloan, E.K.; Zong, H. Integrating Priorities at the Intersection of Cancer and Neuroscience. Cancer Cell 2025, 43, 1–5. [Google Scholar] [CrossRef] [PubMed]
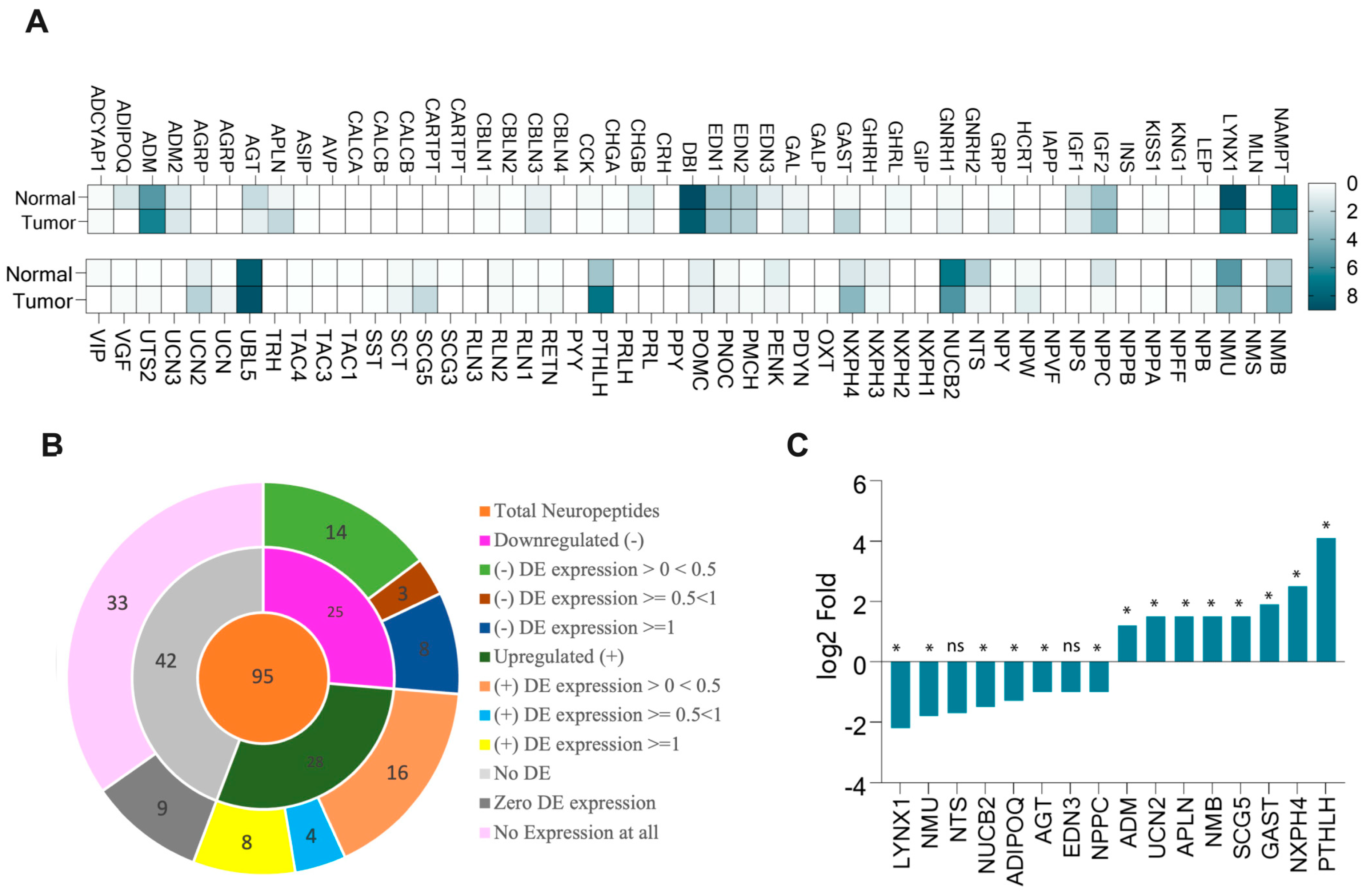


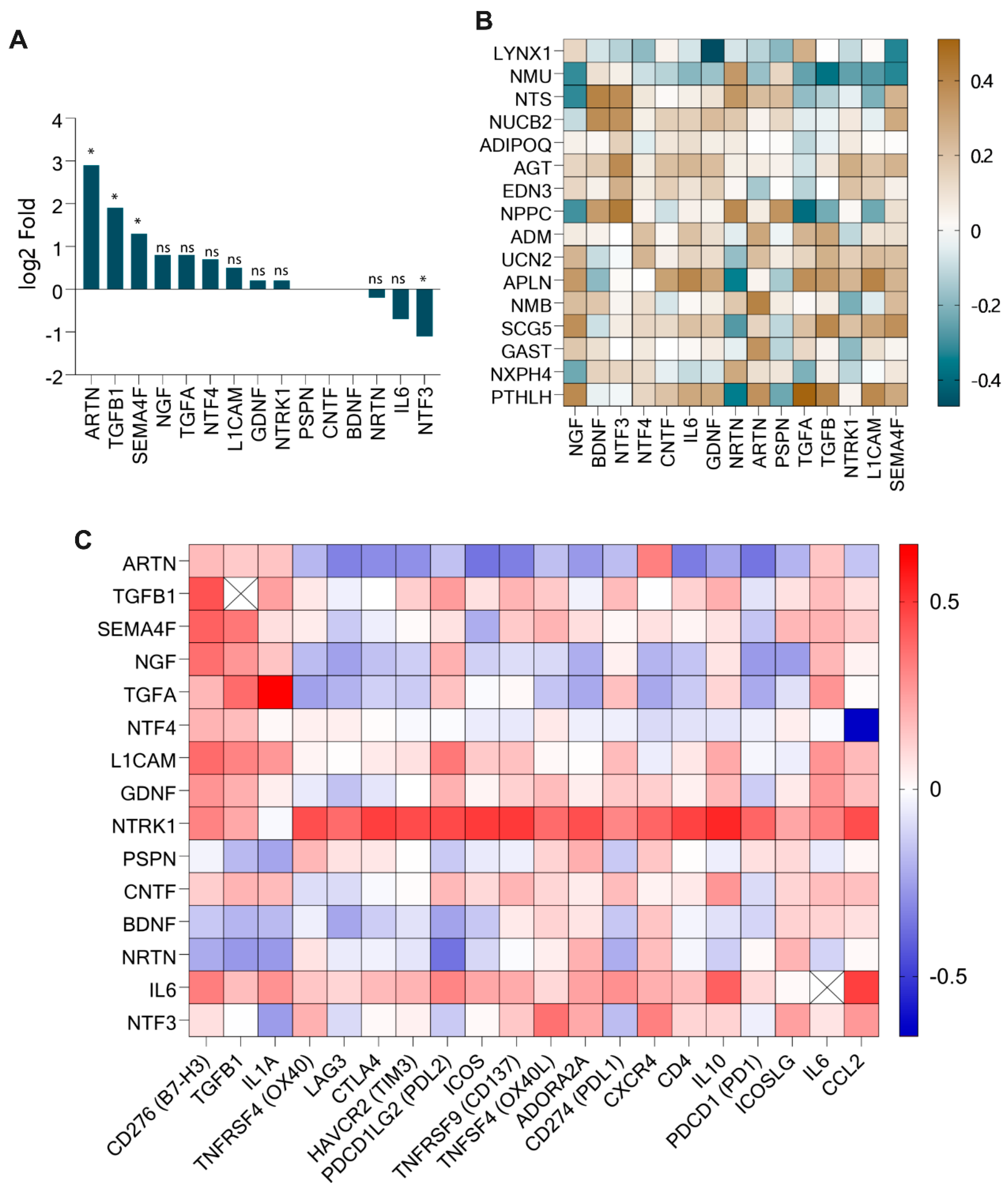
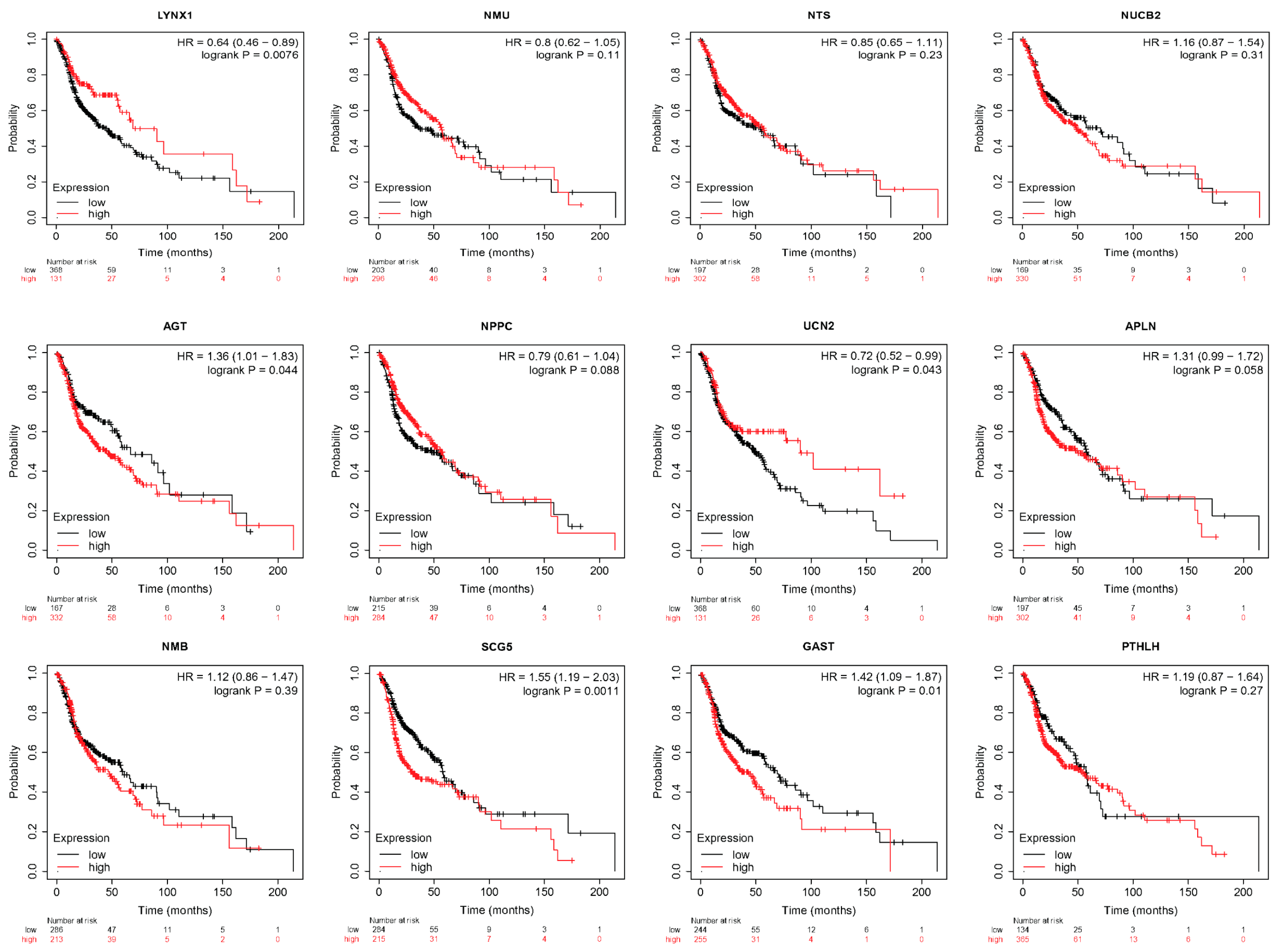
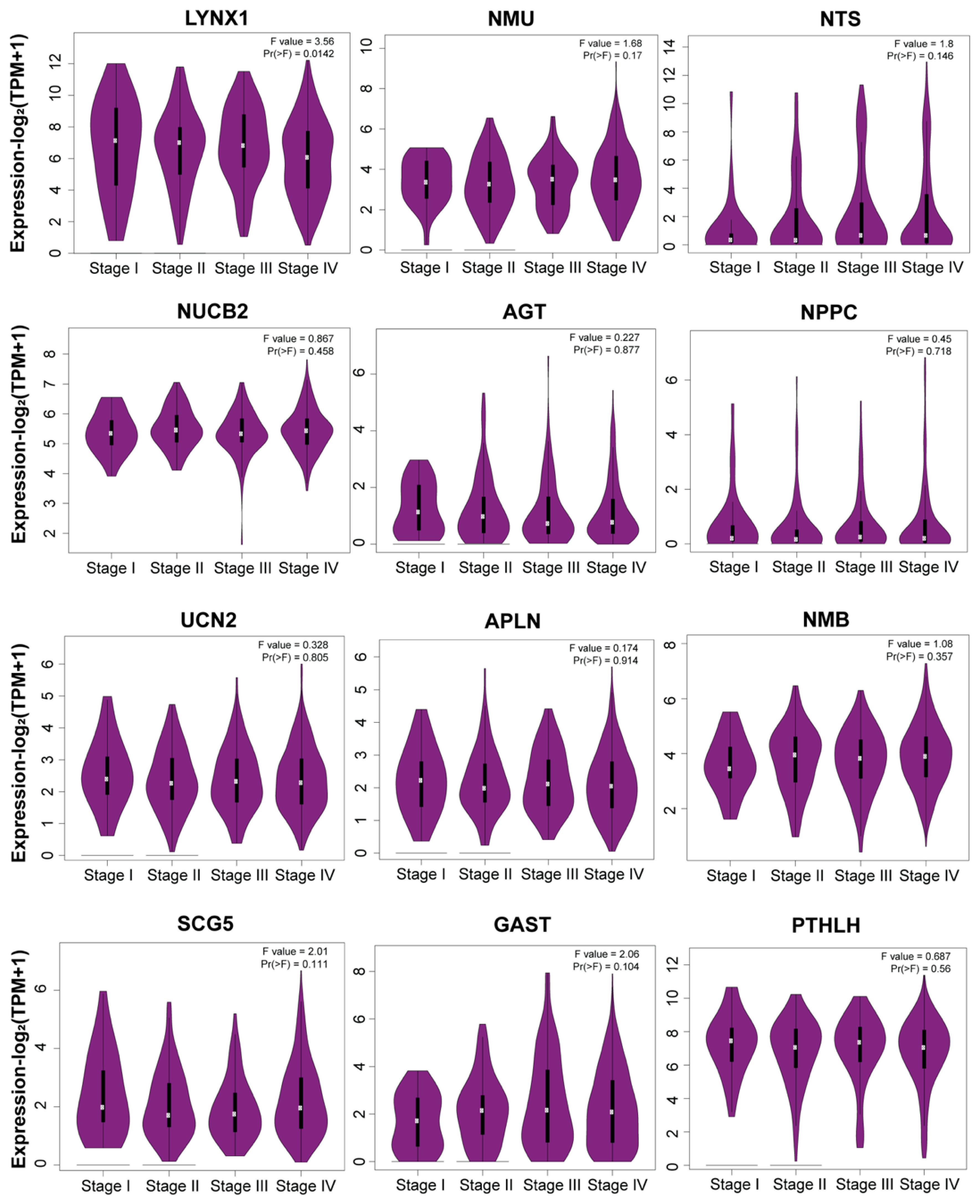
| Neuropeptide Genes | Upregulated Checkpoint Gene (Correlation Value) | Downregulated Immune Checkpoint Gene (Correlation Value) | |
|---|---|---|---|
| Upregulated | PTHLH | CD276/B7H3 (0.322), TGFB1 (0.407), IL1A (0.44), ADORA21 (−0.331), PD1 (−0.345) | |
| GAST | CTLA4 (−0.304), TIM3 (−0.305), ICOS (−0.304), CD137 (−0.349), ADORA2A (−0.349), CXCR4 (−0.324), CD4 (−0.354), PD1 (−0.342) | ||
| SCG5 | B7H3 (0.528), TGFB1 (0.390) | ||
| NMB | LAG3 (−0.396), CTLA4 (−0.397), TIM3 (−0.378), PDL2 (−0.392), ICOS (−0.449), CD137 (−0.341), PDL1 (−0.372), CD4 (−0.365), PD1 (−0.403) | ||
| APLN | B7H3 (0.466), TGFB1 (0.337), IL1A (0.368), PDL2 (0.318) | IL6 (0.396) | |
| UCN2 | B7H3 (0.323) | ||
| Downregulated | NPPC | OX40 (0.327), ADORA2A (0.398), CXCR4 (0.425) IL1A (−0.317) | ICOSLG (0.413) |
| AGT | OX40L (0.387), ADORA2A (0.325), CXCR4 (0.321), | CCL2 (0.4240) | |
| NUCB2 | CXCR4 (0.309), ADORA2A (0.3) | ||
| NMU | B7H3 (−0.364), TGFB1 (−0.375), PDL2 (−0.3) | ||
| LYNX1 | B7H3 (−0.319), OX40L (−0.319) | ||
| Neuropeptide Genes | Upregulated Neurotrophic Genes (Correlation Value) | Non-Differentially Expressed Neurotrophic Genes (Correlation Value) | Downregulated Neurotrophic Genes (Correlation Value) | |
|---|---|---|---|---|
| Upregulated | PTHLH | ARTN (0.362), NGF (0.388), TGFB (0.407), TGFA (0.510), L1CAM (0.390) | NRTN (−0.348) | |
| GAST | ARTN (0.356) | |||
| SCG5 | TGFB (0.390), NGF (0.370), SEMA4F (0.366), L1CAM (0.3) | |||
| NMB | ARTN (0.410) | |||
| APLN | TGFB (0.337), NGF (0.338), TGFA (0.370), L1CAM (0.405) | NRTN (−0.396) | ||
| Downregulated | NPPC | TGFA (−0.388) | PSPN (0.353), BDNF (0.331) | NRTN (0.387), NTF3 (0.434) |
| AGT | NTF3 (0.382) | |||
| NUCB2 | BDNF (0.377) | NTF3 (0.361) | ||
| NTS | NGF (−0.316) | BDNF (0.411) | NRTN (0.344), NTF3 (0.38) | |
| NMU | TGFB (−0.375), SEMA4F (−0.323), NGF (−0.310) | NRTN (0.339) | ||
| LYNX1 | SEMA4F (−0.327), GDNF (−0.470) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishan, R.; Zhang, G.; Yang, W.; Su, Y. Bioinformatics Screening of Tumor-Derived Neuropeptides Mediating Neuroimmune Axis of Head and Neck Cancer. Cancers 2025, 17, 2464. https://doi.org/10.3390/cancers17152464
Kishan R, Zhang G, Yang W, Su Y. Bioinformatics Screening of Tumor-Derived Neuropeptides Mediating Neuroimmune Axis of Head and Neck Cancer. Cancers. 2025; 17(15):2464. https://doi.org/10.3390/cancers17152464
Chicago/Turabian StyleKishan, Ravi, Gao Zhang, Weifa Yang, and Yuxiong Su. 2025. "Bioinformatics Screening of Tumor-Derived Neuropeptides Mediating Neuroimmune Axis of Head and Neck Cancer" Cancers 17, no. 15: 2464. https://doi.org/10.3390/cancers17152464
APA StyleKishan, R., Zhang, G., Yang, W., & Su, Y. (2025). Bioinformatics Screening of Tumor-Derived Neuropeptides Mediating Neuroimmune Axis of Head and Neck Cancer. Cancers, 17(15), 2464. https://doi.org/10.3390/cancers17152464





