Late-Onset Immune-Related Adverse Events in Patients with Advanced Melanoma: The LATENT Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Characteristics
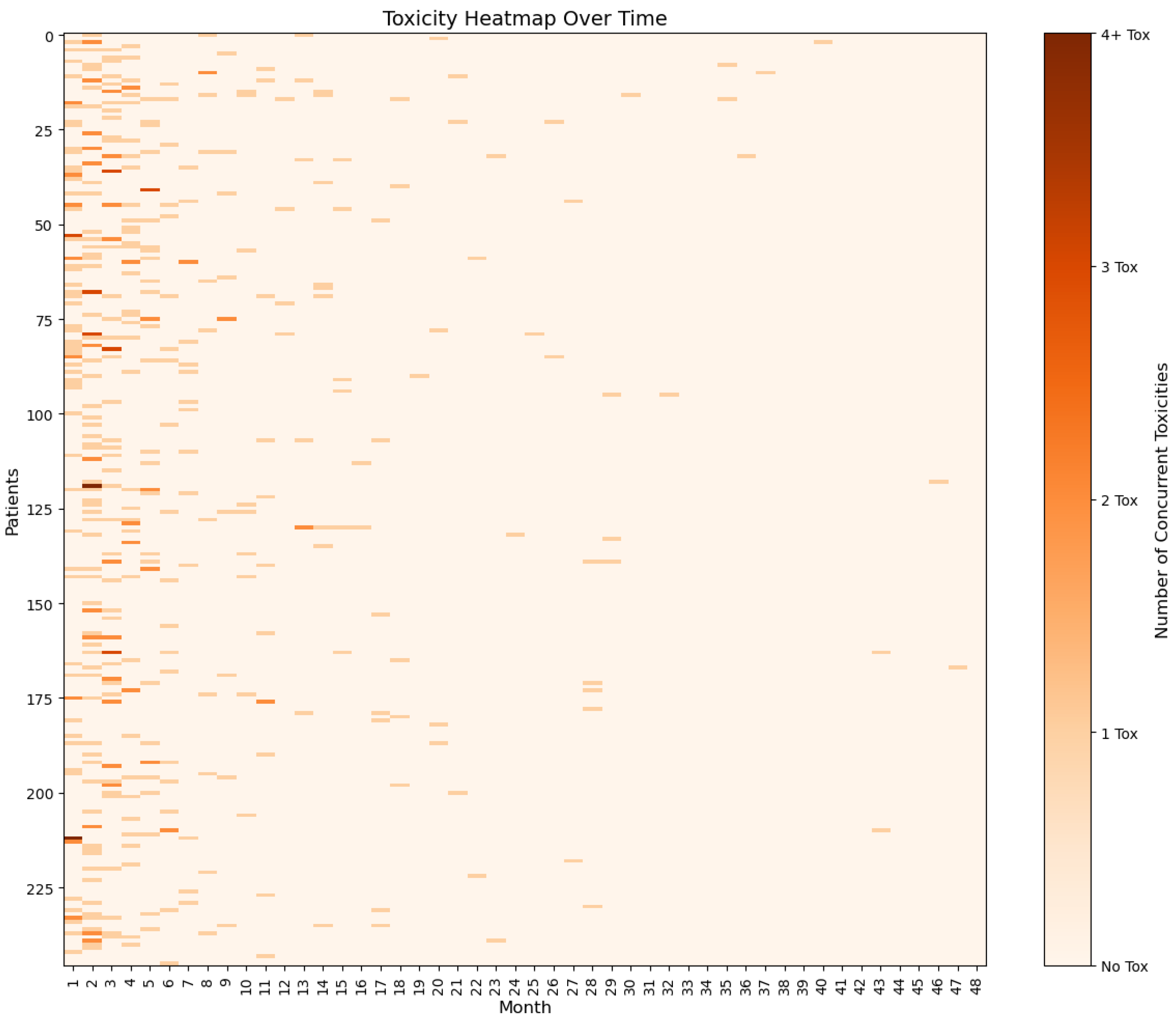
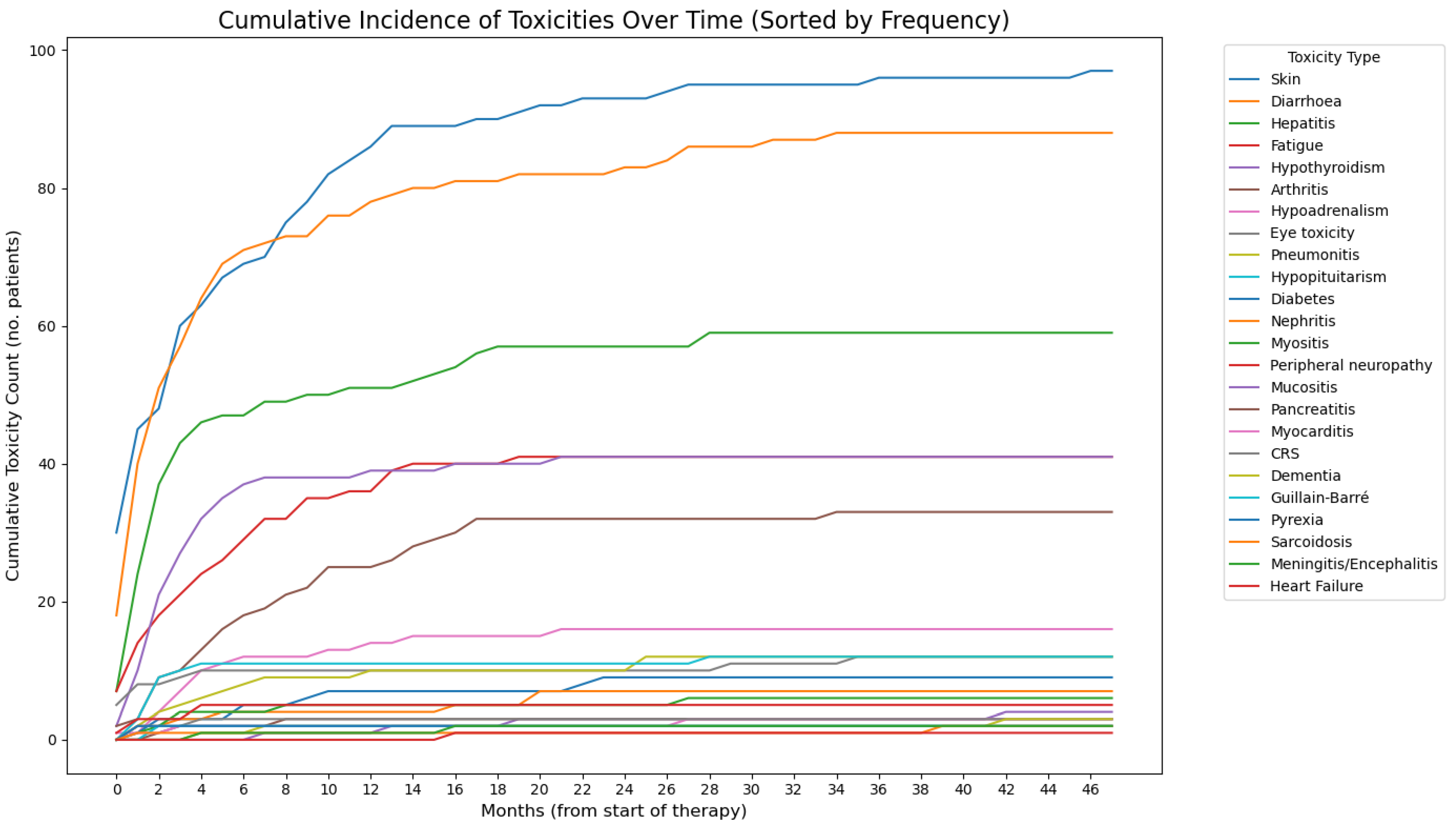
3.2. Development of irAEs in the Study Cohort
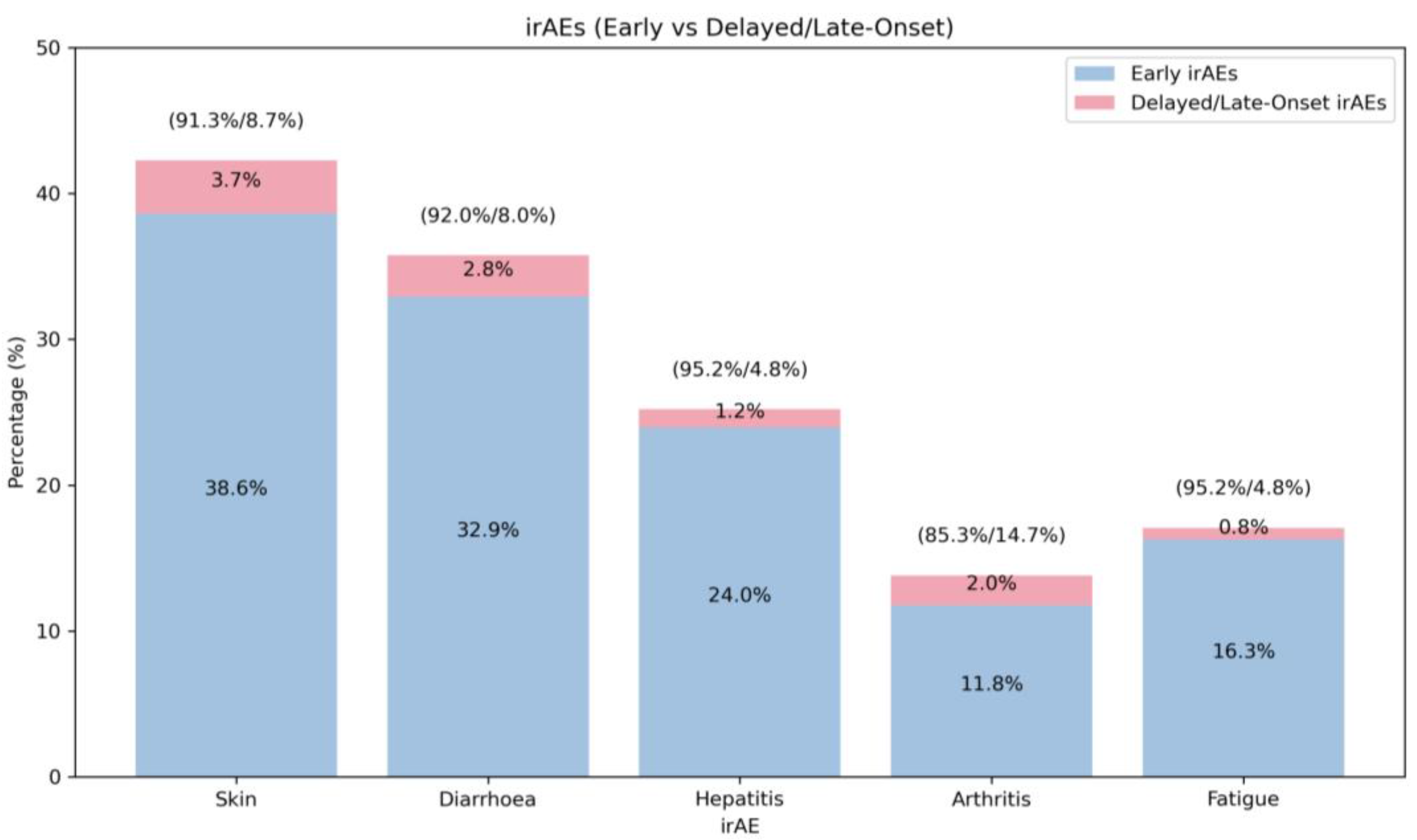
3.3. Late-Onset irAEs
3.4. Ultra-Late-Onset irAEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICI | Immune checkpoint inhibitor |
| CTLA-4 | Cytotoxic T-lymphocyte antigen-4 |
| PD-1 | Programmed cell death-1 |
| irAE | Immune-related adverse event |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CNS | Central nervous system |
| TKI | Tyrosine kinase inhibitors |
| CRS | Cytokine release syndrome |
| NA | Not applicable |
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; Menezes, J.J.D.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wu, L.; Han, L.; Zheng, X.; Tong, R.; Li, L.; Bai, L.; Bian, Y. Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Review. Front. Immunol. 2023, 14, 1167975. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Murphy, C.; Atkins, M.B.; Brahmer, J.R.; Champiat, S.; Feltquate, D.; Krug, L.M.; Moslehi, J.; Pietanza, M.C.; Riemer, J.; et al. Society for Immunotherapy of Cancer (SITC) Consensus Definitions for Immune Checkpoint Inhibitor-Associated Immune-Related Adverse Events (irAEs) Terminology. J. Immunother. Cancer 2023, 11, e006398. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.N.; Allayous, C.; Callaghan, S.; Martínez-Vila, C.; Wallace, R.; Bhave, P.; Reijers, I.L.M.; et al. Delayed Immune-Related Adverse Events with Anti-PD-1-Based Immunotherapy in Melanoma. Ann. Oncol. 2021, 32, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Somekawa, K.; Horita, N.; Kaneko, A.; Tagami, Y.; Fukuda, N.; Matsumoto, H.; Namkoong, H.; Fujiwara, Y.; Minegishi, K.; Fukumoto, T.; et al. Adverse Events Induced by Nivolumab and Ipilimumab Combination Regimens. Ther. Adv. Med. Oncol. 2022, 14, 17588359211058393. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Bedussi, F.; Giunchi, V.; Fusaroli, M.; Raschi, E.; Ceschi, A. Reporting of Late-Onset Immune-Related Adverse Events with Immune Checkpoint Inhibitors in VigiBase. J. Immunother. Cancer 2024, 12, e009902. [Google Scholar] [CrossRef] [PubMed]
- Angeles, A.; Sonke, E.; Nguyen, T.P.; Bahl, G.; Poon, V.I.; Bernstein, V.; Weppler, A.M.; Savage, K.J. Delayed Immune-Related Adverse Events in Patients with Advanced Melanoma Treated with Immune Checkpoint Inhibitors. JCO 2024, 42, 12047. [Google Scholar] [CrossRef]
- Nigro, O.; Pinotti, G.; De Galitiis, F.; Di Pietro, F.R.; Giusti, R.; Filetti, M.; Bersanelli, M.; Lazzarin, A.; Bordi, P.; Catino, A.; et al. Late Immune-Related Adverse Events in Long-Term Responders to PD-1/PD-L1 Checkpoint Inhibitors: A Multicentre Study. Eur. J. Cancer 2020, 134, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, E.; Wicky, A.; Bouchaab, H.; Imbimbo, M.; Delyon, J.; Gautron Moura, B.; Gérard, C.L.; Latifyan, S.; Özdemir, B.C.; Caikovski, M.; et al. Late-Onset and Long-Lasting Immune-Related Adverse Events from Immune Checkpoint-Inhibitors: An Overlooked Aspect in Immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Tian, J.; Ma, L.; Wu, Y.; Luo, Q.; Luo, Y. Delayed Immune-Related Adverse Events Profile Associated with Immune Checkpoint Inhibitors: A Real-World Analysis. Front. Pharmacol. 2024, 15, 1453429. [Google Scholar] [CrossRef] [PubMed]
- Couey, M.A.; Bell, R.B.; Patel, A.A.; Romba, M.C.; Crittenden, M.R.; Curti, B.D.; Urba, W.J.; Leidner, R.S. Delayed Immune-Related Events (DIRE) after Discontinuation of Immunotherapy: Diagnostic Hazard of Autoimmunity at a Distance. J. Immunother. Cancer 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, P.T.; Gaggadi, M.; Kassi, E.; Benopoulou, O.; Anastasopoulou, A.; Gogas, H. Late-Onset Nivolumab-Mediated Pneumonitis in a Patient with Melanoma and Multiple Immune-Related Adverse Events. Melanoma Res. 2017, 27, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Vincelette, N.D.; Mansour, I.; Hariri, D.; Motamed, S. Late Onset Ipilimumab-Induced Pericarditis and Pericardial Effusion: A Rare but Life Threatening Complication. Case Rep. Oncol. Med. 2015, 2015, 794842. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, M.T.; Nelson, K.C.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/Sarcoid-like Lesions Associated with Checkpoint Inhibitors: A Marker of Therapy Response in a Subset of Melanoma Patients. J. Immunother. Cancer 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.; Yadala, V.; Khan, N.A.J.; Graffeo, V.; Denning, K.; Lebowicz, Y. Immunotherapy-Induced Sarcoid-Like Reaction: A Shrewd Imitator. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211009400. [Google Scholar] [CrossRef] [PubMed]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; van Akkooi, A.; Arance, A.; Blank, C.U.; Sileni, V.C.; Donia, M.; et al. ESMO Consensus Conference Recommendations on the Management of Metastatic Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 31, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Otsuka, T.; Inoue, T.; Nawa, T.; Hatano, K.; Yamamoto, Y.; Nagahara, A.; Nakayama, M.; Kakimoto, K.; Nishimura, K. Two Cases of Delayed Onset of Immune-related Adverse Events after Discontinuation of Nivolumab in Patients with Metastatic Renal Cell Cancer. IJU Case Rep. 2021, 4, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Elkrief, A.; Calabrese, C.; Lapointe, R.; Hudson, M.; Routy, B.; Miller, W.H.; Calabrese, L. Moving towards Personalized Treatments of Immune-Related Adverse Events. Nat. Rev. Clin. Oncol. 2020, 17, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Reschke, R.; Sullivan, R.J.; Lipson, E.J.; Enk, A.H.; Gajewski, T.F.; Hassel, J.C. Targeting Molecular Pathways to Control Immune Checkpoint Inhibitor Toxicities. Trends Immunol. 2025, 46, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, S.C.; Pisetsky, D.S. Mechanisms of Immune-Related Adverse Events during the Treatment of Cancer with Immune Checkpoint Inhibitors. Rheumatology 2019, 58, vii59–vii67. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Dougan, S.K.; Dougan, M. Immune Mechanisms of Toxicity from Checkpoint Inhibitors. Trends. Cancer 2023, 9, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, S.; Droney, J.; Jaganathan, P.P.; Howell, P.; Doherty, A.M.-; Young, K.; Cruickshank, S. Identification of the PROMs Used to Assess ICI Toxicities and HRQoL in Patients Receiving Immune Checkpoint Inhibitor Treatment in Cancer Care and Their Suitability: A Systematic Review. Cancer Treat. Rev. 2025, 135, 102862. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Allouche, S.; Dupas, Q.; Gervais, R.; Madelaine, J.; Sassier, M.; Plane, A.-F.; Comoz, F.; Cohen, A.A.; et al. Late Cardiac Adverse Events in Patients with Cancer Treated with Immune Checkpoint Inhibitors. J. Immunother. Cancer 2020, 8, e000261. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients (n = 246) (%) | |
|---|---|---|
| Gender—no. (%) | ||
| Male | 145 (58.9) | |
| Female | 101 (41.1) | |
| Age—median (range) | 63 (18–92) | |
| Melanoma subtype—no. (%) | ||
| Cutaneous | 152 (85.9) | |
| Mucosal | 8 (4.5) | |
| Acral | 4 (2.3) | |
| Other | 13 (7.3) | |
| Mutational profile—no. (%) | ||
| BRAF mutant | 79 (32.1) | |
| NRAS mutant | 24 (9.8) | |
| Extent of disease at stage IV diagnosis—no. (%) | ||
| Brain metastases | 36 (14.6) | |
| Liver metastases | 49 (19.9) | |
| Number of lines—no. (%) | ||
| 1 | 191 (77.6) | |
| 2 | 43 (17.5) | |
| 3 | 8 (3.3) | |
| 4 | 4 (1.6) | |
| First-line treatment regimens—no. (%) | ||
| Ipilimumab/nivolumab | 111 (45.1) | |
| Pembrolizumab | 60 (24.4) | |
| Ipilimumab | 23 (9.3) | |
| Nivolumab | 21 (8.5) | |
| Dacarbazine | 8 (3.3) | |
| Dabrafenib/trametinib | 8 (3.3) | |
| Melphalan | 4 (1.6) | |
| Vemurafenib | 3 (1.2) | |
| Cisplatin/dacarbazine | 3 (1.2) | |
| Bevacizumab | 1 (0.4) | |
| Temozolomide | 1 (0.4) | |
| MEK inhibitor (clinical trial) | 1 (0.4) | |
| Docetaxel | 1 (0.4) | |
| Dabrafenib | 1 (0.4) | |
| Last line of treatment—no. (%) | ||
| Ipilimumab/nivolumab | 115 (46.7) | |
| Pembrolizumab | 87 (35.4) | |
| Nivolumab | 31 (12.6) | |
| Ipilimumab | 13 (5.3) | |
| Variable | Patients (n = 246) (%) | |
|---|---|---|
| Any irAE—no. (%) | 199 (80.9) | |
| Most frequent irAEs (>10%)—no. (%) | ||
| Rash | 104 (42.3) | |
| Diarrhoea | 88 (35.8) | |
| Hepatitis | 62 (25.2) | |
| Fatigue | 42 (17.1) | |
| Hypothyroidism | 41 (16.7) | |
| Arthritis/arthralgia | 34 (13.8) | |
| Any G3/G4 irAEs—no. (%) | 97 (39.4) | |
| Most frequent G3/4 irAEs (>5%)—no. (%) | ||
| Diarrhoea | 43 (17.5) | |
| Hepatitis | 36 (14.6) | |
| Rash | 13 (5.28) | |
| Time of onset (months)—median (range) | 3 (0–46) | |
| Rash | 3 (0–46) | |
| Diarrhoea | 2 (0–34) | |
| Hepatitis | 2 (0–28) | |
| Fatigue | 3 (0–19) | |
| Hypothyroidism | 2 (0–21) | |
| Arthritis/arthralgia | 6 (0–34) | |
| Referral to specialist (among patients with G3/G4 irAEs)—no. (%) | ||
| Rash | 5 (38.5) | |
| Diarrhoea | 20 (46.5) | |
| Hepatitis | 8 (22.2) | |
| Arthritis/arthralgia | 1 (20) | |
| Median time to referral (months)—median (range) | 0 (0–22) | |
| Variable | Patients (n = 199) | |||
|---|---|---|---|---|
| Total | Oral | IV | ||
| Systemic steroids—no. (%) | 122 (61.3%) | 118 (59.4%) | 68 (34.2%) | |
| Rash (n = 104) | 24 (23.1%) | 24 (23.1%) | 5 (4.81%) | |
| Diarrhoea (n = 88) | 66 (75%) | 65 (73.9%) | 48 (54.6%) | |
| Hepatitis (n = 62) | 29 (25.2%) | 24 (38.7%) | 6 (9.7%) | |
| Arthritis (n = 34) | 30 (88.2%) | 30 (88.2%) | 1 (2.94%) | |
| Pneumonitis (n = 12) | 9 (75%) | 9 (75%) | 0 | |
| Ocular toxicity (n = 12) | 4 (33.3%) | 4 (33.3%) | 1 (8.3%) | |
| Nephritis (n = 7) | 4 (57.1%) | 4 (57.1%) | 2 (28.6%) | |
| Myositis (n = 6) | 4 (66.7%) | 4 (66.7%) | 0 | |
| Peripheral neuropathy (n = 5) | 2 (40%) | 2 (40%) | 2 (40%) | |
| Myocarditis (n = 3) | 2 (66.7%) | 2 (66.7%) | 2 (66.7%) | |
| CRS (n = 3) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | |
| Pancreatitis (n = 3) | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | |
| Memory loss (n = 3) | 1 (33.3%) | 1 (33.3%) | 0 | |
| Meningitis/encephalitis (n = 2) | 2 (100%) | 2 (100%) | 2 (100%) | |
| Guillain–Barré (n = 2) | 2 (100%) | 2 (100%) | 1 (50%) | |
| Sarcoidosis (n = 2) | 1 (50%) | 1 (50%) | 0 | |
| Other immunosuppressants—no. (%) | 55 (27.6%) | |||
| Rash (n = 104) | 3 (2.9%) | |||
| Diarrhoea (n = 88) | 29 (33%) | |||
| Hepatitis (n = 62) | 18 (29%) | |||
| Arthritis (n = 34) | 9 (26.5%) | |||
| Pneumonitis (n = 12) | 1 (8.33%) | |||
| CRS (n = 3) | 1 (33.3%) | |||
| Pancreatitis (n = 3) | 1 (33.3%) | |||
| Guillain–Barré (n = 2) | 1 (50%) | |||
| Variable | Value (n = 246) (%) | |
|---|---|---|
| Late-onset irAEs—no. (%) | 36 (14.6) | |
| Rash | 9 (3.7) | |
| Diarrhoea | 7 (2.8) | |
| Arthritis | 5 (2) | |
| Hepatitis | 3 (1.2) | |
| Memory loss | 3 (1.2) | |
| Nephritis | 2 (0.8) | |
| Hypoadrenalism | 2 (0.8) | |
| Myositis | 2 (0.8) | |
| Mucositis | 2 (0.8) | |
| Fatigue | 2 (0.8) | |
| Pneumonitis | 1 (0.4) | |
| Sarcoidosis | 1 (0.4) | |
| Diabetes | 1 (0.4) | |
| Eye toxicity | 1 (0.4) | |
| Heart failure | 1 (0.4) | |
| Myocardial infarction | 1 (0.4) | |
| Ultra-late-onset irAEs—no. (%) | 5 (2) | |
| Pneumonitis | 1 (0.4) | |
| Rash | 1 (0.4) | |
| Hepatitis | 1 (0.4) | |
| Sarcoidosis | 1 (0.4) | |
| Ocular toxicity | 1 (0.4) | |
| Dementia | 1 (0.4) | |
| Patient | Gender, Age | BRAF | Primary | Baseline LDH (U/L) | Treatment | Toxicity | Time from Last Treatment to Toxicity Presentation | Toxicity | Time from Last Treatment | Other Toxicities |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M, 30 | Mut | Cutaneous | 117 | Ipi/Nivo | Sarcoidosis | 15 months | – | – | Hypothyroidism, hepatitis, skin rash |
| 2 | M, 67 | WT | Mucosal | 195 | Nivolumab | Papillitis | 17 months | – | – | Skin rash, diarrhoea, fatigue, diabetes |
| 3 | F, 58 | WT | Cutaneous | NA | Ipi/Nivo | Hepatitis | 18 months | Pneumonitis | 18 months | Arthritis, hypothyroidism, hypoadrenalism |
| 4 | F, 71 | WT | Mucosal | 361 | Ipi/Nivo | Skin rash | 44 months | – | – | Diarrhoea |
| 5 | M, 75 | WT | Unknown | 243 | Pembrolizumab | Dementia | 38 months | – | – | Hypothyroidism, nephritis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozas, J.; Cheruvu, S.; Jaganathan, P.P.; Ganesan, P.; Modi, A.; Larkin, J.; Cossar, L.; Olsson-Brown, A.; Johnson, A.; Garbutt, N.; et al. Late-Onset Immune-Related Adverse Events in Patients with Advanced Melanoma: The LATENT Study. Cancers 2025, 17, 2461. https://doi.org/10.3390/cancers17152461
Pozas J, Cheruvu S, Jaganathan PP, Ganesan P, Modi A, Larkin J, Cossar L, Olsson-Brown A, Johnson A, Garbutt N, et al. Late-Onset Immune-Related Adverse Events in Patients with Advanced Melanoma: The LATENT Study. Cancers. 2025; 17(15):2461. https://doi.org/10.3390/cancers17152461
Chicago/Turabian StylePozas, Javier, Sowmya Cheruvu, Poorni Priya Jaganathan, Priya Ganesan, Arjun Modi, James Larkin, Laura Cossar, Anna Olsson-Brown, Alexandra Johnson, Nicholas Garbutt, and et al. 2025. "Late-Onset Immune-Related Adverse Events in Patients with Advanced Melanoma: The LATENT Study" Cancers 17, no. 15: 2461. https://doi.org/10.3390/cancers17152461
APA StylePozas, J., Cheruvu, S., Jaganathan, P. P., Ganesan, P., Modi, A., Larkin, J., Cossar, L., Olsson-Brown, A., Johnson, A., Garbutt, N., Lee, R., Jones, J., Macklin-Doherty, A., Young, K., & LATENT Study Investigators. (2025). Late-Onset Immune-Related Adverse Events in Patients with Advanced Melanoma: The LATENT Study. Cancers, 17(15), 2461. https://doi.org/10.3390/cancers17152461







