Targeting Wnt Signaling in Acute Lymphoblastic Leukemia
Simple Summary
Abstract
1. Introduction to Acute Leukemias
1.1. Acute Lymphoblastic Leukemia
1.2. Acute Myeloid Leukemia
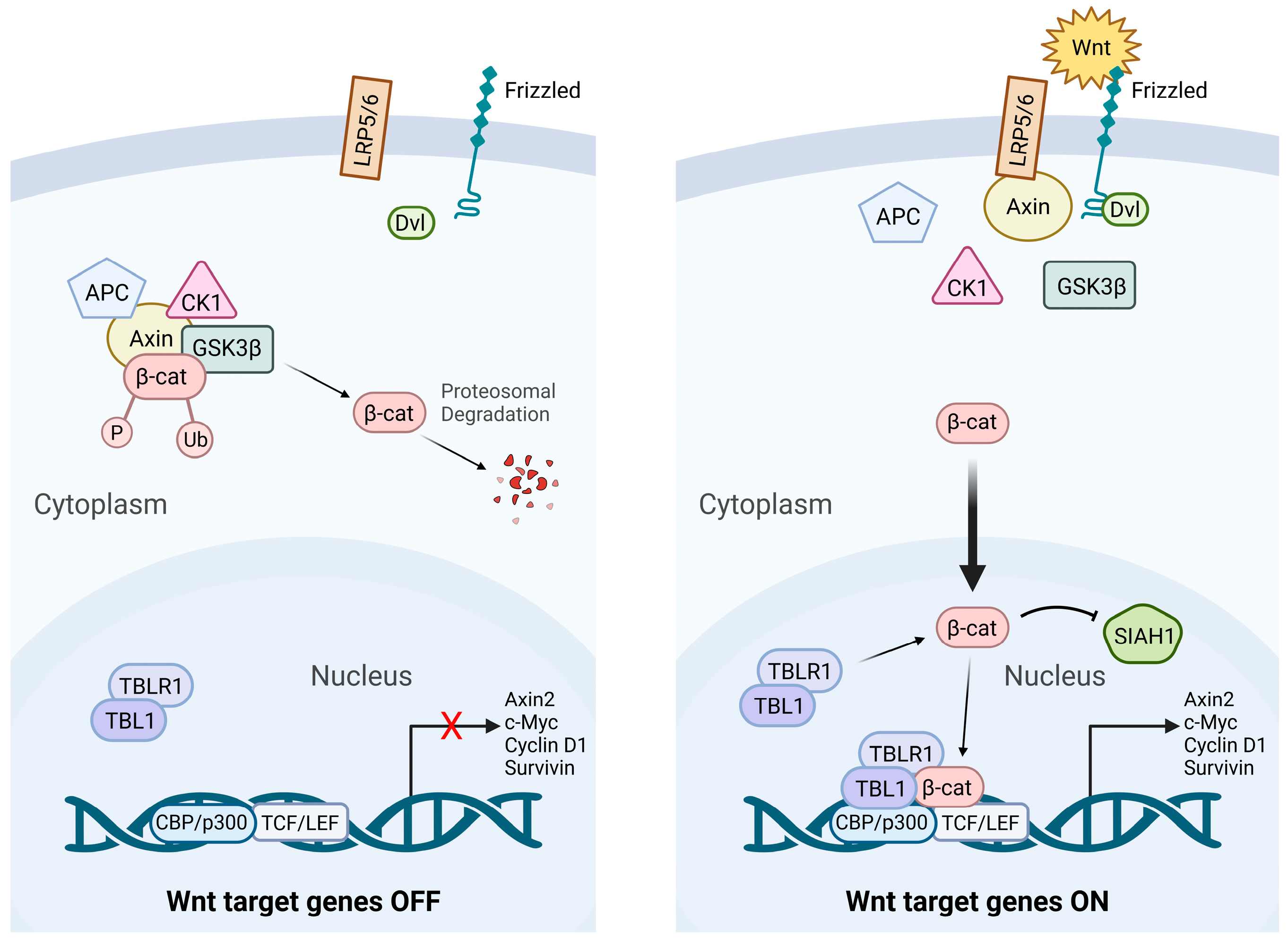
2. Wnt Signaling
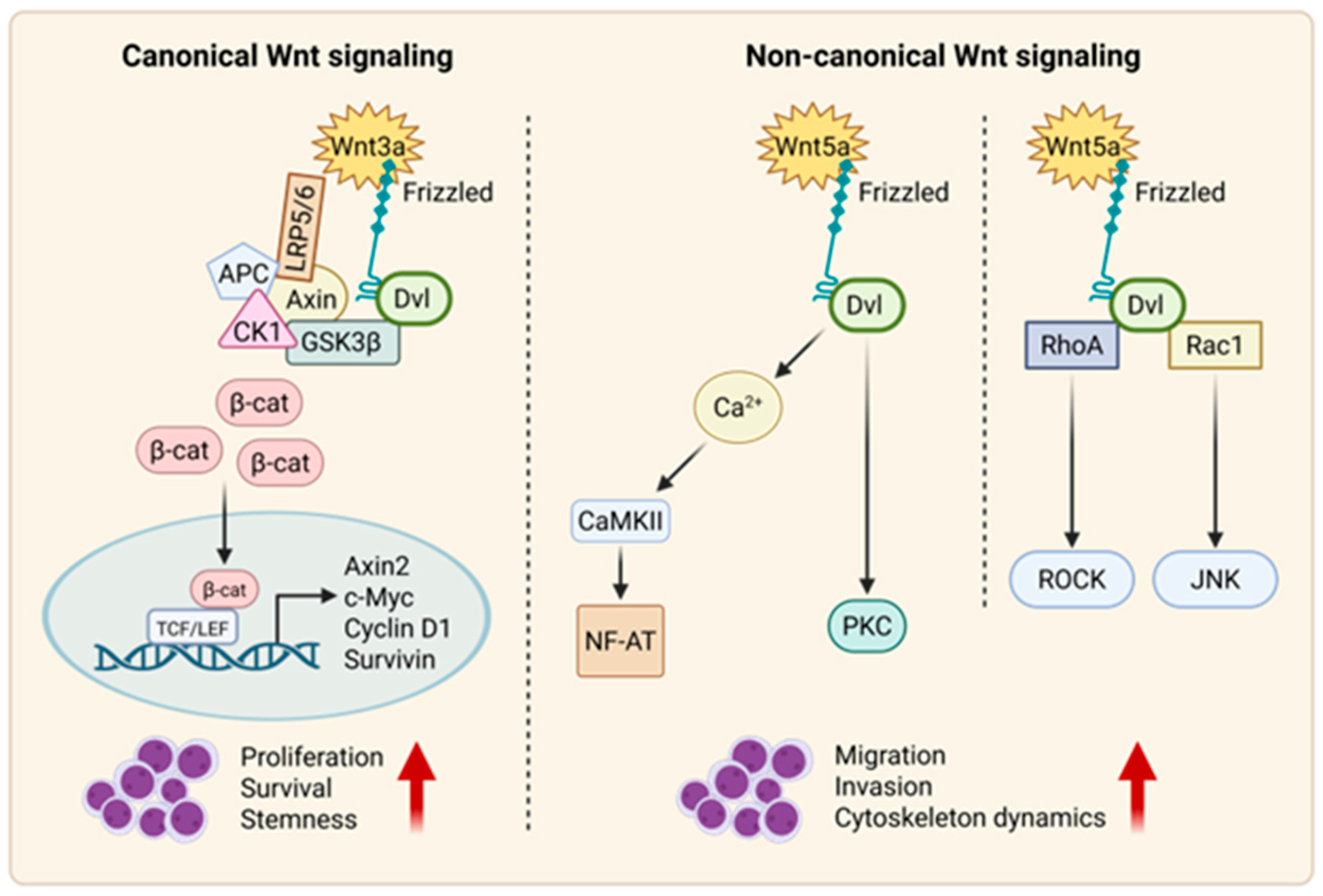
2.1. Canonical Wnt Signaling
2.2. Non-Canonical Wnt Signaling
2.2.1. Wnt–PCP Pathway
2.2.2. Wnt–Ca2+ Pathway
3. Wnt Ligand Activation
3.1. Canonical Wnt Ligands in ALL
3.1.1. Wnt1 and Wnt2b
3.1.2. Wnt3a
3.1.3. Wnt10a and Wnt10b
3.1.4. Wnt16b
3.2. Non-Canonical Wnt Ligands in ALL
3.2.1. Wnt5a
3.2.2. Wnt9a
3.2.3. Wnt11
3.2.4. Non-Wnt Ligands Modulating Wnt Signaling
4. Wnt in Acute Lymphoblastic Leukemia
4.1. Wnt Signaling in B-ALL
4.2. Wnt Signaling in T-ALL
4.3. Wnt Signaling Aberrations in ALL Pathogenesis
5. Leukemia Stem Cells
5.1. Leukemic Stem Cells in B-ALL
5.2. Leukemic Stem Cells in T-ALL
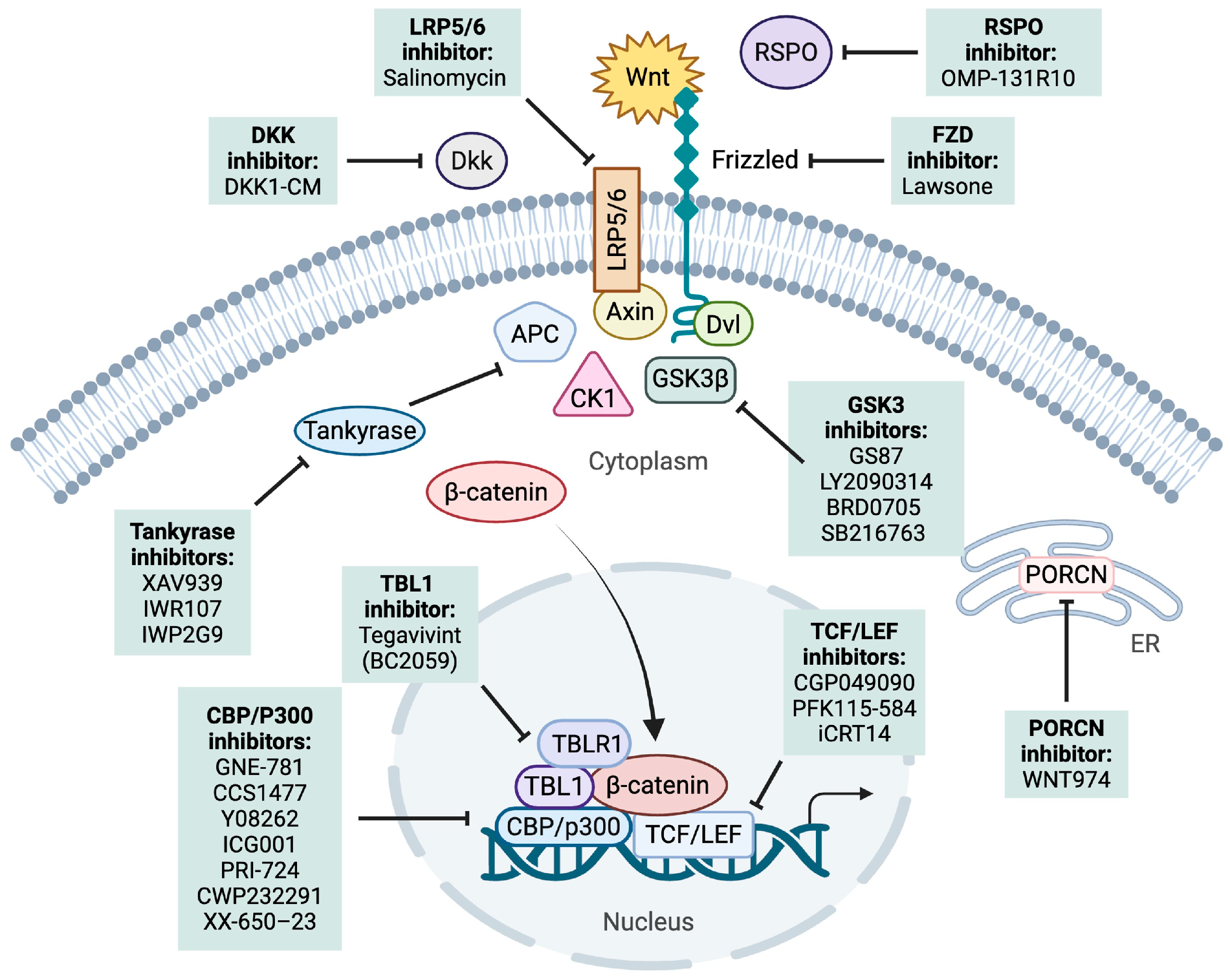
6. Targeting Wnt Signaling in B-ALL and T-ALL
6.1. Upstream Effector Targeting
6.1.1. PORCN
6.1.2. DKK1
6.1.3. LRP5/6
6.1.4. RSPO-LGR4
6.1.5. FZD
6.1.6. GSK-3
6.2. Promoting β-Catenin Degradation
Tankyrase
6.3. Inhibiting β-Catenin–TCF Interaction
6.3.1. TCF
6.3.2. LEF-1
6.3.3. CBP/P300
6.3.4. TBL1
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiarini, F.; Paganelli, F.; Martelli, A.M.; Evangelisti, C. The Role Played by Wnt/β-Catenin Signaling Pathway in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1098. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Faderl, S.; O’Brien, S.; Pui, C.H.; Stock, W.; Wetzler, M.; Hoelzer, D.; Kantarjian, H.M. Adult acute lymphoblastic leukemia: Concepts and strategies. Cancer 2010, 116, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Passet, M.; Kim, R.; Clappier, E. Genetic subtypes of B-cell acute lymphoblastic leukemia in adults. Blood 2025, 145, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Lilljebjörn, H.; Fioretos, T. New oncogenic subtypes in pediatric B-cell precursor acute lymphoblastic leukemia. Blood 2017, 130, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Vadillo, E.; Dorantes-Acosta, E.; Pelayo, R.; Schnoor, M. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018, 32, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.S.; Muffly, L.S. Predicting relapse in acute lymphoblastic leukemia. Leuk. Lymphoma 2024, 65, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; DeAngelo, D.J.; Luskin, M.R. Nelarabine: When and how to use in the treatment of T-cell acute lymphoblastic leukemia. Blood Adv. 2024, 8, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.J.; Teachey, D.T. SOHO State of the Art Updates and Next Questions|Novel Approaches to Pediatric T-cell ALL and T-Lymphoblastic Lymphoma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Saultz, J.N.; Garzon, R. Acute Myeloid Leukemia: A Concise Review. J. Clin. Med. 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.T.; Ahmed, A.; Singh, H.; Mouchati, C.; Al Omari, O.; Bhatt, P.S.; Sharma, R.; Farooq, M.; Liu, W.; Shalhoub, J.; et al. Burden of AML, 1990–2019: Estimates From the Global Burden of Disease Study. JCO Glob. Oncol. 2023, 9, e2300229. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.D.; Zwaan, C.M.; van den Heuvel-Eibrink, M. Pediatric AML: From Biology to Clinical Management. J. Clin. Med. 2015, 4, 127–149. [Google Scholar] [CrossRef] [PubMed]
- De Kouchkovsky, I.; Abdul-Hay, M. ‘Acute myeloid leukemia: A comprehensive review and 2016 update’. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef] [PubMed]
- Bernt, K.M.; Hunger, S.P. Current concepts in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia. Front. Oncol. 2014, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Luis, T.C.; Ichii, M.; Brugman, M.H.; Kincade, P.; Staal, F.J. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia 2012, 26, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Drożak, P.; Bryliński, Ł.; Zawitkowska, J. A Comprehensive Overview of Recent Advances in Epigenetics in Pediatric Acute Lymphoblastic Leukemia. Cancers 2022, 14, 5384. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Famili, F.; Garcia Perez, L.; Pike-Overzet, K. Aberrant Wnt Signaling in Leukemia. Cancers 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.; Bock, F.; Grziwok, S.; Oostendorp, R.A.; Istvanffy, R. Regulation of hematopoiesis by activators and inhibitors of Wnt signaling from the niche. Ann. N. Y. Acad. Sci. 2014, 1310, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Si, L.; Zhuang, Y.; Zhang, A.; Sun, N.; Li, D.; Hao, B.; Ju, X. Wnt/beta-catenin inhibition reverses multidrug resistance in pediatric acute lymphoblastic leukemia. Oncol. Rep. 2019, 41, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.H.; Erbilgin, Y.; Firtina, S.; Celkan, T.; Karakas, Z.; Aydogan, G.; Turkkan, E.; Yildirmak, Y.; Timur, C.; Zengin, E.; et al. Deregulated WNT signaling in childhood T-cell acute lymphoblastic leukemia. Blood Cancer J. 2014, 4, e192. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Yu, M.; Fan, J.; Wang, H.; Zhao, P.; Zhao, G.; Zeng, W.; Chen, C.; Wang, Y.; Wang, A.; et al. Canonical and noncanonical Wnt signaling: Multilayered mediators, signaling mechanisms and major signaling crosstalk. Genes Dis. 2024, 11, 103–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Choi, K.C.; Yoo, J.Y.; Song, M.; Ko, S.J.; Kim, C.H.; Ahn, J.H.; Chun, K.H.; Yook, J.I.; Yoon, H.G. Reversible SUMOylation of TBL1-TBLR1 regulates beta-catenin-mediated Wnt signaling. Mol. Cell 2011, 43, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.Y. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat. Cell Biol. 2008, 10, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef] [PubMed]
- Federici, A.; Ciccone, M.; Gattullo, D.; Losano, G. Systolic and diastolic changes in human coronary blood flow during Valsalva manoeuvre. Clin. Physiol. 2000, 20, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.E.; Mieszczanek, J.; Novcic, N.; Bienz, M. Wnt-Dependent Inactivation of the Groucho/TLE Co-repressor by the HECT E3 Ubiquitin Ligase Hyd/UBR5. Mol. Cell 2017, 67, 181–193.e5. [Google Scholar] [CrossRef] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Moon, R.T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.G.; Rodriguez, D.A.; Valenzuela, M.; Calderon, C.; Urzua, U.; Munroe, D.; Rosas, C.; Lemus, D.; Diaz, N.; Wright, M.C.; et al. Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced beta-catenin/Tcf-Lef dependent transcription. Mol. Cancer 2014, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Schussler, O.; Hebert, J.L.; Vallee, A. Multiple Targets of the Canonical WNT/beta-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Kim, H.N.; Ogana, H.; Kim, Y.M. Wnt Signaling in Leukemia and Its Bone Marrow Microenvironment. Int. J. Mol. Sci. 2020, 21, 6247. [Google Scholar] [CrossRef] [PubMed]
- Kokolus, K.; Nemeth, M.J. Non-canonical Wnt signaling pathways in hematopoiesis. Immunol. Res. 2010, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Janovska, P.; Bryja, V. Wnt signalling pathways in chronic lymphocytic leukaemia and B-cell lymphomas. Br. J. Pharmacol. 2017, 174, 4701–4715. [Google Scholar] [CrossRef] [PubMed]
- Mochmann, L.H.; Bock, J.; Ortiz-Tanchez, J.; Schlee, C.; Bohne, A.; Neumann, K.; Hofmann, W.K.; Thiel, E.; Baldus, C.D. Genome-wide screen reveals WNT11, a non-canonical WNT gene, as a direct target of ETS transcription factor ERG. Oncogene 2011, 30, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Sarabia-Sanchez, M.A.; Robles-Flores, M. WNT Signaling in Stem Cells: A Look into the Non-Canonical Pathway. Stem Cell Rev. Rep. 2024, 20, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, A.; Hussein, M.S.; Prasad, P.D.; Manicassamy, S. Wnt Signaling Cascade in Dendritic Cells and Regulation of Anti-tumor Immunity. Front. Immunol. 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Chaklader, M.; Law, S. Aberrant Wnt Signaling Pathway in the Hematopoietic Stem/Progenitor Compartment in Experimental Leukemic Animal. J. Cell Commun. Signal. 2019, 13, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lento, W.; Congdon, K.; Voermans, C.; Kritzik, M.; Reya, T. Wnt signaling in normal and malignant hematopoiesis. Cold Spring Harb. Perspect. Biol. 2013, 5, a008011. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Stachura, D.L.; Traver, D. Cell signaling pathways involved in hematopoietic stem cell specification. Exp. Cell Res. 2014, 329, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Traver, D.; Willert, K. The role of Wnt signaling in hematopoietic stem cell development. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Moon, J.; Dodge, M.E.; Pan, X.; Zhang, L.; Hanson, J.M.; Tuladhar, R.; Ma, Z.; Shi, H.; Williams, N.S.; et al. The development of highly potent inhibitors for porcupine. J. Med. Chem. 2013, 56, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Luis, T.C.; Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008, 8, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Chae, W.J.; Bothwell, A.L.M. Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends. Immunol. 2018, 39, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Z.; Tang, Y.; Xiao, Q. The involvement of noncanonical Wnt signaling in cancers. Biomed. Pharmacother. 2021, 133, 110946. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grandage, V.L.; Linch, D.C.; Khwaja, A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene 2005, 24, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Echeverry, A.; Prada-Arismendy, J. Deciphering the role of Wnt signaling in acute myeloid leukemia prognosis: How alterations in DNA methylation come into play in patients’ prognosis. J. Cancer Res. Clin. Oncol. 2020, 146, 3097–3109. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Bradstock, K.F.; Bendall, L.J. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br. J. Haematol. 2007, 138, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Niwa, Y.; Abe, A.; Hayakawa, F.; Naoe, T. Wnt Signaling Is Associated with Anti-Apoptosis in the Interaction Between Acute Myeloid Leukemia Cells and Stromal Cells. Blood 2012, 120, 1366. [Google Scholar] [CrossRef]
- Nygren, M.K.; Dosen, G.; Hystad, M.E.; Stubberud, H.; Funderud, S.; Rian, E. Wnt3A activates canonical Wnt signalling in acute lymphoblastic leukaemia (ALL) cells and inhibits the proliferation of B-ALL cell lines. Br. J. Haematol. 2007, 136, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi-Ihara, N.; Murohashi, I.; Nara, N.; Tohda, S. Promotion of the self-renewal capacity of human acute leukemia cells by Wnt3A. Anticancer Res. 2008, 28, 2701–2704. [Google Scholar] [PubMed]
- Beghini, A.; Corlazzoli, F.; Del Giacco, L.; Re, M.; Lazzaroni, F.; Brioschi, M.; Valentini, G.; Ferrazzi, F.; Ghilardi, A.; Righi, M.; et al. Regeneration-associated WNT signaling is activated in long-term reconstituting AC133bright acute myeloid leukemia cells. Neoplasia 2012, 14, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Soares-Lima, S.C.; Pombo-de-Oliveira, M.S.; Carneiro, F.R.G. The multiple ways Wnt signaling contributes to acute leukemia pathogenesis. J. Leukoc. Biol. 2020, 108, 1081–1099. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cheng, Z.; Fricke, D.R.; Zhao, H.; Huang, W.; Zhong, Q.; Zhu, P.; Zhang, W.; Wu, Z.; Lin, Q.; et al. Prognostic role of Wnt and Fzd gene families in acute myeloid leukaemia. J. Cell Mol. Med. 2021, 25, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Chen, K.; Deng, H.; Rao, H.; Huang, H.; Liao, Y.; Sun, X.; Lu, S.; Yuan, Z.; Xie, D.; et al. MicroRNA-374b Suppresses Proliferation and Promotes Apoptosis in T-cell Lymphoblastic Lymphoma by Repressing AKT1 and Wnt-16. Clin. Cancer Res. 2015, 21, 4881–4891. [Google Scholar] [CrossRef] [PubMed]
- McWhirter, J.R.; Neuteboom, S.T.; Wancewicz, E.V.; Monia, B.P.; Downing, J.R.; Murre, C. Oncogenic homeodomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc. Natl. Acad. Sci. USA 1999, 96, 11464–11469. [Google Scholar] [CrossRef] [PubMed]
- Diakos, C.; Xiao, Y.; Zheng, S.; Kager, L.; Dworzak, M.; Wiemels, J.L. Direct and indirect targets of the E2A-PBX1 leukemia-specific fusion protein. PLoS ONE 2014, 9, e87602. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; You, L.; He, B.; Xu, Z.; Lee, A.Y.; Mikami, I.; McCormick, F.; Jablons, D.M. Inhibition of Wnt16 in human acute lymphoblastoid leukemia cells containing the t(1;19) translocation induces apoptosis. Oncogene 2005, 24, 5396–5400. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Tu, Z.; Xiong, M.; Tembo, K.; Zhou, L.; Liu, P.; Pan, S.; Xiong, J.; Yang, X.; Leng, J.; et al. Wnt5a and CCL25 promote adult T-cell acute lymphoblastic leukemia cell migration, invasion and metastasis. Oncotarget 2017, 8, 39033–39047. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Ko, N.L.; Lee, M.J.; Yao, Y.; Waldmann, T.A.; Trepel, J.B.; Nicot, C. Adult T-cell leukemia cells overexpress Wnt5a and promote osteoclast differentiation. Blood 2013, 121, 5045–5054. [Google Scholar] [CrossRef] [PubMed]
- Ide, A.D.; Grainger, S. WNT9A and WNT9B in Development and Disease. Differentiation 2025, 142, 100820. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Stanley, E.G.; Ng, E.S.; Elefanty, A.G.; Traver, D.; Willert, K. WNT9A Is a Conserved Regulator of Hematopoietic Stem and Progenitor Cell Development. Genes 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.; Richter, J.; Palazon, R.E.; Pouget, C.; Lonquich, B.; Wirth, S.; Grassme, K.S.; Herzog, W.; Swift, M.R.; Weinstein, B.M.; et al. Wnt9a Is Required for the Aortic Amplification of Nascent Hematopoietic Stem Cells. Cell Rep. 2016, 17, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Yokota, C.; Puck, H.; Kofron, M.; Birsoy, B.; Yan, D.; Asashima, M.; Wylie, C.C.; Lin, X.; Heasman, J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 2005, 120, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol. Cancer 2010, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Cruciat, C.M.; Niehrs, C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013, 5, a015081. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Hsieh, F.L.; Zebisch, M.; Harlos, K.; Elegheert, J.; Jones, E.Y. Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. Elife 2015, 4, e06554. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Harikumar, K.G.; Erice, C.; Chen, C.; Gu, X.; Wang, L.; Parker, N.; Cheng, Z.; Xu, W.; Williams, B.O.; et al. Structure and function of Norrin in assembly and activation of a Frizzled 4-Lrp5/6 complex. Genes Dev. 2013, 27, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Chen, X.; Lin, Z.; Fang, D.; He, X. The structural basis of R-spondin recognition by LGR5 and RNF43. Genes Dev. 2013, 27, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Frenquelli, M.; Tonon, G. WNT Signaling in Hematological Malignancies. Front. Oncol. 2020, 10, 615190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krivtsov, A.V.; Sinha, A.U.; North, T.E.; Goessling, W.; Feng, Z.; Zon, L.I.; Armstrong, S.A. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 2010, 327, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Kincade, P.W. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell 2009, 4, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kirstetter, P.; Anderson, K.; Porse, B.T.; Jacobsen, S.E.; Nerlov, C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006, 7, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Jenkins, C.E.; Lam, S.H.; Hoofd, C.; Belmonte, M.; Wang, X.; Gusscott, S.; Gracias, D.; Weng, A.P. Leukemia stem cells in T-ALL require active Hif1alpha and Wnt signaling. Blood 2015, 125, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, E.; Takada, T.; Maekawa, T. Targeting the canonical Wnt/beta-catenin pathway in hematological malignancies. Cancer Sci. 2015, 106, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, S.; Romanos-Sirakis, E.; Pais, F.; Bhatla, T.; Jones, C.; Bourgeois, W.; Hunger, S.P.; Raetz, E.A.; Hermiston, M.L.; Dasgupta, R.; et al. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br. J. Haematol. 2014, 167, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gang, E.J.; Hsieh, Y.T.; Pham, J.; Zhao, Y.; Nguyen, C.; Huantes, S.; Park, E.; Naing, K.; Klemm, L.; Swaminathan, S.; et al. Small-molecule inhibition of CBP/catenin interactions eliminates drug-resistant clones in acute lymphoblastic leukemia. Oncogene 2014, 33, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Hogan, L.E.; Meyer, J.A.; Yang, J.; Wang, J.; Wong, N.; Yang, W.; Condos, G.; Hunger, S.P.; Raetz, E.; Saffery, R.; et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood 2011, 118, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; O’Riordan, M.; Okamura, R.; Devaney, E.; Willert, K.; Nusse, R.; Grosschedl, R. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 2000, 13, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ranheim, E.A.; Kwan, H.C.; Reya, T.; Wang, Y.K.; Weissman, I.L.; Francke, U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood 2005, 105, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.S.; Angelova, M.; Lobelle-Rich, P.A.; Levy, L.S. Disruption of pre-B-cell receptor signaling jams the WNT/beta-catenin pathway and induces cell death in B-cell acute lymphoblastic leukemia cell lines. Leuk. Res. 2015, 39, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Gang, E.J.; Hsieh, Y.T.; Schaefer, P.; Chae, S.; Klemm, L.; Huantes, S.; Loh, M.; Conway, E.M.; Kang, E.S.; et al. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood 2011, 118, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Hogan, L.E.; Condos, G.; Bhatla, T.; Germino, N.; Moskowitz, N.P.; Lee, L.; Bhojwani, D.; Horton, T.M.; Belitskaya-Levy, I.; et al. Endogenous knockdown of survivin improves chemotherapeutic response in ALL models. Leukemia 2012, 26, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Jemal, A.M.; Thayer, M.; Druker, B.J.; Chang, B.H. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia 2012, 26, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Gang, E.J.; Kahn, M. CBP/Catenin antagonists: Targeting LSCs’ Achilles heel. Exp. Hematol. 2017, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mallampati, S.; Sun, B.; Zhang, J.; Kim, S.B.; Lee, J.S.; Gong, Y.; Cai, Z.; Sun, X. Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett. 2013, 333, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Weerkamp, F.; van Dongen, J.J.; Staal, F.J. Notch and Wnt signaling in T-lymphocyte development and acute lymphoblastic leukemia. Leukemia 2006, 20, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.; Hoofd, C.; Weng, A.P.; Giambra, V. Targeting leukemia stem cells: Which pathways drive self-renewal activity in T-cell acute lymphoblastic leukemia? Curr. Oncol. 2016, 23, 34–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernandes, J.C.; Rodrigues Alves, A.P.N.; Machado-Neto, J.A.; Scopim-Ribeiro, R.; Fenerich, B.A.; da Silva, F.B.; Simoes, B.P.; Rego, E.M.; Traina, F. IRS1/beta-Catenin Axis Is Activated and Induces MYC Expression in Acute Lymphoblastic Leukemia Cells. J. Cell Biochem. 2017, 118, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, R.; Liu, J.; Li, M.; Song, C.; Dovat, S.; Li, J.; Ge, Z. Characterization of LEF1 High Expression and Novel Mutations in Adult Acute Lymphoblastic Leukemia. PLoS ONE 2015, 10, e0125429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, J.; Feng, Y.; Khadka, B.; Fang, Z.; Gu, J.; Tang, B.; Xiao, R.; Pan, G.; Liu, J.J. A Regulatory Loop Involving Notch and Wnt Signaling Maintains Leukemia Stem Cells in T-Cell Acute Lymphoblastic Leukemia. Front. Cell Dev. Biol. 2021, 9, 678544. [Google Scholar] [CrossRef] [PubMed]
- Weerkamp, F.; Baert, M.R.; Naber, B.A.; Koster, E.E.; de Haas, E.F.; Atkuri, K.R.; van Dongen, J.J.; Herzenberg, L.A.; Staal, F.J. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc. Natl. Acad. Sci. USA 2006, 103, 3322–3326. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Weerkamp, F.; Baert, M.R.; van den Burg, C.M.; van Noort, M.; de Haas, E.F.; van Dongen, J.J. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J. Immunol. 2004, 172, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.; Langerak, A.W. Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica 2008, 93, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Groen, R.W.; Oud, M.E.; Schilder-Tol, E.J.; Overdijk, M.B.; ten Berge, D.; Nusse, R.; Spaargaren, M.; Pals, S.T. Illegitimate WNT pathway activation by beta-catenin mutation or autocrine stimulation in T-cell malignancies. Cancer Res. 2008, 68, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dose, M.; Kovalovsky, D.; Chang, R.; O’Neil, J.; Look, A.T.; von Boehmer, H.; Khazaie, K.; Gounari, F. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood 2007, 109, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Arambilet, D.; Guillen, Y.; Lobo-Jarne, T.; Maqueda, M.; Gekas, C.; Gonzalez, J.; Iglesias, A.; Vega-Garcia, N.; Sentis, I.; et al. beta-Catenin activity induces an RNA biosynthesis program promoting therapy resistance in T-cell acute lymphoblastic leukemia. EMBO Mol. Med. 2023, 15, e16554. [Google Scholar] [CrossRef] [PubMed]
- Bigas, A.; Guillen, Y.; Schoch, L.; Arambilet, D. Revisiting beta-Catenin Signaling in T-Cell Development and T-Cell Acute Lymphoblastic Leukemia. Bioessays 2020, 42, e1900099. [Google Scholar] [CrossRef] [PubMed]
- Gekas, C.; D’Altri, T.; Aligue, R.; Gonzalez, J.; Espinosa, L.; Bigas, A. beta-Catenin is required for T-cell leukemia initiation and MYC transcription downstream of Notch1. Leukemia 2016, 30, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Schubbert, S.; Cardenas, A.; Chen, H.; Garcia, C.; Guo, W.; Bradner, J.; Wu, H. Targeting the MYC and PI3K pathways eliminates leukemia-initiating cells in T-cell acute lymphoblastic leukemia. Cancer Res. 2014, 74, 7048–7059. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.M.F.; Colak, S.; Buikhuisen, J.; Koster, J.; Cameron, K.; de Jong, J.H.; Tuynman, J.B.; Prasetyanti, P.R.; Fessler, E.; van den Bergh, S.P.; et al. Methylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell 2011, 9, 476–485. [Google Scholar] [CrossRef]
- Roderick, J.E.; Tesell, J.; Shultz, L.D.; Brehm, M.A.; Greiner, D.L.; Harris, M.H.; Silverman, L.B.; Sallan, S.E.; Gutierrez, A.; Look, A.T.; et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood 2014, 123, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.Y.; Jun, Y.; Kim, J.Y.; Nam, J.S. Roles of Wnt Target Genes in the Journey of Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1604. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Han, R.; Gan, R. The Wnt/β-catenin signalling pathway in Haematological Neoplasms. Biomark Res 2022, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Agirre, X.; Jiménez-Velasco, A.; José-Eneriz, E.S.; Cordeu, L.; Gárate, L.; Vilas-Zornoza, A.; Castillejo, J.A.; Heiniger, A.; Prósper, F.; et al. Methylation status of Wnt signaling pathway genes affects the clinical outcome of Philadelphia-positive acute lymphoblastic leukemia. Cancer Sci. 2008, 99, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Roman-Gomez, J.; Jimenez-Velasco, A.; Cordeu, L.; Vilas-Zornoza, A.; San Jose-Eneriz, E.; Garate, L.; Castillejo, J.A.; Martin, V.; Prosper, F.; Heiniger, A.; et al. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur. J. Cancer 2007, 43, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.; Duque, M.; Gonçalves, J.; Akkapeddi, P.; Oliveira, M.L.; Cabrita, R.; Yunes, J.A.; Durum, S.K.; Barata, J.T.; Fragoso, R. NRARP displays either pro- or anti-tumoral roles in T-cell acute lymphoblastic leukemia depending on Notch and Wnt signaling. Oncogene 2020, 39, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Bernt, K.M.; Armstrong, S.A. Leukemia stem cells and human acute lymphoblastic leukemia. Semin. Hematol. 2009, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- le Viseur, C.; Hotfilder, M.; Bomken, S.; Wilson, K.; Rottgers, S.; Schrauder, A.; Rosemann, A.; Irving, J.; Stam, R.W.; Shultz, L.D.; et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell 2008, 14, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Mastelaro de Rezende, M.; Ferreira, A.T.; Paredes-Gamero, E.J. Leukemia stem cell immunophenotyping tool for diagnostic, prognosis, and therapeutics. J. Cell Physiol. 2020, 235, 4989–4998. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.V.; Evely, R.S.; Oakhill, A.; Pamphilon, D.H.; Goulden, N.J.; Blair, A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood 2004, 104, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.V.; Diamanti, P.; Evely, R.S.; Kearns, P.R.; Blair, A. Expression of CD133 on leukemia-initiating cells in childhood ALL. Blood 2009, 113, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Kuci, S. CD133-Positive Hematopoietic Stem Cells: From Biology to Medicine. Adv. Exp. Med. Biol. 2013, 777, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Gupta, R.; Ancliff, P.; Atzberger, A.; Brown, J.; Soneji, S.; Green, J.; Colman, S.; Piacibello, W.; Buckle, V.; et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science 2008, 319, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yoshida, S.; Saito, Y.; Doi, T.; Nagatoshi, Y.; Fukata, M.; Saito, N.; Yang, S.M.; Iwamoto, C.; Okamura, J.; et al. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia 2008, 22, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Bardini, M.; Woll, P.S.; Corral, L.; Luc, S.; Wittmann, L.; Ma, Z.; Lo Nigro, L.; Basso, G.; Biondi, A.; Cazzaniga, G.; et al. Clonal variegation and dynamic competition of leukemia-initiating cells in infant acute lymphoblastic leukemia with MLL rearrangement. Leukemia 2015, 29, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hansen, Q.; Bachas, C.; Smit, L.; Cloos, J. Characteristics of leukemic stem cells in acute leukemia and potential targeted therapies for their specific eradication. Cancer Drug Resist. 2022, 5, 344–367. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Zhang, J.; Kasper, L.H.; Lerach, S.; Payne-Turner, D.; Phillips, L.A.; Heatley, S.L.; Holmfeldt, L.; Collins-Underwood, J.R.; Ma, J.; et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 2011, 471, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mullighan, C.G.; Harvey, R.C.; Wu, G.; Chen, X.; Edmonson, M.; Buetow, K.H.; Carroll, W.L.; Chen, I.M.; Devidas, M.; et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 2011, 118, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.V.; Martin, H.M.; Kearns, P.R.; Virgo, P.; Evely, R.S.; Blair, A. Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukemia. Blood 2007, 109, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr. The co-receptor function of CD4. Semin. Immunol. 1991, 3, 153–160. [Google Scholar] [PubMed]
- Gerby, B.; Clappier, E.; Armstrong, F.; Deswarte, C.; Calvo, J.; Poglio, S.; Soulier, J.; Boissel, N.; Leblanc, T.; Baruchel, A.; et al. Expression of CD34 and CD7 on human T-cell acute lymphoblastic leukemia discriminates functionally heterogeneous cell populations. Leukemia 2011, 25, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.P.; Jiang, H.; Dick, J.E. Leukemia-initiating cells in human T-lymphoblastic leukemia exhibit glucocorticoid resistance. Blood 2010, 116, 5268–5279. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Tremblay, C.S.; Herblot, S.; Aplan, P.D.; Hebert, J.; Perreault, C.; Hoang, T. Modeling T-cell acute lymphoblastic leukemia induced by the SCL and LMO1 oncogenes. Genes Dev. 2010, 24, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.Y.; Shestova, O.; Xu, L.; Aster, J.C.; Pear, W.S. Divergent effects of supraphysiologic Notch signals on leukemia stem cells and hematopoietic stem cells. Blood 2013, 121, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Piovan, E.; Yu, J.; Tosello, V.; Herranz, D.; Ambesi-Impiombato, A.; Da Silva, A.C.; Sanchez-Martin, M.; Perez-Garcia, A.; Rigo, I.; Castillo, M.; et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell 2013, 24, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Panelli, P.; De Santis, E.; Colucci, M.; Tamiro, F.; Sansico, F.; Miroballo, M.; Murgo, E.; Padovano, C.; Gusscott, S.; Ciavarella, M.; et al. Noncanonical beta-catenin interactions promote leukemia-initiating activity in early T-cell acute lymphoblastic leukemia. Blood 2023, 141, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Lasky, J.L.; Chang, C.J.; Mosessian, S.; Lewis, X.; Xiao, Y.; Yeh, J.E.; Chen, J.Y.; Iruela-Arispe, M.L.; Varella-Garcia, M.; et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 2008, 453, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal. Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Pecina-Slaus, N.; Anicic, S.; Bukovac, A.; Kafka, A. Wnt Signaling Inhibitors and Their Promising Role in Tumor Treatment. Int. J. Mol. Sci. 2023, 24, 6733. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pepe, F.; Bill, M.; Papaioannou, D.; Karunasiri, M.; Walker, A.; Naumann, E.; Snyder, K.; Ranganathan, P.; Dorrance, A.; Garzon, R. Targeting Wnt signaling in acute myeloid leukemia stem cells. Haematologica 2022, 107, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Roulston, G.D.; Burt, C.L.; Kettyle, L.M.; Matchett, K.B.; Keenan, H.L.; Mulgrew, N.M.; Ramsey, J.M.; Dougan, C.; McKiernan, J.; Grishagin, I.V.; et al. Low-dose salinomycin induces anti-leukemic responses in AML and MLL. Oncotarget 2016, 7, 73448–73461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, L.; Li, Y.; Lu, L.-H.; Chang, Y.; Wang, W.-P.; Li, X.; Xu, X.-R.; Gao, J.-Z. [Expression and Significance of Low-Density Lipoprotein-Related Receptors 5 and 6 in the Wnt/β-Catenin Signaling Pathway in Childhood Acute Lymphoblastic Leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pan, W. GSK3: A multifaceted kinase in Wnt signaling. Trends. Biochem. Sci. 2010, 35, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P. GSK-3 as a novel prognostic indicator in leukemia. Adv. Biol. Regul. 2017, 65, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ueda, M.; Stetson, L.; Ignatz-Hoover, J.; Moreton, S.; Chakrabarti, A.; Xia, Z.; Karan, G.; de Lima, M.; Agrawal, M.K.; et al. A Novel Glycogen Synthase Kinase-3 Inhibitor Optimized for Acute Myeloid Leukemia Differentiation Activity. Mol. Cancer Ther. 2016, 15, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Rizzieri, D.A.; Cooley, S.; Odenike, O.; Moonan, L.; Chow, K.H.; Jackson, K.; Wang, X.; Brail, L.; Borthakur, G. An open-label phase 2 study of glycogen synthase kinase-3 inhibitor LY2090314 in patients with acute leukemia. Leuk. Lymphoma 2016, 57, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Jing, Z.; Sun, P.; Wang, Y.; Dong, P.; Liu, J. TARDBP drives T-cell acute lymphoblastic leukemia progression by binding MDM2 mRNA, involving beta-catenin pathway. FASEB J. 2024, 38, e70110. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001, 276, 36337–36343. [Google Scholar] [CrossRef] [PubMed]
- Ratti, A.; Buratti, E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J. Neurochem. 2016, 138 (Suppl. 1), 95–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dodge, M.E.; Tang, W.; Lu, J.; Ma, Z.; Fan, C.W.; Wei, S.; Hao, W.; Kilgore, J.; Williams, N.S.; et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Minke, K.S.; Staib, P.; Puetter, A.; Gehrke, I.; Gandhirajan, R.K.; Schlosser, A.; Schmitt, E.K.; Hallek, M.; Kreuzer, K.A. Small molecule inhibitors of WNT signaling effectively induce apoptosis in acute myeloid leukemia cells. Eur. J. Haematol. 2009, 82, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Spencer, G.J.; Brooks, N.; Amaral, F.M.R.; Basma, N.J.; Chadwick, J.A.; Revell, B.; Wingelhofer, B.; Maiques-Diaz, A.; Sinclair, O.; et al. Therapeutic targeting of EP300/CBP by bromodomain inhibition in hematologic malignancies. Cancer Cell 2023, 41, 2136–2153. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wu, T.; Zhang, C.; Wang, C.; Xu, H.; Hu, Q.; Hu, J.; Luo, G.; Zhuang, X.; Wu, X.; et al. Discovery of a potent and selective CBP bromodomain inhibitor (Y08262) for treating acute myeloid leukemia. Bioorg. Chem. 2024, 142, 106950. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Chiarini, F.; Cappellini, A.; Paganelli, F.; Fini, M.; Santi, S.; Martelli, A.M.; Neri, L.M.; Evangelisti, C. Targeting Wnt/beta-catenin and PI3K/Akt/mTOR pathways in T-cell acute lymphoblastic leukemia. J. Cell Physiol. 2020, 235, 5413–5428. [Google Scholar] [CrossRef] [PubMed]
- Moisse, K.; Volkening, K.; Leystra-Lantz, C.; Welch, I.; Hill, T.; Strong, M.J. Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: Implications for TDP-43 in the physiological response to neuronal injury. Brain. Res. 2009, 1249, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Toh, S.H.; Chan, Z.L.; Quah, J.Y.; Chooi, J.Y.; Tan, T.Z.; Chong, P.S.Y.; Zeng, Q.; Chng, W.J. A loss-of-function genetic screening reveals synergistic targeting of AKT/mTOR and WTN/beta-catenin pathways for treatment of AML with high PRL-3 phosphatase. J. Hematol. Oncol. 2018, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Wu, W.; Davidson, G.; Marhold, J.; Li, M.; Mechler, B.M.; Delius, H.; Hoppe, D.; Stannek, P.; Walter, C.; et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 2002, 417, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Salik, B.; Yi, H.; Hassan, N.; Santiappillai, N.; Vick, B.; Connerty, P.; Duly, A.; Trahair, T.; Woo, A.J.; Beck, D.; et al. Targeting RSPO3-LGR4 Signaling for Leukemia Stem Cell Eradication in Acute Myeloid Leukemia. Cancer Cell 2020, 38, 263–278.e6. [Google Scholar] [CrossRef] [PubMed]
- Hamdoun, S.; Fleischer, E.; Klinger, A.; Efferth, T. Lawsone derivatives target the Wnt/beta-catenin signaling pathway in multidrug-resistant acute lymphoblastic leukemia cells. Biochem. Pharmacol. 2017, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, M.; Li, Y.; Zhao, L.; Wei, Q. Glycogen synthase kinase-3: A potential immunotherapeutic target in tumor microenvironment. Biomed. Pharmacother 2024, 173, 116377. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I.; Ladenson, P.W. Organ-specific effects of tiratricol: A thyroid hormone analog with hepatic, not pituitary, superagonist effects. J. Clin. Endocrinol. Metab. 1992, 75, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, L.; van Laar, T.; van Dam, H.; Ten Dijke, P. GSK3beta inactivation induces apoptosis of leukemia cells by repressing the function of c-Myb. Mol. Biol. Cell 2011, 22, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Smith, K.S.; Murphy, M.; Piloto, O.; Somervaille, T.C.; Cleary, M.L. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature 2008, 455, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.F.; Benajiba, L.; Campbell, A.J.; Weiwer, M.; Sacher, J.R.; Gale, J.P.; Ross, L.; Puissant, A.; Alexe, G.; Conway, A.; et al. Exploiting an Asp-Glu “switch” in glycogen synthase kinase 3 to design paralog-selective inhibitors for use in acute myeloid leukemia. Sci. Transl. Med. 2018, 10, eaam8460. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.; O’Brien, T.A.; Knight, R.; Lindeman, R.; Shen, S.; Song, E.; Symonds, G.; Dolnikov, A. Glycogen synthase kinase-3beta inhibition preserves hematopoietic stem cell activity and inhibits leukemic cell growth. Stem Cells 2008, 26, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Kang, X.; Lu, J.; Zhang, Y.; Wu, X.; Wu, G.; Zheng, J.; Tuladhar, R.; Shi, H.; Wang, Q.; et al. Installation of a cancer promoting WNT/SIX1 signaling axis by the oncofusion protein MLL-AF9. EBioMedicine 2019, 39, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.; Daniels, G.; Wang, D.; Deng, F.M.; Lee, P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am. J. Cancer Res. 2017, 7, 1389–1406. [Google Scholar] [PubMed]
- Giotopoulos, G.; Chan, W.I.; Horton, S.J.; Ruau, D.; Gallipoli, P.; Fowler, A.; Crawley, C.; Papaemmanuil, E.; Campbell, P.J.; Gottgens, B.; et al. The epigenetic regulators CBP and p300 facilitate leukemogenesis and represent therapeutic targets in acute myeloid leukemia. Oncogene 2016, 35, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.A.; Murray, J.; Lai, K.W.; Tsui, V.; Albrecht, B.K.; An, L.; Beresini, M.H.; de Leon Boenig, G.; Bronner, S.M.; Chan, E.W.; et al. GNE-781, A Highly Advanced Potent and Selective Bromodomain Inhibitor of Cyclic Adenosine Monophosphate Response Element Binding Protein, Binding Protein (CBP). J. Med. Chem. 2017, 60, 9162–9183. [Google Scholar] [CrossRef] [PubMed]
- Katavolos, P.; Cain, G.; Farman, C.; Romero, F.A.; Magnuson, S.; Ly, J.Q.; Choo, E.F.; Katakam, A.K.; Andaya, R.; Maher, J. Preclinical Safety Assessment of a Highly Selective and Potent Dual Small-Molecule Inhibitor of CBP/P300 in Rats and Dogs. Toxicol. Pathol. 2020, 48, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Bigas, A.; Guiu, J.; Gama-Norton, L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013, 51, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Mak, P.Y.; Mu, H.; Tao, W.; Mak, D.H.; Kornblau, S.; Zhang, Q.; Ruvolo, P.; Burks, J.K.; Zhang, W.; et al. Disruption of Wnt/beta-Catenin Exerts Antileukemia Activity and Synergizes with FLT3 Inhibition in FLT3-Mutant Acute Myeloid Leukemia. Clin. Cancer Res. 2018, 24, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Benoit, Y.D.; Mitchell, R.R.; Risueno, R.M.; Orlando, L.; Tanasijevic, B.; Boyd, A.L.; Aslostovar, L.; Salci, K.R.; Shapovalova, Z.; Russell, J.; et al. Sam68 Allows Selective Targeting of Human Cancer Stem Cells. Cell Chem. Biol. 2017, 24, 833–844.e9. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Chiu, C.C.; Dahms, H.U.; Chou, C.K.; Cheng, C.M.; Chang, W.T.; Cheng, K.C.; Wang, H.D.; Lin, I.L. Unfolded Protein Response (UPR) in Survival, Dormancy, Immunosuppression, Metastasis, and Treatments of Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2518. [Google Scholar] [CrossRef] [PubMed]
- Kharabi Masouleh, B.; Chevet, E.; Panse, J.; Jost, E.; O’Dwyer, M.; Bruemmendorf, T.H.; Samali, A. Drugging the unfolded protein response in acute leukemias. J. Hematol. Oncol. 2015, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Jung, J.E.; Emami, K.; Briaud, I.; Tenzin, F.; Pyon, Y.; Lee, D.; Chung, J.U.; Lee, J.H.; Oh, S.W.; et al. Abstract LB-176: Discovery of CWP232291: A novel and potent small molecule inhibitor of Wnt signaling in hematological malignacies. Cancer Res. 2010, 70, LB-176. [Google Scholar] [CrossRef]
- Lee, J.H.; Faderl, S.; Pagel, J.M.; Jung, C.W.; Yoon, S.S.; Pardanani, A.D.; Becker, P.S.; Lee, H.; Choi, J.; Lee, K.; et al. Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv. 2020, 4, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Mitton, B.; Chae, H.D.; Hsu, K.; Dutta, R.; Aldana-Masangkay, G.; Ferrari, R.; Davis, K.; Tiu, B.C.; Kaul, A.; Lacayo, N.; et al. Small molecule inhibition of cAMP response element binding protein in human acute myeloid leukemia cells. Leukemia 2016, 30, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Duque-Afonso, J.; Lin, C.H.; Han, K.; Morgens, D.W.; Jeng, E.E.; Weng, Z.; Jeong, J.; Wong, S.H.K.; Zhu, L.; Wei, M.C.; et al. CBP Modulates Sensitivity to Dasatinib in Pre-BCR(+) Acute Lymphoblastic Leukemia. Cancer Res. 2018, 78, 6497–6508. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.; Ogana, H.; Wan, Z.; Navid, F.; Bhojwani, D.; Kim, Y.M. TBL1 Inhibitor, Tegavivint, Supresses Cell Survival and Promotes Sensitivity to Chemotherapy in ALL and AML. Blood 2022, 140, 5995. [Google Scholar] [CrossRef]
- Fiskus, W.; Sharma, S.; Saha, S.; Shah, B.; Devaraj, S.G.; Sun, B.; Horrigan, S.; Leveque, C.; Zu, Y.; Iyer, S.; et al. Pre-clinical efficacy of combined therapy with novel beta-catenin antagonist BC2059 and histone deacetylase inhibitor against AML cells. Leukemia 2015, 29, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
| Wnt Ligand | Signaling Type | Role in ALL |
|---|---|---|
| Wnt1 | Canonical | Drives β-catenin activation; involved in proliferation and differentiation |
| Wnt2b | Canonical | Linked to leukemogenesis and methylation-mediated gene regulation in B-ALL |
| Wnt3a | Canonical | Promotes proliferation, β-catenin activation, and self-renewal |
| Wnt10a | Canonical | Prognostic marker in AML; not well-studied in ALL |
| Wnt10b | Canonical | Linked to HSC regeneration and leukemia stemness |
| Wnt16b | Canonical | Promotes survival in pre-B-ALL with E2A-Pbx1 fusion |
| Wnt5a | Non-Canonical | Enhances migration and invasion in T-ALL |
| Wnt9a | Canonical/Non-Canonical | Supports leukemic cell survival via stromal interaction |
| Wnt11 | Canonical/Non-Canonical | Induces morphological changes and invasion |
| Targets | Compound | Leukemia Type | Clinical Trial (Number) | PMID | ||
|---|---|---|---|---|---|---|
| Targeting Upstream Effectors | PORCN | WNT974 | AML | Preclinical | [144] | |
| DKK1 | DKK1-conditioned medium | B-ALL | Preclinical | [24] | ||
| LRP5/6 | Salinomycin | AML | Preclinical | [145] | ||
| RSPO | OMP-131R10 (rosmantuzumab) | AML | Preclinical | [162] | ||
| Frizzled | Lawsone | T-ALL | Preclinical | [163] | ||
| GSK3 | GS87 | AML | Preclinical | [149] | ||
| LY2090314 | AML | NCT01214603, Phase II | [167] | |||
| BRD0705 | T-ALL | Preclinical | [168] | |||
| SB216763 | T-ALL | [166] | ||||
| Promotingβ-catenin Degradation | Tankyrase | XAV939 | B-ALL, T-ALL | Preclinical | [95,151] | |
| IWR107 | AML | [170] | ||||
| IWP2G9 | AML | [170] | ||||
| Inhibiting β-catenin– T-cell factor (TCF) Interaction | TCF | iCRT14 | B-ALL, T-ALL | Preclinical | [85] | |
| LEF1 | CGP049090 | AML | Preclinical | [155] | ||
| [155] | ||||||
| PFK115-584 | AML | |||||
| T-ALL | [108] | |||||
| CBP/P300 | GNE-781 | AML | Preclinical | [173] | ||
| CCS1477 | NCT04068597 Phase 1/2a | [156] | ||||
| CBP | ICG-001 | T-ALL | Preclinical | [158] | ||
| B-ALL | [86] | |||||
| AML | [160] | |||||
| PRI-724 (C-82 pro-drug) | AML | NCT01606579 Phase I/II | [176] | |||
| CWP232291 | AML | NCT01398462 Phase I | [180] | |||
| XX-650–23 | AML, B-ALL | Preclinical | [182,183] | |||
| Y08262 | AML | Preclinical | [157] | |||
| TBL1 | BC2059 | AML | NCT04874480 Phase I | [185] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurwitz, S.; Lee, K.J.; Fourfouris, T.; Choi, I.; Parikh, K.; Friedmann, R.; Zarrabi, M.; Kim, Y.-M. Targeting Wnt Signaling in Acute Lymphoblastic Leukemia. Cancers 2025, 17, 2456. https://doi.org/10.3390/cancers17152456
Hurwitz S, Lee KJ, Fourfouris T, Choi I, Parikh K, Friedmann R, Zarrabi M, Kim Y-M. Targeting Wnt Signaling in Acute Lymphoblastic Leukemia. Cancers. 2025; 17(15):2456. https://doi.org/10.3390/cancers17152456
Chicago/Turabian StyleHurwitz, Samantha, Ki Jun Lee, Tatiana Fourfouris, Irene Choi, Krishan Parikh, Rachel Friedmann, Maiah Zarrabi, and Yong-Mi Kim. 2025. "Targeting Wnt Signaling in Acute Lymphoblastic Leukemia" Cancers 17, no. 15: 2456. https://doi.org/10.3390/cancers17152456
APA StyleHurwitz, S., Lee, K. J., Fourfouris, T., Choi, I., Parikh, K., Friedmann, R., Zarrabi, M., & Kim, Y.-M. (2025). Targeting Wnt Signaling in Acute Lymphoblastic Leukemia. Cancers, 17(15), 2456. https://doi.org/10.3390/cancers17152456






