Clinicopathologic Predictors of Survival Following Oral Cancer Surgery: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Adjuvant Treatment
2.5. Follow–Up Assessment
2.6. Histopathological Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Assessment of Clinical Variables
3.3. Evaluation of Certain Pathological Variables
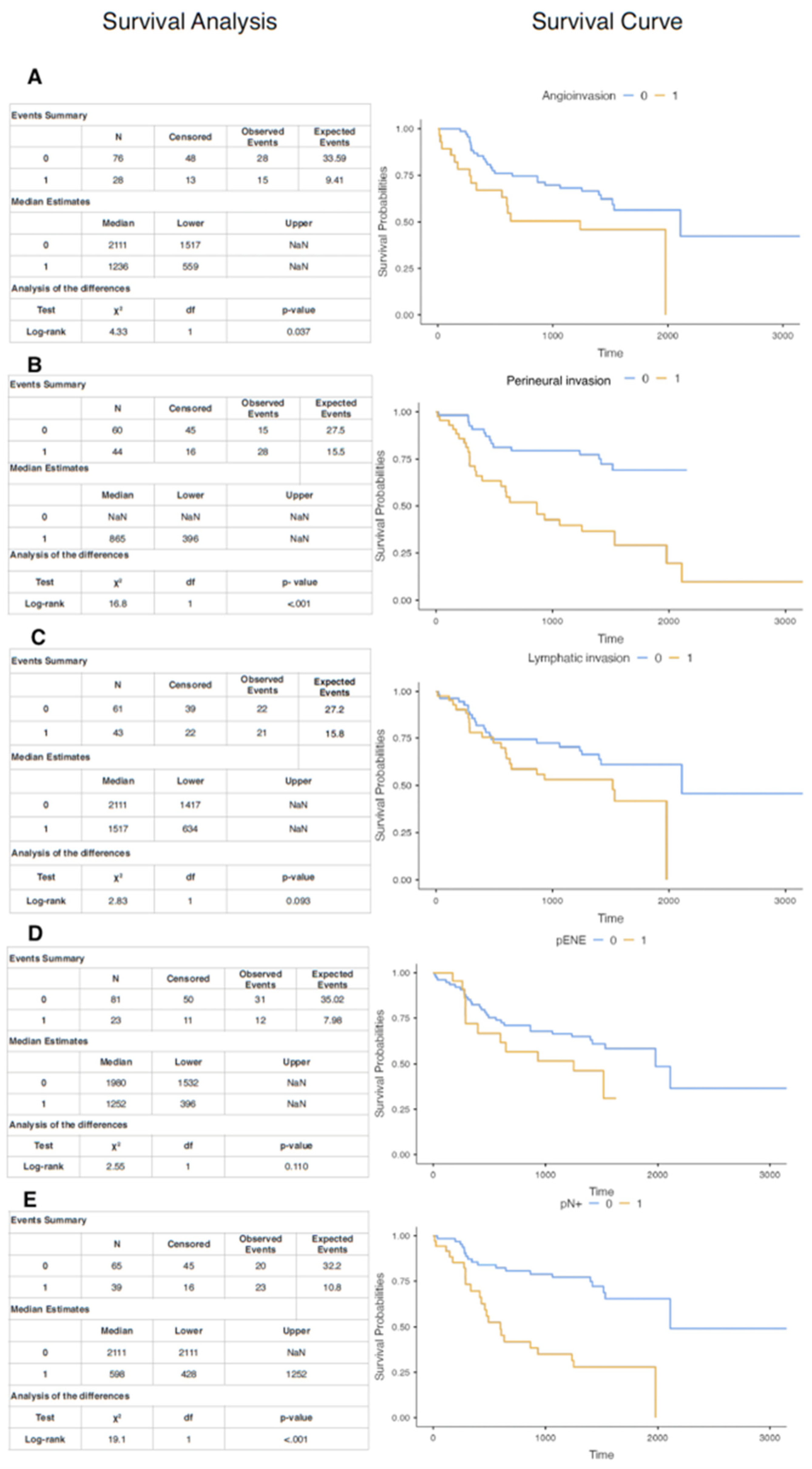
3.4. Univariate Analysis
3.5. Multivariate Analysis
3.6. Cox Regression Analysis
3.7. Kaplan–Meyer Curves
3.8. Combined Perineural, Vascular, and Lymphatic Invasion: Clinical Implications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC/CAP | American Joint Committee on Cancer/College of American Pathologists |
| CHTH | Chemotherapy |
| CI | Confidence Interval |
| DOD | Death of Disease |
| DOI | Depth of Invasion |
| DM | Diabetes Mellitus |
| DSS | Disease-Specific Survival |
| ECOG | Eastern Cooperative Oncology Group |
| H&E | Hematoxylin & Eosin |
| HPV | Human Papillomavirus |
| HR | Hazard Ratio |
| IARC | International Agency for Research on Cancer |
| IHC | Immunohistochemistry |
| IRB | Institutional Review Board |
| IMRT | Intensity-Modulated Radiation Therapy |
| M | Mean |
| NCCN | National Comprehensive Cancer Network |
| OR | Odds Ratio |
| OS | Overall Survival |
| OSCC | Oral Squamous Cell Carcinoma |
| pENE | Pathological Extranodal Expression |
| PORT | Postoperative Radiotherapy |
| RFS | Recurrence Free Survival |
| RTH | Radiotherapy |
| SCC | Squamous Cell Carcinoma |
| SD | Standard Deviation BMC Cancer 2024 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention, Volume 19: Oral Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2023. [Google Scholar]
- Fridman, E.; Na’ARa, S.; Agarwal, J.; Amit, M.; Bachar, G.; Villaret, A.B.; Brandao, J.; Cernea, C.R.; Chaturvedi, P.; Clark, J.; et al. The role of adjuvant treatment in early-stage oral cavity squamous cell carcinoma: An international collaborative study. Cancer 2018, 124, 2948–2955. [Google Scholar] [CrossRef] [PubMed]
- Alim, N.; Elsheikh, M.; Satti, A.A.; Tabassum, N.; Suleiman, A.M. Recurrence of oral squamous cell carcinoma in surgically treated patients at Khartoum Teaching Dental Hospital retrospective cross-sectional study. BMC Cancer 2024, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Gupta, N.; Kb, J. Clinicopathological prognostic implicators of oral squamous cell carcinoma: Need to understand and revise. North Am. J. Med Sci. 2013, 5, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lindenblatt, R.d.C.R.; Martinez, G.L.; Silva, L.E.; Faria, P.S.; Camisasca, D.R.; Lourenço, S.d.Q.C. Oral squamous cell carcinoma grading systems–analysis of the best survival predictor. J. Oral Pathol. Med. 2011, 41, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Alemany, L.; Quer, M.; Halec, G.; Quirós, B.; Tous, S.; Clavero, O.; Alòs, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, djv403. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, V.; Reid, M.; Hatton, E.; Merzianu, M.; Rigual, N.; Marshall, J.; Gill, S.; Frustino, J.; Wilding, G.; Loree, T.; et al. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: A meta-analysis, 1985–2010. Oral Oncol. 2011, 47, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pandey, M.; Dhiman, V.K.; Sharma, A.; Pandey, H.; Verma, S.K.; Pandey, R. Personalized medicine: An alternative for cancer treatment. Cancer Treat. Res. Commun. 2024, 42, 100860. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.T.; Chau, C.H.; Price, D.K.; Figg, W.D. Precision Oncology Medicine: The Clinical Relevance of Patient-Specific Biomarkers Used to Optimize Cancer Treatment. J. Clin. Pharmacol. 2016, 56, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, Y.; Nashef, A.; Sidransky, D.; Abdelraziq, M.; Alkeesh, K.; Araidy, S.; Koch, W.; Brait, M.; Abu El-Naaj, I. Clinical and Prognostic Significance of the Eighth Edition Oral Cancer Staging System. Cancers 2022, 14, 4632. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancer (Version 2.2025). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 10 June 2025).
- Rizzo, A.; Mollica, V.; Cimadamore, A.; Santoni, M.; Scarpelli, M.; Schiavina, R.; Cheng, L.; Lopez-Beltran, A.; Brunocilla, E.; Montironi, R.; et al. TNM staging towards a personalized approach in metastatic urothelial carcinoma: What will the future be like?—A narrative review. Transl. Androl. Urol. 2021, 10, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, L.; Sacco, R.; Papa, S.; Carr, B.I. Understanding the Drawbacks of the Current Tumor Staging Systems: How to Improve? Cancers 2023, 15, 1242. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.R.B.; Jeffus, S.K.; Gehlot, A.; Stone, R.; Erickson, S.W.; Kelly, T.; Quick, C.M.; Post, S.R. Perineural Invasion Is an Independent Pathologic Indicator of Recurrence in Vulvar Squamous Cell Carcinoma. Am. J. Surg. Pathol. 2015, 39, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Mariani, P.; Caponio, V.C.A.; Russo, L.L.; Fiorillo, L.; Zhurakivska, K.; Muzio, L.L.; Laino, L.; Troiano, G. Development and Validation of Prognostic Models for Oral Squamous Cell Carcinoma: A Systematic Review and Appraisal of the Literature. Cancers 2021, 13, 5755. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Piazza, C. Editorial: Oral Oncology: From Precise Surgery to Precision Medicine and Surgery. Front. Oral Heal. 2022, 3, 913172. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakami, H.A.; Al-Talhi, A.A.; AlRajhi, B.; Alshareef, M.A.; Awad, B.I.; Hussain, T.; Al-Garni, M. Oncological outcomes, survival analysis, and failure patterns in patients with resectable squamous cell carcinoma of the oral tongue treated with glossectomy. Egypt. J. Otolaryngol. 2025, 41, 1–11. [Google Scholar] [CrossRef]
- Tranby, E.P.; Heaton, L.J.; Tomar, S.L.; Kelly, A.L.; Fager, G.L.; Backley, M.; Frantsve-Hawley, J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.-F.; Kauke, M.; Grandoch, A.; Nickenig, H.-J.; Zöller, J.E.; Kreppel, M. Analysis of clinicopathological risk factors for locoregional recurrence of oral squamous cell carcinoma–Retrospective analysis of 517 patients. J. Cranio-Maxillofac. Surg. 2017, 45, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, I.; Glaun, M.D.; Prabhash, K.; Busheri, A.; Lai, S.Y.; Noronha, V.; Hosni, A. Current Treatment Strategies and Risk Stratification for Oral Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389810. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraf, M. Treatment of Locally Advanced Head and Neck Cancer: Historical and Critical Review. Cancer Control. 2002, 9, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, D.; George, N.A.; Thomas, S.; Iype, E.M. Factors associated with delay in diagnosis of oral cancers. Cancer Treat. Res. Commun. 2024, 40, 100831. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, R.; Vilén, S.-T.; Keinänen, A.; Salo, T.; Qannam, A.; Bello, I.O.; Snäll, J. Oral squamous cell carcinoma: Effect of tobacco and alcohol on cancer location. Tob. Induc. Dis. 2024, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Colares, N.; Rodrigues, D.F.S.; Freitas, M.O.; Dantas, T.S.; Cunha, M.D.P.S.S.; Sousa, F.B.; Silva, P.G.d.B. Smoking History Decreases Survival in Patients with Squamous Cell Carcinoma of the Mouth: A Retrospective Study with 15 Years of Follow-up. Asian Pac. J. Cancer Prev. 2019, 20, 1781–1787. [Google Scholar] [CrossRef]
- Katsi, V.; Papakonstantinou, I.; Tsioufis, K. Atherosclerosis, Diabetes Mellitus, and Cancer: Common Epidemiology, Shared Mechanisms, and Future Management. Int. J. Mol. Sci. 2023, 24, 11786. [Google Scholar] [CrossRef] [PubMed]
- Remschmidt, B.; Pau, M.; Gaessler, J.; Zemann, W.; Jakse, N.; Payer, M.; Végh, D. Diabetes Mellitus and Oral Cancer: A Retrospective Study from Austria. Anticancer. Res. 2022, 42, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Ogden, G.R. Alcohol and mouth cancer. Br. Dent. J. 2018, 225, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Mores, A.L.; Bonfim-Alves, C.G.; López, R.V.M.; Rodrigues-Oliveira, L.; Palmier, N.R.; Mariz, B.A.L.A.; Migliorati, C.A.; Kowalski, L.P.; Santos-Silva, A.R.; Brandão, T.B.; et al. Prognostic Factors in Head and Neck Cancer: A Retrospective Cohort Study of 3052 Patients in Brazil. Oral Dis. 2024, 31, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Das, A.; Dhal, I.; Shankar, R.; Bhavya, B.; Singh, N.; Tripathi, P.; Daga, D.; Rai, A.; Gupta, M.; et al. Worst pattern of invasion in oral squamous cell carcinoma is an independent prognostic factor. J. Oral Biol. Craniofacial Res. 2022, 12, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.M.V.O.; Dedivitis, R.A.; Kowalski, L.P. Prognostic impact of perineural invasion in oral cancer: A systematic review. Acta Otorhinolaryngol. Ital. 2022, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-W.; Lin, L.-H.; Lin, H.-P.; Liu, C.-J. Perineural Invasion Unveiled: Deciphering the Prognostic Impact of Diameter and Quantity Subcategories in Oral Cancer. J. Otolaryngol.–Head Neck Surg. 2025, 54, 19160216251316219. [Google Scholar] [CrossRef] [PubMed]
- Misztal, C.I.; Green, C.; Mei, C.; Bhatia, R.; Torres, J.M.V.; Kamrava, B.; Moon, S.; Nicolli, E.; Weed, D.; Sargi, Z.; et al. Molecular and Cellular Mechanisms of Perineural Invasion in Oral Squamous Cell Carcinoma: Potential Targets for Therapeutic Intervention. Cancers 2021, 13, 6011. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar, I.N.; Alonso, S.E.; Serrano, F.A.; Herrera, I.H.; León, J.J.Z.; Vera, J.L.D.C.P.d.; López, A.M.L.; Muela, C.M.; de Frutos, G.A.; Caicoya, S.O.; et al. Depth of Invasion: Influence of the Latest TNM Classification on the Prognosis of Clinical Early Stages of Oral Tongue Squamous Cell Carcinoma and Its Association with Other Histological Risk Factors. Cancers 2023, 15, 4882. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Kao, H.-K.; Hsu, C.-L.; Huang, J.-J.; Lee, L.-Y.; Huang, Y.; Browne, T.; Tsang, N.-M.; Chang, Y.-L.; Chang, K.-P. Evaluation of Lymphatic and Vascular Invasion in Relation to Clinicopathological Factors and Treatment Outcome in Oral Cavity Squamous Cell Carcinoma. Medicine 2015, 94, e1510. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.-C.; Lee, T.-L.; Li, W.-Y.; Chang, C.-F.; Chu, P.-Y.; Wang, Y.-F.; Tai, S.-K. Perineural invasion/lymphovascular invasion double positive predicts distant metastasis and poor survival in T3–4 oral squamous cell carcinoma. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, S.; Gerken, M.; Fischer, R.; Mamilos, A.; Spoerl, S.; Wolf, S.; Pohl, F.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; et al. Lymphatic and vascular invasion in oral squamous cell carcinoma: Implications for recurrence and survival in a population-based cohort study. Oral Oncol. 2020, 111, 105009. [Google Scholar] [CrossRef] [PubMed]
- Mijatov, I.; Kiralj, A.; Ilić, M.P.; Vučković, N.; Spasić, A.; Nikolić, J.; Tadić, A.; Mijatov, S. Pathological tumor volume as a simple quantitative predictive factor of survival in oral squamous cell carcinoma. Oncol. Lett. 2023, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, E.; Cercenelli, L.; Maiolo, V.; Bortolani, B.; Marcelli, E.; Tarsitano, A. Pretreatment Tumor Volume and Tumor Sphericity as Prognostic Factors in Patients with Oral Cavity Squamous Cell Carcinoma: A Prospective Clinical Study in 95 Patients. J. Pers. Med. 2023, 13, 1601. [Google Scholar] [CrossRef] [PubMed]
- Viswanatha, S.C.; Hedne, N.; Hasan, S. Correlation between histological grading, LVI and PNI of carcinoma oral tongue to lymph node metastasis. Int. J. Otorhinolaryngol. Head Neck Surg. 2018, 5, 159–164. [Google Scholar] [CrossRef]
- Williams, H.K. Molecular pathogenesis of oral squamous carcinoma. Mol. Pathol. 2000, 53, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Haidari, S.; Obermeier, K.T.; Kraus, M.; Otto, S.; Probst, F.A.; Liokatis, P. Nodal Disease and Survival in Oral Cancer: Is Occult Metastasis a Burden Factor Compared to Preoperatively Nodal Positive Neck? Cancers 2022, 14, 4241. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Gontu, G.S.S.R.; Aasumi, K.; Das, R.J.; Das, A.; Rahman, T.; Das, A.K.; Kakati, K. Occult Metastasis: Incidence, Pattern, and Impact on Survival in Patients with Oral Cancer, pN0 vs pN1 in a Cohort of cN0. A Prospective Cohort Study. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 5312–5318. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Rinaldo, A.; Devaney, K.O.; MacLennan, K.; Myers, J.N.; Petruzzelli, G.J.; Shaha, A.R.; Genden, E.M.; Johnson, J.T.; de Carvalho, M.B.; et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncol. 2002, 38, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Lin, Y.-T.; Chuang, H.-C.; Huang, T.-L.; Lu, H.; Chien, C.-Y.; Fang, F.-M. Prognostic Value of Pathologically Positive Nodal Number in p16-Negative Oropharyngeal and Hypopharyngeal Squamous Cell Carcinoma with pN3b Status. Diagnostics 2022, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-S.; Chen, C.-C.; Liu, Y.-C.; Wang, C.-C.; Chou, Y.-S. Peri-Neural Invasion Is an Important Prognostic Factor of T2N0 Oral Cancer. Medicina 2022, 58, 1809. [Google Scholar] [CrossRef] [PubMed]
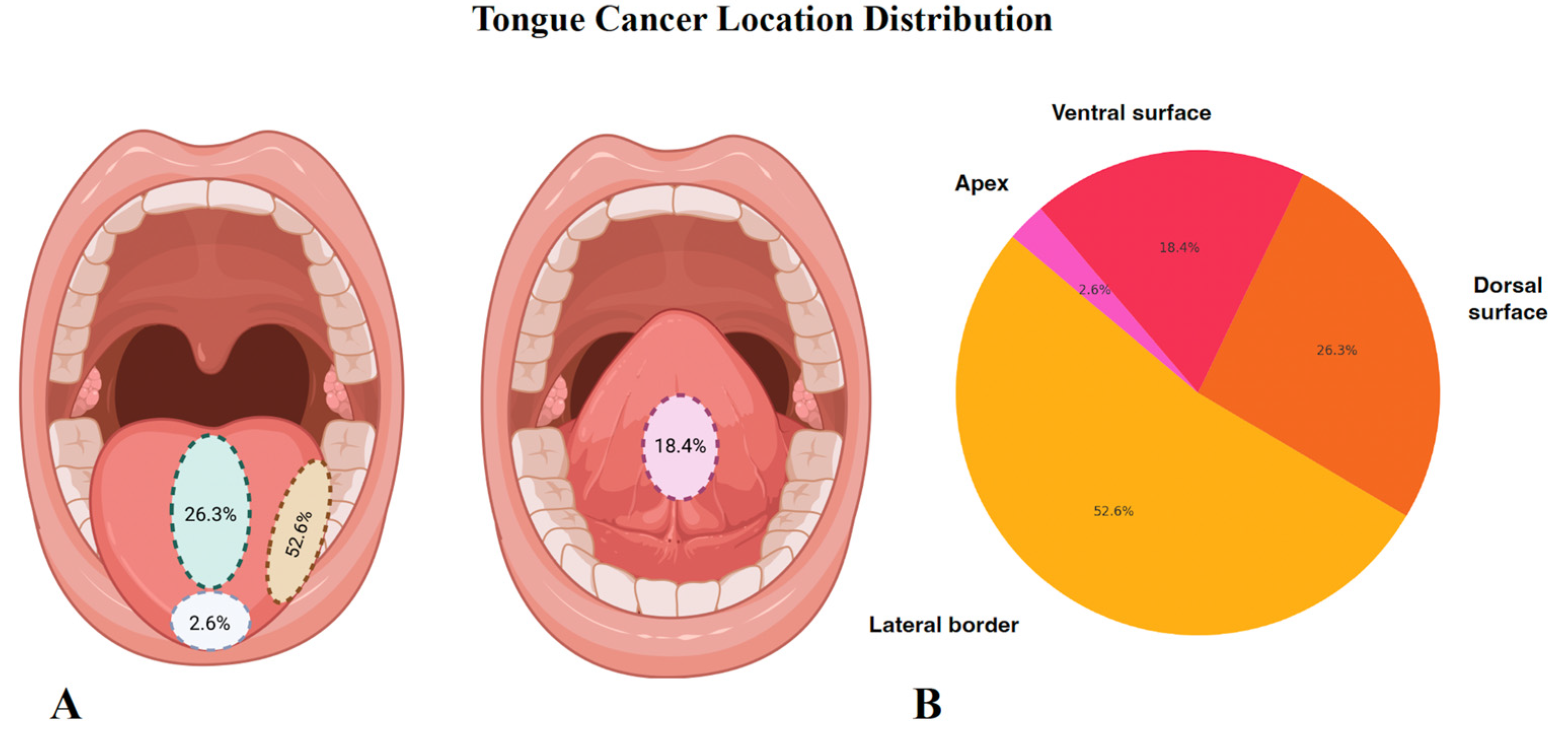
| Variable | N (%) |
|---|---|
| Age | 62.09 ± 10.28 (M ± SD) |
| Gender M | 57 (57.0%) |
| F | 43 (43.0%) |
| DM 2 | 15 (15%) |
| Smoking | 60 (60%) |
| Smoking-years | 25.73 ± 9.65 1(M ± SD) |
| Alcohol use | 45 (45%) |
| Alcohol-years | 8.72 ± 4.79 (M ± SD) |
| Peripheral atherosclerosis | 40 (40%) |
| RTH post-surgery | 64 (64%) |
| RTH mean dose | 62.15 ± 2.90 (M ± SD) |
| CHTH post-surgery | 8 (8%) |
| CHTH mean dose | 244.44 ± 101.38 (M ± SD) |
| Overall Survival | 46 (46 %) |
| DSS | 43 (43.0%) |
| Recurrence | 29 (29%) |
| Local recurrence | 23 (79.3%) |
| Regional recurrence | 6 (20.7%) |
| Recurrence time (months) | 14.4 ± 11.9 (M ± SD) |
| ECOG 0 | 33 (33%) |
| ECOG 1 | 67 (67%) |
| Variable | N (%) |
|---|---|
| Tumor histology | |
| SCC | 100 (100%) |
| Tumor volume in cm3 | 34.55 ± 59.38 (M ± SD) |
| Angioinvasion | 28 (28%) |
| Lymphatic invasion | 42 (42%) |
| Perineural invasion | 44 (44%) |
| pENE | 23 (23%) |
| pN+ | 37 (37%) |
| Grading | |
| G1 | 8 (8%) |
| G2 | 68 (64%) |
| G3 | 24 (24%) |
| TNM | |
| T1 | 6 (6%) |
| T2 | 25 (25%) |
| T3 | 27 (27%) |
| T4 | 42 (42%) |
| N0 | 54 (54%) |
| N1 | 19 (19%) |
| N2 | 25 (25%) |
| N3 | 2 (2%) |
| N (%) | Age (Mean ± SD) | Male (%) | Female (%) | Smoking Years (Mean ± SD) | Alcohol Years (Mean ± SD) | RTH (%) | CHTH (%) | T1 (%) | T2 (%) | T3 (%) | T4 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gum | 11 (11%) | 62.9 ± 6.9 | 7 (64%) | 4 (36%) | 30.6 ± 8.1 | 6.8 ± 2.6 | 8 (73%) | 0 (0%) | 0 (0%) | 2 (18%) | 1 (9%) | 8 (73%) |
| Tongue | 38 (38%) | 59.9 ± 10.5 | 24 (63%) | 14 (37%) | 27.9 ± 8.4 | 9.7 ± 5.5 | 21 (55%) | 3 (8%) | 2 (5%) | 15 (39%) | 10 (26%) | 9 (24%) |
| Floor of the mouth | 29 (29%) | 62.1 ± 9.7 | 13 (45%) | 16 (55%) | 21.7 ± 11.3 | 8.6 ± 4.9 | 23 (79%) | 4 (14%) | 2 (7%) | 5 (17%) | 6 (21%) | 15 (52%) |
| Hard palate | 2 (2%) | 47.0 ± 32.5 | 2 (100%) | 0 (0%) | nan ± nan | 15.0 ± nan | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | 1 (50%) |
| Cheek mucosa | 13 (13%) | 68.1 ± 7.9 | 6 (46%) | 7 (54%) | 22.5 ± 8.7 | 5.8 ± 2.0 | 9 (69%) | 1 (8%) | 1 (8%) | 3 (23%) | 5 (38%) | 4 (31%) |
| Lower lip | 6 (6%) | 63.3 ± 3.4 | 5 (83%) | 1 (17%) | 21.7 ± 7.6 | 15.0 ± nan | 3 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) | 4 (67%) |
| Upper lip | 1 (1%) | 80.0 ± nan | 0 (0%) | 1 (100%) | 30.0 ± nan | 8.0 ± nan | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) |
| Clinical Variable | Perineural Invasion | Angioinvasion | Lymphatic |
|---|---|---|---|
| Age (mean) | −0.057 | 0.077 | 0.096 |
| Gender | 0.037 | 0.002 | 0.043 |
| Smoking | −0.099 | −0.082 | 0.033 |
| Smoking-years | −0.045 | 0.214 | 0.141 |
| Alcohol | −0.032 | 0.152 | 0.086 |
| Alcohol-years | −0.245 | 0.041 | −0.099 |
| DM 2 | −0.09 | −0.012 | −0.017 |
| Peripheral atherosclerosis | 0.063 | 0.011 | −0.104 |
| post-RTH | 0.059 | −0.009 | 0.072 |
| post-RTH mean dose | 0.127 | −0.132 | 0.684 |
| post-CHTH | 0.11 | 0.309 | 0.048 |
| post-CHTH mean dose | −0.164 | 0.329 | 0.286 |
| Death from disease | 0.403 | 0.134 | 0.081 |
| Disease recurrence | 0.188 | 0.141 | 0.081 |
| Pathological Variable | Neuroinvasion | Angioinvasion | Lymphatic Invasion |
|---|---|---|---|
| Tumor volume | 0.0697 | −0.0809 | 0.3992 |
| T1 | −0.0543 | −0.0638 | −0.215 |
| T2 | −0.0202 | −0.1665 | −0.0887 |
| T3 | −0.0853 | 0.1224 | −0.0155 |
| T4 | −0.0378 | 0.0624 | 0.1686 |
| pN+ | 0.238 | 0.4625 | 0.193 |
| G1 | −0.1129 | −0.1839 | 0.1225 |
| G2 | −0.0397 | −0.2406 | −0.0243 |
| G3 | 0.1151 | 0.3796 | −0.0512 |
| pENE | 0.0536 | −0.1414 | 0.5186 |
| Gum | −0.077 | −0.077 | −0.077 |
| Tongue | 0.062 | 0.062 | 0.062 |
| Floor of the mouth | −0.006 | −0.006 | −0.006 |
| Hard palate | 0.07 | 0.07 | 0.07 |
| Cheek mucosa | −0.042 | −0.042 | −0.042 |
| Lower lip | 0.03 | 0.03 | 0.03 |
| Upper lip | −0.063 | −0.063 | −0.063 |
| Angioinvasion | 0.3412 | 0.2809 | |
| Neuroinvasion | 0.3412 | 0.2159 | |
| Lymphatic invasion | 0.2159 | 0.2809 |
| Variable | Odds Ratio | CI Lower | CI Upper | p-Value |
| pN+ | 3.841 | 1.61 | 9.161 | 0.0024 |
| Angioinvasion | 1.826 | 0.751 | 4.443 | 0.1841 |
| Perineural invasion | 5.63 | 2.362 | 13.42 | 0.0001 |
| Lymphatic invasion | 1.39 | 0.626 | 3.084 | 0.4183 |
| Tumor volume in cm3 | 1.002 | 0.996 | 1.009 | 0.4839 |
| pENE | 1.405 | 0.55 | 3.59 | 0.4769 |
| Odds Ratio | CI Lower | CI Upper | p-Value | |
|---|---|---|---|---|
| pN+ | 4.837 | 1.974 | 11.852 | 0.0006 |
| Perineural invasion | 3.079 | 1.215 | 7.804 | 0.0178 |
| Variable | Category 0 (n, %) | Category 1 (n, %) | HR (Univariable) | HR (Multivariable) |
|---|---|---|---|---|
| pN+ | 25 (46.3%) | 29 (53.7%) | 1.69 (0.88–3.24), p = 0.117 | 1.68 (0.88–3.24), p = 0.118 |
| Perineural invasion | 20 (37.0%) | 34 (63.0%) | 1.05 (0.54–2.08), p = 0.877 | 1.02 (0.52–2.02), p = 0.944 |
| Variable | Value |
|---|---|
| Gender | |
| Male | 11 (78.6%) |
| Female | 3 (21.4%) |
| Age | |
| Mean ± SD | 59.6 ± 10.3 years |
| Tumor volume | |
| Mean ± SD | 37.8 ± 20.5 cm3 |
| Reccurence | |
| Yes | 9 (64.3%) |
| No | 5 (35.7%) |
| Death | |
| Deceased | 10 (71.4%) |
| Statistic | Value |
| Chi-square | 5.191 |
| p-value | 0.0227 |
| Degrees of Freedom | 1.0 |
| Logistic Regression | Patients with all three invasions |
| Coefficient | 1.838 |
| Odds Ratio | 6.285 |
| CI Lower | 1.326 |
| CI Upper | 29.778 |
| p-value | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarz, K.; Bieńkowska-Pluta, K.; Galazka, A.; Gorzelnik, A.; Durzynska, M.; Misiak-Galazka, M.; Stawarz, G.; Zwolinski, J. Clinicopathologic Predictors of Survival Following Oral Cancer Surgery: A Retrospective Cohort Study. Cancers 2025, 17, 2454. https://doi.org/10.3390/cancers17152454
Stawarz K, Bieńkowska-Pluta K, Galazka A, Gorzelnik A, Durzynska M, Misiak-Galazka M, Stawarz G, Zwolinski J. Clinicopathologic Predictors of Survival Following Oral Cancer Surgery: A Retrospective Cohort Study. Cancers. 2025; 17(15):2454. https://doi.org/10.3390/cancers17152454
Chicago/Turabian StyleStawarz, Katarzyna, Karolina Bieńkowska-Pluta, Adam Galazka, Anna Gorzelnik, Monika Durzynska, Magdalena Misiak-Galazka, Grzegorz Stawarz, and Jakub Zwolinski. 2025. "Clinicopathologic Predictors of Survival Following Oral Cancer Surgery: A Retrospective Cohort Study" Cancers 17, no. 15: 2454. https://doi.org/10.3390/cancers17152454
APA StyleStawarz, K., Bieńkowska-Pluta, K., Galazka, A., Gorzelnik, A., Durzynska, M., Misiak-Galazka, M., Stawarz, G., & Zwolinski, J. (2025). Clinicopathologic Predictors of Survival Following Oral Cancer Surgery: A Retrospective Cohort Study. Cancers, 17(15), 2454. https://doi.org/10.3390/cancers17152454






