Advances in Vulvar Cancer: A Radiation Oncology Perspective
Simple Summary
Abstract
1. Introduction
2. Surgically Resectable Cancer of the Vulva
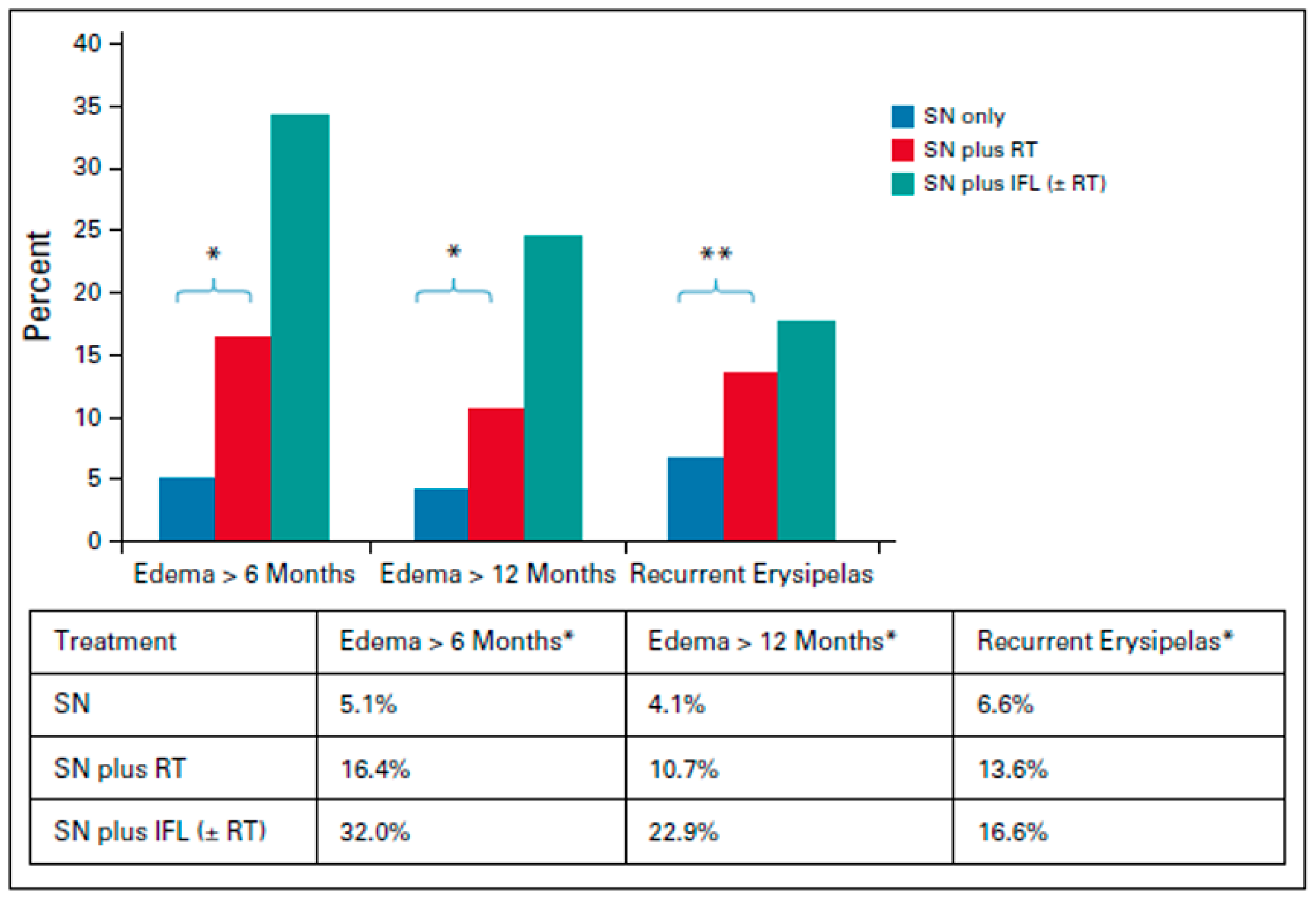
Adjuvant Radiotherapy for the Primary Tumor and Local Regional Lymph Nodes
3. Non-Operative Cancer of the Vulva
4. Vulvar Cancer with Distant Metastatic Disease
5. Future Directions in Clinical Research
5.1. Surgery
5.2. Systemic Therapy
5.3. Radiotherapy
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Key Statistics & Cancer. Available online: https://seer.cancer.gov/statfacts/html/vulva.html (accessed on 1 May 2025).
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Alemany, L.; Ordi, J.; Tous, S.; Alejo, M.; Bigby, S.M.; Joura, E.A.; Maldonado, P.; Laco, J.; Bravo, I.G.; et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer 2013, 49, 3450–3461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Zhang, Z. Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0204162. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.L.; Sand, F.L.; Fredericksen, M.F.; Andersen, K.K.; Kjaer, S.K. Does HPV status influence survival after vulvar cancer? Int. J. Cancer 2018, 142, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; Allo, G.; Cuartero, J.; Pintilie, M.; Kamel-Reid, S.; Murphy, J.; Mackay, H.; Clarke, B.; Fyles, A.; Milosevic, M. Prognostic Significance of Human Papilloma Virus and p16 Expression in Patients with Vulvar Squamous Cell Carcinoma who Received Radiotherapy. Clin. Oncol. 2018, 30, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, M.C.; Visser, P.J.; Overbeek, L.I.; van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Parra-Herran, C.; Nucci, M.R.; Singh, N.; Rakislova, N.; Howitt, B.E.; Hoang, L.; Gilks, C.B.; Bosse, T.; Watkins, J.C. HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: A review of nomenclature and the journey to characterize verruciform and acanthotic precursor lesions of the vulva. Mod. Pathol. 2022, 35, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. World Health Organization Classification of Tumours—Female Genital Tumours, 5th ed.; IARC: Lyon, France, 2020. [Google Scholar]
- Heller, D.S.; Day, T.; Allbritton, J.I.; Scurry, J.; Radici, G.; Welch, K.; Preti, M.; ISSVD Difficult Pathologic Diagnoses Committee. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J. Low. Genit. Tract. Dis. 2021, 25, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.S.; Olawaiye, A.; Borger, D.; Growdonb, W.B.; Krasnerb, C.N.; Matulonisa, U.A.; Liua, J.F.; Leea, J.; Brardc, L.; Dizon, D.S. Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol. Oncol. 2012, 127, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtsh. Frauenheilk. 2021, 81, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Garganese, G.; Inzani, F.; Fragomeni, S.M.; Mantovani, G.; Della Corte, L.; Piermattei, A.; Santoro, A.; Angelico, G.; Giacò, L.; Corrado, G.; et al. The Vulvar Immunohistochemical Panel (VIP) Project: Molecular Profiles of Vulvar Squamous Cell Carcinoma. Cancers 2021, 13, 6373. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Mannel, R.; Khalifa, M.; Walker, J.L.; Wren, M.; Min, K.-W.; Benbrook, D.M. Epidermal growth factor receptor in vulvar malignancies and its relationship to metastasis and patient survival. Gynecol. Oncol. 1997, 65, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Growdon, W.B.; Boisvert, S.L.; Akhavanfard, S.; Oliva, E.; Dias-Santagata, D.C.; Kojiro, S.; Horowitz, N.S.; Iafrate, A.J.; Borger, D.R.; Rueda, B.R. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol. Oncol. 2008, 111, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; de Bock, G.H.; van der Veen, D.J.; Ten Hoor, K.A.; de Hullu, J.A.; Hollema, H.; van der Zee, A.G. EGFR expression is associated with groin node metastases in vulvar cancer, but does not improve their prediction. Gynecol. Oncol. 2007, 104, 109–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Howitt, B.E.; Sun, H.H.; Roemer, M.G.M.; Kelley, A.; Chapuy, B.; Aviki, E.; Pak, C.; Connelly, C.; Gjini, E.; Shi, Y.; et al. Genetic Basis for PD-L1 Expression in Squamous Cell Carcinomas of the Cervix and Vulva. JAMA Oncol. 2016, 2, 518–522. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Vulva Version 1.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025; Available online: https://www.nccn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 1 May 2025).
- Wagner, M.M.; van der Zee, A.G.J.; Oonk, M.H.M. History and Updates of the GROINSS-V Studies. Cancers 2022, 14, 1956. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.J.; Chundury, A.; Schwarz, J.K.; Hassanzadeh, C.; DeWees, T.; Mullen, D.; Powell, M.A.; Mutch, D.G.; Grigsby, P.W. Intensity modulated radiation therapy for squamous cell carcinoma of the vulva: Treatment technique and outcomes. Adv. Radiat. Oncol. 2017, 2, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, D.K.; King, B.; Viswanathan, A.N.; Barkati, M.; Beriwal, S.; Eifel, P.; Erickson, B.; Fyles, A.; Goulart, J.; Harkenrider, M.; et al. Consensus Recommendations for Radiation Therapy Contouring and Treatment of Vulvar Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H. Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: Are two therapies better than one? Gynecol. Oncol. 2009, 113, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Hacker, N.F.; Van der Velden, J. Conservative management of early vulvar cancer. Cancer 1993, 71 (Suppl. S4), 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Stehman, F.B.; Bundy, B.N.; Dvoretsky, P.M.; Creasman, W.T. Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: A prospective study of the Gynecologic Oncology Group. Obstet. Gynecol. 1992, 79, 490–497. [Google Scholar] [PubMed]
- Gaarenstroom, K.N.; Kenter, G.G.; Trimbos, J.B.; Agous, I.; Amant, F.; Peters, A.A.W.; Vergote, I. Postoperative Complications After Vulvectomy and Inguinofemoral Lymphadenectomy using Separate Groin Incisions. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2003, 13, 522–527. [Google Scholar] [CrossRef]
- Rouzier, R.; Haddad, B.; Dubernard, G.; Dubois, P.; Paniel, B.-J. Inguinofemoral dissection for carcinoma of the vulva: Effect of modifications of extent and technique on morbidity and survival. J. Am. Coll. Surg. 2003, 196, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; van Hemel, B.M.; Hollema, H.; de Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; et al. Size of Sentinel-Node Metastasis and Chances of Non-Sentinel-Node Involvement and Survival in Early Stage Vulvar Cancer: Results from GROINSS-V, a Multicentre Observational Study. Lancet Oncol. 2010, 11, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Vander Zee, A.G.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel Node Dissection is Safe in the Treatment of Early-Stage Vulvar Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Te Grootenhuis, N.C.; van der Zee, A.G.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brannstrom, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Heaps, J.M.; Fu, Y.S.; Montz, F.J.; Hacker, N.F.; Berek, J.S. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol. Oncol. 1990, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Sugiyama, V.; Pham, H.; Gu, M.; Rutgers, J.; Osann, K.; Cheung, M.K.; Berman, M.L.; Disaia, P.J. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol. Oncol. 2007, 104, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.M.; Mirmow, D.; Huang, Q.; Gerszten, K.; Day, R.; Jones, M.W. Adjuvant radiation for vulvar carcinoma: Improved local control. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Pinto, A.P.; Schultz, D.; Berkowitz, R.; Crum, C.P. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol. Oncol. 2013, 130, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Choschzick, M.; Eulenburg, C.; Hager, M.; Jaenicke, F.; Gieseking, F.; Kock, L.; Ihnen, M.; Petersen, C.; Schwarz, J.; et al. Prognostic Value of Pathological Resection Margin Distance in Squamous Cell Cancer of the Vulva. Ann. Surg. Oncol. 2011, 18, 3811–3818. [Google Scholar] [CrossRef] [PubMed]
- Bedell, S.; Hedberg, C.; Griffin, A.; Pearson, H.; Wilhite, A.; Rubin, N.; Erickson, B.K. Role of adjuvant radiation or re-excision for early stage vulvar squamous cell carcinoma with positive or close surgical margins. Gynecol. Oncol. 2019, 154, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Te Grootenhuis, N.C.; Pouwer, A.W.; de Bock, G.H.; Hollema, H.; Bulten, J.; van der Zee, A.G.J.; de Hullu, J.A.; Oonk, M.H.M. Margin status revisited in vulvar squamous cell carcinoma. Gynecol. Oncol. 2019, 154, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerova, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.V.; Gill, B.S.; Viswanathan, A.N.; Balasubramani, G.K.; Sukumvanich, P.; Beriwal, S. Adjuvant radiotherapy for margin-positive vulvar squamous cell carcinoma: Defining the ideal dose-response using the National Cancer Data Base. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Adcock, L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obs. Gynecol. 1986, 68, 733–740. [Google Scholar]
- Mahner, S.; Jueckstock, J.; Hilpert, F.; Neuser, P.; Harter, P.; de Gregorio, N.; Hasenburg, A.; Sehouli, J.; Habermann, A.; Hillemanns, P.; et al. Adjuvant therapy in lymph node–positive vulvar cancer: The AGO-CaRE-1 study. J. Natl. Cancer Inst. 2015, 107, dju426. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Prieske, K.; Eulenburg, C.Z.; Corradini, S.; Petersen, C.; Bommert, M.; Blankenstein, T.; Hilpert, F.; de Gregorio, N.; Iborra, S.; et al. Adjuvant radiotherapy and local recurrence in vulvar cancer—A subset analysis of the AGO-CaRE-1 study. Gynecol. Oncol. 2022, 164, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cheung, M.K.; Osann, K.; Husain, A.; Teng, N.N.; Berek, J.S.; Kapp, D.S.; Chan, J.K. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol. Oncol. 2006, 103, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Bernard, M.E.; Lin, J.F.; Balasubramani, G.K.; Rajagopalan, M.S.; Sukumvanich, P.; Krivak, T.C.; Olawaiye, A.B.; Kelley, J.L.; Beriwal, S. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynecol. Oncol. 2015, 137, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Kim, D.H.; Higgins, S.A.; Carcangiu, M.L.; BM, K. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Rydzewski, N.R.; Kanis, M.J.; Donnelly, E.D.; Lurain, J.R.; Strauss, J.B. Role of adjuvant external beam radiotherapy and chemotherapy in one versus two or more node-positive vulvar cancer: A National Cancer Database study. Radiother. Oncol. 2018, 129, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Boronow, R.C. Combined therapy as an alternative to exenteration for locally advanced vulvo-vaginal cancer: Rationale and results. Cancer 1982, 49, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Levin, W.; Goldberg, G.; Altaras, M.; Bloch, B.; Shelton, M.G. The use of concomitant chemotherapy and radiotherapy prior to surgery in advanced stage carcinoma of the vulva. Gynecol. Oncol. 1986, 25, 20–25. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3732915 (accessed on 1 May 2025). [CrossRef] [PubMed]
- Moore, D.H.; Thomas, G.M.; Montana, G.S.; Saxer, A.; Gallup, D.G.; Olt, G. Preoperative chemoradiation for advanced vulvar cancer: A phase II study of the Gynecologic Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H.; Ali, S.; Koh, W.-J.; Michael, H.; Barnes, M.N.; McCourt, C.K.; Homesley, H.D.; Walker, J.L. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol. Oncol. 2012, 124, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.S.; Deng, W.; Peterson, I.; Mannel, R.S.; Thompson, S.; Lokich, E.; Myers, T.; Hanjani, P.; O’Malley, D.M.; Chung, K.Y.; et al. Phase II Trial of Cisplatin, Gemcitabine, and Intensity-Moulated Radiation Therapy for Locally Advanced Vulvar Squamous Cell Carcinoma: NRG Oncology/GOG Study 279. JCO 2024, 42, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Horne, Z.D.; Dohopolski, M.J.; Pradhan, D.; Bhargava, R.; Edwards, R.P.; Kelley, J.L.; Comerci, J.T.; Olawaiye, A.B.; Courtney-Brooks, M.B.; Bockmeier, M.M.; et al. Human papillomavirus infection mediates response and outcome of vulvar squamous cell carcinomas treated with radiation therapy. Gynecol. Oncol. 2018, 151, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Howitt, B.; Catalano, P.; Tanaka, C.; Murphy, R.; Cimbak, N.; DeMaria, R.; Bu, P.; Crum, C.; Horowitz, N.; et al. Prognostic importance of human papillomavirus (HPV) and p16 positivity in squamous cell carcinoma of the vulva treated with radiotherapy. Gynecol. Oncol. 2016, 142, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, P.O.; van der Velden, J.; Vergote, I.; Guerra, C.; Scarabeli, C.; Coens, C.; Demonty, G.; Reed, N. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: A study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer—Gynaecological Cancer Group). Ann. Oncol. 2009, 20, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, J.T.; Blessing, J.A.; Homesley, H.D.; Lewis, G.C., Jr. Phase II trials of cisplatin and piperazinedione in advanced or recurrent squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol. Oncol. 1986, 23, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Cormio, G.; Loizzi, V.; Gissi, F.; Serrati, G.; Panzarino, M.; Carriero, C.; Selvaggi, L. Cisplatin and Vinorelbine Chemotherapy in Recurrent Vulvar Carcinoma. Oncology 2009, 77, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-cell inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Shapira-Frommer, R.; Mileshkinb, L.; Manzyukc, L.; Peneld, N.; Burgee, M.; Piha-Paul, S.A.; Girda, E.; Martin, J.A.L.; van Dongen, M.G.J.; Italiano, A.; et al. Efficacy and safety of pembrolizumab for patients with previously treated advanced vulvar squamous cell carcinoma: Results from the phase 2 KEYNOTE-158 study. Gynecol. Oncol. 2022, 166, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.-J.; Oaknin, A.; Kerger, J.; Lopez-Pocazo, J.M.; Machiels, J.-P.; Delord, J.-P.; Evans, T.R.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results From the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, G.; Thayer, E.G.; Patel, A.B.; Weirich, M.L.; Starbuck, K.D. Induction chemotherapy with cemiplimab in a patient with coexistent vulvar cancer and autoimmune disease: A case report. Gynecol. Oncol. Rep. 2024, 55, 101487. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with cemiplimab in recurrent cervical cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Klavans, M.R.; Erickson, S.H.; Modesitt, S.C. Neoadjuvant chemotherapy with paclitaxel/carboplatin/bevacizumab in advanced vulvar cancer: Time to rethink standard of care? Gynecol. Oncol. Rep. 2020, 34, 100631. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.M.; Ahmad, S.; Recio, F.O.; Awada, A.; McKenzie, N.D.; Kendrick, J.E.; Keller, A.; Holloway, R.W. Neoadjuvant chemotherapy with bevacizumab for locally advanced vulvar cancer. Int. J. Gynecol. Cancer 2024, 34, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Choschzick, M.; Hess, S.; Grob, T.; Burandt, E.; Linn Wolber, L.; Simon, R.; Sauter, G. HER 2 amplification in squamous cell carcinomas of the vulva. Histopathology 2013, 62, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; Gonzalez-Martin, A.; Jung, K.H.; Lugowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. J. Clin. Oncol. 2023, 41, LBA3000. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ctv.veeva.com/study/stratification-of-vulvar-squamous-cell-carcinoma-by-hpv-and-p53-status-to-guide-excision (accessed on 1 May 2025).
- Sardi, J.; Sananes, C.; Giaroli, A.; Bayo, J.; Rueda, N.G.; Vighi, S.; Guardado, N.; Paniceres, G.; Snaidas, L.; Vico, C.; et al. Results of a Prospective Randomized Trial with Neoadjuvant Chemotherapy in Stage IB, Bulky, Squamous Carcinoma of the Cervix. Gynecol Oncol. 1993, 49, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Treatment of Locally Advanced VULvar CArcinoma in a Neoadjuvant Setting With Carboplatin and Paclitaxel Chemotherapy (VULCANize); The Netherlands Cancer Institute: Amsterdam, Netherlands. 2022. Available online: https://connect.careboxhealth.com/en-US/trial/listing/243736 (accessed on 1 May 2025).
- Yeku, O.O.; Russo, A.L.; Bregar, A.; Brower, J.V.; Atwal, D.; Bouberhan, S.; Shea, M.; Widick, P.; Jang, J.W.; Colella, T.; et al. Primary results of a phase 2 study of cisplatin-sensitized radiation therapy and pembrolizumab for unresectable vulvar cancer Meeting Abstract: JCO. 2025 ASCO Annual Meeting I. J. Clin. Oncol. 2025, 43, 16. [Google Scholar]
- Swanick, C.W.; Eifel, P.J.; Huo, J.; Meyer, L.A.; Smith, G.L. Challenges to Delivery and Effectiveness of Adjuvant Radiation Therapy in Elderly Patients with Node-Positive Vulvar Cancer. Gynecol. Oncol. 2017, 146, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, S.; Crafton, S.M.; Miller, E.M.; Krivak, T.C.; Glaser, S.M.; Teterichko, S.R.; Sukumvanich, P.; Viswanathan, A.N.; Beriwal, S.; Horne, Z.D. Optimal overall treatment time for adjuvant therapy for completely resected, node-positive vulvar cancer. Gynecol. Oncol. 2021, 161, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Zhao, X.; Ponzini, M.; Leiserowitz, G.; Brooks, R.A. Time to completion of radiation treatment in LAVC and the impact on survival. Gynecol. Oncol. 2022, 167, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.E.T.; Lit, M.; Gupta, V.; Zakashansky, K.; Zeligs, K.; Kolev, V. Upfront boost to gross disease followed by elective pelvic radiation improves compliance to radiation therapy delivery metrics in locally advanced vulvar cancer. Gynecol. Oncol. Rep. 2024, 52, 101362. [Google Scholar] [CrossRef] [PubMed]
- van Triest, B.; Rasing, M.; van der Velden, J.; de Hullu, P.O.; Witteveenm, J.C.; Beaukema, E.; van der Steen-Banasik, H.; Westerveld, A.; Snyers, M.; Peters, C.L.; et al. Phase II study of definitive chemoradiation for locally advanced squamous cell cancer of the vulva: An efficacy study. Gyn. Oncol. 2021, 163, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.M.; Ferioli, M.; Argnani, L.; De Terlizzi, F.; Pirovano, C.; Covarelli, P.; Dondi, G.; Tesei, M.; De Crescenzo, E.; Ravegnini, G.; et al. Quality of Life with Vulvar Carcinoma Treated with Palliative Electrochemotherapy: The ELECHTRA (ELEctroCHemoTherapy vulvaR cAncer) Study. Cancers 2021, 13, 1622. [Google Scholar] [CrossRef] [PubMed]

| Factor | Keratinizing Squamous Carcinomas | Basaloid Squamous Carcinomas |
|---|---|---|
| Prevalence | 80% | 20% |
| Age | Older | Younger |
| Related Disease | LS and other vulvar dystrophy | HPV infection, other anogenital lesions, VIN, multifocality |
| p16 | often (−) | often (+) |
| p53 | either (+) or (−) | Often (−) |
| Side-Effects | IFN Dissection | SLN Surgery | |
|---|---|---|---|
| Wound breakdown | 34% | 11.7% | p < 0.0001 |
| Cellulitis | 21.3% | 4.5% | p < 0.0001 |
| Lymphedema | 25.2% | 1.9% | p < 0.0001 |
| UCLA/CoH Heaps et al. [32] | Irvine Chan et al. [33] | Pittsburgh Faul et al. [34] | BWH/DFCI Viswanathan et al. [35] | |
|---|---|---|---|---|
| Study period | 1957–1985 | 1984–2000 | 1980–2004 | 1980–2009 |
| No: of patients | 135 | 90 | 62 | 205 |
| No: of patients with close/positive | 44 | 60 | 62 | 116 |
| Close margin | <8 mm | <8 mm | <8 mm | <5 mm |
| % patients receiving RT | 0% | 20% | 50% | 30% |
| Local recurrence | 47.7% | 23% | 58% no RT 16% with RT | 38.9% |
| GOG 101 [50] Phase II | GOG 205 [51] Phase II | GOG 279 [52] Phase II | |
|---|---|---|---|
| Protocol | 2 cycles of 5FU + cisplatin 47.6 Gy × 1.7 G fx. Split course RT with break Biopsy/Surgery 4–8 weeks later | Weekly cisplatin 45 Gy with boost 57.6 Gy to gross disease Biopsy/Surgery 4–8 weeks later | Weekly cisplatin + gemcitabine IMRT 45 Gy with boost 64 Gy to gross primary/nodes Imaging evaluation 4–6 weeks with FNA to confirm path status |
| Evaluable # of patients | 71 | 58 | 52 |
| Clinical complete response (CCR) | 34 (48%) | 37 (64%) | 37 (71%) |
| Complete pathologic response (CPR) | 22 (31%) | 29 (50%) | 38 (73%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Peacock, D.N.; Chadha, M. Advances in Vulvar Cancer: A Radiation Oncology Perspective. Cancers 2025, 17, 2415. https://doi.org/10.3390/cancers17152415
Ayala-Peacock DN, Chadha M. Advances in Vulvar Cancer: A Radiation Oncology Perspective. Cancers. 2025; 17(15):2415. https://doi.org/10.3390/cancers17152415
Chicago/Turabian StyleAyala-Peacock, Diandra N., and Manjeet Chadha. 2025. "Advances in Vulvar Cancer: A Radiation Oncology Perspective" Cancers 17, no. 15: 2415. https://doi.org/10.3390/cancers17152415
APA StyleAyala-Peacock, D. N., & Chadha, M. (2025). Advances in Vulvar Cancer: A Radiation Oncology Perspective. Cancers, 17(15), 2415. https://doi.org/10.3390/cancers17152415






