Lateral Flow Assay to Detect Carbonic Anhydrase IX in Seromas of Breast Implant-Associated Anaplastic Large Cell Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seroma Samples and Cell Lines
2.2. Preparation of LFA Strips and Conjugation of Latex Detection Particles with CA9 Antibodies
2.3. Lateral Flow Assay (LFA)
2.4. Image Analysis
2.5. Immunostaining
2.6. Enzyme Linked Immunosorbant Assay (ELISA)
2.7. Statistical Analysis
3. Results
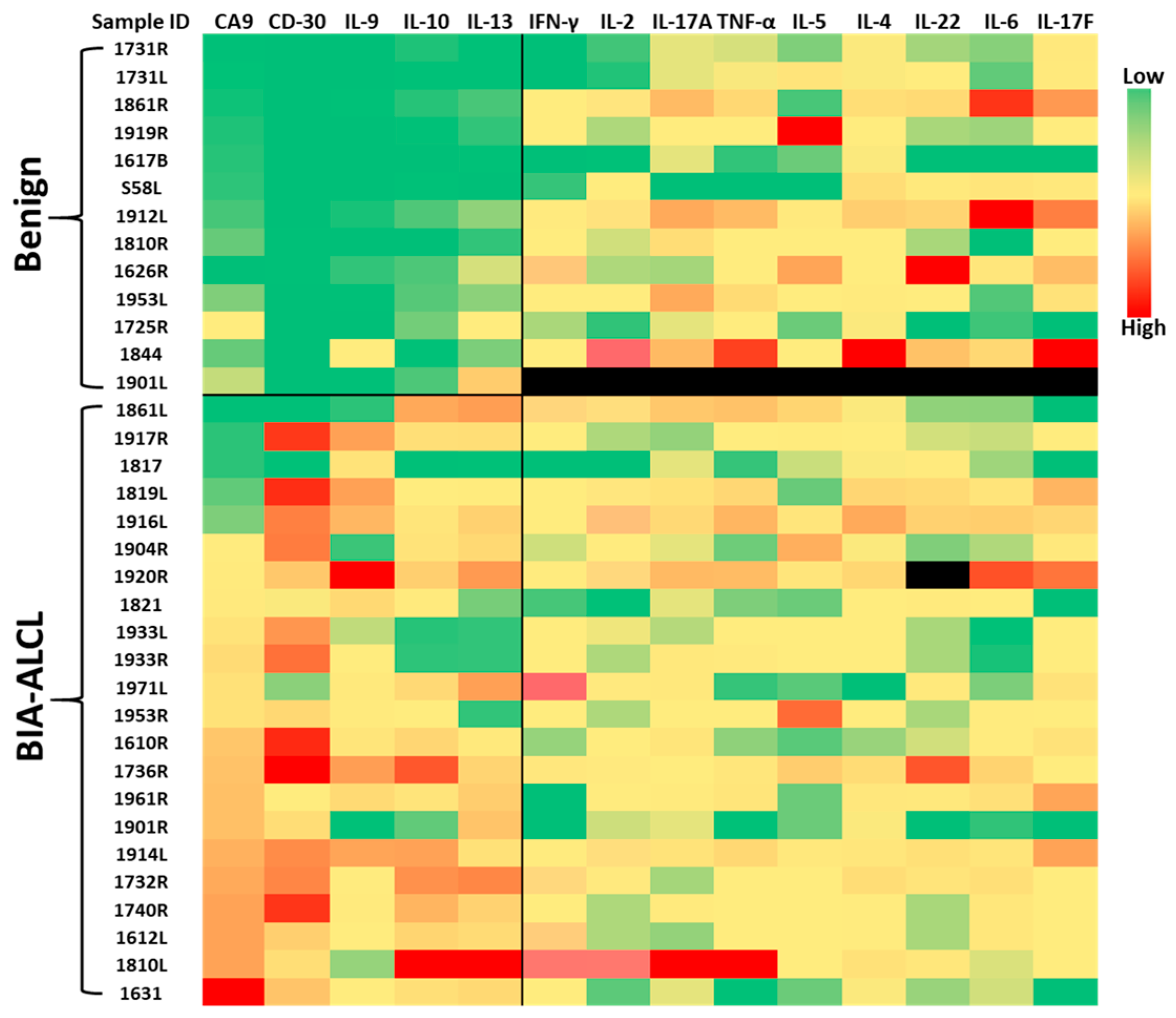
3.1. CA9, CD30, and Cytokines Differentiate BIA-ALCL from Benign Seromas
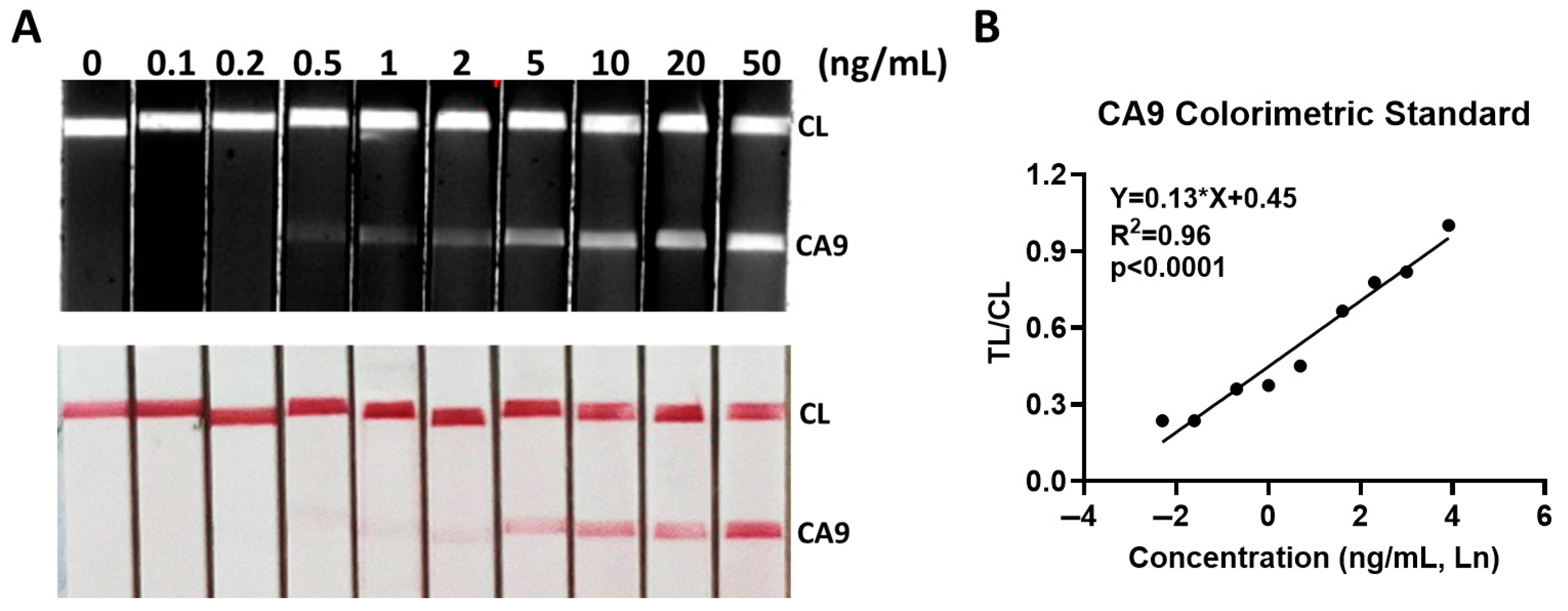
3.2. Analytical Sensitivity of CA9 LFA
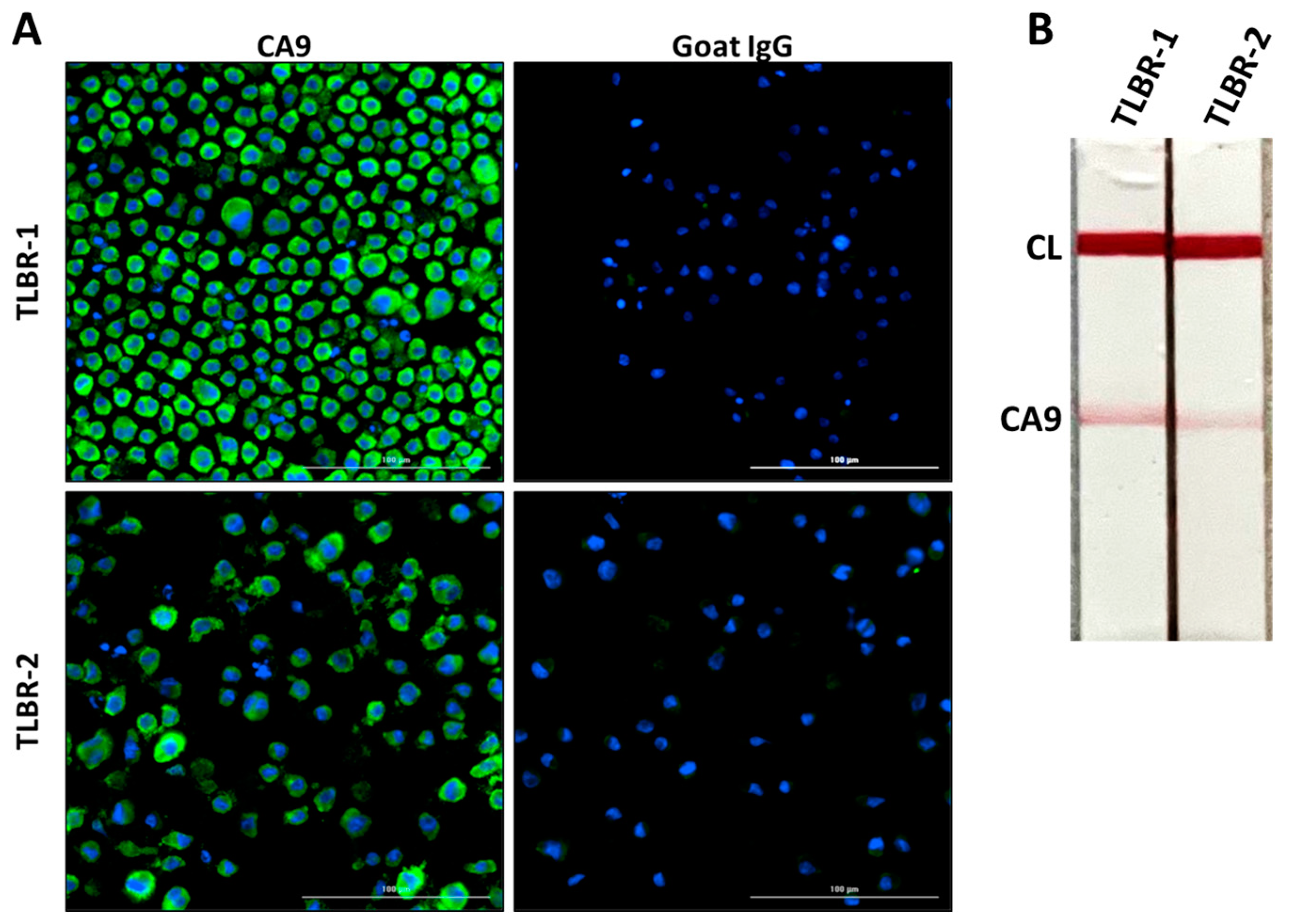
3.3. CA9 Expression in TLBR BIA-ALCL Cell Lines
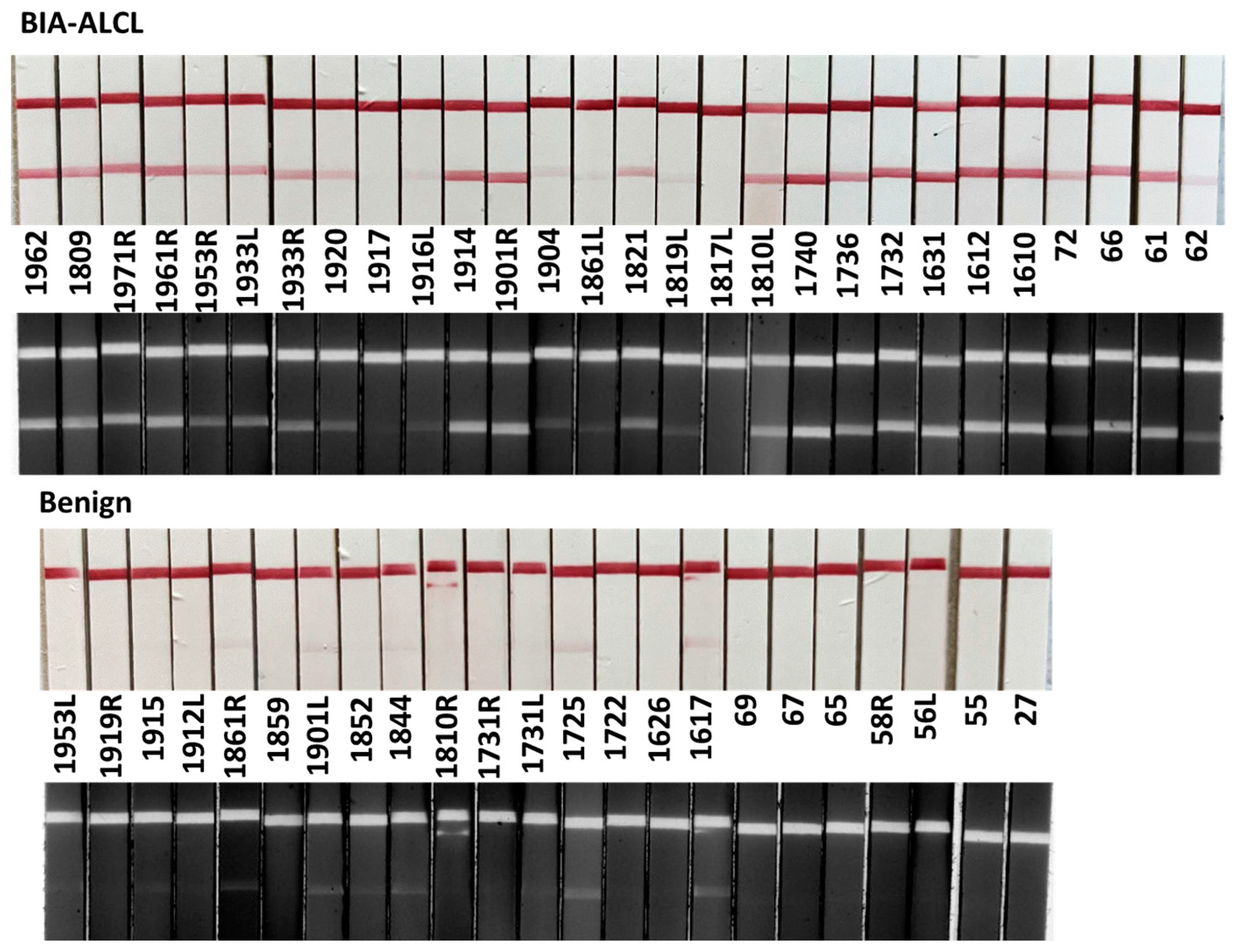
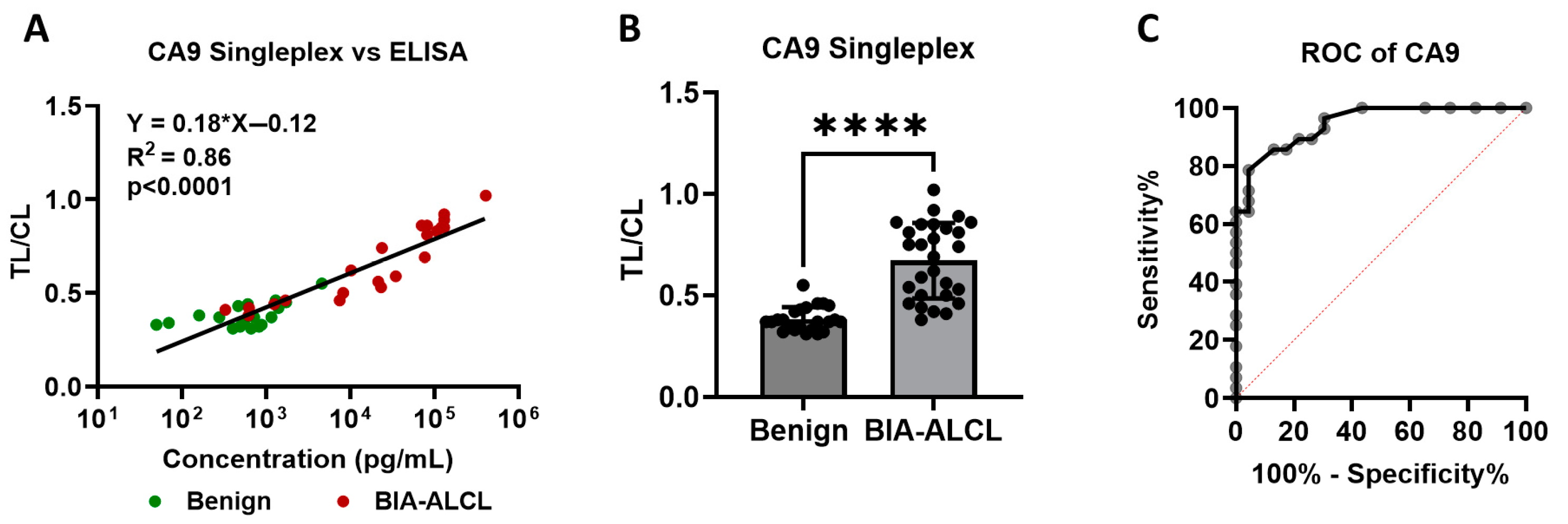
3.4. Detection of CA9 in Clinical Seroma Samples
3.5. Image-Based Quantitative Analysis
3.6. Diagnostic Performance of CA9 Lateral Flow Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LFA | Lateral Flow Assay |
| CA9 | Carbonic Anhydrase IX |
| BIA-ALCL | Breast Implant Associated Anaplastic Large Cell Lymphoma |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
References
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Bellizzi, A.; Caldeira, S.; Albarani, V.; Malanchi, I.; Poignee, M.; Alunni-Fabbroni, M.; Casavola, V.; Tommasino, M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000, 14, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Harris, A.L. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 49, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Wigfield, S.; Cobden, P.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. Tumor-associated carbonic anhydrase 9 spatially coordinates intracellular pH in three-dimensional multicellular growths. J. Biol. Chem. 2008, 283, 20473–20483. [Google Scholar] [CrossRef] [PubMed]
- White, K.A.; Grillo-Hill, B.K.; Barber, D.L. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J. Cell Sci. 2017, 130, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Opavsky, R.; Pastorekova, S.; Zelnik, V.; Gibadulinova, A.; Stanbridge, E.J.; Zavada, J.; Kettmann, R.; Pastorek, J. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: Structure and exon to protein domain relationships. Genomics 1996, 33, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Parkkila, A.K.; Opavsky, R.; Zelnik, V.; Saarnio, J.; Pastorek, J. Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997, 112, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Marques-Piubelli, M.L.; Lyapichev, K.A.; Fnu, A.; Adrada, B.; Stewart, J.; Hunt, K.K.; Clemens, M.W.; Iyer, S.; Wu, Y.; El Hussein, S.; et al. The Spectrum of Non-neoplastic Changes Associated With Breast Implants: Histopathology, Imaging, and Clinical Significance. Am. J. Surg. Pathol. 2024, 48, e43–e64. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Hundal, T.; Phillips, J.L.; Dasari, S.; Hu, G.; Viswanatha, D.S.; He, R.; Mai, M.; Jacobs, H.K.; Ahmed, N.H.; et al. Molecular profiling reveals a hypoxia signature in breast implant-associated anaplastic large cell lymphoma. Haematologica 2020, 106, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shklovskaya, E.; Deva, A.; Xu, H.; Fan, K.; Brosamer, K.; Willson, R.; Khan, I.; Sinha, M.; Kadin, M.E. Diagnosis of breast implant associated anaplastic large cell lymphoma by analysis of cytokines in peri-implant seromas. Am. J. Hematol. 2023, 98, E312–E314. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, G.; Giacalone, M.; Calicchia, A.; Gagliano, E.; Vannucchi, L.; Vanni, G.; Buonomo, O.C.; Cervelli, V.; Longo, B. BIA-ALCL and BIA-SCC: Updates on Clinical Features and Genetic Mutations for Latest Recommendations. Medicina 2024, 60, 793. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; Dabic, S.; Mehrara, B.J.; Cordeiro, P.G.; Disa, J.J.; Pusic, A.L.; Matros, E.; Dayan, J.H.; Allen, R.J., Jr.; Coriddi, M.; et al. Breast Implant-associated Anaplastic Large Cell Lymphoma Incidence: Determining an Accurate Risk. Ann. Surg. 2020, 272, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.W.; Horwitz, S.M. NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Aesthet. Surg. J. 2017, 37, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.W.; Medeiros, L.J.; Butler, C.E.; Hunt, K.K.; Fanale, M.A.; Horwitz, S.; Weisenburger, D.D.; Liu, J.; Morgan, E.A.; Kanagal-Shamanna, R.; et al. Complete Surgical Excision Is Essential for the Management of Patients with Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J. Clin. Oncol. 2015, 34, 2. [Google Scholar] [CrossRef] [PubMed]
- Longo, B.; Di Napoli, A.; Curigliano, G.; Veronesi, P.; Pileri, S.; Martelli, M.; De Vita, R.; Felici, N.; Cirillo, P.; Bernardi, C.; et al. Clinical recommendations for diagnosis and treatment according to current updated knowledge on BIA-ALCL. Breast 2022, 66, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Ashar, B.S.; Clemens, M.W.; Feldman, A.L.; Gaulard, P.; Miranda, R.N.; Sohani, A.R.; Stenzel, T.; Yoon, S.W. Best Practices Guideline for the Pathologic Diagnosis of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Delas, A.; Gaulard, P.; Haioun, C.; Moreau, A.; Zerri, L.; Traverse-Glehen, A.; Rousset, T.; Quintin-Rouse, I.; Petrellas, T.; et al. Breast implant associated anaplastic large cell lymphoma: Two distinct clinicopathological variants with different outcomes. Ann. Oncol. 2016, 27, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Luong-Player, A.; Liu, H.; Wang, H.L.; Lin, F. Immunohistochemical reevaluation of carbonic anhydrase IX (CA IX) expression in tumors and normal tissues. Am. J. Clin. Pathol. 2014, 141, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Courcier, J.; de la Taille, A.; Nourieh, M.; Leguerney, I.; Lassau, N.; Ingels, A. Carbonic Anhydrase IX in Renal Cell Carcinoma, Implications for Disease Management. Int. J. Mol. Sci. 2020, 21, 7146. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.H.; Seligson, D.; Han, K.R.; Pantuck, A.J.; Dorey, F.J.; Huang, Y.; Horvath, S.; Leibovich, B.C.; Chopra, S.; Liao, S.Y.; et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: Implications for prognosis and therapy. Clin. Cancer Res. 2003, 9, 802–811. [Google Scholar] [PubMed]
| Benign | BIA-ALCL | p Value | ||
|---|---|---|---|---|
| Number of Subjects | 23 | 28 | ||
| Age | 46.9 ± 10 | 48.7 ± 9 | Ns | |
| Implant Duration | 7.8 ± 4.3 | 8.6 ± 4.3 | Ns | |
| Implant Surface grade | 4 | 2 | 8 | |
| 3 | 8 | 14 | ||
| 2 | 9 | 5 | 0.02 * | |
| Unknown | 4 | 1 | ||
| Implant Manufacturer | Silimed/Amostra | 2 | 8 | |
| McGhan | 4 | 6 | ||
| Mentor | 5 | 4 | ||
| Natrelle/Allergan | 4 | 7 | ||
| Others | 6 | 2 | 0.01 * | |
| Unknown | 2 | 1 | ||
| CA9 | BIA-ALCL | Benign | Total |
|---|---|---|---|
| Positive | 26 | 5 | 31 |
| Negative | 2 | 18 | 20 |
| Total | 28 | 23 | 51 |
| Sensitivity | 93% | PPV | 84% |
| Specificity | 78% | NPV | 90% |
| CA9 | BIA-ALCL | Benign | Total |
|---|---|---|---|
| Positive | 22 | 1 | 23 |
| Negative | 6 | 22 | 28 |
| Total | 28 | 23 | 51 |
| Sensitivity | 79% | PPV | 96% |
| Specificity | 96% | NPV | 79% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Kourentzi, K.; Willson, R.; Hu, H.; Deva, A.; Campbell, C.; Kadin, M. Lateral Flow Assay to Detect Carbonic Anhydrase IX in Seromas of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Cancers 2025, 17, 2405. https://doi.org/10.3390/cancers17142405
Xu P, Kourentzi K, Willson R, Hu H, Deva A, Campbell C, Kadin M. Lateral Flow Assay to Detect Carbonic Anhydrase IX in Seromas of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Cancers. 2025; 17(14):2405. https://doi.org/10.3390/cancers17142405
Chicago/Turabian StyleXu, Peng, Katerina Kourentzi, Richard Willson, Honghua Hu, Anand Deva, Christopher Campbell, and Marshall Kadin. 2025. "Lateral Flow Assay to Detect Carbonic Anhydrase IX in Seromas of Breast Implant-Associated Anaplastic Large Cell Lymphoma" Cancers 17, no. 14: 2405. https://doi.org/10.3390/cancers17142405
APA StyleXu, P., Kourentzi, K., Willson, R., Hu, H., Deva, A., Campbell, C., & Kadin, M. (2025). Lateral Flow Assay to Detect Carbonic Anhydrase IX in Seromas of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Cancers, 17(14), 2405. https://doi.org/10.3390/cancers17142405









