Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
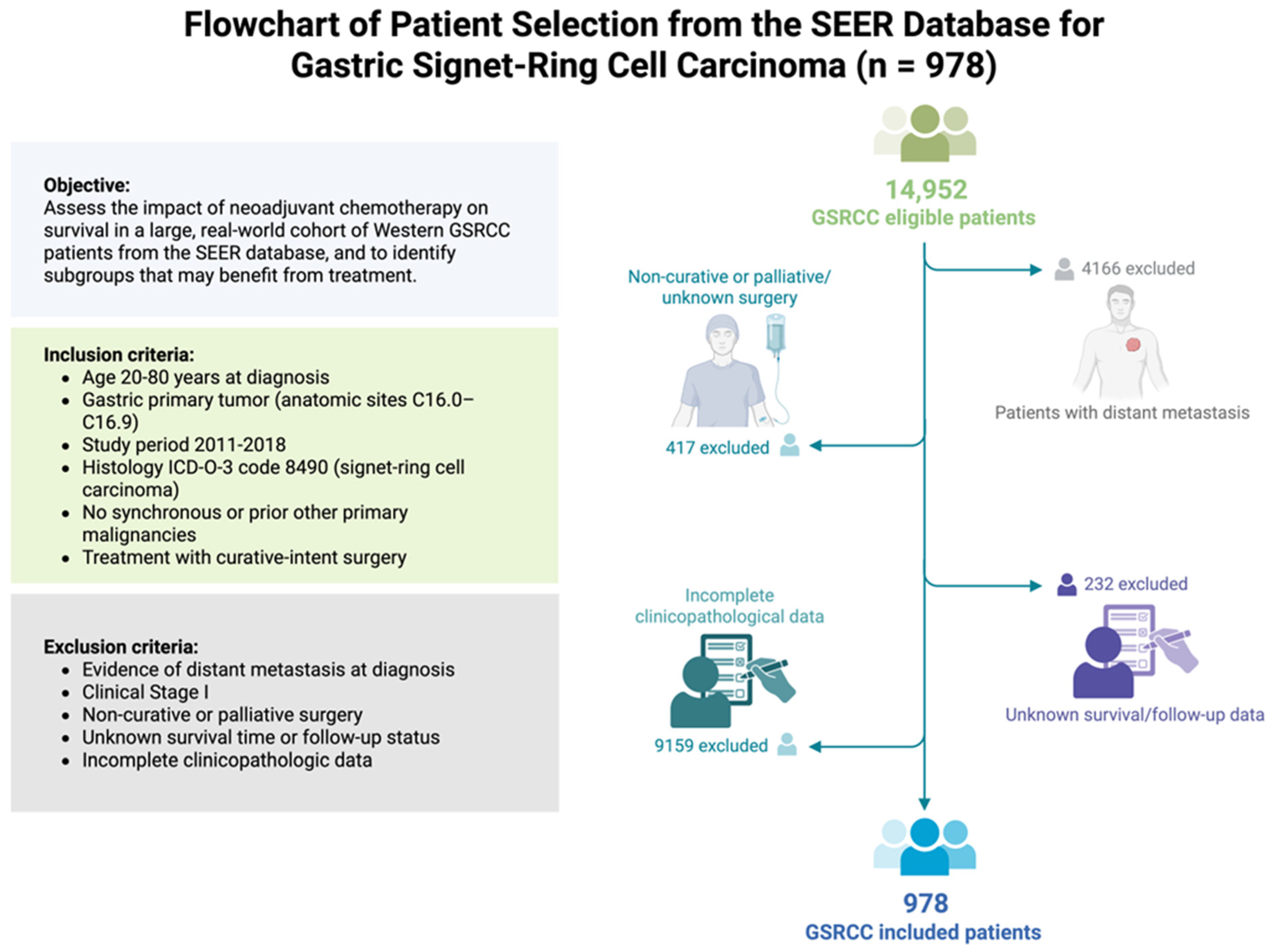
2.1. Study Population
2.2. Treatment Definitions
2.3. Outcome and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Histopathological Findings
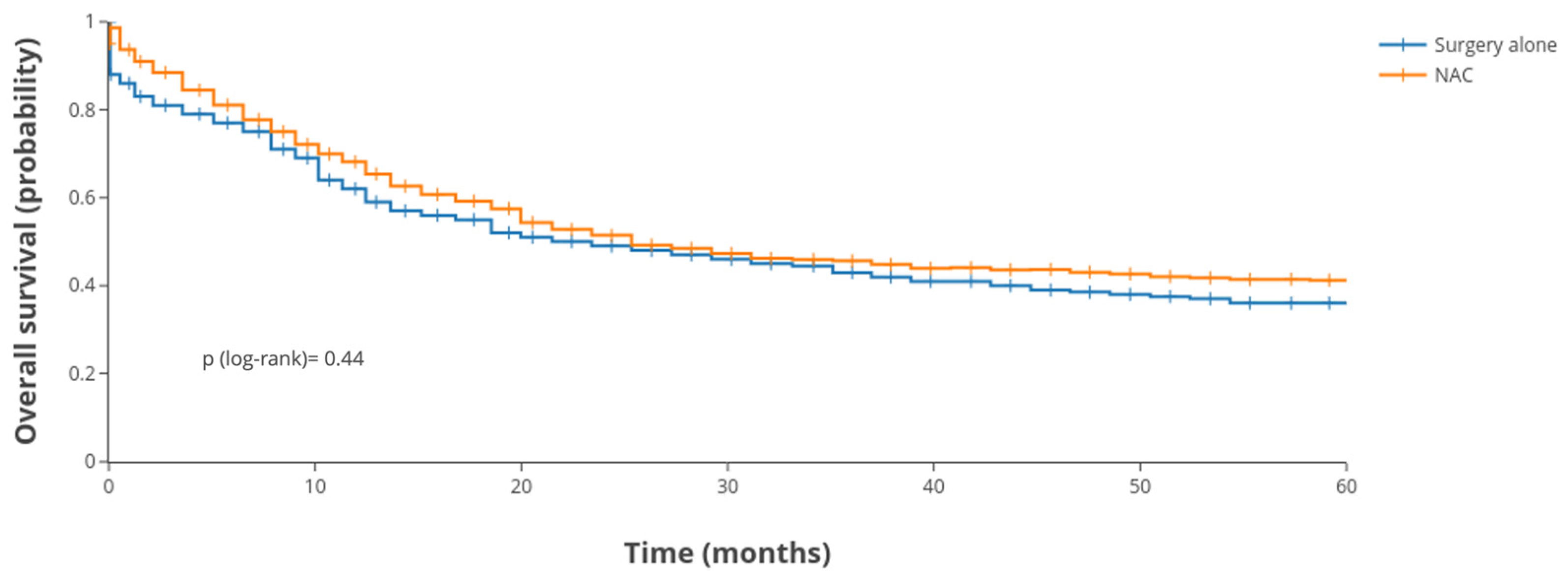
3.3. Survival Outcomes
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GSRCC | Gastric Signet-Ring Cell Carcinoma |
| SEER | Surveillance, Epidemiology, and End Results |
| NAC | Neoadjuvant Chemotherapy |
| OS | Linear dichroism |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| TNM | Tumor–Node–Metastasis (staging system) |
| KM | Kaplan–Meier |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-Ring Cell Carcinoma of the Stomach: Impact on Prognosis and Specific Therapeutic Challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Ma, F.; Xue, L.; Tian, Y. Gastric Signet Ring Cell Carcinoma: Current Management and Future Challenges. Cancer Manag. Res. 2020, 12, 7973–7981. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Ambrosio, M.R.; Resca, L.; Carbone, L.; Carpineto Samorani, O.; Petrioli, R.; Savelli, V.; Costantini, M.; Malaspina, L.; Polom, K.; et al. The Percentage of Signet Ring Cells Is Inversely Related to Aggressive Behavior and Poor Prognosis in Mixed-Type Gastric Cancer. Front. Oncol. 2022, 12, 897218. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C. The Impact of Perioperative Chemotherapy on Survival in Patients with Gastric Signet Ring Cell Adenocarcinoma: A Multicenter Comparative Study. Ann. Surg. 2011, 254, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Takiuchi, H.; Hirata, I.; Kawabe, S.I.; Egashira, Y.; Katsu, K.I. Immunohistochemical Expression of Vascular Endothelial Growth Factor Can Predict Response to 5-Fluorouracil and Cisplatin in Patients with Gastric Adenocarcinoma. Oncol. Rep. 2000, 7, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, E.C.; van der Veen, A.; Brenkman, H.J.F.; Brosens, L.A.A.; van der Post, R.S.; Verhoeven, R.H.A.; Luijten, J.C.H.B.M.; Vissers, P.A.J.; Vegt, E.; van Hillegersberg, R.; et al. Multimodal Therapy Versus Primary Surgery for Gastric and Gastroesophageal Junction Diffuse Type Carcinoma, with a Focus on Signet Ring Cell Carcinoma: A Nationwide Study. Ann. Surg. Oncol. 2024, 31, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, F.H.; Xue, L.Y.; Tian, Y.T. Neoadjuvant Chemotherapy vs Upfront Surgery for Gastric Signet Ring Cell Carcinoma: A Retrospective, Propensity Score-Matched Study. World J. Gastroenterol. 2020, 26, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Dal Cero, M.; Bencivenga, M.; Liu, D.H.W.; Sacco, M.; Alloggio, M.; Kerckhoffs, K.G.P.; Filippini, F.; Saragoni, L.; Iglesias, M.; Tomezzoli, A.; et al. Clinical Features of Gastric Signet Ring Cell Cancer: Results from a Systematic Review and Meta-Analysis. Cancers 2023, 15, 5191. [Google Scholar] [CrossRef] [PubMed]
- Heger, U.; Sisic, L.; Nienhüser, H.; Blank, S.; Hinz, U.; Haag, G.M.; Ott, K.; Ulrich, A.; Büchler, M.W.; Schmidt, T. Neoadjuvant Therapy Improves Outcomes in Locally Advanced Signet-Ring-Cell Containing Esophagogastric Adenocarcinomas. Ann. Surg. Oncol. 2018, 25, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Piessen, G.; Messager, M.; Le Malicot, K.; Robb, W.B.; Di Fiore, F.; Guilbert, M.; Moreau, M.; Christophe, V.; Adenis, A.; Mariette, C. Phase II/III Multicentre Randomised Controlled Trial Evaluating a Strategy of Primary Surgery and Adjuvant Chemotherapy versus Peri-Operative Chemotherapy for Resectable Gastric Signet Ring Cell Adenocarcinomas—PRODIGE 19—FFCD1103—ADCI002. BMC Cancer 2013, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/ (accessed on 4 May 2025).
- Gillespie, B.W.; Chen, Q.; Reichert, H.; Franzblau, A.; Hedgeman, E.; Lepkowski, J.; Adriaens, P.; Demond, A.; Luksemburg, W.; Garabrant, D.H. Estimating Population Distributions When Some Data Are below a Limit of Detection by Using a Reverse Kaplan-Meier Estimator. Epidemiology 2010, 21 (Suppl. 4), S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Piessen, G.; Messager, M.; Leteurtre, E.; Jean-Pierre, T.; Mariette, C. Signet Ring Cell Histology Is an Independent Predictor of Poor Prognosis in Gastric Adenocarcinoma Regardless of Tumoral Clinical Presentation. Ann. Surg. 2009, 250, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, S.; Crnovrsanin, N.; Kalkum, E.; Vey, J.A.; Nienhüser, H.; Rompen, I.F.; Haag, G.M.; Müller-Stich, B.; Billmann, F.; Schmidt, T.; et al. Is Neoadjuvant Chemotherapy Followed by Surgery the Appropriate Treatment for Esophagogastric Signet Ring Cell Carcinomas? A Systematic Review and Meta-Analysis. Front. Surg. 2024, 11, 1382039. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.A.N.; Jose, A.; Usman, N.; Rajan, K.; Munisamy, M.; Shetty, P.S.; Rao, M. Signet Ring Cell Cancer of Stomach and Gastro-Esophageal Junction: Molecular Alterations, Stage-Stratified Treatment Approaches, and Future Challenges. Langenbecks Arch. Surg. 2021, 407, 87. [Google Scholar] [CrossRef] [PubMed]
- Bickenbach, K.; Strong, V.E. Comparisons of Gastric Cancer Treatments: East vs. West. J. Gastric Cancer 2012, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.R.; Cervantes, A.; van de Velde, C.J.H. Gastric Cancer: Epidemiology, Pathology and Treatment. Ann. Oncol. 2003, 14 (Suppl. 2), ii31–ii36. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular Analysis of Gastric Cancer Identifies Subtypes Associated with Distinct Clinical Outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; Hinoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, Y.; Ma, Y.; Ning, Y.; Chen, G.; Liu, Y. Survival Benefits from Neoadjuvant Treatment in Gastric Cancer: A Systematic Review and Meta-Analysis. Syst. Rev. 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Marano, L.; Rossetti, R.R.A.; Rizzo, M.R.; di Martino, N.; Paolisso, G. Serum CD26 Levels in Patients with Gastric Cancer: A Novel Potential Diagnostic Marker. BMC Cancer 2015, 15, 703. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Marano, L.; Ambrosio, M.R.; Resca, L.; D’Ignazio, A.; Petrelli, F.; Petrioli, R.; Costantini, M.; Polom, K.; Macchiarelli, R.; et al. Signet Ring Cell Percentage in Poorly Cohesive Gastric Cancer Patients: A Potential Novel Predictor of Survival. Eur. J. Surg. Oncol. 2022, 48, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Carneiro, F.; Grabsch, H.I.; van der Post, R.S.; Allum, W.; de Manzoni, G.; Luca, B.G.; Maria, B.; Jean-Francois, F.; Uberto, F.; et al. Consensus on the Pathological Definition and Classification of Poorly Cohesive Gastric Carcinoma. Gastric Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mathias-Machado, M.C.; de Jesus, V.H.F.; Jácome, A.; Donadio, M.D.; Aruquipa, M.P.S.; Fogacci, J.; Cunha, R.G.; da Silva, L.M.; Peixoto, R.D. Claudin 18.2 as a New Biomarker in Gastric Cancer—What Should We Know? Cancers 2024, 16, 679. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Al-Batran, S.E.; Arnold, D.; Borner, M.; Bruns, C.J.; Eisterer, W.; Faber, G.; Gockel, I.; Köberle, D.; Lorenzen, S.; et al. German, Austrian, and Swiss Guidelines for Systemic Treatment of Gastric Cancer. Gastric Cancer 2023, 27, 6. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Category | SEER (n = 978) |
|---|---|---|
| Gender | Male | 517 (52.9%) |
| Female | 461 (47.1%) | |
| Age at diagnosis | ≤60 years | 500 (51.1%) |
| >60 years | 478 (48.9%) | |
| Race | Black | 130 (13.3%) |
| White | 640 (65.4%) | |
| Asian (Chinese) | 37 (3.8%) | |
| Other/unspecified | 171 (17.5%) | |
| Tumor location * | Proximal (cardia/fundus) | 174 (17.8%) |
| Mid (corpus/antrum) ** | 278 (28.4%) | |
| Distal (antrum/pylorus) | 290 (29.7%) | |
| Overlapping regions *** | 91 (9.3%) | |
| Unknown | 63 (6.4%) | |
| Tumor size | ≤5 cm | 450 (46.0%) |
| >5 cm | 248 (25.4%) | |
| Unknown | 281 (28.7%) | |
| Year of surgery | 2011–2014 | 503 (51.4%) |
| 2015–2018 | 475 (48.6%) | |
| Clinical TNM stage | II | 202 (20.7%) |
| III | 776 (79.3%) |
| Treatment Variable | Category | SEER (n = 978) |
|---|---|---|
| Neoadjuvant therapy | Received chemotherapy (NAC) | 436 (44.6%) |
| None (surgery first) | 542 (55.4%) | |
| Adjuvant therapy | Chemotherapy (post-op) | 163 (16.7%) |
| Chemoradiotherapy (post-op) | 214 (21.9%) | |
| Radiotherapy only (post-op) | 30 (3.0%) | |
| None | 571 (58.4%) |
| Pathologic Variable | Category | SEER (n = 978) |
|---|---|---|
| Pathologic T stage | T1 (mucosa/submucosa) | 277 (28.3%) |
| T2 (muscularis propria) | 164 (16.8%) | |
| T3 (subserosa/adjacent) | 271 (27.7%) | |
| T4 (serosa or adjacent structures) | 266 (27.2%) | |
| Pathologic N stage | N0 (0 positive nodes) | 395 (40.4%) |
| N1 (1–2 positive nodes) | 236 (24.1%) | |
| N2 (3–6 positive nodes) | 185 (18.9%) | |
| N3 (≥7 positive nodes) | 162 (16.6%) | |
| Lymph nodes examined | Mean ± SD | 27.2 (±39.7) |
| Lymph nodes positive | Mean ± SD | 18.7 (±23.4) |
| Variable (Reference) | Univariable HR (95% CI) | p | Multivariable HR (95% CI) | p |
|---|---|---|---|---|
| Sex (Male ref) | ||||
| Female vs. Male | 1.07 (0.91–1.26) | 0.397 | 1.09 (0.91–1.30) | 0.334 † |
| Age (≤60 ref) | ||||
| >60 vs. ≤60 | 1.03 (0.87–1.22) | 0.723 | 1.05 (0.88–1.26) | 0.605 † |
| Race (Black ref) | ||||
| White vs. Black | 0.92 (0.72–1.18) | 0.505 | 0.95 (0.74–1.22) | 0.695 |
| Asian (Chinese) vs. Black | 0.56 (0.34–0.91) | 0.019 | 0.58 (0.35–0.96) | 0.033 |
| Tumor size (≤5 cm ref) | ||||
| >5 cm vs. ≤5 cm | 2.57 (2.14–3.09) | <0.001 | 1.67 (1.37–2.04) | <0.001 |
| Tumor location (Proximal ref) | ||||
| Mid vs. Proximal | 1.11 (0.89–1.37) | 0.346 | 1.06 (0.85–1.33) | 0.589 |
| Distal vs. Proximal | 1.14 (0.91–1.42) | 0.265 | 1.12 (0.89–1.42) | 0.318 |
| Overlapping vs. Proximal | 1.12 (0.83–1.50) | 0.457 | 1.08 (0.79–1.48) | 0.625 |
| Clinical TNM stage (II ref) | ||||
| Stage III vs. II | 3.38 (2.03–5.62) | <0.001 | 2.98 (1.59–5.58) | 0.001 |
| Pathologic T stage (T2 ref) | ||||
| T3 vs. T2 | 2.74 (2.14–3.52) | <0.001 | 1.52 (1.08–2.15) | 0.017 |
| T4 vs. T2 | 4.61 (3.54–6.01) | <0.001 | 2.13 (1.45–3.13) | <0.001 |
| Pathologic N stage (N0 ref) | ||||
| N1 vs. N0 | 1.85 (1.45–2.37) | <0.001 | 1.34 (1.02–1.77) | 0.035 |
| N2 vs. N0 | 2.01 (1.53–2.64) | <0.001 | 1.25 (0.89–1.75) | 0.197 |
| N3 vs. N0 | 3.09 (2.40–3.97) | <0.001 | 1.64 (1.20–2.25) | 0.002 |
| Year of surgery (2011–14 ref) | ||||
| 2015–2018 vs. 2011–2014 | 0.93 (0.78–1.11) | 0.419 | 0.89 (0.74–1.07) | 0.229 |
| Neoadjuvant therapy (NAC vs. none) | ||||
| No NAC vs. NAC | 0.94 (0.77–1.15) | 0.557 | 0.91 (0.74–1.13) | 0.397 |
| Adjuvant therapy (Yes ref) | ||||
| No adjuvant vs. Yes | 1.08 (0.81–1.43) | 0.610 | 1.17 (0.88–1.57) | 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrenti, S.; Malerba, S.; Lori, E.; Pironi, D.; Polom, K.; Skokowski, J.; Girnyi, S.; Cwalinski, T.; Prete, F.P.; Vashist, Y.K.; et al. Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma. Cancers 2025, 17, 2400. https://doi.org/10.3390/cancers17142400
Sorrenti S, Malerba S, Lori E, Pironi D, Polom K, Skokowski J, Girnyi S, Cwalinski T, Prete FP, Vashist YK, et al. Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma. Cancers. 2025; 17(14):2400. https://doi.org/10.3390/cancers17142400
Chicago/Turabian StyleSorrenti, Salvatore, Silvia Malerba, Eleonora Lori, Daniele Pironi, Karol Polom, Jaroslaw Skokowski, Sergii Girnyi, Tomasz Cwalinski, Francesco Paolo Prete, Yogesh K. Vashist, and et al. 2025. "Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma" Cancers 17, no. 14: 2400. https://doi.org/10.3390/cancers17142400
APA StyleSorrenti, S., Malerba, S., Lori, E., Pironi, D., Polom, K., Skokowski, J., Girnyi, S., Cwalinski, T., Prete, F. P., Vashist, Y. K., Testini, M., & Marano, L. (2025). Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma. Cancers, 17(14), 2400. https://doi.org/10.3390/cancers17142400









