The Roles of Tripartite Motif Proteins in Urological Cancers: A Systematic Review
Simple Summary
Abstract
1. Introduction
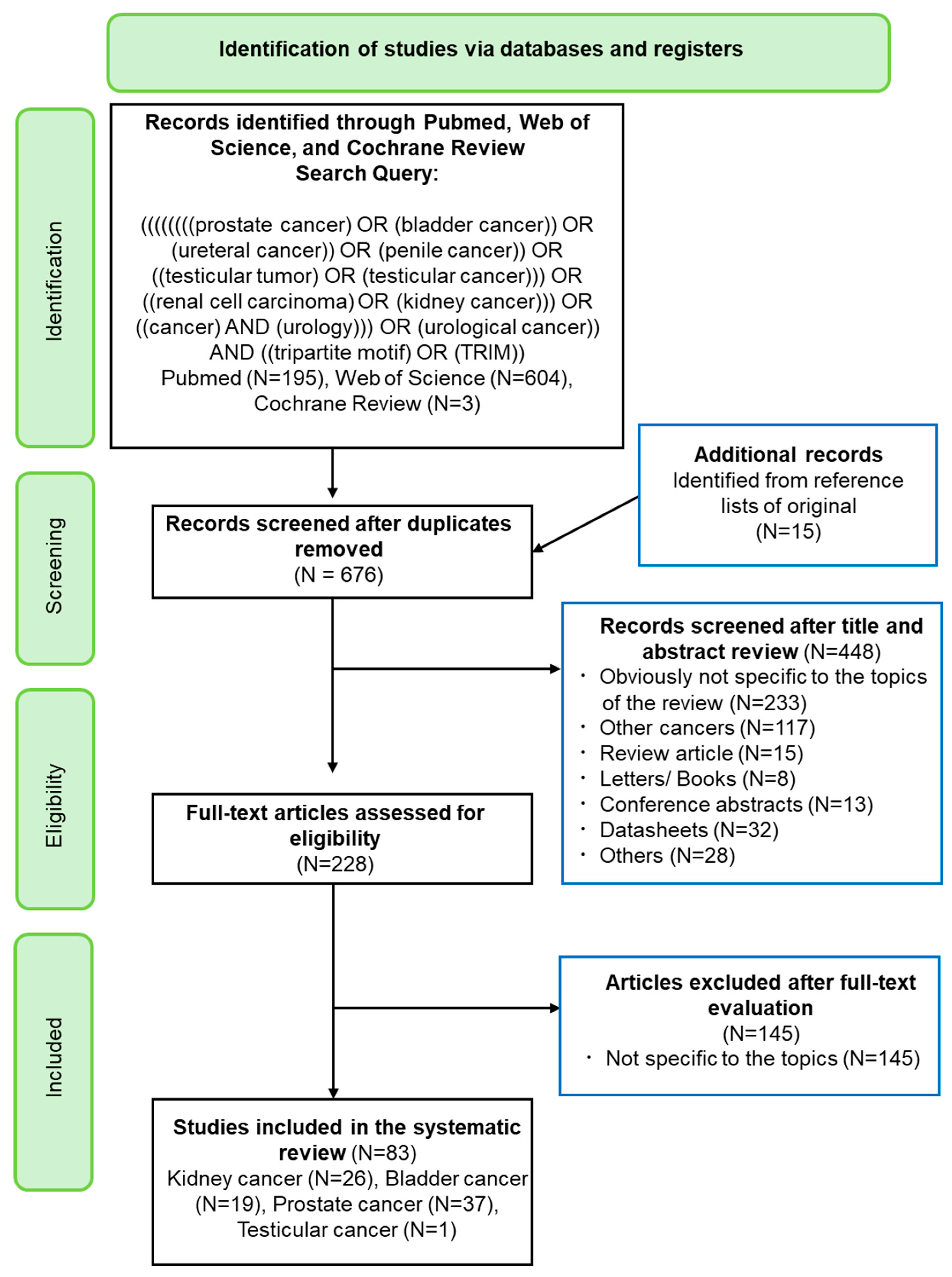
2. Materials and Methods
3. Evidence Acquisition
4. Risk of Bias Assessment
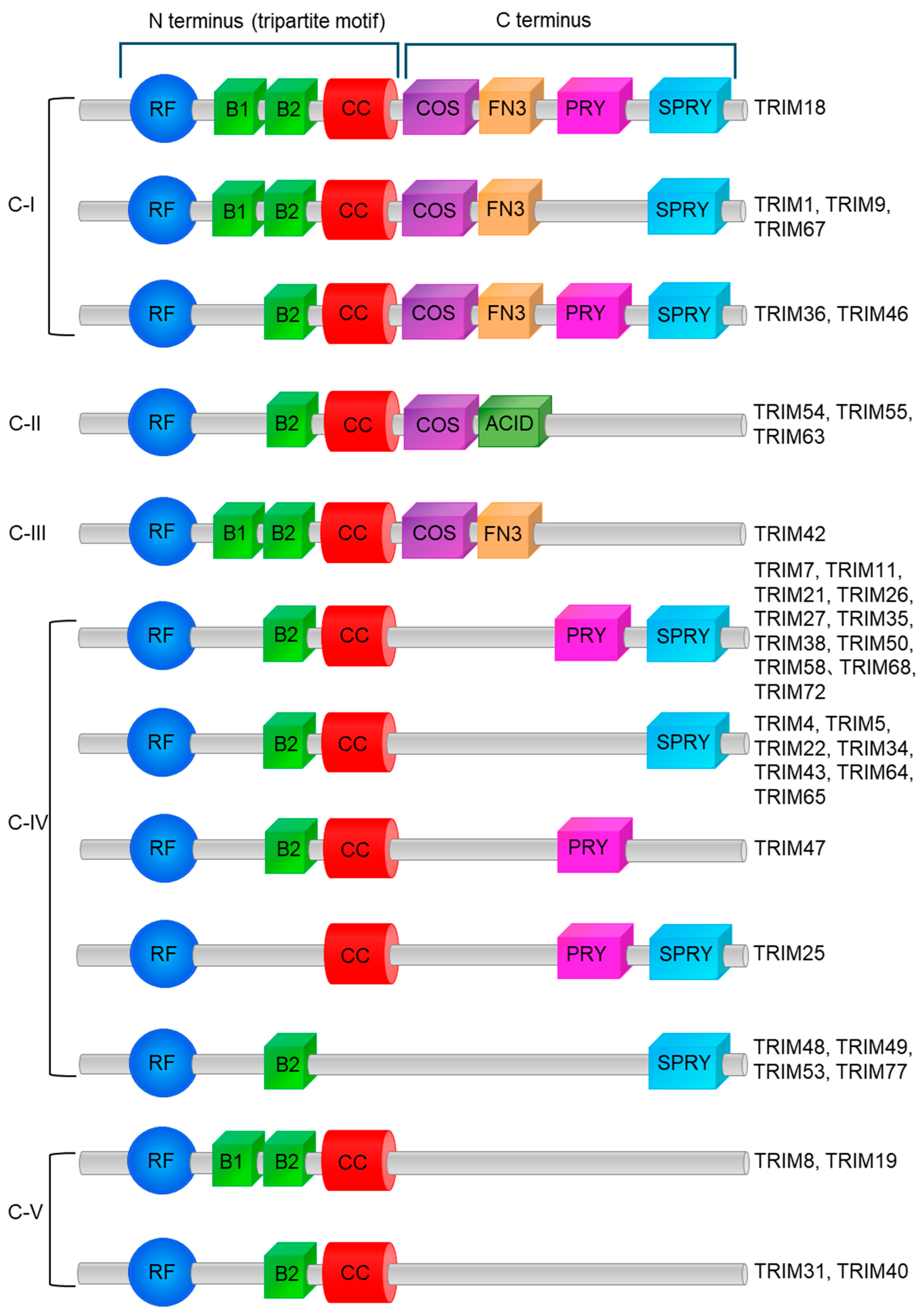
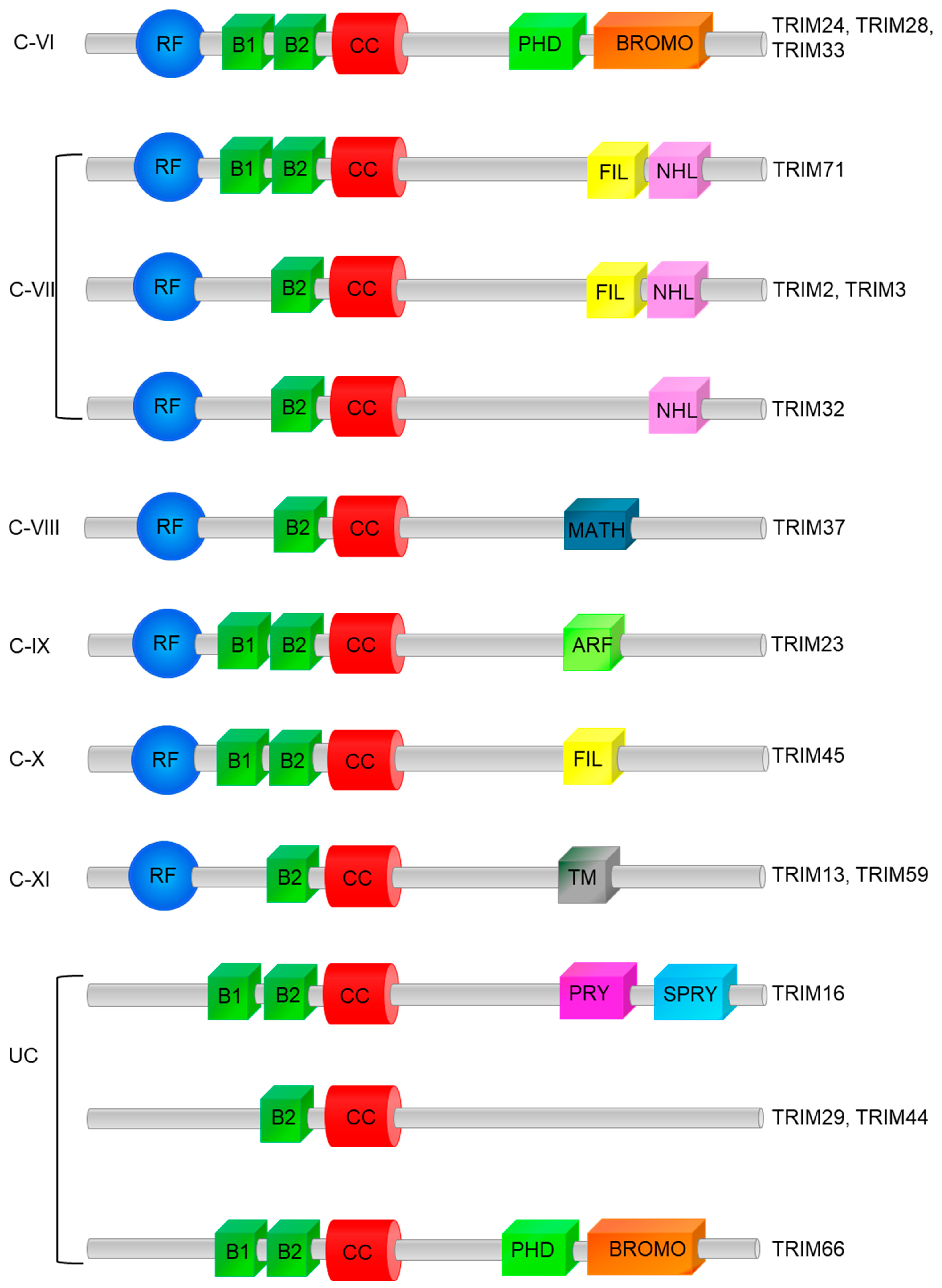
5. Categorization of Classes, Structures of TRIM, and Function of Domains
6. The Role of TRIM Proteins in Kidney Cancer
7. The Role of TRIM Proteins in Bladder Cancer
8. The Role of TRIM Proteins in Prostate Cancer
9. The Role of TRIM Proteins in Castration-Resistant Prostate Cancer
10. The Role of TRIM Proteins in Testicular Cancer
11. The Role of TRIM Proteins in Other Types of Urological Malignancies
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. TRIM proteins and cancer. Nat. Rev. Cancer 2011, 11, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hatakeyama, S. TRIM proteins and diseases. J. Biochem. 2017, 161, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, R.B. The ubiquitin system: From cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.4. Available online: https://training.cochrane.org/handbook/current (accessed on 31 May 2024).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Short, K.M.; Hopwood, B.; Yi, Z.; Cox, T.C. MID1 and MID2 homo- and heterodimerise to tether the rapamycin-sensitive PP2A regulatory subunit, alpha 4, to microtubules: Implications for the clinical variability of X-linked Opitz GBBB syndrome and other developmental disorders. BMC Cell Biol. 2002, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Gushchina, L.V.; Kwiatkowski, T.A.; Bhattacharya, S.; Weisleder, N.L. Conserved structural and functional aspects of the tripartite motif gene family point towards therapeutic applications in multiple diseases. Pharmacol. Ther. 2018, 185, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Du, H.; Massiah, M.A. Detection and characterization of the In Vitro e3 ligase activity of the human MID1 protein. J. Mol. Biol. 2011, 407, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.N.; Luisi, B.; Milner, A.; Elliott, M.; Everett, R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. A new structural class of zinc-finger. J. Mol. Biol. 1994, 237, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Spyroulias, G.A. RING finger E(3) ubiquitin ligases: Structure and drug discovery. Curr. Pharm. Des. 2009, 15, 3716–3731. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Lin, T.C.; McCarty, N. TRIM44 mediated p62 deubiquitination enhances DNA damage repair by increasing nuclear FLNA and 53BP1 expression. Oncogene 2021, 40, 5116–5130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, M.; Yan, S.; Liu, Y.; Fan, W.; Cui, Y.; Tian, F.; Gu, R.; Cui, Y.; Zhan, Y.; et al. TRIM44 promotes BRCA1 functions in HR repair to induce Cisplatin Chemoresistance in Lung Adenocarcinoma by Deubiquitinating FLNA. Int. J. Biol. Sci. 2022, 18, 2962–2979. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Usui, T.; Takeda, S.; Ikeda, K.; Okada, A.; Ishida, Y.; Iwayanagi, T.; Otomo, J.; Ouchi, Y.; Inoue, S. TRIM44 interacts with and stabilizes terf, a TRIM ubiquitin E3 ligase. Biochem. Biophys. Res. Commun. 2009, 383, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Dudley-Fraser, J.; Garza-Garcia, A.; Rittinger, K. Divergent self-association properties of paralogous proteins TRIM2 and TRIM3 regulate their E3 ligase activity. Nat. Commun. 2022, 13, 7583. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Pearson, A.N.; Ashley, M.D.; Jessick, V.; Murphy, B.M.; Gafken, P.; Henshall, D.C.; Morris, K.T.; Simon, R.P.; Meller, R. Identification of a novel Bcl-2-interacting mediator of cell death (Bim) E3 ligase, tripartite motif-containing protein 2 (TRIM2), and its role in rapid ischemic tolerance-induced neuroprotection. J. Biol. Chem. 2011, 286, 19331–19339. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, X.; Zhu, L.; Huang, J.; Huang, Y. TRIM2 directly deubiquitinates and stabilizes Snail1 protein, mediating proliferation and metastasis of lung adenocarcinoma. Cancer Cell Int. 2020, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.; Végh, M.J.; Dawitz, J.; Kroon, T.; Loos, M.; Labonté, D.; Li, K.W.; Van Nierop, P.; Van Diepen, M.T.; De Zeeuw, C.I.; et al. Ubiquitin ligase TRIM3 controls hippocampal plasticity and learning by regulating synaptic γ-actin levels. J. Cell Biol. 2015, 211, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhang, Y.; Xia, Y.; Cheng, F. A Novel Gene Signature of Tripartite Motif Family for Predicting the Prognosis in Kidney Renal Clear Cell Carcinoma and Its Association with Immune Cell Infiltration. Front. Oncol. 2022, 17, 840410. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, X.; Wang, T.; Xing, J. TRIM2 downregulation in clear cell renal cell carcinoma affects cell proliferation, migration, and invasion and predicts poor patients’ survival. Cancer Manag. Res. 2018, 10, 5951–5964. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Dong, Y.; Chen, X.; Ren, X.; Li, G.; Wang, Y.; Wang, Y.; Zhang, T.; Wang, S.; Qin, C.; et al. Construction of circRNA-based ceRNA network to reveal the role of circRNAs in the progression and prognosis of metastatic clear cell renal cell carcinoma. Aging 2020, 12, 24184–24207. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, R.; Chen, Y.; Fang, Z.; Tang, J.; Yao, J.; Gao, J.; Zhou, W.; Chen, X. Comprehensive analysis of expression profiles and prognosis of TRIM genes in human kidney clear cell carcinoma. Aging 2022, 14, 4606–4617. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liu, J.; Liu, L.; Jia, H.; Gao, Q.; Wang, X.; Zhao, J. TRIM7 suppresses cell invasion and migration through inhibiting HIF-1α accumulation in clear cell renal cell carcinoma. Cell Biol. Int. 2022, 46, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, Y.; Li, L.; Li, J.; Ruan, N.; Dong, J.; Si, Z.; Xia, Q.; Wang, Q. Tripartite-motif family genes associated with cancer stem cells affect tumor progression and can assist in the clinical prognosis of kidney renal clear cell carcinoma. Int. J. Med. Sci. 2020, 17, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Caratozzolo, M.F.; Valletti, A.; Gigante, M.; Aiello, I.; Mastropasqua, F.; Marzano, F.; Ditonno, P.; Carrieri, G.; Simonnet, H.; D’Erchia, A.M.; et al. TRIM8 anti-proliferative action against chemo-resistant renal cell carcinoma. Oncotarget 2014, 5, 7446–7457. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, L.; Zhou, R.; Wu, Y.; Zhou, S.; Zhang, Y.; Cheng, B.; Ni, J.; Huang, H.; Hou, J. TRIM13 inhibits cell migration and invasion in clear-cell renal cell carcinoma. Nutr. Cancer 2020, 72, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lu, L.T.; Chen, H.Y.; Duan, X.; Lin, X.; Feng, X.H.; Tang, M.J.; Chen, R.H. SCP phosphatases suppress renal cell carcinoma by stabilizing PML and inhibiting mTOR/HIF signaling. Cancer Res. 2014, 74, 6935–6946. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Menegazzi, C.; Fracassi, C.; Biffi, C.C.; Genova, F.; Tenace, N.P.; Lucianò, R.; Raimondi, A.; Tacchetti, C.; Brugarolas, J.; et al. PML restrains p53 activity and cellular senescence in clear cell renal cell carcinoma. EMBO Mol. Med. 2024, 16, 1324–1351. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Yong, H.; Wang, W.; Wang, D.; Chu, S.; Li, M.; Hou, P.; Zheng, J.; Bai, J. Trim21-mediated HIF-1α degradation attenuates aerobic glycolysis to inhibit renal cancer tumorigenesis and metastasis. Cancer Lett. 2021, 508, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, H.; Chen, Q.; Cao, L.; He, Y.; Gao, X.; Chen, W.; Zhang, H. Trim24 prompts tumor progression via inducing EMT in renal cell carcinoma. Open Med. 2020, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.P.; Cai, L.C.; Wang, X.Y.; Cheng, S.Y.; Zhang, D.M.; Jian, W.G.; Wang, T.D.; Yang, J.K.; Yang, K.B.; Zhang, C. BMP8A promotes survival and drug resistance via Nrf2/TRIM24 signaling pathway in clear cell renal cell carcinoma. Cancer Sci. 2020, 111, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ning, J.; Deng, H.; Ruan, Y.; Cheng, F. TRIM26 inhibits clear cell renal cell carcinoma progression through destabilizing ETK and thus inactivation of AKT/mTOR signaling. J. Transl. Med. 2024, 22, 481. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhang, W.; Hua, M.; Chen, H.; Yang, B.; Wang, Y.; Yang, Q. TRIM27 interacts with Iκbα to promote the growth of human renal cancer cells through regulating the NF-κB pathway. BMC Cancer 2021, 21, 841. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Lv, S.; Ma, X.; Zhao, X.; Fan, L.; Zou, Q.; Li, N.; Yan, Y.; Zhang, W.; Sun, L. TRIM28 represses renal cell carcinoma cell proliferation by inhibiting TFE3/KDM6A-regulated autophagy. J. Biol. Chem. 2023, 299, 104621. [Google Scholar] [CrossRef] [PubMed]
- Jingushi, K.; Ueda, Y.; Kitae, K.; Hase, H.; Egawa, H.; Ohshio, I.; Kawakami, R.; Kashiwagi, Y.; Tsukada, Y.; Kobayashi, T.; et al. miR-629 Targets TRIM33 to Promote TGFβ/Smad Signaling and Metastatic Phenotypes in ccRCC. Mol. Cancer Res. 2015, 13, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, G.; Zhang, J.; Li, J.; Ruan, N.; Zhang, J.; Zhang, Z.; Chen, Y.; Zhang, Q.; Xia, Q. TRIM33 Overexpression Inhibits the Progression of Clear Cell Renal Cell Carcinoma In Vivo and In Vitro. BioMed. Res. Int. 2020, 2020, 8409239. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Liang, C.; Li, P.; Liu, B.; Qin, C.; Yuan, H.; Liu, Y.; Zhu, J.; Cui, Y.; Xu, A.; et al. TRIM37 orchestrates renal cell carcinoma progression via histone H2A ubiquitination-dependent manner. J. Exp. Clin. Cancer Res. 2021, 40, 195. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kimura, N.; Takayama, K.I.; Sato, Y.; Suzuki, T.; Azuma, K.; Fujimura, T.; Ikeda, K.; Kume, H.; Inoue, S. TRIM44 promotes cell proliferation and migration by inhibiting FRK in renal cell carcinoma. Cancer Sci. 2020, 111, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.B.; Zhao, J.; Liang, X.F.; Guo, X.D.; Jiang, S.B.; Xiang, Y.Z. Identification TRIM46 as a Potential Biomarker and Therapeutic Target for Clear Cell Renal Cell Carcinoma Through Comprehensive Bioinformatics Analyses. Front. Med. 2021, 8, 785331. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xu, D.; Cao, J.W.; Zuo, L.; Han, Z.T.; Tian, Y.J.; Chu, C.M.; Zhou, W.; Pan, X.W.; Cui, X.G. TRIM47 promotes malignant progression of renal cell carcinoma by degrading P53 through ubiquitination. Cancer Cell Int. 2021, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Cao, C.; Li, A.; Song, H.; Kuang, G.; Ma, B.; Zhang, Q.; Zhang, Q. Silencing of the TRIM58 Gene by Aberrant Promoter Methylation is Associated with a Poor Patient Outcome and Promotes Cell Proliferation and Migration in Clear Cell Renal Cell Carcinoma. Front. Mol. Biosci. 2021, 8, 655126. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Zhao, M.J.; Wang, W.X.; Xu, C.W.; Wang, G.D. TRIM59 is a key regulator of growth and migration inrenal cell carcinoma. Cell. Mol. Biol. 2017, 63, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Y.; Mannan, R.; Skala, S.L.; Rangaswamy, R.; Chinnaiyan, A.; Su, F.; Cao, X.; Zelenka-Wang, S.; McMurry, L.; et al. TRIM63 is a sensitive and specific biomarker for MiT family aberration-associated renal cell carcinoma. Mod. Pathol. 2021, 34, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhu, Q.; Xie, T.; Xiao, Y.; Zhang, F.; Li, N.; Deng, K.; Xin, H.; Huang, X. TRIM65 promotes renal cell carcinoma through ubiquitination and degradation of BTG3. Cell Death Dis. 2024, 15, 355. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Yuan, J.L.; Ye, H.; Yi, K.; Qie, M.R.; Hou, M.M. TRIM44 facilitates ovarian cancer proliferation, migration, and invasion by inhibiting FRK. Neoplasma 2021, 68, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Y.; Ma, M.; Chang, L. Knockdown of TRIM44 inhibits the proliferation and invasion in papillary thyroid cancer cells through suppressing the Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2017, 96, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, C.; Luo, P.; Yao, J.; Li, J.; Wang, W.; Liu, F. Circular RNA circWDR27 Promotes Papillary Thyroid Cancer Progression by Regulating miR-215-5p/TRIM44 Axis. OncoTargets Ther. 2021, 14, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Jin, C.; Ye, X.; Qiu, B.; Jianjun, X.; Zhu, S.; Xiang, L.; Wu, H.; Yongbing, W. TRIM44 promotes human esophageal cancer progression via the AKT/mTOR pathway. Cancer Sci. 2018, 109, 3080–3092. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Komatsu, S.; Ichikawa, D.; Hirajima, S.; Nishimura, Y.; Konishi, H.; Shiozaki, A.; Fujiwara, H.; Okamoto, K.; Tsuda, H.; et al. Overexpression of TRIM44 is related to invasive potential and malignant outcomes in esophageal squamous cell carcinoma. Tumour Biol. 2017, 39, 1010428317700409. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.A.; Shapiro, J.; Nason, K.S.; Davison, J.M.; Liu, X.; Ross-Innes, C.; O’Donovan, M.; Dinjens, W.N.; Biermann, K.; Shannon, N.; et al. Three-gene immunohistochemical panel adds to clinical staging algorithms to predict prognosis for patients with esophageal adenocarcinoma. J. Clin. Oncol. 2013, 31, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Sun, Y.; Chu, Y.; Liu, F.; Chen, C. TRIM44 regulates tumor immunity in gastric cancer through LOXL2-dependent extracellular matrix remodeling. Cell. Oncol. 2023, 46, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Goshayeshi, L.; Afshar, J.; Mehrad-Majd, H. Clinical Significance of TRIM44 Expression in Patients with Gastric Cancer. Asian Pac. J. Cancer Prev. 2022, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Kashimoto, K.; Komatsu, S.; Ichikawa, D.; Arita, T.; Konishi, H.; Nagata, H.; Takeshita, H.; Nishimura, Y.; Hirajima, S.; Kawaguchi, T.; et al. Overexpression of TRIM44 contributes to malignant outcome in gastric carcinoma. Cancer Sci. 2012, 103, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Meng, Q.; Chen, X.; Zhao, Y.; Liu, W.; Hu, J.; Xue, F.; Wang, X.; Cai, L. TRIM44 promotes proliferation and metastasis in non small cell lung cancer via mTOR signaling pathway. Oncotarget 2016, 7, 30479–30491. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Lin, H.; Ye, X.; Huang, J.; Lu, S.; Xu, L. Trim44 facilitates the migration and invasion of human lung cancer cells via the NF-κB signaling pathway. Int. J. Clin. Oncol. 2015, 20, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tao, R.; Sun, L.Y.; Xu, X.L.; Ling, W. Down-regulation of long non-coding RNA DUXAP8 suppresses proliferation, metastasis and EMT by modulating miR-498 through TRIM44-mediated AKT/mTOR pathway in non-small-cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3152–3165. [Google Scholar] [PubMed]
- Ma, Q.; Huai, B.; Liu, Y.; Jia, Z.; Zhao, Q. Circular RNA circ_0020123 Promotes Non-Small Cell Lung Cancer Progression Through miR-384/TRIM44 Axis. Cancer Manag. Res. 2021, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, J.; He, B.; Zhou, D. CircFBXW8 Acts an Oncogenic Role in the Malignant Progression of Non-small Cell Lung Carcinoma by miR-370-3p-Dependent Regulation of TRIM44. Biochem. Genet. 2022, 60, 1313–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, Y.; Miao, X.; Li, C.; Yin, H.; Yang, S.; Lu, X.; Liu, Y.; Chen, Y.; Shen, R.; et al. High expression of TRIM44 is associated with enhanced cell proliferation, migration, invasion, and resistance to doxorubicin in hepatocellular carcinoma. Tumour Biol. 2016, 37, 14615–14628. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Hu, H.; Yang, X.J.; Huang, C.Q.; Yu, X.Q. TRIM44 Promotes Colorectal Cancer Proliferation, Migration, and Invasion Through the Akt/mTOR Signaling Pathway. Onco Targets Ther. 2019, 12, 10693–10701. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, W.; Ma, X.; Luan, H. Long Noncoding RNA LINC00265 Promotes Glycolysis and Lactate Production of Colorectal Cancer through Regulating of miR-216b-5p/TRIM44 Axis. Digestion 2020, 101, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Sha, D.; Wang, F.; He, T.; He, X. The Novel Target of Colorectal Carcinoma: TRIM44 Regulates Cell Migration and Invasion via Activation of CXCR4/NF-κB Signaling. Cell Biochem. Biophys. 2021, 79, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ding, J.; Xu, W.; Luo, C.; Chen, X.; Zhang, R.; Sui, L.; Hu, Y.; Liu, S.; Shi, G.; et al. Knockdown of TRIM44 inhibits the progression of ovarian cancer and is related to the FOXM1-EZH2 signaling pathway. Transl. Cancer Res. 2022, 11, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, H.; Ji, H.; Zhu, J.; Ma, R. Overexpression of TRIM44 is an independent marker for predicting poor prognosis in epithelial ovarian cancer. Exp. Ther. Med. 2018, 16, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, S.; Sun, J.; Wang, Y.; Xie, L.; Guo, Y.; Li, J.; Han, F. Overexpression of TRIM44 mediates the NF-κB pathway to promote the progression of ovarian cancer. Genes Genom. 2024, 46, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, F.; Ding, J.; Ji, H.; Lin, M.; Zhu, J.; Ma, R. High TRIM44 expression as a valuable biomarker for diagnosis and prognosis in cervical cancer. Biosci. Rep. 2019, 39, BSR20181639. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Azuma, K.; Ikeda, K.; Sugitani, I.; Kinowaki, K.; Fujii, T.; Osaki, A.; Saeki, T.; Horie-Inoue, K.; Inoue, S. TRIM44 Is a Poor Prognostic Factor for Breast Cancer Patients as a Modulator of NF-κB Signaling. Int. J. Mol. Sci. 2017, 18, 1931. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yao, H.; Hu, J.; Liu, L. Knockdown of TRIM44 Inhibits the Proliferation and Invasion in Prostate Cancer Cells. Oncol. Res. 2017, 25, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, G.; Feng, Z.; Zhu, B.; Zhou, L.; Zhang, Y.; Mai, J.; Jiang, C.; Zeng, J. YTHDF1 promotes the proliferation.; migration.; and invasion of prostate cancer cells by regulating TRIM44. Genes Genom. 2021, 43, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Takayama, K.I.; Fujimura, T.; Ashikari, D.; Obinata, D.; Takahashi, S.; Ikeda, K.; Kakutani, S.; Urano, T.; Fukuhara, H.; et al. A novel prognostic factor TRIM44 promotes cell proliferation and migration, and inhibits apoptosis in testicular germ cell tumor. Cancer Sci. 2017, 108, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.A.; Shannon, N.B.; Ross-Innes, C.S.; O’Donovan, M.; Rueda, O.M.; Hu, D.E.; Kettunen, M.I.; Walker, C.E.; Noorani, A.; Hardwick, R.H.; et al. Amplification of TRIM44: Pairing a prognostic target with potential therapeutic strategy. J. Natl. Cancer Inst. 2014, 106, dju050. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, X.; Zhang, S.; Dang, S.; Zhang, Y.; Zhang, G. Expression of TRIM44 and its correlation with TLR4 in laryngeal squamous cell carcinoma. Cell. Mol. Biol. 2022, 68, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, Y.; Ma, P.; Wang, N.; Yang, D.; Tu, Q.; Sun, B.; Xiang, T.; Zhao, X.; Hou, Z.; et al. TRIM44 is indispensable for glioma cell proliferation and cell cycle progression through AKT/p21/p27 signaling pathway. J. Neurooncol 2019, 145, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Alkhathami, A.G.; Sahib, A.S.; Al Fayi, M.S.; Fadhil, A.A.; Jawad, M.A.; Shafik, S.A.; Sultan, S.J.; Almulla, A.F.; Shen, M. Glycolysis in human cancers: Emphasis circRNA/glycolysis axis and nanoparticles in glycolysis regulation in cancer therapy. Environ. Res. 2023, 234, 116007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Zhao, H.F.; Sun, Z.; Li, Y.; Zhong, M.L.; Wang, B.H.; Jiang, X.Z. Tripartite motif-containing 9 promoted proliferation and migration of bladder cancer cells through CEACAM6-Smad2/3 axis. J. Cell Commun. Signal 2023, 17, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Nan, X.; Chang, K.S.; Wang, Y.; Chung, L.W. Overexpression of the promyelocytic leukemia gene suppresses growth of human bladder cancer cells by inducing G1 cell cycle arrest and apoptosis. Chin. Med. J. (Engl.) 2003, 116, 1394–1398. [Google Scholar] [PubMed]
- Xue, Y.; Li, L.; Zhang, D.; Wu, K.; Guo, P.; Zeng, J.; Wang, X.; He, D. Telomerase suppression initiates PML-dependent p53 activation to inhibit bladder cancer cell growth. Oncol. Rep. 2010, 24, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, D.; He, H.; Wang, X.; Zhang, L.; Luo, Y.; Nan, X. Overexpression of PML induced apoptosis in bladder cancer cell by caspase dependent pathway. Cancer Lett. 2006, 236, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.S.; Jou, Y.C.; Tsai, H.T.; Shiau, A.L.; Wu, C.L.; Tzai, T.S. Prothymosin-α enhances phosphatase and tensin homolog expression and binds with tripartite motif-containing protein 21 to regulate Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling in human bladder cancer. Cancer Sci. 2019, 110, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Wei, W.; Duan, J.; Chen, R.; Wang, N.; He, L.; Peng, Y.; Ma, X.; Wu, Z.; Liu, J.; et al. ZHX3 promotes the progression of urothelial carcinoma of the bladder via repressing of RGS2 and is a novel substrate of TRIM21. Cancer Sci. 2021, 112, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Peng, S.; Lu, J.; Zhou, T.; Hong, X.; Chen, S.; Liu, G.; Li, H.; Huang, J.; Chen, X.; et al. UBE2S interacting with TRIM21 mediates the K11-linked ubiquitination of LPP to promote the lymphatic metastasis of bladder cancer. Cell Death Dis. 2023, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhang, X.; Zhang, X.; Liu, J.; Li, N.; Liu, C.; Liu, Y.; Wang, P. Clinical significance and biological roles of TRIM24 in human bladder carcinoma. Tumour Biol. 2015, 36, 6849–6855. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Rinaldetti, S.; Cheikh, B.B.; Zhou, Q.; Hass, E.P.; Jones, R.T.; Joshi, M.; LaBarbera, D.V.; Knott, S.R.V.; Cech, T.R.; et al. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2102423118. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, X.; Jiang, L.; Liu, Z.; Chen, L.; Chen, J.; Deng, M.; Zhou, F.; Zheng, X.; Liu, Z. RITA1 drives the growth of bladder cancer cells by recruiting TRIM25 to facilitate the proteasomal degradation of RBPJ. Cancer Sci. 2022, 113, 3071–3084. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, H.; Pan, J.; Han, X. Knockdown of TRIM26 inhibits the proliferation, migration and invasion of bladder cancer cells through the Akt/GSK3β/β-catenin pathway. Chem. Biol. Interact. 2021, 337, 109366. [Google Scholar] [CrossRef] [PubMed]
- Palmbos, P.L.; Wang, L.; Yang, H.; Wang, Y.; Leflein, J.; Ahmet, M.L.; Wilkinson, J.E.; Kumar-Sinha, C.; Ney, G.M.; Tomlins, S.A.; et al. ATDC/TRIM29 Drives Invasive Bladder Cancer Formation through miRNA-Mediated and Epigenetic Mechanisms. Cancer Res. 2015, 75, 5155–5166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Zhou, C.K.; Chen, S.Z.; Yang, Y.; Shi, B.K. Identification of hub genes and pathways associated with bladder cancer based on co-expression network analysis. Oncol. Lett. 2017, 14, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, H.; Rui, W.; Zhang, N.; Zhu, Y.; Xie, X. TRIM38 triggers the uniquitination and degradation of glucose transporter type 1 (GLUT1) to restrict tumor progression in bladder cancer. J. Transl. Med. 2021, 19, 508. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, K.; Miao, C.; Xu, A.; Zhang, J.; Zhu, J.; Su, S.; Wang, Z. Silencing Trim59 inhibits invasion/migration and epithelial-to-mesenchymal transition via TGF-β/Smad2/3 signaling pathway in bladder cancer cells. OncoTargets Ther. 2017, 10, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Qiu, Y.; Deng, L.; Nie, L.; Ge, L.; Zheng, X.; Jin, D.; Jin, K.; Zhou, X.; Su, X.; et al. Cell softness reveals tumorigenic potential via ITGB8/AKT/glycolysis signaling in a mice model of orthotopic bladder cancer. Chin. Med. J. (Engl.) 2024, 137, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.S.; Chen, X.; Guo, L.Y.; Li, X.D.; Deng, M.H.; Yuan, G.J.; He, L.Y.; Li, Y.H.; Zhang, Z.L.; Jiang, L.J.; et al. TRIM65 supports bladder urothelial carcinoma cell aggressiveness by promoting ANXA2 ubiquitination and degradation. Cancer Lett. 2018, 435, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xu, L.; Zheng, B.; Wu, Z.; Chen, J. Tripartite motif containing 66 promotes malignant progression of bladder cancer by activating matrix metallopeptidase 11. J. Biomed. Nanotechnol. 2023, 19, 1550–1557. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Q.; Zhou, Y. The construction and validation of an RNA binding protein-related prognostic model for bladder cancer. BMC Cancer 2021, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Offermann, A.; Kang, D.; Watermann, C.; Weingart, A.; Hupe, M.C.; Saraji, A.; Stegmann-Frehse, J.; Kruper, R.; Schüle, R.; Pantel, K.; et al. Analysis of tripartite motif (TRIM) family gene expression in prostate cancer bone metastases. Carcinogenesis 2021, 42, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, R.; Chen, H.; Chen, W.; Wu, K.; Lv, J. Expression of Tripartite Motif-Containing Proteactiin 11 (TRIM11,) is Associated with the Progression of Human Prostate Cancer and is Downregulated by MicroRNA-5193. Med. Sci. Monit. 2019, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, H.; Lu, F. TRIM11 Posttranscriptionally Modulated by miR-5193 Facilitates Tumor Growth and Metastasis of Prostate Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231178639. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Deng, C.; Liang, T.; Ye, X.; Li, Z.; Song, W.; Yan, D. Tripartite Motif-Containing Protein 11 Reverses Paclitaxel Resistance in Prostate Cancer Drug-Resistant Cells by Mediating Family with Sequence Similarity 46B Expression. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Spirina, L.V.; Kovaleva, I.V.; Usynin, E.A.; Goorbunov, A.K.; Kondakova, I.V. Progesterone Receptor Expression in the Benign Prostatic Hyperplasia and Prostate Cancer Tissues, Relation with Transcription, Growth Factors, Hormone Reception and Components of the AKT/mTOR Signaling Pathway. Asian Pac. J. Cancer Prev. 2020, 21, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Buczek, M.E.; Miles, A.K.; Green, W.; Johnson, C.; Boocock, D.J.; Pockley, A.G.; Rees, R.C.; Hulman, G.; van Schalkwyk, G.; Parkinson, R.; et al. Cytoplasmic PML promotes TGF-β-associated epithelial-mesenchymal transition and invasion in prostate cancer. Oncogene 2016, 35, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Birch, S.E.; Kench, J.G.; Takano, E.; Chan, P.; Chan, A.L.; Chiam, K.; Veillard, A.S.; Stricker, P.; Haupt, S.; Haupt, Y.; et al. Expression of E6AP and PML predicts for prostate cancer progression and cancer-specific death. Ann. Oncol. 2014, 25, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Chatterjee, U.; Ghosh, M.K. Activation of protein kinase CK2 attenuates FOXO3a functioning in a PML-dependent manner: Implications in human prostate cancer. Cell Death Dis. 2013, 4, e543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Melamed, J.; Wei, P.; Cox, K.; Frankel, W.; Bahnson, R.R.; Robinson, N.; Pyka, R.; Liu, Y.; Zheng, P. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003, 3, 2. [Google Scholar] [PubMed]
- He, D.; Mu, Z.M.; Le, X.; Hsieh, J.T.; Pong, R.C.; Chung, L.W.; Chang, K.S. Adenovirus-mediated expression of PML suppresses growth and tumorigenicity of prostate cancer cells. Cancer Res. 1997, 57, 1868–1872. [Google Scholar] [PubMed]
- Yang, L.; Yeh, S.D.; Xie, S.; Altuwaijri, S.; Ni, J.; Hu, Y.C.; Chen, Y.T.; Bao, B.Y.; Su, C.H.; Chang, C. Androgen suppresses PML protein expression in prostate cancer CWR22R cells. Biochem. Biophys. Res. Commun. 2004, 314, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Höflmayer, D.; Fraune, C.; Hube-Magg, C.; Simon, R.; Schroeder, C.; Büscheck, F.; Möller, K.; Dum, D.; Weidemann, S.; Wittmer, C.; et al. Overexpression of the TRIM24 E3 Ubiquitin Ligase is Linked to Genetic Instability and Predicts Unfavorable Prognosis in Prostate Cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 29, e29–e38. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; He, C.; Shi, S.; Wang, M.; Ma, J.; Yang, P.; Dong, Y.; Mou, X.; Han, S. Linc00963 Promote Cell Proliferation and Tumor Growth in Castration-Resistant Prostate Cancer by Modulating miR-655/TRIM24 Axis. Front. Oncol. 2021, 11, 636965. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Guan, X.; An, H.; Baihetiya, A.; Wang, W.; Shao, W.; Yang, H.; Wang, Y. Epigenetic silencing of miR-137 induces resistance to bicalutamide by targeting TRIM24 in prostate cancer cells. Am. J. Transl. Res. 2019, 11, 3226–3237. [Google Scholar] [PubMed]
- Wang, S.; Kollipara, R.K.; Humphries, C.G.; Ma, S.H.; Hutchinson, R.; Li, R.; Siddiqui, J.; Tomlins, S.A.; Raj, G.V.; Kittler, R. The ubiquitin ligase TRIM25 targets ERG for degradation in prostate cancer. Oncotarget 2016, 7, 64921–64931. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.I.; Suzuki, T.; Tanaka, T.; Fujimura, T.; Takahashi, S.; Urano, T.; Ikeda, K.; Inoue, S. TRIM25 enhances cell growth and cell survival by modulating p53 signals via interaction with G3BP2 in prostate cancer. Oncogene 2018, 37, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dou, P.; Lu, X.; Guan, P.; Lin, Z.; Zhou, Y.; Lu, X.; Lin, X.; Xu, G. Identification and Validation of TRIM25 as a Glucose Metabolism Regulator in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 9325. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ren, Y. Long Noncoding RNA Small Nucleolar RNA Host Gene 3 Mediates Prostate Cancer Migration, Invasion, and Epithelial-Mesenchymal Transition by Sponging miR-487a-3p to Regulate TRIM25. Cancer Biother. Radiopharm. 2022, 37, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.W.; Zhao, J.C.; Song, B.; Zheng, B.; Yu, J. TRIM28 protects TRIM24 from SPOP-mediated degradation and promotes prostate cancer progression. Nat. Commun. 2018, 9, 5007. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zuo, Q.; Chang, J.; Wu, X. The correlation between TRIM28 expression and immune checkpoints in CRPC. FASEB J. 2024, 38, e23663. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Watanabe, M.; Kimura, T.; Nonomura, K.; Tanaka, S.; Hatakeyama, S. TRIM29 as a novel prostate basal cell marker for diagnosis of prostate cancer. Acta Histochem. 2014, 116, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wu, C.; Tang, Q.; Zhou, X.; He, B.; Chang, P.; He, X. Silencing LINC00491 inhibits prostate cancer development through the miR-384/TRIM44 axis. J. Biochem. Mol. Toxicol. 2023, 37, e23370. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lingadahalli, S.; Narwade, N.; Lei, K.M.K.; Liu, S.; Zhao, Z.; Zheng, Y.; Lu, Q.; Tang, A.H.N.; Poon, T.C.W.; et al. TRIM33 drives prostate tumor growth by stabilizing androgen receptor from Skp2-mediated degradation. EMBO Rep. 2022, 23, e53468. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Inoue, S.; Urano, T.; Takayama, K.; Yamada, Y.; Ikeda, K.; Obinata, D.; Ashikari, D.; Takahashi, S.; Homma, Y. Increased Expression of Tripartite Motif (TRIM) 47 Is a Negative Prognostic Predictor in Human Prostate Cancer. Clin. Genitourin. Cancer 2016, 14, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Yamada, Y.; Takayama, K.I.; Fujimura, T.; Takahashi, S.; Kume, H.; Inoue, S. Androgen-responsive tripartite motif 36 enhances tumor-suppressive effect by regulating apoptosis-related pathway in prostate cancer. Cancer Sci. 2018, 109, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, T.; Wang, Y.; Bao, M.; Ni, C.; Ding, L.; Sun, S.; Dong, H.; Li, J.; Liang, C. Trigred motif 36 regulates neuroendocrine differentiation of prostate cancer via HK2 ubiquitination and GPx4 deficiency. Cancer Sci. 2023, 114, 2445–2459. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Wang, H.; Song, X.; Zhang, S.X.; Zhou, P.S.; Sun, J.M.; Li, J.S. Knockdown of tripartite motif 59 (TRIM59) inhibits tumor growth in prostate cancer. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4864–4873. [Google Scholar] [PubMed]
- Fan, L.; Gong, Y.; He, Y.; Gao, W.Q.; Dong, X.; Dong, B.; Zhu, H.H.; Xue, W. TRIM59 is suppressed by androgen receptor and acts to promote lineage plasticity and treatment-induced neuroendocrine differentiation in prostate cancer. Oncogene 2023, 42, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, Y.; Ge, Q.; Huang, Y. MicroRNA-300 inhibits the metastasis of prostate cancer through the regulation of TRIM63. Trop. J. Pharma Res. 2022, 21, 479–484. [Google Scholar] [CrossRef]
- Cao, H.; Gao, R.; Chen, L.; Feng, Y. TRIM66 promotes malignant progression of prostate carcinoma through the JAK/STAT pathway. FEBS Open Bio 2020, 10, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, N.; Maruyama, S.; Bohgaki, M.; Kano, S.; Shigemura, M.; Shinohara, N.; Nonomura, K.; Hatakeyama, S. TRIM68 regulates ligand-dependent transcription of androgen receptor in prostate cancer cells. Cancer Res. 2008, 68, 3486–3494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Ahmad, A.; Bao, B.; Dyson, G.; Sarkar, F.H. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics 2012, 7, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Wu, X.; Huang, Y.; Guo, T.; Kuang, J.; Du, C. RNA N6-methyladenosine-modified-binding protein YTHDF1 promotes prostate cancer progression by regulating androgen function-related gene TRIM68. Eur. J. Med. Res. 2023, 28, 552. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Tanaka, H.; Nishimune, Y. Haprin, a novel haploid germ cell-specific RING finger protein involved in the acrosome reaction. J. Biol. Chem. 2003, 278, 44417–44423. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Ikeda, K.; Gotoh, N.; Inoue, S.; Horie, K. Efp promotes growth of triple-negative breast cancer cells. Biochem. Biophys. Res. Commun. 2022, 624, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, S.; Liao, L.; Li, Y.; Li, H.; Li, Z.; Lin, L.; Wan, X.; Yang, X.; Chen, L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat. Commun. 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Xue, Y.; Dai, T.; Li, X.; Zheng, N. Tripartite motif containing 25 promotes proliferation and invasion of colorectal cancer cells through TGF-β signaling. Biosci. Rep. 2017, 37, BSR20170805. [Google Scholar] [CrossRef] [PubMed]
- Horie-Inoue, K.; Inoue, S. Epigenetic and proteolytic inactivation of 14-3-3sigma in breast and prostate cancers. Semin. Cancer Biol. 2006, 16, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, S.P.; Saxena, N.; Zhang, F.; Beraldi, E.; Huang, J.N.; Gentle, C.; Fazli, L.; Thi, M.; Sorensen, P.H.; Gleave, M. Regulation of AR mRNA translation in response to acute AR pathway inhibition. Nucleic Acids Res. 2022, 50, 1069–1091. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, Y.; Yamada, Y.; Kawai, T.; Nakamura, M.; Takeshima, Y.; Iwaki, T.; Teshima, T.; Kinoshita, Y.; Fujii, Y.; Akiyama, Y.; et al. Time to castration resistance is a novel prognostic factor of cancer-specific survival in patients with nonmetastatic castration-resistant prostate cancer. Sci. Rep. 2022, 12, 16202. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, Y.; Yamada, Y.; Takeshima, Y.; Taguchi, S.; Kawai, T.; Nakamura, M.; Iwaki, T.; Teshima, T.; Kinoshita, Y.; Akiyama, Y.; et al. Low hemoglobin and PSA kinetics are prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients. Sci. Rep. 2023, 13, 2672. [Google Scholar] [CrossRef] [PubMed]
| Authors | Journals, Year | TRIM | Tumor-Promoting (P)/ Tumor-Suppressive (S) | Involved Signal/ Pathway | Summary |
|---|---|---|---|---|---|
| Zheng [23] | Front Oncol, 2022 | TRIM1, TRIM2, TRIM13, TRIM26, TRIM27, TRIM35, TRIM47, TRIM55 | NA | NA | Prognostic signature was developed using 8 TRIM family proteins. Higher risk scores were associated with higher level of immune infiltration by plasma cells, follicular helper T cells, and NK cells and a lower level of immune infiltration by memory resting CD4 T cells, M1 and M2 macrophages, and resting dendritic cells. |
| Xiao [24] | Cancer Manag Res, 2018 | TRIM2 | S | NA | Low expression of TRIM2 was correlated with poor prognosis. Overexpression of TRIM2 promoted cell proliferation, migration, and invasion in RCC cell line. |
| Wei [25] | Aging, 2020 | TRIM2 | S | NA | TRIM2 was related to clinical stage and pathological grade and was an independent prognostic factor. KEGG and GO indicated ubiquitin mediated protein hydrolysis, cell adhesion molecules, and Th17 cell differentiation signaling pathway. |
| Shen [26] | Aging, 2022 | TRIM4, TRIM7, TRIM27, TRIM58, TRIM65, TRIM72 | NA | NA | These TRIM proteins showed positive correlation with worse survival in kidney clear cell carcinoma patients. |
| Yuan [27] | Cell Biology Int, 2022 | TRIM7 | S | HIF-1ɑ, Src; PI3K/AKT/mTOR pathway | TRIM7 acts as a tumor suppressor which inhibits HIF-1ɑ through degrading Src protein and regulating PI3K/AKT/mTOR pathway or reactive oxygen species production. |
| Wu [28] | Int J Med Sci, 2020 | TRIM8, TRIM11, TRIM16, TRIM19, TRIM27 | NA | NA | high mRNA expression levels of these genes were correlated with poor prognosis. |
| TRIM24, TRIM32 | NA | NA | Low mRNA expression levels of these genes were correlated with poor prognosis. | ||
| Caratozzolo [29] | Oncotarget, 2014 | TRIM8 | S | p53 | TRIM8 upregulation restores p53 tumor suppressor response to chemotherapeutic drug treatments in renal cell carcinoma cell lines. |
| Li [30] | Nutr Cancer, 2020 | TRIM13 | S | NFκβ, MMP-9, p-AKT | Upregulation of TRIM13 inhibited NFκβ, MMP-9, and p-AKT and suppressed cell migration and invasion in RCC cell line. |
| Lin [31] | Cancer Res, 2014 | TRIM19 | S | SCP1; mTOR-HIF pathway | SCP1 inhibits the degradation of PML that inhibits the cell proliferation, migration, invasion, angiogenesis, tumor growth, and mTOR-HIF pathway in clear cell renal cell carcinoma. |
| Simoni [32] | EMBO Mol Med, 2024 | TRIM19 | P | p53, cell cycle | PML knockdown leads to accumulation of G0/G1 distribution in RCC cell lines and inhibition of tumor growth in vivo. |
| Chen [33] | Cancers Letters, 2021 | TRIM21 | S | HIF-1α | TRIM21 inhibits RCC cell glycolysis via the ubiquitination-mediated degradation of HIF-1ɑ leading to inhibition of cell proliferation, tumorigenesis, migration, and metastasis of RCC cells in vitro and in vivo. |
| Jiang [34] | Open Medicine, 2020 | TRIM24 | P | MMP-2, MMP-9, fibronectin, SNAIL, vimentin, N-cadherin, β-catenin; EMT | TRIM24 promoted cell proliferation, migration, and invasion of RCC cell line. |
| Yu [35] | Cancer Sci, 2020 | TRIM24 | P | Wnt/beta catenin pathway | TRIM24 promoted the invasion and migration of clear cell RCC cells through the Wnt/beta catenin pathway. |
| Zheng [36] | J Transl Med, 2024 | TRIM26 | S | ETK, AKT/mTOR | Low expression of TRIM26 is associated with worse overall survival. TRIM26 inhibits cell proliferation, migration, and invasion by degrading ETK resulting in inhibition of AKT/mTOR signaling pathway in clear cell RCC cel line. |
| Xiao [37] | BMC Cancer, 2021 | TRIM27 | P | lκβα | High expression of TRIM27 was correlated with poor prognosis. TRIM27 interacted with lκβɑ that promoted NFκβ. |
| Song [38] | J Biol Chem, 2023 | TRIM28 | S | TFE3, histone H3K27 demethylase KDM6A | TRIM28 inhibits TFE3 that interacts with and recruits histone H3K27 demethylase KDM6A for autophagic gene upregulation and suppresses tumor growth. |
| Jingushi [39] | Mol Cancer Res, 2015 | TRIM33 | S | miR-629, TGF-β; SMAD signaling, EMT | miR-629 inhibits TGF-beta-induced SMAD activation via upregulation of TRIM33. |
| Xu [40] | Biomed Res Int, 2020 | TRIM33 | S | β-catenin, cyclin D1, c-myc | Low expression of TRIM33 was related to poor prognosis. TRIM33 overexpression inhibited cell proliferation, migration, and invasion of RCC cell lines and reduced β-catenin, cyclin D1, and c-myc, and inhibited tumor growth in vivo. |
| Miao [41] | J Exp Clin Cancer Res, 2021 | TRIM37 | P | TGF-β1 signaling, EMT | TRIM37 promoted ubiquitination of histone H2A and promoted EMT and cancer progression through activating TGF-β1 signaling. |
| Yamada [42] | Cancer Sci, 2020 | TRIM44 | P | FRK | TRIM44 overexpression was associated with clinical M stage, clear cell type, lymphatic invasion, and cancer-specific survival. TRIM44 promotes cell proliferation via regulating FRK in RCC cell line. |
| Ren [43] | Front Med, 2021 | TRIM46 | P | tumor immunity several pathways | TRIM46 upregulation was associated with unfavorable prognosis based on the bioinformatics analyses using the data from TCGA and GEO databases. TRIM46 was positively correlated with NUMBL, CACNB1, THBS3, ROBO3, MAP3K12, ANKRD13B, and PCNX2. |
| Chen [44] | Cancer Cell Int, 2021 | TRIM47 | P | p53 | TRIM47 promoted RCC proliferation in vitro and in vivo via degrading p53 by ubiquitination. |
| Gan [45] | Front Mol Biosci, 2021 | TRIM58 | S | NA | TRIM58-specific DNA demethyltransferase promotes demethylation of TRIM58 CpG islands and activates the TRIM58 transcription that leads to inhibition of cell proliferation and migration in RCC cell lines. |
| Hu [46] | Cell Mol Biol, 2017 | TRIM59 | P | NA | TRIM59 knockdown inhibited cell migration in 786-O cells line and inhibited tumor growth in vivo. |
| Wang [47] | Mod Pathol, 2021 | TRIM63 | P | TFE3 | TRIM63 was highly expressed in MiT family aberration-associated RCC. TRIM63 RNA-ISH was strongly positive in TFE3 FISH false-negative cases. |
| Zhang [48] | Cell Death Dis, 2024 | TRIM65 | P | BTG3; cell cycle | TRIM65 promoted cell proliferation by degrading BTG3 and suppressing G2/M phase cell cycle arrest. |
| Authors | Journals, Year | TRIM | Tumor-Promoting (P)/ Tumor-Suppressive (S) | Involved Signal/ Pathway | Summary |
|---|---|---|---|---|---|
| Zhang [79] | J Cell Commun Sigaling, 2023 | TRIM9 | P | CEACAM6 | TRIM9 promoted cell proliferation and migration of bladder cancer cells. TRIM9 modulated CEACAM6 upregulation, which facilitated Smad2/3-MMP2 signaling pathway in vitro and in vivo. Overexpression of TRIM9 reduced the chemosensitivity to mitomycin C and gemcitabine in bladder cancer cell line. |
| He [80] | Chin Med J, 2003 | TRIM19 | S | NA | PML inhibited cell proliferation by inducing G1 cell cycle arrest and apoptosis in vitro and in vivo. |
| Xue [81] | Oncol Rep, 2010 | TRIM19 | S | p53 | Inhibition of teromerase activates p53 via PML regulation. |
| Li [82] | Cancer Lett, 2006 | TRIM19 | S | Caspase-dependent pathway | PML induced apoptosis of bladder cancer cell by promoting Caspase-dependent pathway. |
| Tsai [83] | Cancer Sci, 2019 | TRIM21 | S | PTEN, Nrf2 signaling | PTMA upregulates PTEN and interacts with TRIM21 to regulate Nrf2 signaling. |
| Deng [84] | Cancer Sci, 2021 | TRIM21 | S | ZHX3 | TRIM21 regulates ZHX3, which inhibits RGS2. |
| Xiao [85] | Cell Death Dis, 2023 | TRIM21 | P | UBE2S | UBE2 interacting with TRIM21 degrades LPP through k11-linked ubiquitination and promotes the lymphatic metastasis of bladder cancer. |
| Xue [86] | Tumour Biol, 2015 | TRIM24 | P | NFκβ, AKT pathway | TRIM24 promotes cell proliferation and invasion. TRIM24 upregulated cyclinD1, cyclinE, p-lκβα, and p-AKT. |
| Agarwal [87] | Proc Natl Acad Sci U S A, 2021 | TRIM24 | S | TRIM28 | TRIM28 activates hTERT and promotes bladder cancer cell growth. TRIM24 interacts with TRIM28 and inhibits its activity. |
| TRIM28 | P | hTERT | |||
| Tang [88] | Cancer Sci, 2022 | TRIM25 | P | RBPJ | RITA1 promotes bladder cancer cell proliferation by interacting with TRIM25 and degrading RBPJ. |
| Xie [89] | Chem Biol Interact, 2021 | TRIM26 | P | p-AKT, p-GSK3β, β-catenin, c-Myc | TRIM26 upregulates bladder cancer cells via the AKT/GSK3β/β-catenin pathway. |
| Palmbos [90] | Cancer Res, 2015 | TRIM29 | P | TRIM29 is highly expressed in huma bladder cancer and correlates with invasive disease. TRIM29 promotes cell proliferation and invasion. | |
| Zhang [91] | Oncol Lett, 2017 | TRIM31 | NA | NA | TRIM31 as well as LGALS4, PTPRN2, TMPRSS11E, and KCND3 were identified as 5 hub genes associated with bladder cancer. |
| Wang [92] | J Transl Med, 2021 | TRIM38 | S | GLUT1 | TRIM38 degrades GLUT1 and act as tumor suppressor in vitro and in vivo |
| Chen [93] | Onco Targets Ther, 2017 | TRIM59 | P | TGF-β/Smad2/3 signaling pathway | TRIM59 induced EMT via activation of TGFβ/Smad2/3 signaling pathway. |
| Qiu [94] | Chinese Med J, 2024 | TRIM59 | P | NA | 3D Matrigel activated the F-actin/ITGB8/TRIM59/AKT/mTOR/glycolysis pathways to promote softness of tumor cells. |
| Wei [95] | Cancer Lett, 2018 | TRIM65 | P | ANXA2, EMT | TRIM65 induces EMT of urothelial carcinoma of the bladder cells by degrading ANXA2. |
| Xiao [96] | J Biomed Nanotechnol, 2023 | TRIM66 | P | MMP-11 | Knockdown of TRIM66 inhibits bladder cancer cell proliferation and migration by downregulating MMP-11. |
| Chen [97] | BMC Cancer, 2021 | TRIM71 | NA | NA | Higher mRNA expression level of TRIM71 based on the RNA sequence data from TCGA database. |
| Authors | Journals, Year | TRIM | Tumor-Promoting (P)/ Tumor-Suppressive (S) | Involved Signal/ Pathway | Summary |
|---|---|---|---|---|---|
| Offermann [98] | Carcinogenesis, 2021 | TRIM1, TRIM5, TRIM21, TRIM23, TRIM33, TRIM36, TRIM44, TRIM4, TRIM10, TRIM40, TRIM42, TRIM50, TRIM66, TRIM67, TRIM71, TRIM77 | NA | TNF, TGF-β, PI3K/AKT, HIF-1 signaling pathway | Transcriptome analysis was carried out in 59 patients, including localized and bone metastatic prostate cancer. A total of 7 TRIM genes (TRIM1, TRIM5, TRIM21, TRIM23, TRIM33, TRIM36, and TRIM44) were lower expressed and 9 TRIM genes (TRIM 4, TRIM10, TRIM40, TRIM42, TRIM50, TRIM66, TRIM67, TRIM71, and TRIM77) were overexpressed in bone metastatic prostate cancer compared to localized cancer. |
| Pan [99] | Med Sci Monit, 2019 | TRIM11 | P | NA | TRIM11 was upregulated in prostate cancer tissue and was associated with poor prognosis. TRIM11 overexpression promoted cell proliferation and was inhibited by miR-5193. |
| Pan [100] | Technol Cancer Res Treat, 2023 | TRIM11 | P | MEK1/2, ERK1/2 | miR-5193 suppresses cell proliferation by inhibiting TRIM11 and MEK1/2 and ERK1/2 pathway in vitro. miR-5193 also inhibited tumor growth in vivo by modulating TRIM11. |
| Guo [101] | Crit Rev Eukaryot Gene Exp, 2022 | TRIM11 | P | FAM46B | TRIM11 was upregulated in paclitaxel-resistant cells and inhibited FAM46B that led to promotion of cell proliferation, migration, and invasion in these cells. |
| Spirina [102] | Asian Pac J Cancer Prev, 2020 | TRIM16 | NA | NA | Higher expression of progesterone receptor (PR) was observed in cancer tissues. Low PR level was associated with high expression of TRIM16. |
| Buczek [103] | Oncogene, 2016 | TRIM19 | P | TGF-β, SMAD2/3; EMT | Cytoplasmic PML promotes EMT by activating TGF-β canonical signaling pathway through the induction of SMAD2/3 phosphorylation. |
| Birch [104] | Ann Oncol, 2014 | TRIM19 | NA | NA | Low expression levels of PML protein were associated with cancer-specific death. |
| Chatterjee [105] | Cell Death Dis, 2013 | TRIM19 | S | CK2, PHLPP2, FOXO3a, pAKT; AKT pathway | Inhibition of CK2 promotes PML elevation, which interacts with PHLPP2 in the nucleus and increases FOXO3a activity, leading to inhibition of cell proliferation and promotion of apoptosis in prostate cancer cells. |
| Zhang [106] | Cancer Immun, 2003 | TRIM19 | NA | NA | Low expression levels of PML protein were observed in prostate cancer tissue by IHC. |
| He [107] | Cancer Res, 1997 | TRIM19 | S | NA | PML suppresses tumor growth in prostate cancer cells |
| Yang [108] | Biochem Biophys Res Commun, 2004 | TRIM19 | S | p21, p53, AR | PML inhibits androgen receptor (AR) transactivation and promotes p21 and p53 activity. Knockdown of PML promotes AR activity and cell proliferation. |
| Höflmayer [109] | Appl immunohistochem Mol Morphol, 2021 | TRIM24 | NA | NA | TRIM24 upregulation was associated with high Gleason grade, advanced pathological T stage, lymph node metastasis, higher preoperative PSA level, increased cell proliferation, and genomic instability. |
| Bai [110] | Front Oncol, 2021 | TRIM24 | P | miR-655, Linc00963 | Lin00963 interacts with miR-655 and upregulates TRIM24, thereby activating PI3K/AKT signaling and promoting cell proliferation in CRPC cells. |
| Guan [111] | Am J Trans Res, 2019 | TRIM24 | P | MeCP2, DNMT, miRNa-137; glutamine metabolism | MeCP2 and DNMTs interacted to promote epigenetic silencing of miRNA-137, which led to promotion in TRIM24 upregulation and glutamine metabolism in bicalutamide-resistant prostate cancer cells. |
| Wang [112] | Oncotarget, 2016 | TRIM25 | S | ERG | TRIM25 degrades ERG by ubiquitination. |
| Takayama [113] | Oncogene, 2018 | TRIM25 | P | G3BP2 | TRIM25 promoted cell proliferation and inhibited apoptosis by modulating p53 signals via regulation of G3BP2. |
| Li [114] | Int J Mol Sci, 2022 | TRIM25 | NA | IDH1, FH | TRIM25 is involved in glucose metabolism by regulating IDH1 and FH. TRIM25 expression level was positively associated with Gleason scores. |
| Yu [115] | Cancer Biother Radiopharm, 2022 | TRIM25 | P | SNHG3, miR-487 | SNHG3 mediates migration, invasion, and EMT in Pca cells by sponging miR-487 a-3p to regulate TRIM25. |
| Fong [116] | Nat Commun, 2018 | TRIM28 | P | TRIM24 | TRIM28 stabilizes TRIM24 by blocking SPOP-mediated degradation and promotes CRPC tumor growth. |
| Xue [117] | FASEB J, 2024 | TRIM28 | P | T cell immune system | Knockdown of TRIM28 reduces proportions of M2 macrophages, enhanced infiltration of CD8+ T cells, and reduced tumor growth of CRPC cells. |
| Kanno [118] | Acta histochem, 2014 | TRIM29 | NA | NA | TRIM29 is selectively expressed in basal cells of normal prostate. |
| Zhou [119] | J Exp Clin Cancer Res, 2023 | TRIM32 | S | TSPAN18, STIM1 | TSPAN18 promotes bone metastasis by interacting with STIM1 that blocked from TRIM32-mediated ubiquitination and degradation. |
| Chen [120] | EMBO Rep, 2022 | TRIM33 | P | AR | TRIM33 promotes tumor growth by stabilizing AR from Skp-mediated degradation in prostate cancer cells. |
| Fujimura [121] | Clin Cancer Res, 2014 | TRIM36 | NA | NA | Lower mRNA expression of TRIM36 was correlated with lower cancer-specific survival. |
| Kimura [122] | Cancer Sci, 2018 | TRIM36 | S | BAX, TNFSF10; apoptosis-related pathway, T-cell receptor signaling pathway | High expression of TRIM36 is associated with favorable prognosis. TRIM36 promotes apoptosis and suppresses cell proliferation and migration in prostate cancer cell lines. TRIM36 acts as tumor suppressor by upregulating BAX and TNFSF10. |
| Zhao [123] | Cancer Sci, 2023 | TRIM36 | S | lys-48, HK2, GPX4; glycolysis pathway | TRIM36 was expressed low in neuroendocrine prostate cancer. TRIM36 inhibited the glycolysis pathway by promoting K48-linked ubiquitination of HK2. This loss of HK2 activity leads to downregulation of GPX4, which then makes the cancer cells vulnerable to ferroptosis. |
| Tan [72] | Oncol Res, 2017 | TRIM44 | P | PI3K/AKT pathway | TRIM44 was upregulated in prostate cancer cell lines. Knockdown of TRIM44 inhibited proliferation and invasion of prostate cancer cells in vitro and reduced tumor growth in vivo. Knockdown of TRIM44 also reduced the levels of phosphorylated PI3K and Akt in PC-3 cells. |
| Li [73] | Genes Genomics, 2021 | TRIM44 | P | YTHDF1 | YTHDF1 promotes cell proliferation, migration, and invasion by modulating TRIM44. |
| Zhou [119] | J Biochem Mol Toxicol, 2023 | TRIM44 | P | NA | Knockdown of long non-coding RNA, LINC00491, inhibited cell proliferation in vitro and reduced tumor growth in vivo. LINC00491 is a tumor promoter that upregulates TRIM44 by sponging miR-384 to facilitate cancer progression. |
| Fujimura [121] | Clin Genitourin Cancer, 2016 | TRIM47 | NA | NA | Based on immunohistochemical findings, high expression levels of TRIM47 were associated with ≥pT3 stage and worse cancer-specific survival. |
| Lin [124] | Eur Rev Med Pharmacol Sci, 2016 | TRIM59 | P | CDC25A, CDC2, cyclin B1; cell cycle | TRIM59 was highly expressed in prostate cancer tissues. TRIM59 promotes cell proliferation by upregulating CDC25A, CDC2, and cyclin B1. |
| Fan [125] | Oncogene, 2023 | TRIM59 | P | AR, RB1, p53 | AR inhibits TRIM59, which is highly expressed in CRPC. TRIM59 is associated with poor prognosis. TRIM 59 knockdown suppresses CRPC cell proliferation, migration, and tumor growth in vitro and in vivo. TRIM59 promotes degradation of RB1 and p53. |
| Ma [126] | Tropical J Pharma Res, 2022 | TRIM63 | P | microRNA-300 | microRNA-300 suppresses cell proliferation and migration by targeting TRIM63. |
| Cao [127] | FEBS Open Bio, 2020 | TRIM66 | P | JAK/STAT pathway | TRIM66 promoted STAT2 and IL-2 and cell proliferation and migration in prostate cancer cells. |
| Miyajima [128] | Cancer Res, 2008 | TRIM68 | P | AR, TIP60, p300 | TRIM68 regulates AR-mediated transcription. Knockdown of TRIM68 inhibited colony formation of LNCaP cells. |
| Li [129] | Epigenetics, 2012 | TRIM68 | P | miR29a and miR-1256 | Isoflavone increased the levels of miR29a and miR-1256, which resulted in a decreased expression of TRIM68, thereby inhibiting cell growth and invasion. |
| Nie [130] | Eur J Med Res, 2023 | TRIM68 | P | YTHDF1 | YTHDF1 promotes cancer progression by regulating TRIM68 in vitro and in vivo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Kimura, N.; Maki, K.; Hakozaki, Y.; Urabe, F.; Kimura, S.; Fujimura, T.; Inoue, S.; Kume, H. The Roles of Tripartite Motif Proteins in Urological Cancers: A Systematic Review. Cancers 2025, 17, 2367. https://doi.org/10.3390/cancers17142367
Yamada Y, Kimura N, Maki K, Hakozaki Y, Urabe F, Kimura S, Fujimura T, Inoue S, Kume H. The Roles of Tripartite Motif Proteins in Urological Cancers: A Systematic Review. Cancers. 2025; 17(14):2367. https://doi.org/10.3390/cancers17142367
Chicago/Turabian StyleYamada, Yuta, Naoki Kimura, Kazuki Maki, Yuji Hakozaki, Fumihiko Urabe, Shoji Kimura, Tetsuya Fujimura, Satoshi Inoue, and Haruki Kume. 2025. "The Roles of Tripartite Motif Proteins in Urological Cancers: A Systematic Review" Cancers 17, no. 14: 2367. https://doi.org/10.3390/cancers17142367
APA StyleYamada, Y., Kimura, N., Maki, K., Hakozaki, Y., Urabe, F., Kimura, S., Fujimura, T., Inoue, S., & Kume, H. (2025). The Roles of Tripartite Motif Proteins in Urological Cancers: A Systematic Review. Cancers, 17(14), 2367. https://doi.org/10.3390/cancers17142367









