Effectiveness of Surgical Treatment on Survival of Patients with Malignant Pleural Mesothelioma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Variables
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Mesothelioma Fact Sheet, Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/18-Mesothelioma-fact-sheet.pdf (accessed on 21 October 2023).
- Milano, M.T.; Zhang, H. Malignant Pleural Mesothelioma: A Population-Based Study of Survival. J. Thorac. Oncol. 2010, 5, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin Versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644, Erratum in J. Clin. Oncol. 2023, 41, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Taioli, E.; Wolf, A.S.; Camacho-Rivera, M.; Kaufman, A.; Lee, D.S.; Nicastri, D.; Rosenzweig, K.; Flores, R.M. Determinants of survival in malignant pleural mesothelioma: A SEER study of 14,228 patients. PLoS ONE 2015, 10, e0145039. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomized feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Waller, D.; Lau, K.; Steele, J.; Pope, A.; Ali, C.; Bilancia, R.; Keni, M.; Popat, S.; O’BRien, M.; et al. Extended pleurectomy decortication and chemotherapy versus chemotherapy alone for pleural mesothelioma (MARS 2): A phase 3 randomised controlled trial. Lancet Respir. Med. 2024, 12, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., 3rd; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef] [PubMed]
- Lang-Lazdunski, L.; Bille, A.; Lal, R.; Cane, P.; McLean, E.; Landau, D.; Steele, J.; Spicer, J. Pleurectomy/Decortication is Superior to Extrapleural Pneumonectomy in the Multimodality Management of Patients with Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2012, 7, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.M.; Pass, H.I.; Seshan, V.E.; Dycoco, J.; Zakowski, M.; Carbone, M.; Bains, M.S.; Rusch, V.W. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 620–626.e3. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Q.; Yan, T.D.; Bannon, P.G.; McCaughan, B.C. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J. Thorac. Oncol. 2010, 5, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Madrid, G.; Pesch, B.; Calderón-Aranda, E.S.; Burek, K.; Jiménez-Ramírez, C.; Juárez-Pérez, C.A.; Ochoa-Vázquez, M.D.; Torre-Bouscoulet, L.; Acosta-Saavedra, L.C.; Sada-Ovalle, I.; et al. Biomarkers for Predicting Malignant Pleural Mesothelioma in a Mexican Population. Int. J. Med. Sci. 2018, 15, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Rusch, V.; Pass, H.; Asamura, H.; Nakano, T.; Edwards, J.; Giroux, D.J.; Hasegawa, S.; Kernstine, K.H.; Waller, D.; et al. Recommendations for Uniform Definitions of Surgical Techniques for Malignant Pleural Mesothelioma: A Consensus Report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J. Thorac. Oncol. 2011, 6, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tian, D.; Park, J.; Allan, J.; Pataky, K.A.; Yan, T.D. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014, 83, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.M. Pleurectomy decortication for mesothelioma: The procedure of choice when possible. J. Thorac. Cardiovasc. Surg. 2016, 151, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.M.; Pass, H.I.; Rusch, V.W.; Kindler, H.L.; Sugarbaker, D.J.; Rosenzweig, K.E.; Flores, R.; Friedberg, J.S.; Pisters, K.; Monberg, M.; et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tian, D.; Manganas, C.; Matthews, P.; Yan, T.D. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann. Cardiothorac. Surg. 2012, 1, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Stahel, R.A.; Riesterer, O.; Xyrafas, A.; Opitz, I.; Beyeler, M.; Ochsenbein, A.; Früh, M.; Cathomas, R.; Nackaerts, K.; Peters, S.; et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAAKK 17/04): A randomized, international, multicentre phase 2 trial. Lancet Oncol. 2015, 16, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Fiore, M.; Filippini, T.; Tumino, R.; Sabbioni, M.; Spatola, C.; Polosa, R.; Vigneri, P.; Nardini, M.; Castorina, S.; et al. Comparison of video-assisted pleurectomy/decortication surgery plus hyperthermic intrathoracic chemotherapy with VATS talc pleurodesis for the treatment of malignant pleural mesothelioma: A pilot study. Heliyon 2023, 9, e16685. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.G.; Kutywayo, K.; Mohammed, S.B.; Fennell, D.A.; Nakas, A. Cytoreductive surgery with hyperthermic intrathoracic chemotherapy for malignant pleural mesothelioma: A systematic review. Thorax 2022, 78, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Halstead, J.; Lim, E.; Venkateswaran, R.; Charman, S.; Goddard, M.; Ritchie, A. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur. J. Surg. Oncol. 2005, 31, 314–320. [Google Scholar] [CrossRef] [PubMed]

| Variables | Study Population N = 122 | Surgery n = 16 (13.11%) | Chemotherapy or Supportive Care n = 106 (86.9%) | p Value a |
|---|---|---|---|---|
| Age | 63 (12) | 56 (8) | 64 (12) | 0.019 b |
| Sex, men n (%) | 87 (71%) | 10 (62.5%) | 77 (72.6) | 0.403 |
| Any type of exposure to asbestos | 85 (69.7%) | 9 (56.3%) | 76 (71.7%) | 0.210 |
| Current or past smoking n (%) | 72 (59%) | 8 (50%) | 64 (60.4%) | 0.431 |
| Smoking index b | 7.5 (1.5–20) | 12.7 (1.5–20) | 7.2 (1.5–20) | 0.295 |
| Wood smoke n (%) | 42 (34.4%) | 4 (25%) | 38 (35.9%) | 0.395 |
| Wood smoke index | 42.5 (20–146) | 20 (6.5–35) | 54 (30–150) | 0.051 c |
| Comorbidities | ||||
| Any comorbidity | 59 (48.4%) | 3 (18.8%) | 56 (52.8%) | 0.011 |
| Diabetes | 26 (21.3%) | 2 (12.5%) | 24 (22.6%) | 0.356 |
| Hipertension | 34 (27.9%) | 1 (6.3%) | 33 (31.1%) | 0.039 |
| Time from onset of symptoms to diagnosis (days) b | 149 (87–257) | 175 (92–435.5) | 125 (82–244) | 0.216 |
| Clinical laboratory studies | ||||
| Lymphopenia | 21 (17.2%) | 1 (6.3%) | 20 (18.9%) | 0.213 |
| Neutrophil/lymphocyte ratio b | 3.9 (2.8–6.1) | 2.3 (1.8–3.41) | 4.3 (3.1–6.4) | 0.001 |
| Hemoglobin < 10 g/dL | 10 (8.2%) | 1 (6.3%) | 9 (8.5%) | 0.761 |
| Albumin ≤ 3 g/dL | 46 (37.7%) | 4 (25%) | 42 (39.6%) | 0.261 |
| ECOG ≥ 2 | 107 (87.7%) | 14 (87.5%) | 93 (87.7%) | 0.979 |
| Deaths | 50 (41%) | 4 (25%) | 46 (43.4%) | 0.163 |
| Variables | Study Population N = 122 | Surgery n = 16 (13.11%) | Chemotherapy or Supportive Care n = 106 (86.9%) | p Value a |
|---|---|---|---|---|
| TNM classification | ||||
| T1 | 6 (4.9%) | 2 (12.5%) | 4 (3.8%) | |
| T2 | 35 (28.7%) | 10 (62.5%) | 25 (23.6%) | 0.001 |
| T3 | 41 (33.6%) | 4 (25%) | 37 (34.9%) | |
| T4 | 40 (32.8%) | 40 (37.7%) | ||
| N0 | 23 (18.9%) | 8 (50%) | 15 (14.2%) | |
| N1 | 20 (16.4%) | 3 (18.8%) | 17 (16%) | 0.005 |
| N2 | 72 (59%) | 5 (31.2%) | 67 (63.2%) | |
| N3 | 7 (5.7%) | 7 (6.6%) | ||
| M0 | 89 (73%) | 16 (100%) | 73 (68.9%) | |
| M1 | 27 (22.1%) | 27 (25.5%) | 0.033 | |
| Mx | 6 (4.9%) | 6 (5.6%) | ||
| Clinical stage | ||||
| IB | 2 (1.6%) | 1 (6.2%) | 1 (0.9%) | |
| II | 13 (10.7%) | 7 (43.8%) | 6 (5.7%) | 0.000 |
| IIIA | 47 (38.5%) | 8 (50%) | 39 (36.8%) | |
| IV | 60 (49.2%) | 60 (56.6%) |
| Total Population N = 122 | Surgery n = 16 (16.4%) | Chemotherapy or Supportive Care n = 106 (83.6%) | |
|---|---|---|---|
| Chemotherapy | 97 (79.5%) | 16 (100%) | 83 (78.3%) |
| Pemetrexed-based chemotherapy | 41 (42.3%) | 8/16 (50%) | 33/83 (39.8%) |
| Chemotherapy lines a | 2 (1–3) Min–max (1–6) | 2 (1–4) Min–max (1–5) | 2 (1–3) Min–max (1–6) |
| Chemotherapy complications b | 48/97 (49.5%) | 5/14 (35.7%) | 43/83 (51.8%) |
| Time in days from the start of oncological treatment to progression, median (IQR) a | 199 (112–336) | 345 (99–592) | 177 (112–291) |
| Radiotherapy | 8 (6.6%) | 7 (43.8%) | 1 (0.9%) |
| Type of surgery | |||
| Pleurectomy/Decortication | 8 (50%) | ||
| Extrapleural pneumonectomy | 8 (50%) | ||
| Time in months from diagnosis to death a | 9.5 (2.3–17.9) | 24.2 (11.6–46.3) | 7.7 (1.5–15.5) |
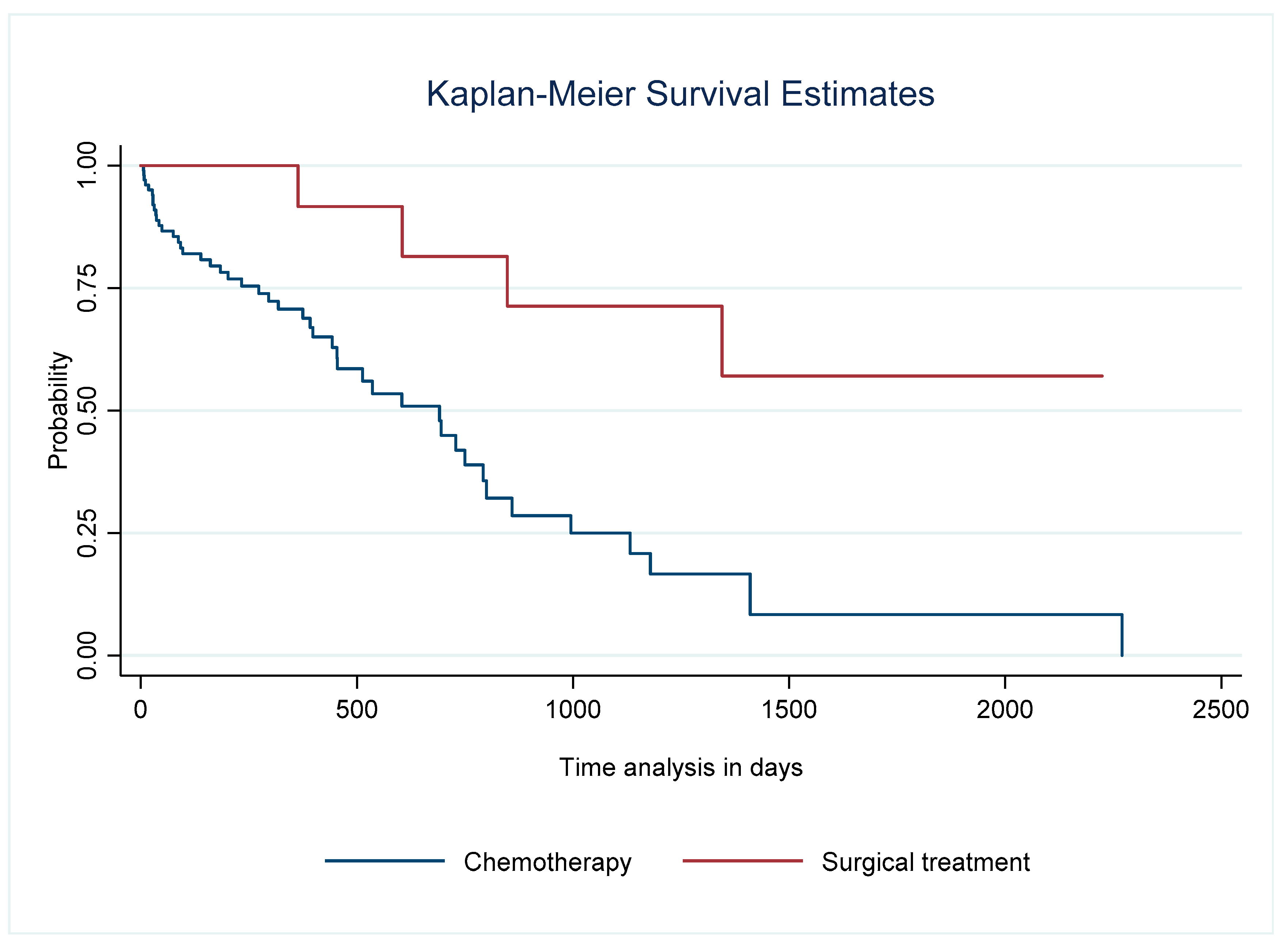
| 1 Year | 2 Years | 3 Years | 4–5 Years | 6 Years | |
|---|---|---|---|---|---|
| Survival (%) (95% CI) | |||||
| Total population n = 122 | 74 (65–82) | 52 (40–63) | 35 (22–48) | 19 (8–34) | 6 (0–32) |
| Surgery n = 16 | 100 | 82 (45–95) | 71 (34–90) | 53 (15–81) | 53 (15–81) |
| Chemotherapy n = 97 | 79 (68–86) | 59 (45–70) | 41 (27–55) | 23 (10–40) | 8 (0–36) |
| Pleurectomy/ Decortication n = 8 | 100 | 83 (27–97) | 67 (19–90) | 67 (19–90) | ̶ |
| Extrapleural pneumonectomy n = 8 | 100 | 80 (20–97) | 80 (20–97) | 40 (1–83) | 40 (1–83) |
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| Age > 65 | 1.24 | 0.87–1.79 | 0.230 |
| Sex | 0.93 | 0.62–1.38 | 0.710 |
| Any comorbitidy | 1.21 | 0.84–1.73 | 0.304 |
| Diabetes | 1.20 | 0.78–1.87 | 0.404 |
| Hipertension | 1.04 | 0.69–1.55 | 0.860 |
| Time in months from onset of symptoms to diagnosis | 1.03 | 0.99–1.06 | 0.064 |
| Hemoglobin < 10 g | 2.89 | 1.46–5.70 | 0.002 |
| Lymphopenia | 2.87 | 1.41–3.31 | <0.001 |
| Neutrophil/lymphocyte ratio ≥ 6 | 2.15 | 1.41–3.31 | 0.000 |
| Albumin ≤ 3 g/dL | 2.30 | 1.56–3.37 | <0.001 |
| LDH in pleural fluid > 300 IU/L | 2.33 | 1.45–3.75 | 0.000 |
| pH in pleural fluid ≤ 7.2 | 1.47 | 0.89–2.42 | 0.136 |
| Histological types | |||
| Epithelioid | 1 | ||
| Sarcomatoid | 3.59 | 1.10–11.62 | 0.033 |
| Mixed or biphasic | 0.68 | 0.17–2.78 | 0.595 |
| Clinical stage IV versus I, II and III | 2.0 | 1.39–2.92 | 0.000 |
| ECOG ≥ 2 | 1.53 | 0.89–2.65 | 0.123 |
| Chemotherapy | 0.24 | 0.15–0.38 | <0.001 |
| Radiotherapy | 0.35 | 0.17–0.73 | 0.005 |
| Surgical treatment (both PD and EPP) | 0.40 | 0.23–0.70 | 0.001 |
| Type of treatment | |||
| Chemotherapy | 1 | ||
| Pleurectomy/Decortication | 0.35 | 0.17–0.74 | 0.006 |
| Extrapleural pneumonectomy | 0.48 | 0.17–74 | 0.048 |
| Variables | All Patients n = 122 | Only Patients’ Clinical Stage I, II and III n = 62 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Surgery (PD or EPP) | 0.34 | 0.19–0.61 | <0.001 | 0.37 | 0.19–0.72 | 0.003 |
| Age > 65 | 0.92 | 0.63–1.35 | 0.682 | 0.73 | 0.38–1.38 | 0.332 |
| Time in months from onset of symptoms to diagnosis | 1.00 | 0.99–1.00 | 0.249 | 1.00 | 0.99–1.00 | 0.238 |
| Hemoglobin < 10 g | 3.90 | 1.91–7.96 | <0.001 | 4.84 | 1.69–13.9 | 0.003 |
| Neutrophil/lymphocyte ratio ≥ 6 | 2.22 | 1.44–3.42 | <0.001 | 2.19 | 1.09–4.38 | 0.027 |
| ECOG ≥ 2 | 1.70 | 0.97–2.96 | 0.062 | 1.47 | 0.72–3.01 | 0.295 |
| Variables | All Patients n = 122 | Only Patients Clinical Stage I, II and III n = 62 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Type of treatment | ||||||
| Chemotherapy | 1 | 1 | ||||
| Pleurectomy/Decortication | 0.26 | 0.12–0.57 | 0.001 | 0.29 | 0.13–0.67 | 0.003 |
| Extrapleural pneumonectomy | 0.48 | 0.22–1.06 | 0.070 | 0.53 | 0.22–1.23 | 0.140 |
| Age > 65 | 0.94 | 0.64–1.38 | 0.747 | 0.75 | 0.40–1.44 | 0.391 |
| Time in months from onset of symptoms to diagnosis | 1.00 | 0.99–1.00 | 0.296 | 1.00 | 0.99–1.00 | 0.302 |
| Hemoglobin < 10 g/dL | 4.12 | 2.0–8.46 | <0.001 | 5.24 | 1.81–15.19 | 0.002 |
| Neutrophil/lymphocyte ratio | 2.31 | 1.49–3.59 | <0.001 | 2.40 | 1.17–4.9 | 0.017 |
| ECOG ≥ 2 | 1.71 | 0.98–2.98 | 0.060 | 1.47 | 0.72–3.01 | 0.294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Báez-Saldaña, R.; Marmolejo-Torres, M.E.; Iñiguez-García, M.A.; Jiménez-Corona, A.; Berrios-Mejía, J.A. Effectiveness of Surgical Treatment on Survival of Patients with Malignant Pleural Mesothelioma. Cancers 2025, 17, 2360. https://doi.org/10.3390/cancers17142360
Báez-Saldaña R, Marmolejo-Torres ME, Iñiguez-García MA, Jiménez-Corona A, Berrios-Mejía JA. Effectiveness of Surgical Treatment on Survival of Patients with Malignant Pleural Mesothelioma. Cancers. 2025; 17(14):2360. https://doi.org/10.3390/cancers17142360
Chicago/Turabian StyleBáez-Saldaña, Renata, María Esther Marmolejo-Torres, Marco Antonio Iñiguez-García, Aída Jiménez-Corona, and Juan Alberto Berrios-Mejía. 2025. "Effectiveness of Surgical Treatment on Survival of Patients with Malignant Pleural Mesothelioma" Cancers 17, no. 14: 2360. https://doi.org/10.3390/cancers17142360
APA StyleBáez-Saldaña, R., Marmolejo-Torres, M. E., Iñiguez-García, M. A., Jiménez-Corona, A., & Berrios-Mejía, J. A. (2025). Effectiveness of Surgical Treatment on Survival of Patients with Malignant Pleural Mesothelioma. Cancers, 17(14), 2360. https://doi.org/10.3390/cancers17142360






