1. Introduction
Gliomas represent the most prevalent and aggressive primary brain tumors within the central nervous system [
1]. Despite the application of comprehensive treatment such as surgical resection, radiotherapy, and chemotherapy, which can improve the prognosis of glioma, the median survival period remains only about 14 months [
2,
3]. The tumor’s high invasiveness and tendency to recur pose significant clinical challenges for early diagnosis and treatment.
Magnetic resonance imaging (MRI) has become a crucial clinical diagnostic tool due to its advantages of being radiation-free, non-invasive, and providing excellent soft-tissue contrast along with multi-sequence imaging capabilities. In recent years, MRI-based molecular imaging has opened new avenues for glioma diagnosis. This technology integrates MRI with molecular biology techniques, enabling non-invasive observation of pathophysiological changes at the cellular or molecular level in living tissues [
4,
5]. With the rapid advancement of molecular and cellular imaging technologies, it can now dynamically monitor cellular immunotherapy, including non-invasive tracking of cell localization, activation, and fate [
6]. MRI reporter gene imaging is one of the key components of molecular imaging.
Ferritin heavy chain 1 (FTH1), as an MRI reporter gene, has been extensively validated for its imaging efficacy in experimental studies [
7,
8,
9,
10].
FTH1 not only exhibits low cytotoxicity but also enables stable imaging through sustained expression [
11]. Our previous research has shown that
FTH1 expression can induce intracellular iron accumulation detectable by MRI [
12,
13]; however, the expression levels of
FTH1 and the resulting MR imaging effects have not been optimal. Therefore, it is necessary to genetically modify or engineer
FTH1 to enhance its potential as a reporter gene for MR imaging.
We selected three types of gene regulatory elements to modify the
FTH1 gene.
Progression Elevated Gene-3 (PEG3) is a broad-spectrum tumor-specific gene that is significantly overexpressed in human cancer cell lines while exhibiting minimal expression in normal tissue cells. In tumor cells, the activity of the
PEG3 promoter is regulated by the transcription factors
PEA3 and
AP-1, leading to enhanced expression that specifically modulates the translation of downstream target genes within the tumor microenvironment [
14]. The 5′ UTR of
basic fibroblast growth factor-2 (bFGF-2) contains various higher-order structures, with a GC-rich segment that is unraveled in tumor cells due to the overexpression of translation initiation factor 4E (eIF4E), resulting in increased translation of downstream genes [
15]. The woodchuck hepatitis virus post-transcriptional regulatory element (
WPRE) is a potent RNA cis-acting element that regulates post-transcriptional levels by enhancing RNA polyadenylation. Inserting
WPRE into the 3′ UTR of the target gene can significantly boost the expression rate of exogenous genes and prolong their expression duration [
16]. The construction of a chimeric expression system using these components could potentially upregulate
FTH1 expression and its iron-accumulation capacity, thereby improving the detection sensitivity of MRI-based reporter gene imaging.
Tf, TfR, and
FTH1 play distinct roles in iron metabolism, collectively maintaining systemic iron homeostasis. Tf is responsible for iron transport, facilitating the transfer of ferric ions from serum to cells through specific binding to TfR on the cell membrane, followed by endocytosis and storage within
FTH1. Research indicates that
FTH1, as an iron storage protein, when overexpressed, can induce a deficiency of free iron within the cell [
17], leading to an upregulation of TfR expression on the cell membrane and an increase in iron uptake. Based on this mechanism,
FTH1 can serve not only as an MRI reporter gene but also facilitate targeted drug delivery via the Tf-TfR transport system.
Liposomes (LP) are extensively studied drug delivery vehicles that, due to their favorable affinity for cell membranes, hollow structure, biocompatibility, stability, ease of modification, and cost-effectiveness, have emerged as an ideal choice for anticancer drug delivery systems [
18,
19]. Doxorubicin (DOX), a commonly used broad-spectrum anticancer agent, demonstrates significant efficacy; however, its clinical application is limited by severe adverse effects such as cardiotoxicity and myelosuppression [
20,
21,
22]. Therefore, encapsulating DOX within LP carriers (LP-DOX, LPD) to enable tumor-targeted accumulation while minimizing off-target toxicity holds substantial clinical significance. As a nanomedicine delivery system, liposomes often utilize the enhanced permeability and retention (EPR) effect to achieve passive targeting, as seen with PEGylated liposomal doxorubicin (Doxil). Transferrin receptor (TfR)-targeted liposomes offer greater specificity, enabling more precise drug delivery to glioma cells expressing TfR and reducing drug distribution to normal tissues. While EPR-based passive delivery can also promote drug accumulation at tumor sites, it lacks specific targeting, which may result in some degree of drug exposure to healthy tissues [
23].
Based on the aforementioned context, this study proposes to construct an FTH1 expression system regulated by a chimeric element composed of the tumor-specific promoter PEG3, bFGF-2 5′ UTR, and WPRE (PEG3-bFGF2 5′UTR-FTH1-WPRE). Through in vitro and in vivo experiments, we aim to validate the enhancing effect of this chimeric element on the expression of the MRI reporter gene FTH1. Simultaneously, leveraging the mechanism by which FTH1 overexpression induces intracellular iron depletion to activate TfR upregulation, we will utilize the specific binding of TfR and Tf to deliver Tf-modified LPD (Tf-LPD) to glioma cells. This dual-function system is designed to achieve both MRI-based glioma monitoring and targeted therapy, thereby providing a novel integrated diagnostic and therapeutic strategy for glioma management.
2. Materials and Methods
2.1. Cell Preparation
The U251 human glioblastoma cell line was obtained from the Chongqing Key Laboratory of Child Neurodevelopment and Cognitive Disorders (Chongqing, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Brooklyn, NY, USA) supplemented with 10% FBS (Gibco, USA) and 1% penicillin-streptomycin (Gibco, USA) in a 37 °C incubator with 5% CO2. Cells were subcultured regularly using trypsin/EDTA (Meilune, Dalian, China).
2.2. Mice
BALB/C nude female mice aged 4–5 weeks had 16 ± 2 g weights and were obtained from the Animal Experiment Center of Chongqing Medical University. Animal experiments in this study were approved by the Animal Ethics Committee of the Children’s Hospital of Chongqing Medical University, China, on 11 January 2024 (approval No. CHCMU-IACUC20240111010). The experimental protocols and procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed) (National Institutes of Health, 2011).
2.3. Plasmids and Lentivirus
The FTH1 reporter gene, PEG3 promoter, bFGF-2 5′UTR, WPRE, and the lentiviral vector packaging system were all constructed by GeneChem (Shanghai, China).
2.4. Main Reagents
Fetal bovine serum and DMEM culture medium were obtained from Gibco. Ferric ammonium citrate (FAC) was purchased from Sigma (Saint Louis, MO, USA). DSPE-PEG2000-Tf was acquired from Xi’an Ruixi Biological Technology Co., Ltd. (Xi’an, China). Ferritin Heavy Chain Rabbit pAb, GAPDH Mouse mAb (HRP), and Goat Anti-Rabbit IgG H&L (HRP) were all purchased from Zoonbio Biotechnology Co., Ltd. (Nanjing, China).
2.5. Preparation of Recombinant Lentiviral Vector
The FTH1 reporter gene was ligated to the PEG3 promoter, bFGF-2 5′ UTR, and WPRE through restriction enzyme digestion and amplification, resulting in the construction of two lentiviral plasmids: U6-PEG3-FTH1-CMV-EGFP-EF1A-Luciferase-sv40-puromycin and U6-PEG3-bFGF2 5′UTR-FTH1-WPRE-CMV-EGFP-EF1A-Luciferase-sv40-puromycin.Following sequencing validation, these lentiviral plasmids were co-transfected with the packaging plasmids pSPAX2 and pMD2G into 293T cells. Viral supernatants were collected at 48 and 72 h post-transfection, and the resulting viral particles were concentrated via ultracentrifugation, designated as PEG3-FTH1-LV and PEG3-bFGF2 5′UTR-FTH1-WPRE-LV, respectively. The lentiviral particles were aliquoted and stored at −80 °C. A negative control group was established using the NC-LV, while U251 cells that were not subjected to lentiviral transfection served as the blank control group.
2.6. Lentiviral Transduction
U251 cells in the logarithmic growth phase were seeded at a density of 1.0 × 105 cells per well in a 12-well plate and cultured in complete medium for 24 h at 37 °C with 5% CO2 to allow for adherence. The medium was aspirated, and the cells were washed three times with PBS. The complete medium was replaced, and the required lentiviral volume was calculated at an MOI of 5. HitransG P transduction enhancer was added according to the manufacturer’s instructions, and the cells were incubated. After 12–16 h, the supernatant was removed and replaced with complete medium, followed by further incubation. The expression of green fluorescent protein was observed under a fluorescence microscope 48 h post-infection.
2.7. Western Blot
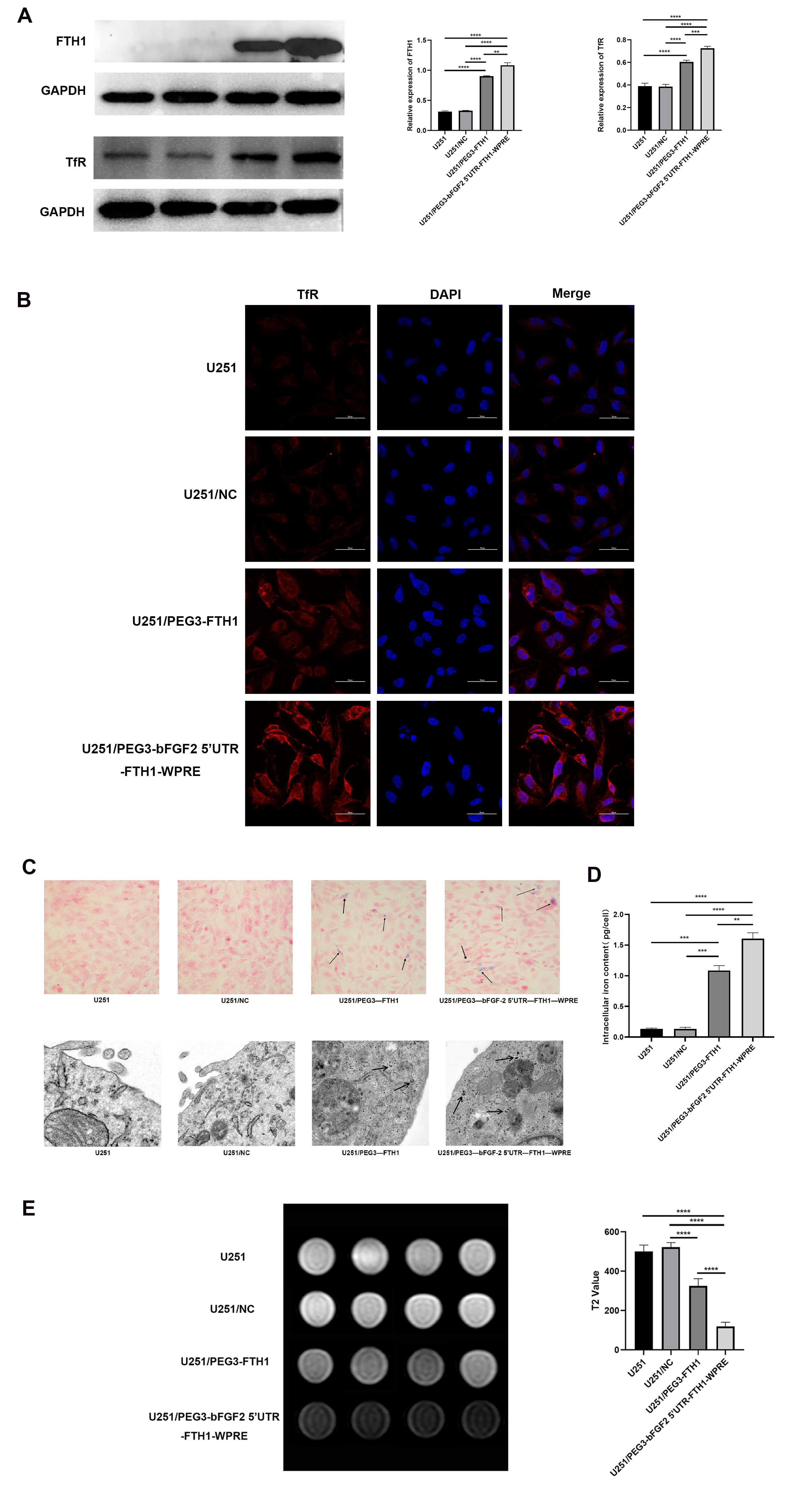
Following the collection of untreated U251 cells and U251/NC, U251/PEG3-FTH1, and U251/PEG3-bFGF2 5′UTR-FTH1-WPRE cells transduced with lentivirus, protein was extracted. Protein concentration was determined via BCA assay. Following SDS-PAGE electrophoresis, proteins were transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk for 1 h, followed by overnight incubation at 4 °C with primary antibodies against GAPDH (1:10,000) (Zen BioScience, Chengdu, Sichuan, China; Cat.R23306), FTH1 (1:2000) (Zen BioScience, Chengdu, Sichuan, China; Cat. 200350-F12), and TfR (1:1000) (Zen BioScience, Chengdu, Sichuan, China; R381603). Membranes were then incubated with secondary antibodies (1:3000) (Zen BioScience, Chengdu, Sichuan, China; Cat.511203) for 1–2 h and visualized using ECL reagent.
2.8. Immunofluorescence Assay
Following logarithmic growth, U251 cells from each group were harvested and seeded into confocal dishes for 24 h. Following PBS washes, cells were fixed with 4% paraformaldehyde (PFA, Beyotime, p0099, Shanghai, China) for 20 min. Post-fixation, cells were blocked with 5% normal goat serum at 37 °C for 1 h. To validate TfR overexpression, cells were incubated overnight at 4 °C with rabbit anti-TfR monoclonal antibody (1:250), followed by 1 h incubation with DyLight 488-conjugated goat anti-rabbit secondary antibody (1:500). Nuclei were counterstained with DAPI (1:1000). Visualization was performed using a laser confocal microscope (Nikon A1R, Shinagawa, Japan) at 600× magnification.
2.9. Prussian Blue Staining and Transmission Electron Microscopy
U251 cells from each group were cultured in complete medium supplemented with ferric ammonium citrate (FAC; Sigma-Aldrich, St. Louis, MO, USA) solution at a final concentration of 0.2 mmol/L. Following 72 h of incubation at 37 °C in a 5% CO2 incubator, the cells were washed three times with PBS. Subsequently, the cells were stained using a Prussian blue staining kit (nuclear fixation method), and the staining characteristics of the cells in each group were observed under a microscope.
U251 cells from each group were cultured in complete medium supplemented with 0.2 mmol/L FAC solution for 72 h at 37 °C in a 5% CO2 incubator. Following three washes with PBS, the supernatant was discarded after centrifugation. Cells were then fixed with 2.5% glutaraldehyde and stored at 4 °C. Samples were prepared, stained, dehydrated, and embedded for transmission electron microscopy (TEM) analysis.
For quantification of the intracellular iron content, 1 × 106 cells per group were centrifuged and dried at 110 °C overnight. The cells were then digested in 500 μL of a nitric acid-perchloric acid mixture (3:1) at 60 °C for 3 h. The iron concentration was measured using an atomic absorption spectrophotometer (Huaguang HG-960 2A, Shenyang, China). Each sample was measured 3 times. The concentration values are presented in units of pg/cell.
2.10. MR Imaging of Cells
A final concentration of 0.2 mmol/L ferric ammonium citrate solution (FAC) (Sigma, Kanagawa, Japan) was added to the complete culture medium of the cells. Following a 72-h incubation with 0.2 mmol/L FAC, U251 cells from each group were washed thrice with PBS. The cells were then collected and allowed to sediment naturally before undergoing T2WI scanning using a 3.0 T clinical MR imaging system (Philips Healthcare, Best, Hoofddorp, The Netherlands). The T2WI scanning parameters were as follows: TR = 2424 ms, TE = 100 ms, FOV = 100 mm × 100 mm, voxel size = 0.620 mm × 0.600 mm, imaging matrix = 160 × 150, signal-to-noise ratio = 1, with a scan duration of 1 min 56 s.
2.11. Preparation of Tf-LPD
Tf-modified liposomes (Tf-LP) were prepared via the thin-film dispersion method. Briefly, HSPC (6 mg), cholesterol (2 mg), and DSPE-PEG2000-Tf (2 mg) (Xi’an ruixi Biological Technology Co., Ltd., Xi’an, China) were dissolved in 3 mL of chloroform and evaporated under reduced pressure to form a thin film. The film was hydrated with ammonium sulfate solution and sonicated. Following sonication and extrusion through a polycarbonate membrane (200 nm pore size), the liposomes were dialyzed using a nano-dialysis device (polycarbonate membrane, 10 nm pore size) to obtain transferrin-modified liposomes (Tf-LP).
Doxorubicin (Innochem, SY034864, Beijing, China) aqueous solution was added, and the mixture was sonicated and incubated at 50 °C for 1 h. Subsequently, the mixture was dialyzed again using a nano-dialysis device (polycarbonate membrane, 10 nm pore size) to yield transferrin-modified doxorubicin liposomes (Tf-LPD).
2.12. Identification of Tf-LPD
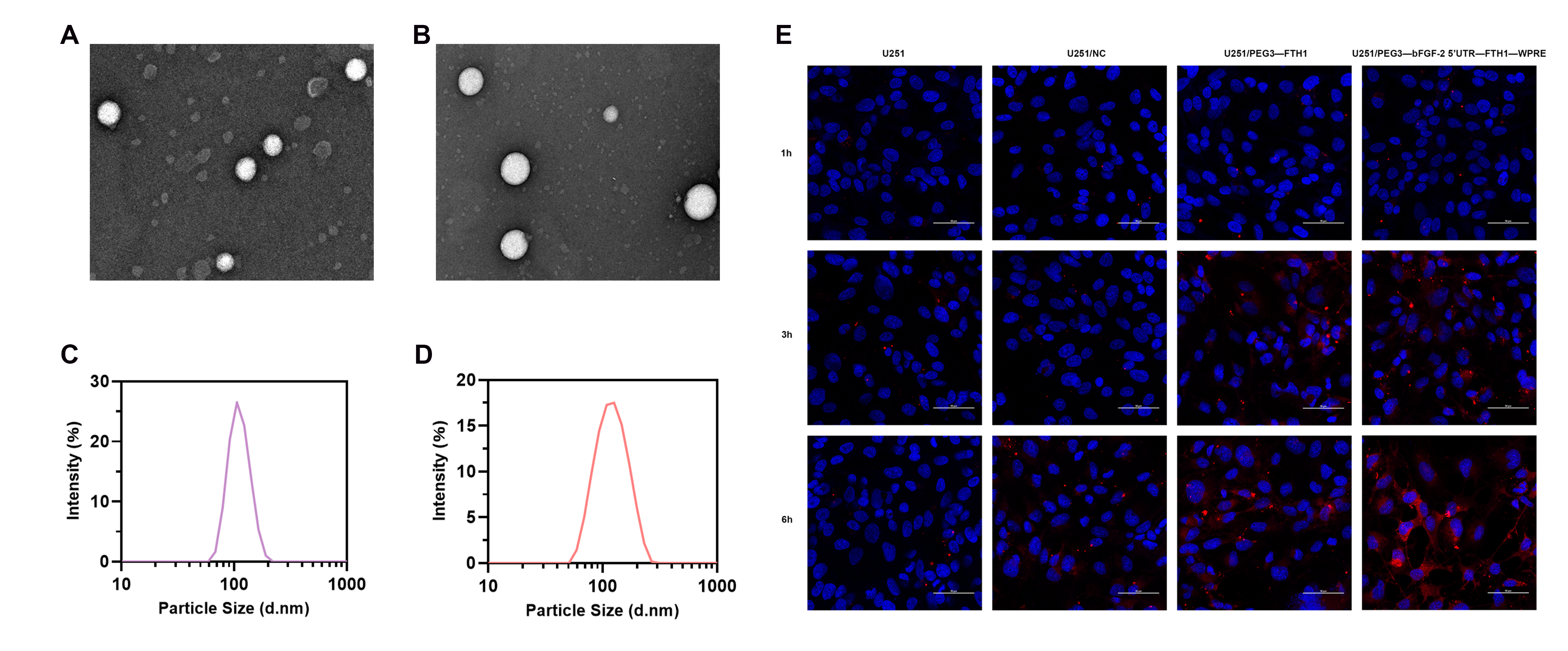
TEM was employed to observe the morphology of each liposome formulation, including Tf-LP and Tf-LPD. Liposome size was analyzed using a Malvern particle size analyzer. Doxorubicin encapsulation efficiency was determined via ultrafiltration centrifugation. Briefly, 300 μL of the Tf-LPD solution was added to an ultrafiltration tube and centrifuged at 5000 rpm for 10 min. The filtrate was collected, diluted with methanol, and the amount of free drug (M1) in the filtrate was quantified. An equal volume of Tf-LPD solution was also treated with methanol to disrupt the liposomes, and the total doxorubicin amount (M0) was determined. Encapsulation efficiency was then calculated.
2.13. Cellular Uptake Assays of Tf-LP
U251 cells from each group were cultured in complete medium (1% double antibody, 10% fetal bovine serum). Cells in the logarithmic growth phase were digested and transferred to confocal dishes for 24 h, followed by the addition of DiI-labeled Tf-LP. After co-incubation for 1, 3, 6, and 12 h, the cells were washed three times with PBS to remove unbound liposomes and fixed with paraformaldehyde. Subsequently, the cells were stained with a nuclear dye, and the binding of cells and liposomes was observed under a laser confocal microscope at 600× magnification.
2.14. Cytotoxicity Assays of Tf-LPD
The cytotoxic effects of Tf-LPD loaded with drugs on U251 cells were assessed using the CCK8 assay. Six wells per group were utilized, with a total of four groups: U251 cells, U251/NC cells (following lentiviral transduction), U251/PEG3-FTH1 cells, and U251/PEG3-bFGF2 5′UTR-FTH1-WPRE cells. Cells were seeded in 96-well plates, and 24 h later, 0, 1, 2, 4, 8, and 16 uL of Tf-LPD solution (1 mg/mL) were added to each well. After a 6-h incubation, CCK-8 reagent was added. Absorbance at 450 nm was measured using a multi-function microplate reader, and cell viability was calculated.
2.15. Flow Cytometry
U251 cells from each group were seeded at a density of 1 × 106 cells per well in 6-well plates and incubated at 37 °C with 5% CO2 for 24 h. Following cell adherence, the culture medium was removed, and the cells were washed with PBS. Subsequently, the Tf-LPD solution at a concentration of 8 μg/mL was added, and incubation continued for 24 h. After PBS washing, trypsin digestion was performed. The PBS wash and digested cell suspension were combined, and cells were collected by centrifugation at 1000 rpm for 5 min. The cells were washed twice with PBS. The cells were resuspended in 500 μL of 1× binding buffer, and the solution was transferred to a 5 mL flow cytometry tube. Annexin-FITC (5 μL) (BioLegend, San Diego, CA, USA) and PI (5 μL) (BioLegend, San Diego, CA, USA) were added, and the cells were mixed and incubated in the dark at room temperature for 10 min. Analysis was performed within 1 h using flow cytometry.
2.16. Establishment of U251 Cell Grafts in Nude Mice
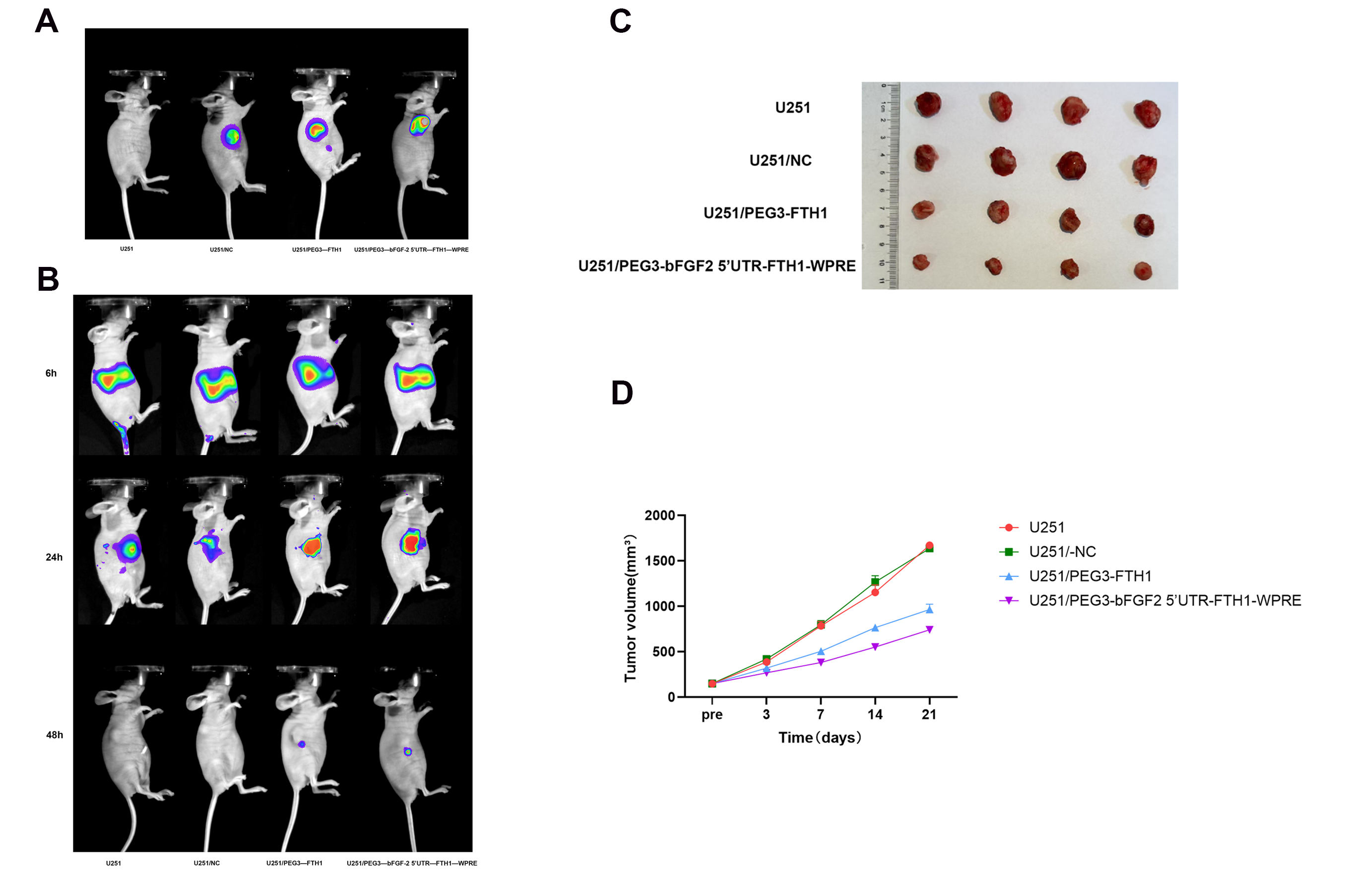
U251 cells were cultured at 37 °C and 5% CO2, and the cells were taken at the logarithmic growth stage to establish the nude mouse U251 subcutaneous graft tumor model. Twenty tumor-bearing nude mice were randomly divided into four groups: (1) U251; (2) U251/NC; (3) U251/PEG3-FTH1; and (4) U251/PEG3-bFGF2 5′UTR-FTH1-WPRE. Intratumoral viral transfections were performed when the tumors of hormonal nude mice grew to a size of approximately 150 mm3. The first group was a control group without any intervention, the second group was injected with NC-LV, the third group was injected with PEG3-FTH1-LV, and the fourth group was injected with PEG3-bFGF2 5′UTR-FTH1-WPRE-LV. Intratumoral injections were administered every three days using the same procedure, for a total of 3–5 injections. Forty-eight hours following the final lentiviral injection, 100 μL of the luciferase (Luc) substrate, luciferin, was administered via intraperitoneal injection. Subsequently, tumor site fluorescence intensity was assessed using an in vivo imaging system.
2.17. In Vivo Biodistribution of Tf-LPD
DiR-labeled Tf-LPD, at a concentration of 5 mg/mL, was dissolved in distilled water. Both the non-transduced tumor-bearing mice and the three groups of tumor-bearing mice that had undergone successful in vivo lentiviral transduction were administered 0.2 mL of DiR-labeled Tf-LPD via tail vein injection. Fluorescence intensity in various tissues was observed at different time points using an in vivo small animal imaging system.
2.18. Tumor Suppression via Tf-LPD
Following intravenous administration via the tail vein, the therapeutic efficacy of drug-loaded liposomes was assessed in tumor-bearing mice. The treatment groups comprised three cohorts of mice that underwent successful in vivo lentiviral transduction and a control group of non-transduced tumor-bearing mice. Drug administration was performed every three days for a total of five administrations, with a dosage of 4 mg/kg per injection. Tumor dimensions, specifically the longest diameter (D1) and the shortest diameter (D2), were measured using a digital caliper. Tumor volume (Vtumor) was calculated using the formula: Vtumor = 1/2 × (D1 × D22). Tumor growth inhibition by the drug-loaded liposomes in the model animals was evaluated by plotting tumor relative growth curves.
2.19. MR Scanning of Animals
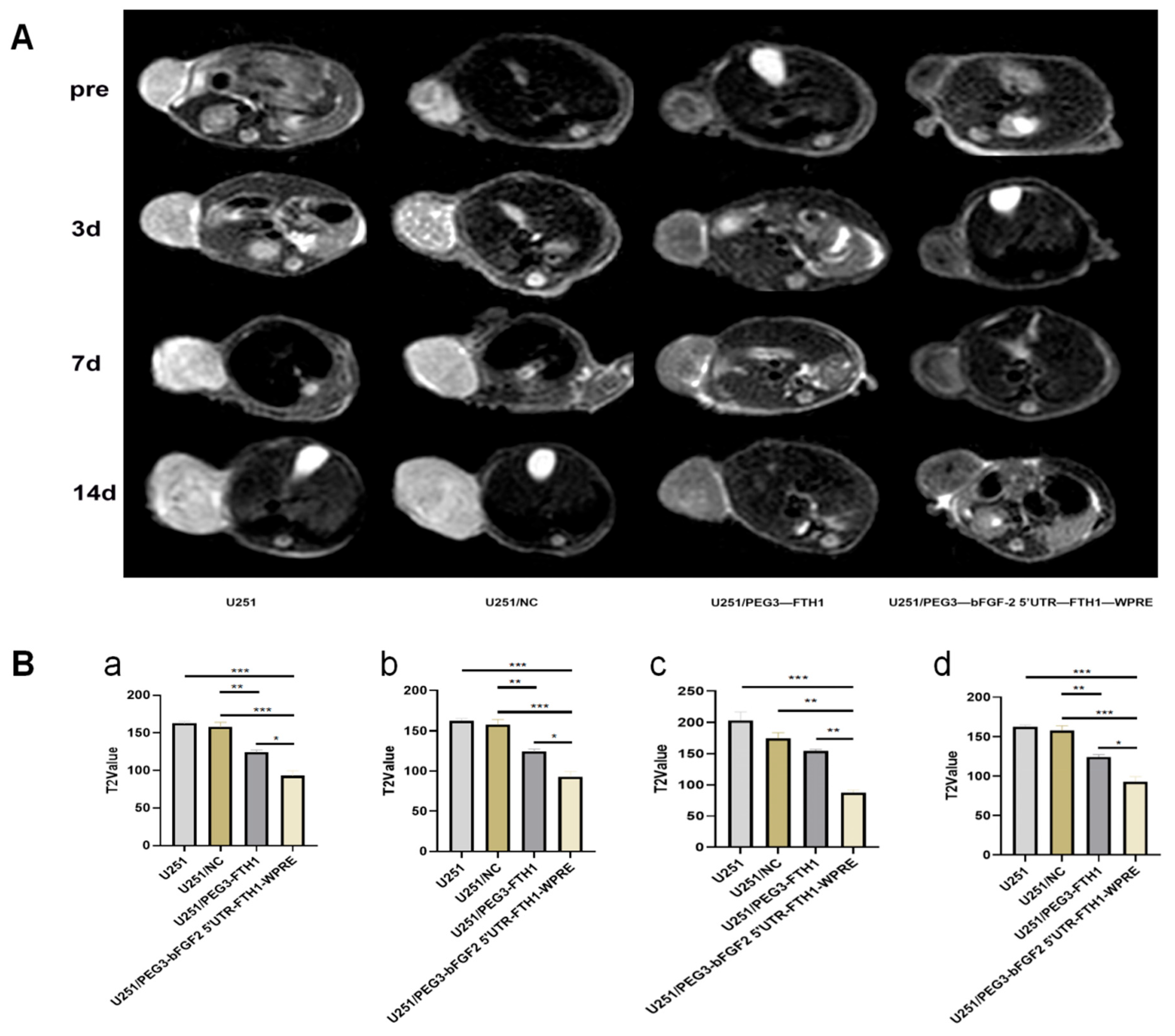
After the plasmid was stably expressed in the tumor of nude mice, T2WI scans were performed on each group of nude mice using a 3.0 T clinical MR imager (Philips Healthcare, Best, The Netherlands). 48 h after plasmid transfection, each group of tumor-bearing nude mice was injected with 200 uL of Tf-LPD solution (at a concentration of 1 mg/mL) via tail vein. Tumor size and morphology were observed by magnetic resonance scanning (MRI) before and on days 1, 3, and 7 after the injection of Tf-LPD solution, and the parameters of T2WI scanning were as follows: TR = 2424 ms, TE = 100 ms, FOV = 100 mm × 100 mm, voxel = 0.62 mm × 0.6 mm, imaging matrix = 160 × 150, signal-to-noise (S/N) ratio = 1, scan time was 1 min 56 s; scan time was 1 min 56 s. 160 × 150, S/N = 1, scan time 1 min 56 s; transverse position: TR = 4414 ms, TE = 80 ms, FOV = 65 mm × 65 mm, voxel = 0.5 mm × 0.5 mm, imaging matrix = 132 × 127, S/N = 1, scan time 5 min 44 s. T2 mapping Scanning parameters were as follows: TR = 2000 ms, TE1 = 1, scan time 5 min 44 s. 2000 ms, TE1 = 13 ms, △TE = 13 ms, TE8 = 104 ms, FOV = 80 mm × 80 mm, voxel = 0.5 mm × 0.55 mm, imaging matrix = 160 × 145, signal-to-noise ratio = 1, and scanning time was 3 min 56 s.
2.20. In Vivo Pharmacodynamics of Tf-LPD
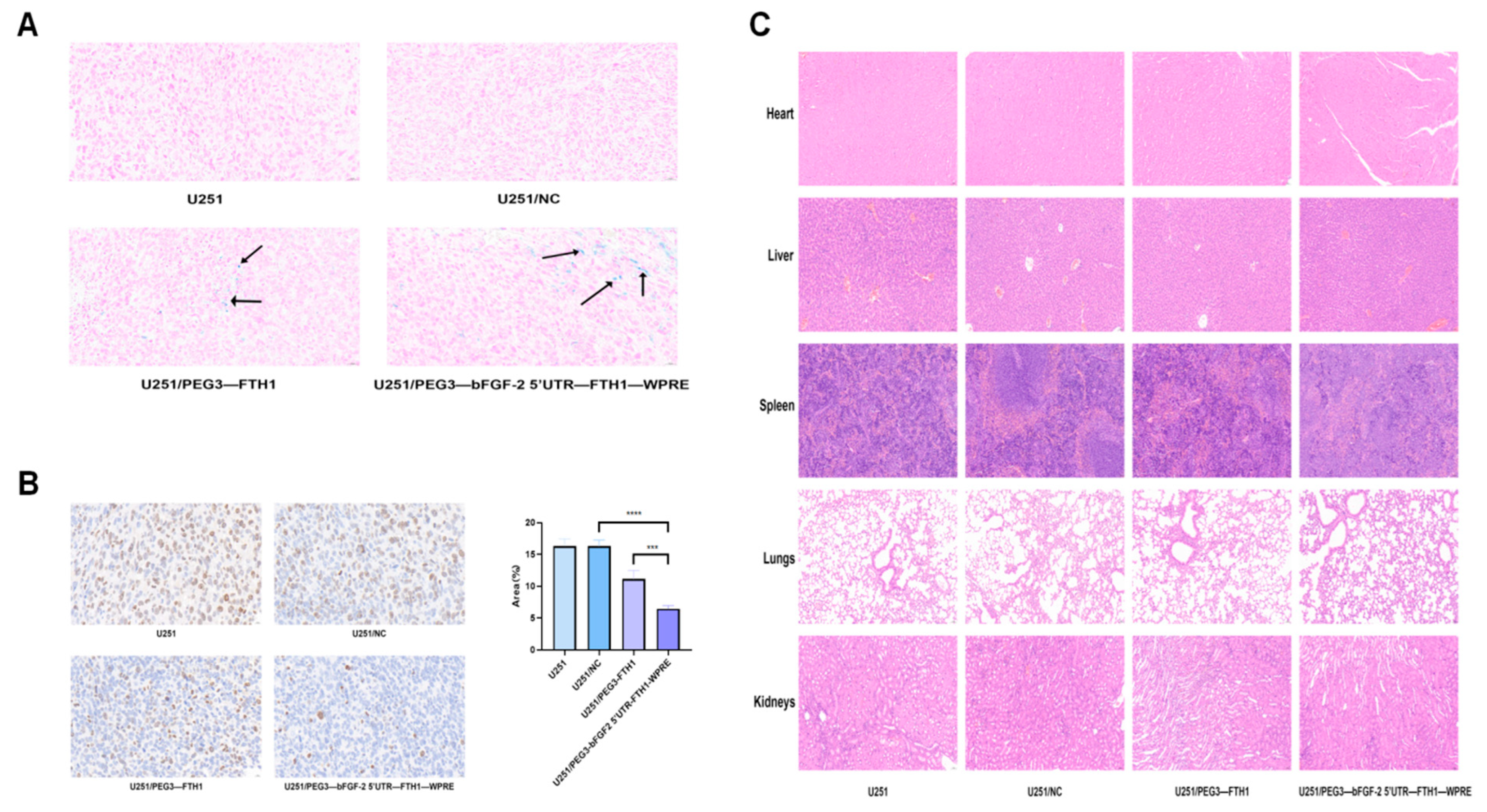
The nude mice in each group of tumor-bearing nude mice were put to death on the 14th day after Tf-LPD injection, and the tumors, hearts, lungs, livers, spleens, and kidneys were excised sequentially. Tumor size was collected from each group for evaluation. Excised tissue specimens were fixed in tissue fixative containing 4% paraformaldehyde to maintain their morphological and structural integrity. After fixation for an appropriate period of time (usually 24 h), the tissue specimens were embedded, and sections were prepared for subsequent immunohistochemistry, Prussian blue staining, and HE staining. Finally, images are captured using a microscope.
2.21. Statistical Analysis
Each test was repeated at least three times; the quantitative data are shown as means ± standard deviations (SDs). These data were analyzed using GraphPad Prism 5.01 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The data were analyzed by paired t-test between two groups and one-way ANOVA for more than two groups, followed by Tukey’s post hoc test. p < 0.05 was considered statistically significant.
4. Discussion
This study successfully established an FTH1 reporter gene expression system regulated by a chimeric element composed of the PEG3 promoter, bFGF-2 5′ UTR, and WPRE. Both in vitro and in vivo experiments confirmed that this chimeric component significantly enhances the specific expression of the MRI reporter gene FTH1 in gliomas. Furthermore, we integrated the FTH1-mediated TfR upregulation mechanism with Tf-modified drug-loaded liposomes (Tf-LPD) for targeted therapy, achieving a unified diagnostic and therapeutic approach for gliomas. The innovation of this strategy lies in: (1) addressing the insufficient expression efficiency of FTH1 through synergistic regulation of multiple components while ensuring specificity of FTH1 expression within tumors and (2) utilizing the bidirectional regulatory mechanism of FTH1-TfR to simultaneously enhance MRI imaging sensitivity and drug targeting. This research enables FTH1-mediated tumor MRI visualization and targeted therapy, providing a multimodal approach for gliomas, which also holds significant reference value for the treatment of pancreatic cancer, breast cancer, and other related malignancies.
The efficacy of MRI reporter imaging is determined by the expression levels of the reporter gene within cells or tissues. This study utilized a chimeric construct comprising the
PEG3 promoter,
bFGF-2 5′ UTR, and
WPRE to achieve specific and high expression of the
FTH1 reporter gene. Compared with the single
PEG3 promoter, the chimeric construct not only ensured specificity of
FTH1 expression in tumor tissues but also significantly enhanced the expression efficiency of
FTH1 at different levels. Firstly, the
PEG3 promoter is activated specifically in tumor cells by the
PEA3/AP-1 transcription factors, regulating the specific expression of
FTH1 at the transcriptional level, thereby ensuring
FTH1 expression is restricted to tumor tissues and absent in normal tissues. Secondly, the
bFGF-2 5′ UTR, rich in “GC” content and highly structured, may participate in the translational control of numerous mRNAs. In normal cells, these structures inhibit the translation of downstream genes; however, in tumor cells, these structures are unwound by the translation initiation factor
eIF4E, losing their original function of blocking downstream gene translation, resulting in the overexpression of downstream genes [
24,
25,
26]. Additionally,
WPRE may enhance the sustained expression of the protein by prolonging the mRNA half-life and promoting polyadenylation, further augmenting
FTH1 expression at the post-transcriptional level [
27]. This chimeric system may not be limited to the
FTH1 reporter gene; it could also be applicable to other reporter genes, such as luciferase used in optical imaging.
The Tf-TfR transport system holds dual implications: (1) Imaging aspect: Increased iron uptake mediated by TfR can enhance the magnetic susceptibility and imaging effects of FTH1. In this study, both in vivo and in vitro MR imaging demonstrated that the chimeric element regulation group exhibited a more pronounced reduction in T2-weighted imaging (T2WI) signals compared to the PEG3 promoter group. (2) Therapeutic aspect: The upregulation of TfR provides a foundation for Tf-mediated targeted drug delivery. Flow cytometry in vitro revealed a significant increase in Tf-LPD uptake in the chimeric element group compared to the control group. Both in vivo and in vitro experiments indicated that the cytotoxic effect of Tf-LPD on gliomas in the chimeric element group was markedly greater than that of the control group. These results suggest that the upregulation of TfR induced by FTH1 overexpression can significantly enhance the efficiency of targeted drug delivery.
In vivo gene delivery is pivotal for gene therapy and diagnostics, utilizing two primary methodologies: viral and non-viral vectors, each characterized by unique mechanisms, benefits, and drawbacks. Non-viral vectors predominantly consist of lipid nanoparticles (LNPs) and extracellular vesicles (EVs). LNPs are extensively researched for their capacity to encapsulate and safeguard genes, thereby enhancing delivery efficacy. They exhibit favorable biocompatibility, yielding non-toxic degradation products. However, their limited aqueous solubility may lead to drug leakage, and their larger size complicates targeting despite surface modifications [
28,
29,
30]. EVs, currently a focal point of research, provide excellent biocompatibility, low immunogenicity, the ability to penetrate the blood-brain barrier, and versatile cargo loading capabilities (e.g., drugs, proteins, mRNA). Nonetheless, challenges in production, low yields, and potential instability hinder their large-scale application [
31,
32]. Viral vectors encompass adenoviruses, adeno-associated viruses (AAVs), and lentiviruses. Adenoviruses are straightforward to prepare and can infect a variety of cell types but elicit robust immune responses and result in transient gene expression [
33,
34]. AAVs circumvent genomic integration, thereby minimizing oncogenic risks, yet their limited genome size restricts insert capacity, and exogenous gene expression is often delayed [
35,
36]. Lentiviruses integrate stably into the host genome, facilitating long-term expression and accommodating larger inserts without generating infectious particles [
37]. Whether in the preclinical research phase or during clinical trials, both the FDA and EMA impose stringent requirements on the production and use of lentiviral vectors. We opted for lentiviruses due to their sustained expression and high safety profile. In this study, we implemented a strategy involving multiple intratumoral injections of lentivirus in vivo, which offers the advantages of direct vector delivery to circumvent the blood-brain barrier limitations, enhancing transfection uniformity through multi-point distribution, and mitigating systemic toxicity risks with localized high titers. In vivo fluorescence imaging revealed significant fluorescence in the tumors of all three groups of tumor-bearing mice transfected with lentivirus, indicating successful in vivo transfection.