Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Leukemia and Lymphoma: Basic Principles
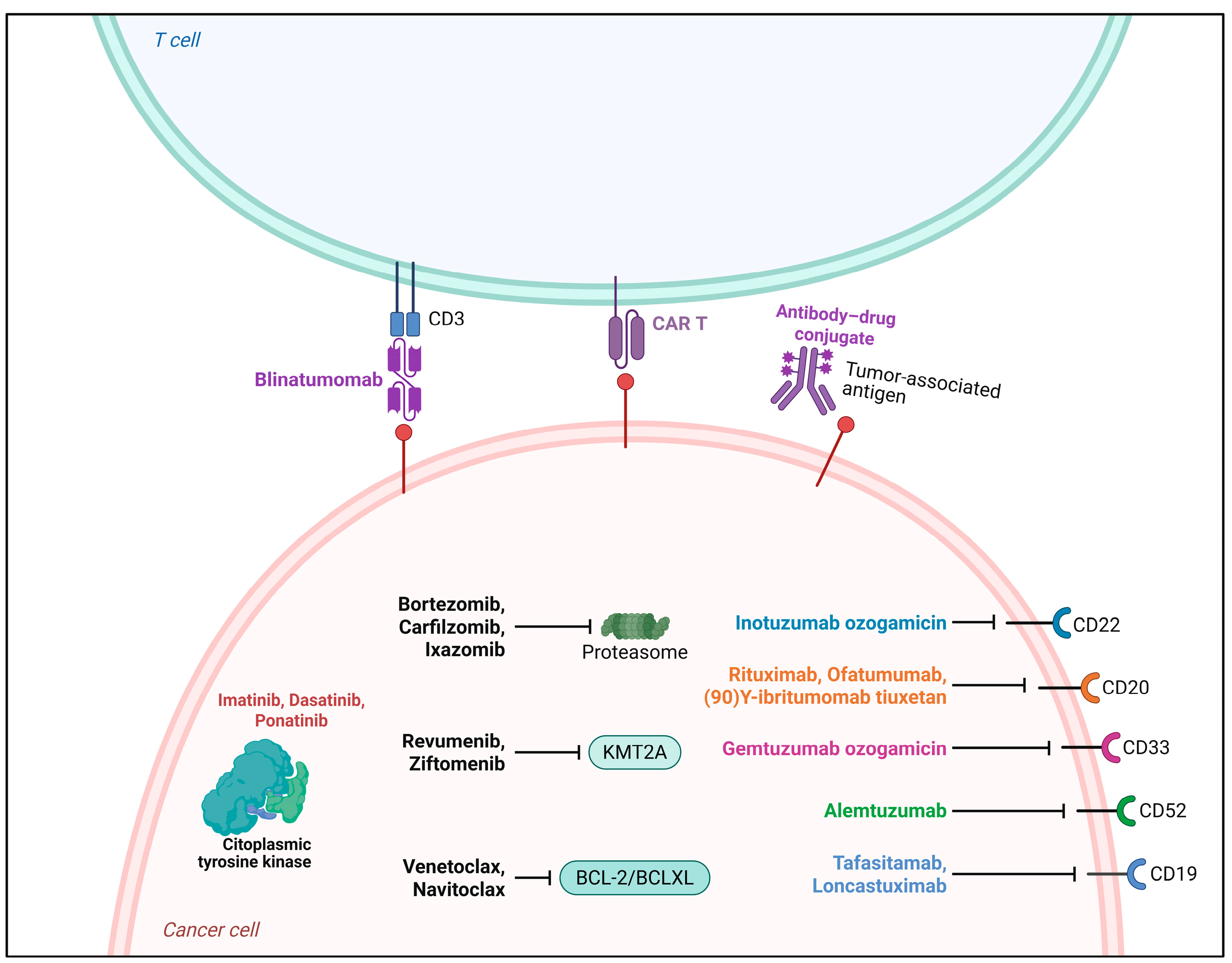
Therapeutic Strategies Available for Leukemia and Lymphoma
| Treatment | Mechanism of Action (Summary) |
|---|---|
| Chemotherapy | Primarily targets rapidly dividing cells by interfering with DNA, RNA, and protein synthesis. Includes classes such as alkylating agents, antimetabolites, topoisomerase inhibitors, and antimicrotubule agents. Induces cell damage leading to apoptosis [54]. |
| Radiation Therapy | Uses ionizing radiation to cause DNA breaks (directly or via free radicals). Triggers cell death and modulates the tumor microenvironment, potentially enhancing immune responses [55]. |
| Targeted Therapy | Focuses on specific molecules or pathways altered in cancer, such as tyrosine kinases, BCL2, or epigenetic regulators. More selective than conventional chemotherapy. Includes monoclonal antibodies and small-molecule inhibitors [56]. |
| Immunotherapy | Enhances or restores the immune system’s ability to recognize and eliminate cancer cells. Includes immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1), CAR-T cells, and BiTEs. Helps reestablish immune surveillance [57,58]. |
| Hematopoietic Stem Cell Transplantation (HSCT) | Replaces bone marrow after myeloablative therapy. Allogeneic HSCT also provides a graft-versus-tumor/leukemia effect, where donor immune cells attack residual malignant cells [59]. |
4. Animal Venoms with Potential Cytotoxic Action Against Leukemia and Lymphoma Cancer Cells
4.1. Emerging Therapeutic Agents Derived from Snake Venom
4.2. Anticancer Effects of Bee-Derived Compounds
4.3. Scorpion Venom Peptides and Components Against Leukemia and Lymphoma
4.4. Cone-Snail-Venom-Derived Conotoxins Against Leukemia and Lymphoma Cells
5. Venoms and Toxins: A Promising Source for the Development of New Drugs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zona Rubio, D.C.; Aragón, D.M.; Almeida Alves, I. Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications. Toxins 2025, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211006071. [Google Scholar] [CrossRef] [PubMed]
- Abidin, S.A.Z.; Liew, A.K.Y.; Othman, I.; Shaikh, F. Animal Venoms as Potential Source of Anticonvulsants. F1000Research 2024, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.M.; Goncalves, B.D.C.; Gomez, M.V.; Vieira, L.B.; Ribeiro, F.M. Animal Toxins as Therapeutic Tools to Treat Neurodegenerative Diseases. Front. Pharmacol. 2018, 9, 145. [Google Scholar] [CrossRef]
- Chatterjee, B. Animal Venoms Have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef]
- Ejaz, S.; Hashmi, F.B.; Malik, W.N.; Ashraf, M.; Nasim, F.U.-H.; Iqbal, M. Applications of Venom Proteins as Potential Anticancer Agents. Protein Pept. Lett. 2018, 25, 688–701. [Google Scholar] [CrossRef]
- Shahzadi, S.K.; Karuvantevida, N.; Banerjee, Y. A Venomics Approach to the Identification and Characterization of Bioactive Peptides From Animal Venoms for Colorectal Cancer Therapy: Protocol for a Proof-of-Concept Study. JMIR Res. Protoc. 2021, 10, e31128. [Google Scholar] [CrossRef]
- Majc, B.; Novak, M.; Lah, T.T.; Križaj, I. Bioactive Peptides from Venoms against Glioma Progression. Front. Oncol. 2022, 12, 965882. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Venom-Derived Bioactive Compounds as Potential Anticancer Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 129–147. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Li, X.; Han, W.; Su, X. Anticancer Potential of Bioactive Peptides from Animal Sources (Review). Oncol. Rep. 2017, 38, 637–651. [Google Scholar] [CrossRef]
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, N.; Javidan, M.; Sheikhi, S.; Taştan, Ö.; Parodi, A.; Liao, Z.; Tayybi Azar, M.; Ganjalıkhani-Hakemi, M. Bioactive Peptides: An Alternative Therapeutic Approach for Cancer Management. Front. Immunol. 2024, 15, 1310443. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, N.; Khan, M.H.; Sahibzada, M.; Khan, S.A.; Syamprabha Vijayan, A.; Ullah, N.; Koodarath, C.; Khalil, H.; Ali, U.A.; Saleem, F.; et al. Recent Developments and Challenges in the Treatment of Acute Leukemia and Myelodysplastic Syndromes: A Systematic Review. Cureus 2024, 16, e72599. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Carrà, G.; Lingua, M.F.; Maffeo, B.; Brancaccio, M.; Taulli, R.; Morotti, A. Tumor Suppressors in Chronic Lymphocytic Leukemia: From Lost Partners to Active Targets. Cancers 2020, 12, 629. [Google Scholar] [CrossRef]
- Puente, X.S.; Jares, P.; Campo, E. Chronic Lymphocytic Leukemia and Mantle Cell Lymphoma: Crossroads of Genetic and Microenvironment Interactions. Blood 2018, 131, 2283–2296. [Google Scholar] [CrossRef]
- Peloquin, S.; Cymbalista, F.; Dreyling, M.; Shah, N.N.; Murray, S.; Del Fiacco, R.; Muehlenbein, C.E.; Lazure, P. Knowledge, Skills, and Confidence Gaps Impacting Treatment Decision Making in Relapsed/Refractory Chronic Lymphocytic Leukemia and Mantle Cell Lymphoma: A Quantitative Survey Study in France, Germany, and the United States. BMC Cancer 2024, 24, 1003. [Google Scholar] [CrossRef]
- Fakhri, B.; Andreadis, C. The Role of Acalabrutinib in Adults with Chronic Lymphocytic Leukemia. Ther. Adv. Hematol. 2021, 12, 2040620721990553. [Google Scholar] [CrossRef]
- Arens, D.K.; Rose, M.A.; Salazar, E.M.; Harvey, M.A.; Huh, E.Y.; Ford, A.A.; Thompson, D.W.; Sanchez, E.E.; Hwang, Y.Y. Doxycycline-Mediated Inhibition of Snake Venom Phospholipase and Metalloproteinase. Mil. Med. 2024, 189, e2430–e2438. [Google Scholar] [CrossRef]
- Anand, P.; Filipenko, P.; Huaman, J.; Lyudmer, M.; Hossain, M.; Santamaria, C.; Huang, K.; Ogunwobi, O.O.; Holford, M. Selective Inhibition of Liver Cancer Cells Using Venom Peptide. Mar. Drugs 2019, 17, 587. [Google Scholar] [CrossRef]
- Ochoa-Mosquera, J.; Montoya-Gómez, A.; Jiménez-Charris, E. Snake Venom Toxins as Potential Therapeutic Agents in the Treatment of Prostate Cancer. Mol. Biol. Rep. 2024, 51, 1153. [Google Scholar] [CrossRef]
- Zainal Abidin, S.A.; Lee, Y.Q.; Othman, I.; Naidu, R. Malaysian Cobra Venom: A Potential Source of Anti-Cancer Therapeutic Agents. Toxins 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Y.; Li, J.; Xu, S.; Liu, C.; Zhu, Y.; Liang, S. The Venom of the Spider Macrothele Raveni Induces Apoptosis in the Myelogenous Leukemia K562 Cell Line. Leuk. Res. 2012, 36, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Luo, W.; Zhang, Z.; Lv, M.; Sang, L.; Wen, Y.; Wang, L.; Ding, C.; Wu, K.; Li, F.; et al. A Lipid-Sensitive Spider Peptide Toxin Exhibits Selective Anti-Leukemia Efficacy through Multimodal Mechanisms. Adv. Sci. 2024, 11, e2404937. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Powsner, E.H.; Harris, J.C.; Day, E.S. Biomimetic Nanoparticles for the Treatment of Hematologic Malignancies. Adv. NanoBiomed Res. 2021, 1, 2000047. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Luo, Q.; Zhang, J.; Huang, M.; Xu, Y.; Huo, D.; Shan, W.; Tie, R.; Zhang, M.; et al. Generating Hematopoietic Cells from Human Pluripotent Stem Cells: Approaches, Progress and Challenges. Cell Regen. 2023, 12, 31. [Google Scholar] [CrossRef]
- Câmara, A.B.; Brandão, I.A. The Non-Hodgkin Lymphoma Treatment and Side Effects: A Systematic Review and Meta-Analysis. Recent. Pat. Anticancer. Drug Discov. 2023, 19, 93–120. [Google Scholar] [CrossRef]
- Munir, F.; Hardit, V.; Sheikh, I.N.; AlQahtani, S.; He, J.; Cuglievan, B.; Hosing, C.; Tewari, P.; Khazal, S. Classical Hodgkin Lymphoma: From Past to Future—A Comprehensive Review of Pathophysiology and Therapeutic Advances. Int. J. Mol. Sci. 2023, 24, 10095. [Google Scholar] [CrossRef]
- The International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-832-4494-3. [Google Scholar]
- Eichenauer, D.A.; Hartmann, S. Nodular Lymphocyte-Predominant Hodgkin Lymphoma: Current Management Strategies and Evolving Approaches to Individualize Treatment. Expert. Rev. Hematol. 2023, 16, 607–615. [Google Scholar] [CrossRef]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care Clin. Off. Pract. 2016, 43, 661–675. [Google Scholar] [CrossRef]
- Montorsi, L.; Siu, J.H.Y.; Spencer, J. B Cells in Human Lymphoid Structures. Clin. Exp. Immunol. 2022, 210, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Gong, Y.; Shi, X.; Shi, H.; Wan, Y.; Wu, Q.; Xu, K. Expression and Regulation of COP1 in Chronic Lymphocytic Leukemia Cells for Promotion of Cell Proliferation and Tumorigenicity. Oncol. Rep. 2016, 35, 1493–1500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, C.; Shi, X.; Gong, Y.; Wan, Y.; Sun, Z.; Shi, H.; Wang, Z.; Marinaccio, C.; Crispino, J.D.; Xu, K. Constitutively Photomorphogenic 1 Reduces the Sensitivity of Chronic Lymphocytic Leukemia Cells to Fludarabine Through Promotion of Ubiquitin-Mediated P53 Degradation. Cell Physiol. Biochem. 2018, 50, 2314–2328. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Dell’Antonio, L.S.; Pessanha, R.M.; Dell’Antonio, C.S.; da Silva, M.I.; de Souza, T.M.; Grassi, J. Completeness and Consistency of Epidemiological Variables from Hospital-Based Cancer Registries in a Brazilian State. Int. J. Environ. Res. Public Health 2022, 19, 12003. [Google Scholar] [CrossRef]
- Dinner, S.; Liedtke, M. Antibody-Based Therapies in Patients with Acute Lymphoblastic Leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 9–15. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Gonçalves, M.V.; Ikoma, M.R.V.; Lorand-Metze, I.; Pereira, A.D.; de Farias, D.L.C.; de Lourdes Lopes Ferrari Chauffaille, M.; Schaffel, R.; Ribeiro, E.F.O.; da Rocham, T.S.; et al. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: Recommendations from the Brazilian Group of Chronic Lymphocytic Leukemia. Rev. Bras. Hematol. Hemoter. 2016, 38, 346–357. [Google Scholar] [CrossRef]
- De Kouchkovsky, I.; Abdul-Hay, M. “Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic Myeloid Leukemia: A Review. J. Am. Med. Assoc. 2025, 333, 1618. [Google Scholar] [CrossRef]
- Terwilliger, T.; Abdul-Hay, M. Acute Lymphoblastic Leukemia: A Comprehensive Review and 2017 Update. Blood Cancer J. 2017, 7, e577. [Google Scholar] [CrossRef]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef]
- Ravandi, F.; Roboz, G.J.; Wei, A.H.; Döhner, H.; Pocock, C.; Selleslag, D.; Montesinos, P.; Sayar, H.; Musso, M.; Figuera-Alvarez, A.; et al. Management of Adverse Events in Patients with Acute Myeloid Leukemia in Remission Receiving Oral Azacitidine: Experience from the Phase 3 Randomized QUAZAR AML-001 Trial. J. Hematol. Oncol. 2021, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y. Structure, Function and Inhibition of Critical Protein–Protein Interactions Involving Mixed Lineage Leukemia 1 and Its Fusion Oncoproteins. J. Hematol. Oncol. 2021, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mahmud, A.R.; Faijanur-Rob-Siddiquee, M.; Shahriar, A.; Biswas, P.; Ebrahim, K.S.; Ahmed, S.Z.; Ema, T.I.; Rahman, N.; Furkanur, R.M.; et al. Role of T Cells in Cancer Immunotherapy: Opportunities and Challenges. Cancer Pathog. Ther. 2022, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.H.; Shafqat, A.; Ahmad, O.; Alkattan, K.; Yaqinuddin, A.; Damlaj, M. Bispecific Antibodies in Hematological Malignancies: A Scoping Review. Cancers 2023, 15, 4550. [Google Scholar] [CrossRef]
- Merino, A.; Maakaron, J.; Bachanova, V. Advances in NK Cell Therapy for Hematologic Malignancies: NK Source, Persistence and Tumor Targeting. Blood Rev. 2023, 60, 101073. [Google Scholar] [CrossRef]
- García-Gutiérrez, V.; Breccia, M.; Jabbour, E.; Mauro, M.; Cortes, J.E. A Clinician Perspective on the Treatment of Chronic Myeloid Leukemia in the Chronic Phase. J. Hematol. Oncol. 2022, 15, 90. [Google Scholar] [CrossRef]
- Shammas, T.; Peiris, M.N.; Meyer, A.N.; Donoghue, D.J. BCR-ABL: The Molecular Mastermind behind Chronic Myeloid Leukemia. Cytokine Growth Factor. Rev. 2025, 83, 45–58. [Google Scholar] [CrossRef]
- Radich, J.P.; Wall, M.; Branford, S.; Campbell, C.D.; Chaturvedi, S.; DeAngelo, D.J.; Deininger, M.; Guinney, J.; Hochhaus, A.; Hughes, T.P.; et al. Molecular Response in Newly Diagnosed Chronic-Phase Chronic Myeloid Leukemia: Prediction Modeling and Pathway Analysis. Haematologica 2023, 108, 1567–1578. [Google Scholar] [CrossRef]
- Zafar, F.; Poombal, F.; Ashraf, L.; Shivakumar, D.; Wankhade, D.; Winayak, R.; Ali Malik, G.M.; Mahapatra, S.S.; Shasan, G.C.; Huynh, T.; et al. Nilotinib Versus Imatinib in Philadelphia Chromosome-Positive Chronic Myeloid Leukemia (Ph+ CML): A Systematic Review and Meta-Analysis of Randomized Controlled Trials (RCTs). Cureus 2025, 17, e82631. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, Z.; Lu, J.; Pan, J.; Yu, Y.; Zhao, Y.; Zhang, H.; Hu, T.; Liu, Q.; et al. Novel Multiple Tyrosine Kinase Inhibitor Ponatinib Inhibits bFGF-Activated Signaling in Neuroblastoma Cells and Suppresses Neuroblastoma Growth in Vivo. Oncotarget 2017, 8, 5874–5884. [Google Scholar] [CrossRef]
- Syed, Y.Y. Revumenib: First Approval. Drugs 2025, 85, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Aldoss, I.; Thirman, M.J.; DiPersio, J.; Arellano, M.; Blachly, J.S.; Mannis, G.N.; Perl, A.; Dickens, D.S.; McMahon, C.M.; et al. Menin Inhibition With Revumenib for KMT2A-Rearranged Relapsed or Refractory Acute Leukemia (AUGMENT-101). J. Clin. Oncol. 2025, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Carlos-Reyes, A.; Muñiz-Lino, M.A.; Romero-Garcia, S.; López-Camarillo, C.; Hernández-de la Cruz, O.N. Biological Adaptations of Tumor Cells to Radiation Therapy. Front. Oncol. 2021, 11, 718636. [Google Scholar] [CrossRef]
- Min, H.-Y.; Lee, H.-Y. Molecular Targeted Therapy for Anticancer Treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Lanier, O.L.; Pérez-Herrero, E.; Andrea, A.P.D.; Bahrami, K.; Lee, E.; Ward, D.M.; Ayala-Suárez, N.; Rodríguez-Méndez, S.M.; Peppas, N.A. Immunotherapy Approaches for Hematological Cancers. iScience 2022, 25, 105326. [Google Scholar] [CrossRef]
- Batlevi, C.L.; Matsuki, E.; Brentjens, R.J.; Younes, A. Novel Immunotherapies in Lymphoid Malignancies. Nat. Rev. Clin. Oncol. 2016, 13, 25–40. [Google Scholar] [CrossRef]
- Ibikunle, S.; Grosso, D.; Gergis, U. The Two-Step Approach to Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2023, 14, 1237782. [Google Scholar] [CrossRef]
- Aureli, A.; Marziani, B.; Sconocchia, T.; Del Principe, M.I.; Buzzatti, E.; Pasqualone, G.; Venditti, A.; Sconocchia, G. Immunotherapy as a Turning Point in the Treatment of Acute Myeloid Leukemia. Cancers 2021, 13, 6246. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, B.; Brandl, C.; Johnson, J.; Wolf, A.; Chow, A.; Doshi, S. Blinatumomab, a Bispecific T-Cell Engager BiTE® for CD-19 Targeted Cancer Immunotherapy: Clinical Pharmacology and Its Implications. Clin. Pharmacokinet. 2016, 55, 1271–1288. [Google Scholar] [CrossRef]
- Lo, M.-Y.; Tsai, X.C.-H.; Lin, C.-C.; Tien, F.-M.; Kuo, Y.-Y.; Lee, W.-H.; Peng, Y.-L.; Liu, M.-C.; Tseng, M.-H.; Hsu, C.-A.; et al. Validation of the Prognostic Significance of the 2022 European LeukemiaNet Risk Stratification System in Intensive Chemotherapy Treated Aged 18 to 65 Years Patients with de Novo Acute Myeloid Leukemia. Am. J. Hematol. 2023, 98, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Rujkijyanont, P.; Inaba, H. Diagnostic and Treatment Strategies for Pediatric Acute Lymphoblastic Leukemia in Low- and Middle-Income Countries. Leukemia 2024, 38, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Ma, Q.; Yin, W.; Ma, X.; He, Z. CRISPR/Cas9 Gene-Editing in Cancer Immunotherapy: Promoting the Present Revolution in Cancer Therapy and Exploring More. Front. Cell Dev. Biol. 2021, 9, 674467. [Google Scholar] [CrossRef] [PubMed]
- Vu, S.H.; Vetrivel, P.; Kim, J.; Lee, M.-S. Cancer Resistance to Immunotherapy: Molecular Mechanisms and Tackling Strategies. Int. J. Mol. Sci. 2022, 23, 10906. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foà, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- de Castro, A.A.C.; de Oliveira, L.A.; de Andrade, D.P.; Carbone, E.K.; Rosati, R. Use of Rituximab in Mature, High-Grade and Advanced-Stage Pediatric B-Lineage Non-Hodgkin Lymphomas: A Systematic Review, Meta-Analysis and the Brazilian Reality. Front. Pediatr. 2025, 13, 1532274. [Google Scholar] [CrossRef]
- Tobinai, K.; Klein, C.; Oya, N.; Fingerle-Rowson, G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv. Ther. 2017, 34, 324–356. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Hashmi, H.; Darwin, A.; Nishihori, T. Therapeutic Roles of Antibody Drug Conjugates (ADCs) in Relapsed/Refractory Lymphomas. Hematol. Oncol. Stem Cell Ther. 2021, 16, 21–34. [Google Scholar] [CrossRef]
- Hasan, H.F.; Mostafa, D.M.; Lotfy, D.M. Concerted Hepatoprotective Effect of Bradykinin Potentiating Factor and Low Dose of γ- Radiation on Naja Haje Envenomed Rats via Bax/Bcl2 Pathway. Toxicol. Mech. Methods 2022, 32, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Badawi, J.K. Bee Venom Components as Therapeutic Tools against Prostate Cancer. Toxins 2021, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Adhami, V.; Sajjadi Alehashem, S.H.; Vatanpour, H.; Sadeghi, L. Iranian Mesobuthus Eupeus Crude Venom Induces Selective Toxicity in Chronic Lymphocytic Leukemia B-Lymphocytes Through Lysosomal/Mitochondrial Dysfunction and Reactive Oxygen Species Formation. Asian Pac. J. Cancer Prev. 2022, 23, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, R.A.; Mohallal, M.E.; Mobarak, Y.M.; Ebaid, H.M.; Haywood-Small, S.; Miller, K.; Strong, P.N.; Abdel-Rahman, M.A. Scorpion Venom Antimicrobial Peptides Induce Caspase-1 Dependant Pyroptotic Cell Death. Front. Pharmacol. 2021, 12, 788874. [Google Scholar] [CrossRef]
- Abdelfatah, S.; Lu, X.; Schmeda-Hirschmann, G.; Efferth, T. Cytotoxicity and Antimitotic Activity of Rhinella Schneideri and Rhinella Marina Venoms. J. Ethnopharmacol. 2019, 242, 112049. [Google Scholar] [CrossRef]
- Sampat, G.H.; Hiremath, K.; Dodakallanavar, J.; Patil, V.S.; Harish, D.R.; Biradar, P.; Mahadevamurthy, R.K.; Barvaliya, M.; Roy, S. Unraveling Snake Venom Phospholipase A2: An Overview of Its Structure, Pharmacology, and Inhibitors. Pharmacol. Rep. 2023, 75, 1454–1473. [Google Scholar] [CrossRef]
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.-M.; Legros, C.; Mattei, C. Snake Venom Components: Tools and Cures to Target Cardiovascular Diseases. Molecules 2021, 26, 2223. [Google Scholar] [CrossRef]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of Snake Venom Enzymatic Toxins: Phospholipase A2 and l-Amino Acid Oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Sagar; Daul, F.; Salvat Lago, M.; Decker, A.; et al. Characterization of Pre-Existing and Induced SARS-CoV-2-Specific CD8+ T Cells. Nat. Med. 2021, 27, 78–85. [Google Scholar] [CrossRef]
- Truong, N.V.; Phan, T.T.T.; Hsu, T.-S.; Phu Duc, P.; Lin, L.-Y.; Wu, W.-G. Action Mechanism of Snake Venom L-Amino Acid Oxidase and Its Double-Edged Sword Effect on Cancer Treatment: Role of Pannexin 1-Mediated Interleukin-6 Expression. Redox Biol. 2023, 64, 102791. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; van Thiel, J.; Cardoso, F.C.; Casewell, N.R.; Gutiérrez, J.-M.; Kool, J.; Vonk, F.J. Tissue Damaging Toxins in Snake Venoms: Mechanisms of Action, Pathophysiology and Treatment Strategies. Commun. Biol. 2024, 7, 358. [Google Scholar] [CrossRef]
- Ullah, A. Structure-Function Studies and Mechanism of Action of Snake Venom L-Amino Acid Oxidases. Front. Pharmacol. 2020, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.F.T.; de Souza Imberg, A.; Mariano, D.O.C.; de Moraes, A.C.; Andrade-Silva, J.; Fernandes, C.M.; Sobral, A.C.; Giannotti, K.C.; Kuwabara, W.M.T.; Pimenta, D.C.; et al. β-Micrustoxin (Mlx-9), a PLA2 from Micrurus Lemniscatus Snake Venom: Biochemical Characterization and Anti-Proliferative Effect Mediated by P53. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210094. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Molinier-Frenkel, V. An Overview of L-Amino Acid Oxidase Functions from Bacteria to Mammals: Focus on the Immunoregulatory Phenylalanine Oxidase IL4I1. Molecules 2017, 22, 2151. [Google Scholar] [CrossRef] [PubMed]
- Lukasheva, E.V.; Babayeva, G.; Karshieva, S.S.; Zhdanov, D.D.; Pokrovsky, V.S. L-Lysine α-Oxidase: Enzyme with Anticancer Properties. Pharmaceuticals 2021, 14, 1070. [Google Scholar] [CrossRef]
- Burin, S.M.; Ghisla, S.; Ouchida, A.T.; Aissa, A.F.; Coelho, M.G.B.; Costa, T.R.; Marsola, A.P.Z.C.; Pinto-Simões, B.; Antunes, L.M.G.; Curti, C.; et al. CR-LAAO Antileukemic Effect against Bcr-Abl+ Cells Is Mediated by Apoptosis and Hydrogen Peroxide. Int. J. Biol. Macromol. 2016, 86, 309–320. [Google Scholar] [CrossRef]
- Cedro, R.C.A.; Menaldo, D.L.; Costa, T.R.; Zoccal, K.F.; Sartim, M.A.; Santos-Filho, N.A.; Faccioli, L.H.; Sampaio, S.V. Cytotoxic and Inflammatory Potential of a Phospholipase A2 from Bothrops Jararaca Snake Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 33. [Google Scholar] [CrossRef]
- Castro-Amorim, J.; Novo de Oliveira, A.; Da Silva, S.L.; Soares, A.M.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. Catalytically Active Snake Venom PLA2 Enzymes: An Overview of Its Elusive Mechanisms of Reaction. J. Med. Chem. 2023, 66, 5364–5376. [Google Scholar] [CrossRef]
- Castro-Amorim, J.; Pinto, A.V.; Mukherjee, A.K.; Ramos, M.J.; Fernandes, P.A. Beyond Fang’s Fury: A Computational Study of the Enzyme-Membrane Interaction and Catalytic Pathway of the Snake Venom Phospholipase A2 Toxin. Chem. Sci. 2025, 16, 1974–1985. [Google Scholar] [CrossRef]
- Mathis, S.; Carla, L.; Duval, F.; Nadal, L.; Solé, G.; Le Masson, G. Acute Peripheral Neuropathy Following Animal Envenomation: A Case Report and Systematic Review. J. Neurol. Sci. 2022, 442, 120448. [Google Scholar] [CrossRef]
- Bezerra, P.H.A.; Ferreira, I.M.; Franceschi, B.T.; Bianchini, F.; Ambrósio, L.; Cintra, A.C.O.; Sampaio, S.V.; de Castro, F.A.; Torqueti, M.R. BthTX-I from Bothrops Jararacussu Induces Apoptosis in Human Breast Cancer Cell Lines and Decreases Cancer Stem Cell Subpopulation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190010. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Hees, C.; Sterzik, A.; Bauernfeind, F.; Mak’Anyengo, R.; Duewell, P.; Lehr, H.-A.; Noessner, E.; Wank, R.; Trauzold, A.; et al. Proapoptotic and Antiapoptotic Proteins of the Bcl-2 Family Regulate Sensitivity of Pancreatic Cancer Cells toward Gemcitabine and T-Cell-Mediated Cytotoxicity. J. Immunother. 2015, 38, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; O’Hare, T.; Deininger, M.W. Mechanisms of Resistance to ABL Kinase Inhibition in CML and the Development of next Generation ABL Kinase Inhibitors. Hematol. Oncol. Clin. North Am. 2017, 31, 589–612. [Google Scholar] [CrossRef]
- Sobrinho, J.C.; Kayano, A.M.; Simões-Silva, R.; Alfonso, J.J.; Gomez, A.F.; Gomez, M.C.V.; Zanchi, F.B.; Moura, L.A.; Souza, V.R.; Fuly, A.L.; et al. Anti-Platelet Aggregation Activity of Two Novel Acidic Asp49-Phospholipases A2 from Bothrops Brazili Snake Venom. Int. J. Biol. Macromol. 2018, 107, 1014–1022. [Google Scholar] [CrossRef]
- Hu, Z.; Slayton, W.B. Integrin VLA-5 and FAK Are Good Targets to Improve Treatment Response in the Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia. Front. Oncol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Almeida, G.O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake Venom Disintegrins Update: Insights about New Findings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230039. [Google Scholar] [CrossRef]
- Almeida, G.O.; Cintra, A.C.O.; Silva, T.A.; de Oliveira, I.S.; Correia, L.I.V.; Torquato, R.J.S.; Ferreira Junior, R.S.; Arantes, E.C.; Sampaio, S.V. Moojecin: The First Disintegrin from Bothrops Moojeni Venom and Its Antitumor Activity in Acute Myeloid Leukemia. Int. J. Biol. Macromol. 2024, 279, 135066. [Google Scholar] [CrossRef]
- Das, V.; Kalyan, G.; Hazra, S.; Pal, M. Understanding the Role of Structural Integrity and Differential Expression of Integrin Profiling to Identify Potential Therapeutic Targets in Breast Cancer. J. Cell Physiol. 2018, 233, 168–185. [Google Scholar] [CrossRef]
- Cavalcante, J.S.; Arruda, S.S.T.; Riciopo, P.M.; Pucca, M.; Ferreira Junior, R.S. Diagnosis of Human Envenoming by Terrestrial Venomous Animals: Routine, Advances, and Perspectives. Toxicon X 2024, 24, 100211. [Google Scholar] [CrossRef]
- Macêdo, J.K.A.; Fox, J.W.; de Souza Castro, M. Disintegrins from Snake Venoms and Their Applications in Cancer Research and Therapy. Curr. Protein Pept. Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, P.; Li, D.; Xia, Z.; Wang, P.; Zhang, X.; Liu, M.; Liao, L.; Jiao, B.; Ren, R. Combination Therapy of BCR-ABL-Positive B Cell Acute Lymphoblastic Leukemia by Tyrosine Kinase Inhibitor Dasatinib and c-JUN N-Terminal Kinase Inhibition. J. Hematol. Oncol. 2020, 13, 80. [Google Scholar] [CrossRef]
- de Carvalho, D.D.; Schmitmeier, S.; Novello, J.C.; Markland, F.S. Effect of BJcuL (a Lectin from the Venom of the Snake Bothrops Jararacussu) on Adhesion and Growth of Tumor and Endothelial Cells. Toxicon 2001, 39, 1471–1476. [Google Scholar] [CrossRef]
- Sartim, M.A.; Pinheiro, M.P.; de Pádua, R.A.P.; Sampaio, S.V.; Nonato, M.C. Structural and Binding Studies of a C-Type Galactose-Binding Lectin from Bothrops jararacussu Snake Venom. Toxicon 2017, 126, 59–69. [Google Scholar] [CrossRef]
- Pires, W.L.; de Castro, O.B.; Kayano, A.M.; da Silva Setúbal, S.; Pontes, A.S.; Nery, N.M.; Paloschi, M.V.; Dos Santos Pereira, S.; Stábeli, R.G.; Fernandes, C.F.C.; et al. Effect of BjcuL, a Lectin Isolated from Bothrops Jararacussu, on Human Peripheral Blood Mononuclear Cells. Toxicol. In Vitro 2017, 41, 30–41. [Google Scholar] [CrossRef]
- Zischler, L.; Cogo, S.C.; Micheau, O.; Elifio-Esposito, S. Evidence That BJcuL, a C-Type Lectin from Bothrops Jararacussu Venom, Influences Deubiquitinase Activity, Resulting in the Accumulation of Anti-Apoptotic Proteins in Two Colorectal Cancer Cell Lines. Int. J. Biol. Macromol. 2022, 209, 1205–1210. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zhao, P.; Ban, X.; Zhi, D.; Li, G.; Wang, F.; Yang, X.; Huai, L. Recognization of Receptors on Bone Marrow-Derived Dendritic Cells Bound with Pholiota nameko Polysaccharides. Int. J. Biol. Macromol. 2015, 72, 649–657. [Google Scholar] [CrossRef]
- Alves, B.F.A.; Ferreira, R.S., Jr. Antineoplastic Properties and Pharmacological Applications of Crotalus durissus terrificus Snake Venom. Rev. Soc. Bras. Med. Trop. 2022, 55, e0323. [Google Scholar] [CrossRef]
- Moraes, V.W.R.; Santos, V.M.; Suarez, E.R.; Ferraz, L.S.; Lopes, R.M.; Mognol, G.P.; Campeiro, J.D.; Machado-Neto, J.A.; Nascimento, F.D.; Hayashi, M.A.F.; et al. Targeting Ca2+ and Mitochondrial Homeostasis by Antipsychotic Thioridazine in Leukemia Cells. Life 2022, 12, 1477. [Google Scholar] [CrossRef]
- Deshwal, A.; Phan, P.; Datta, J.; Kannan, R.; Thallapuranam, S.K. A Meta-Analysis of the Protein Components in Rattlesnake Venom. Toxins 2021, 13, 372. [Google Scholar] [CrossRef]
- Salazar, E.; Rodriguez-Acosta, A.; Lucena, S.; Gonzalez, R.; McLarty, M.C.; Sanchez, O.; Suntravat, M.; Garcia, E.; Finol, H.J.; Giron, M.E.; et al. Biological Activities of a New Crotamine-like Peptide from Crotalus Oreganus Helleri on C2C12 and CHO Cell Lines, and Ultrastructural Changes on Motor Endplate and Striated Muscle. Toxicon 2020, 188, 95–107. [Google Scholar] [CrossRef]
- Gajski, G.; Leonova, E.; Sjakste, N. Bee Venom: Composition and Anticancer Properties. Toxins 2024, 16, 117. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Khan, M.A.; Kumar, R.; Upadhyay, T.K. An Updated Review Summarizing the Anticancer Efficacy of Melittin from Bee Venom in Several Models of Human Cancers. Nutrients 2023, 15, 3111. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Kwon, N.-Y.; Sung, S.-H.; Sung, H.-K.; Park, J.-K. Anticancer Activity of Bee Venom Components against Breast Cancer. Toxins 2022, 14, 460. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvătescu, C.A.; Ifteni, P.; Pleş, L. Anticancer Activity of Toxins from Bee and Snake Venom—An Overview on Ovarian Cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef]
- Ceremuga, M.; Stela, M.; Janik, E.; Gorniak, L.; Synowiec, E.; Sliwinski, T.; Sitarek, P.; Saluk-Bijak, J.; Bijak, M. Melittin—A Natural Peptide from Bee Venom Which Induces Apoptosis in Human Leukaemia Cells. Biomolecules 2020, 10, 247. [Google Scholar] [CrossRef]
- Ullah, A.; Aldakheel, F.M.; Anjum, S.I.; Raza, G.; Khan, S.A.; Tlak Gajger, I. Pharmacological Properties and Therapeutic Potential of Honey Bee Venom. Saudi Pharm. J. 2023, 31, 96–109. [Google Scholar] [CrossRef]
- Chaisakul, J.; Hodgson, W.C.; Kuruppu, S.; Prasongsook, N. Effects of Animal Venoms and Toxins on Hallmarks of Cancer. J. Cancer 2016, 7, 1571–1578. [Google Scholar] [CrossRef]
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Recent Advances on the Interaction of Glycolipid and Lipopeptide Biosurfactants with Model and Biological Membranes. Curr. Opin. Colloid. Interface Sci. 2023, 68, 101748. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Sun, C.; Xu, N.; Liu, W. The Current Landscape of the Antimicrobial Peptide Melittin and Its Therapeutic Potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef]
- Yu, X.; Jia, S.; Yu, S.; Chen, Y.; Zhang, C.; Chen, H.; Dai, Y. Recent Advances in Melittin-Based Nanoparticles for Antitumor Treatment: From Mechanisms to Targeted Delivery Strategies. J. Nanobiotechnol. 2023, 21, 454. [Google Scholar] [CrossRef]
- Cotoraci, C.; Ciceu, A.; Sasu, A.; Miutescu, E.; Hermenean, A. The Anti-Leukemic Activity of Natural Compounds. Molecules 2021, 26, 2709. [Google Scholar] [CrossRef]
- Somwongin, S.; Chantawannakul, P.; Chaiyana, W. Antioxidant Activity and Irritation Property of Venoms from Apis Species. Toxicon 2018, 145, 32–39. [Google Scholar] [CrossRef]
- Borojeni, S.K.; Zolfagharian, H.; Babaie, M.; Javadi, I. Cytotoxic Effect of Bee (A. Mellifera) Venom on Cancer Cell Lines. J. Pharmacopunct. 2020, 23, 212–219. [Google Scholar] [CrossRef]
- Antunovic, M.; Kriznik, B.; Ulukaya, E.; Yilmaz, V.T.; Mihalic, K.C.; Madunic, J.; Marijanovic, I. Cytotoxic Activity of Novel Palladium-Based Compounds on Leukemia Cell Lines. Anticancer. Drugs 2015, 26, 180–186. [Google Scholar] [CrossRef]
- Mukherjee Chatterjee, S.; Jain, C.K.; Singha, S.; Das, P.; Roychoudhury, S.; Majumder, H.K.; Das, S. Activity of CoII-Quinalizarin: A Novel Analogue of Anthracycline-Based Anticancer Agents Targets Human DNA Topoisomerase, Whereas Quinalizarin Itself Acts via Formation of Semiquinone on Acute Lymphoblastic Leukemia MOLT-4 and HCT 116 Cells. ACS Omega 2018, 3, 10255–10266. [Google Scholar] [CrossRef]
- Ryu, J.-M.; Na, H.-H.; Park, Y.-J.; Park, J.-S.; Ahn, B.-S.; Kim, K.-C. Sweet Bee Venom Triggers Multiple Cell Death Pathways or Spurs Acute Cell Rupture According to Its Concentration in THP-1 Monocytic Leukemia Cells. Genes 2022, 13, 223. [Google Scholar] [CrossRef]
- Rady, I.; Siddiqui, I.A.; Rady, M.; Mukhtar, H. Melittin, a Major Peptide Component of Bee Venom, and Its Conjugates in Cancer Therapy. Cancer Lett. 2017, 402, 16–31. [Google Scholar] [CrossRef]
- Małek, A.; Strzemski, M.; Kurzepa, J.; Kurzepa, J. Can Bee Venom Be Used as Anticancer Agent in Modern Medicine? Cancers 2023, 15, 3714. [Google Scholar] [CrossRef]
- Maitip, J.; Mookhploy, W.; Khorndork, S.; Chantawannakul, P. Comparative Study of Antimicrobial Properties of Bee Venom Extracts and Melittins of Honey Bees. Antibiotics 2021, 10, 1503. [Google Scholar] [CrossRef]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological Effects and Mechanisms of Bee Venom and Its Main Components: Recent Progress and Perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Islam, M.; Al-Zahrani, A.M. In Vitro Analysis of the Anticancer Properties of Scorpion Venom in Colorectal and Breast Cancer Cell Lines. Oncol. Lett. 2016, 11, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-Based Peptide Therapy: Insights into Anti-Cancer Mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic Anticancer Properties of Scorpion Venom Peptides: Rhopalurus Princeps Venom as an Anticancer Agent. Drug Des. Dev. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef]
- Hassan, H.; Mirza, M.R.; Jabeen, A.; Alam, M.; Kori, J.A.; Sultan, R.; ur Rahman, S.; Choudhary, M.I. Yellow Scorpion (Buthus sinidicus) Venom Peptides Induce Mitochondrial-Mediated Apoptosis in Cervical, Prostate and Brain Tumor Cell Lines. PLoS ONE 2024, 19, e0296636. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.-F. Natural Compounds as Anticancer Agents: Experimental Evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef]
- Rapôso, C. Scorpion and Spider Venoms in Cancer Treatment: State of the Art, Challenges, and Perspectives. J. Clin. Transl. Res. 2017, 3, 233–249. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Ullah, Z.; Al Balowi, A.; Islam, M. In Vitro Determination of the Efficacy of Scorpion Venoms as Anti-Cancer Agents against Colorectal Cancer Cells: A Nano-Liposomal Delivery Approach. Int. J. Nanomed. 2017, 12, 559–574. [Google Scholar] [CrossRef]
- Bahrami, A.; Khalaji, A.; Bahri Najafi, M.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Khaksary Mahabady, M.; Rahimian, N.; Mirzaei, H. NF-κB Pathway and Angiogenesis: Insights into Colorectal Cancer Development and Therapeutic Targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef]
- Ma, Q.; Hao, S.; Hong, W.; Tergaonkar, V.; Sethi, G.; Tian, Y.; Duan, C. Versatile Function of NF-ĸB in Inflammation and Cancer. Exp. Hematol. Oncol. 2024, 13, 68. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Song, N.; Guo, Y.; Hui, L.; Sang, C. Lappaconitine Sulfate Inhibits Proliferation and Induces Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells through the Reactive Oxygen Species-Dependent Mitochondrial Pathway. Pharmacology 2020, 105, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Wudtiwai, B.; Khawon, P. Induction of Human Hepatocellular Carcinoma HepG2 Cell Apoptosis by Naringin. Asian Pac. J. Cancer Prev. 2016, 17, 3289–3294. [Google Scholar] [PubMed]
- Ju, S.; Zhang, Y.; Guo, X.; Yan, Q.; Liu, S.; Ma, B.; Zhang, M.; Bao, J.; Luo, S.; Fu, Y. Anti-Ovarian Cancer Conotoxins Identified from Conus Venom. Molecules 2022, 27, 6609. [Google Scholar] [CrossRef]
- Salimi, A.; Salehian, S.; Aboutorabi, A.; Vazirizadeh, A.; Adhami, V.; Sajjadi Alehashem, S.H.; Seydi, E.; Pourahmad, J. Cytotoxicity Studies of the Crude venom and Fractions of Persian Gulf Snail (Conus textile) on Chronic Lymphocytic Leukemia and Normal Lymphocytes. Asian Pac. J. Cancer Prev. 2021, 22, 1523–1529. [Google Scholar] [CrossRef]
- Oroz-Parra, I.; Álvarez-Delgado, C.; Cervantes-Luevano, K.; Dueñas-Espinoza, S.; Licea-Navarro, A.F. Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299. Mar. Drugs 2020, 18, 10. [Google Scholar] [CrossRef]
- Sciaccotta, R.; Gangemi, S.; Penna, G.; Giordano, L.; Pioggia, G.; Allegra, A. Potential New Therapies “ROS-Based” in CLL: An Innovative Paradigm in the Induction of Tumor Cell Apoptosis. Antioxidants 2024, 13, 475. [Google Scholar] [CrossRef]
- Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Toxins 2024, 16, 94. [Google Scholar] [CrossRef]
- Luna-Nophal, A.; Díaz-Castillo, F.; Izquierdo-Sánchez, V.; Velázquez-Fernández, J.B.; Orozco-Morales, M.; Lara-Mejía, L.; Bernáldez-Sarabia, J.; Sánchez-Campos, N.; Arrieta, O.; Díaz-Chávez, J.; et al. Preclinical Efficacy and Proteomic Prediction of Molecular Targets for S-Cal14.1b and s-Cal14.2b Conotoxins with Antitumor Capacity in Xenografts of Malignant Pleural Mesothelioma. Mar. Drugs 2025, 23, 32. [Google Scholar] [CrossRef]
- Bao, N.; Le Caer, J.-P.; Vinh, P.T.K. Isolation and characterization of five novel mini-M conotoxins from the venom of mollusk-hunter snail Conus bandanus. Asian Pac. J. Trop. Biomed. 2020, 10, 343. [Google Scholar] [CrossRef]
- Li, R.; Yu, J.; Ye, D.; Liu, S.; Zhang, H.; Lin, H.; Feng, J.; Deng, K. Conotoxins: Classification, Prediction, and Future Directions in Bioinformatics. Toxins 2025, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.; Barreto, N.; Bonfanti, A.P.; Munhoz, J.; Rocha e Silva, T.; Sutti, R.; Verinaud, L.; Pinheiro de Mato, F.C.; Lanfredi, G.P.; Rapôso, C. Isolated Components From Spider Venom Targeting Human Glioblastoma Cells and Its Potential Combined Therapy With Rapamycin. Front. Mol. Biosci. 2022, 9, 752668. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Gómez, A.; Montealegre-Sánchez, L.; García-Perdomo, H.A.; Jiménez-Charris, E. Cervical Cancer and Potential Pharmacological Treatment with Snake Venoms. Mol. Biol. Rep. 2020, 47, 4709–4721. [Google Scholar] [CrossRef]
- Gasanoff, E.; Liu, Y.; Li, F.; Hanlon, P.; Garab, G. Bee Venom Melittin Disintegrates the Respiration of Mitochondria in Healthy Cells and Lymphoblasts, and Induces the Formation of Non-Bilayer Structures in Model Inner Mitochondrial Membranes. Int. J. Mol. Sci. 2021, 22, 11122. [Google Scholar] [CrossRef]
- Sung, S.-H.; Kim, J.-W.; Han, J.-E.; Shin, B.-C.; Park, J.-K.; Lee, G. Animal Venom for Medical Usage in Pharmacopuncture in Korean Medicine: Current Status and Clinical Implication. Toxins 2021, 13, 105. [Google Scholar] [CrossRef]
- Marinho, A.D.; Lucena da Silva, E.; Jullyanne de Sousa Portilho, A.; Lacerda Brasil de Oliveira, L.; Cintra Austregésilo Bezerra, E.; Maria Dias Nogueira, B.; Leitão-Araújo, M.; Lúcia Machado-Alves, M.; Correa Neto, C.; Seabra Ferreira, R.; et al. Three Snake Venoms from the Bothrops Genus Induced Apoptosis and Cell Cycle Arrest in the Human Leukemia Cell Line K562. Toxicon 2024, 238, 107547. [Google Scholar] [CrossRef]
- Prinholato da Silva, C.; Costa, T.R.; Paiva, R.M.A.; Cintra, A.C.O.; Menaldo, D.L.; Antunes, L.M.G.; Sampaio, S.V. Antitumor Potential of the Myotoxin BthTX-I from Bothrops Jararacussu Snake Venom: Evaluation of Cell Cycle Alterations and Death Mechanisms Induced in Tumor Cell Lines. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 44. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Lee, S.-H.; Lee, S.-K.; Ha, S.-H.; Suh, S.-J.; Kwon, K.-M.; Chung, T.-W.; Ha, K.-T.; Chang, Y.-C.; Lee, Y.-C.; et al. Induction of Apoptosis and Antitumor Activity of Eel Skin Mucus, Containing Lactose-Binding Molecules, on Human Leukemic K562 Cells. Mar. Drugs 2015, 13, 3936–3949. [Google Scholar] [CrossRef]
- Almeida, J.R.; Mendes, B.; Lancellotti, M.; Franchi, G.C.; Passos, Ó.; Ramos, M.J.; Fernandes, P.A.; Alves, C.; Vale, N.; Gomes, P.; et al. Lessons from a Single Amino Acid Substitution: Anticancer and Antibacterial Properties of Two Phospholipase A2-Derived Peptides. Curr. Issues Mol. Biol. 2021, 44, 46–62. [Google Scholar] [CrossRef]
- Stábeli, R.G.; Amui, S.F.; Sant’Ana, C.D.; Pires, M.G.; Nomizo, A.; Monteiro, M.C.; Romão, P.R.T.; Guerra-Sá, R.; Vieira, C.A.; Giglio, J.R.; et al. Bothrops Moojeni Myotoxin-II, a Lys49-Phospholipase A2 Homologue: An Example of Function Versatility of Snake Venom Proteins. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 142, 371–381. [Google Scholar] [CrossRef]
- Sánchez, E.E.; González, R.; Lucena, S.; García, S.; Finol, H.J.; Suntravat, M.; Girón, M.E.; Fernández, I.; Rodríguez-Acosta, A. Crotamine-like from Southern Pacific Rattlesnake (Crotalus Oreganus helleri) Venom Acts on Human Leukemia (K-562) Cell Lines and Produces Ultrastructural Changes on Mice Adrenal Gland. Ultrastruct. Pathol. 2018, 42, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Da Silva, J.F.; Boldrini França, J.; Fonseca, F.P.P.; Otaviano, A.R.; Henrique Silva, F.; Hamaguchi, A.; Magro, A.J.; Braz, A.S.K.; Dos Santos, J.I. Structural and Functional Properties of Bp-LAAO, a New l-Amino Acid Oxidase Isolated from Bothrops Pauloensis Snake Venom. Biochimie 2009, 91, 490–501. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.C.; de Morais Ribeiro Silva, L.; de Oliveira, A.M.B.; Lopes, F.S.R.; Sant’Anna, M.B.; Picolo, G. Cytotoxic Effect of Crotoxin on Cancer Cells and Its Antitumoral Effects Correlated to Tumor Microenvironment: A Review. Int. J. Biol. Macromol. 2023, 242, 124892. [Google Scholar] [CrossRef] [PubMed]
- Offor, B.C.; Piater, L.A. Snake Venom Toxins: Potential Anticancer Therapeutics. J. Appl. Toxicol. 2024, 44, 666–685. [Google Scholar] [CrossRef]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake Venom L-Amino Acid Oxidases: Trends in Pharmacology and Biochemistry. Biomed Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef]
- Paloschi, M.V.; Pontes, A.S.; Soares, A.M.; Zuliani, J.P. An Update on Potential Molecular Mechanisms Underlying the Actions of Snake Venom L-Amino Acid Oxidases (LAAOs). Curr. Med. Chem. 2018, 25, 2520–2530. [Google Scholar] [CrossRef]
- Burin, S.M.; Berzoti-Coelho, M.G.; Cominal, J.G.; Ambrosio, L.; Torqueti, M.R.; Sampaio, S.V.; de Castro, F.A. The L-Amino Acid Oxidase from Calloselasma Rhodostoma Snake Venom Modulates apoptomiRs Expression in Bcr-Abl-Positive Cell Lines. Toxicon 2016, 120, 9–14. [Google Scholar] [CrossRef]
- Richard, S.A.; Kampo, S.; Sackey, M.; Hechavarria, M.E.; Buunaaim, A.D.B. The Pivotal Potentials of Scorpion Buthus Martensii Karsch-Analgesic-Antitumor Peptide in Pain Management and Cancer. Evid. Based Complement. Altern. Med. 2020, 2020, 4234273. [Google Scholar] [CrossRef]
- Gupta, S.D.; Gomes, A.; Debnath, A.; Saha, A.; Gomes, A. Apoptosis Induction in Human Leukemic Cells by a Novel Protein Bengalin, Isolated from Indian Black Scorpion Venom: Through Mitochondrial Pathway and Inhibition of Heat Shock Proteins. Chem. Biol. Interact. 2010, 183, 293–303. [Google Scholar] [CrossRef]
- Al-Asmari, A.; Khan, A. Investigation of in Vivo Potential of Scorpion Venom against Skin Tumorigenesis in Mice via Targeting Markers Associated with Cancer Development. Drug Des. Dev. Ther. 2016, 10, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Al-khraisat, I.F.; Jaradat, D.M.M.; Ghanim, B.Y.; Abdallah, Q.M.; Arqoub, D.A.; Sabbah, D.; Al-Sanabra, O.M.; Arafat, T.; Qinna, N.A. Mellitin Peptide Quantification in Seasonally Collected Crude Bee Venom and Its Anticancer Effects on Myelogenous K562 Human Leukaemia Cell Line. BMC Complement. Med. Ther. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Doupnik, C.A.; Luer, C.A.; Walsh, C.J.; Restivo, J.; Brick, J.X. Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets. Pharmaceuticals 2024, 17, 488. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Medina, J.; Mendivil-Perez, M.; Rey-Suarez, P.; Jimenez-Del-Rio, M.; Núñez, V.; Velez-Pardo, C. L-Amino Acid Oxidase Isolated from Micrurus Mipartitus Snake Venom (MipLAAO) Specifically Induces Apoptosis in Acute Lymphoblastic Leukemia Cells Mostly via Oxidative Stress-Dependent Signaling Mechanism. Int. J. Biol. Macromol. 2019, 134, 1052–1062. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion Venom Causes Apoptosis by Increasing Reactive Oxygen Species and Cell Cycle Arrest in MDA-MB-231 and HCT-8 Cancer Cell Lines. J. Evid. Based Integr. Med. 2018, 23, 2156587217751796. [Google Scholar] [CrossRef]
- Song, X.; Zhang, G.; Sun, A.; Guo, J.; Tian, Z.; Wang, H.; Liu, Y. Scorpion Venom Component III Inhibits Cell Proliferation by Modulating NF-κB Activation in Human Leukemia Cells. Exp. Ther. Med. 2012, 4, 146–150. [Google Scholar] [CrossRef]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a Scorpion-Venom Peptide, Exhibits Potent Antitumor Effects against Hepatoma HepG2 Cells via Multi-Mechanisms In Vivo and In Vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef]
- Robinson, S.D.; Undheim, E.A.B.; Ueberheide, B.; King, G.F. Venom Peptides as Therapeutics: Advances, Challenges and the Future of Venom-Peptide Discovery. Expert. Rev. Proteom. 2017, 14, 931–939. [Google Scholar] [CrossRef]
- Smallwood, T.B.; Clark, R.J. Advances in Venom Peptide Drug Discovery: Where Are We at and Where Are We Heading? Expert. Opin. Drug Discov. 2021, 16, 1163–1173. [Google Scholar] [CrossRef]
- Daniel, J.T.; Clark, R.J. G-Protein Coupled Receptors Targeted by Analgesic Venom Peptides. Toxins 2017, 9, 372. [Google Scholar] [CrossRef]
- Chan, Y.W.; Tan, C.H.; Heh, C.H.; Tan, K.Y. An Immunoinformatic Approach to Assessing the Immunogenic Capacity of Alpha-Neurotoxins in Elapid Snake Venoms. Front. Pharmacol. 2023, 14, 1143437. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Lewis, R.J. Structure-Function and Therapeutic Potential of Spider Venom-Derived Cysteine Knot Peptides Targeting Sodium Channels. Front. Pharmacol. 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.E.; Richards, C.M.; Robert-Gostlin, V.N.; Bernath, A.K.; Lindhout, I.A.; Klegeris, A. Potential Neurotoxic Activity of Diverse Molecules Released by Astrocytes. Brain Res. Bull. 2022, 189, 80–101. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) Family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Thakur, S.; Yasmin, R.; Malhotra, A.; Lalremsanga, H.T.; Santra, V.; Giri, S.; Doley, R. Isolation and Functional Characterization of Erythrofibrase: An Alfa-Fibrinogenase Enzyme from Trimeresurus Erythrurus Venom of North-East India. Toxins 2024, 16, 201. [Google Scholar] [CrossRef]
- Liu, C.-C.; Hao, D.-J.; Zhang, Q.; An, J.; Zhao, J.-J.; Chen, B.; Zhang, L.-L.; Yang, H. Application of Bee Venom and Its Main Constituent Melittin for Cancer Treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef]
- Tan, C.H. Snake Venomics: Fundamentals, Recent Updates, and a Look to the Next Decade. Toxins 2022, 14, 247. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, W.; Li, X. Bioactive Peptides for Anticancer Therapies. Biomater. Transl. 2023, 4, 5–17. [Google Scholar] [CrossRef]
- Calvete, J.J. Venomics: Integrative Venom Proteomics and Beyond. Biochem. J. 2017, 474, 611–634. [Google Scholar] [CrossRef]
- Khusro, A.; Aarti, C.; Barbabosa-Pliego, A.; Rivas-Cáceres, R.R.; Cipriano-Salazar, M. Venom as Therapeutic Weapon to Combat Dreadful Diseases of 21st Century: A Systematic Review on Cancer, TB, and HIV/AIDS. Microb. Pathog. 2018, 125, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wang, Y.; Yang, X. Amphibian-Derived Wound Healing Peptides: Chemical Molecular Treasure Trove for Skin Wound Treatment. Front. Pharmacol. 2023, 14, 1120228. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Goles, M.; Daza, A.; Cabas-Mora, G.; Sarmiento-Varón, L.; Sepúlveda-Yañez, J.; Anvari-Kazemabad, H.; Davari, M.D.; Uribe-Paredes, R.; Olivera-Nappa, Á.; Navarrete, M.A.; et al. Peptide-Based Drug Discovery through Artificial Intelligence: Towards an Autonomous Design of Therapeutic Peptides. Brief. Bioinform. 2024, 25, bbae275. [Google Scholar] [CrossRef]
- Romano, J.D.; Li, H.; Napolitano, T.; Realubit, R.; Karan, C.; Holford, M.; Tatonetti, N.P. Discovering Venom-Derived Drug Candidates Using Differential Gene Expression. Toxins 2023, 15, 451. [Google Scholar] [CrossRef]
- Vidya, V.; Achar, R.R.; Himathi, M.U.; Akshita, N.; Somayaji, T.Y.; Kameshwar, V.H.; Byrappa, K.; Ramadas, D. Venom Peptides—A Comprehensive Translational Perspective in Pain Management. Curr. Res. Toxicol. 2021, 2, 329–340. [Google Scholar] [CrossRef]


| Species | Lineage | Action Factor | Effect | Activity |
|---|---|---|---|---|
| Apis mellifera | CCRF-CEM (ALL), K-562 (CML), U937, HL-60 | Melittin | Induces mitochondrial apoptosis (caspase-3/7), downregulates ERK/Akt and NF-κB pathways, and modulates Bcl-2, c-MYC, CDK4, among others | Cytotoxic, pro-apoptotic, and intracellular signaling modulator [155,156] |
| Bothrops erytromelas, Bothrops jararaca, Bothrops alternatus | Leukemia–K562 | X | Reduction of cell viability and proliferation | Morphological alterations, plasma membrane rupture, presence of pyknotic cells, increased membrane permeability, loss of mitochondrial function, higher total DNA damage index, reduction in transcript levels of CCN1, CCNH, CDK2, CDK1, and BCR-ABL1, increased expression of cell cycle inhibitors CDKN1A and WEE1, reduced gene expression of CCNB1, CCNH, CDK1, and BCR-ABL1 [157] |
| Bothrops jararacussu | Acute promyelocytic leukemia–HL-60 | BthTX-I | Reduction of cell viability | Induction of necrosis and apoptosis, 75% to 90% cytotoxicity [158] |
| Bothrops mattogrossensis | Acute T-cell leukemia–JURKAT | BmatTX-I e BmatTX-II | Apoptosis | Changes in the cell membrane, catalytic activity-independent cytotoxic activity [159]. |
| Bothrops moojeni | Chronic myeloid leukemia–K562-S and K562-R Bcr-Abl + | MjTX-I | Reduction in cell viability by up to 65% | Increase from 45.5% to 62% in hypodiploid nuclei, high levels of cell death, reduced expression levels of pro-caspase 3, and increased expression levels of caspase 9 in K562-S lineage, reduced expression of pro-caspase 3, 8, and 9, and higher levels of cleaved PARP in K562-R lineage, reduced level and expression of the anti-apoptotic gene BCL-2, BAD, BAX, CLL-XL, and c-FLIP in K562-S, and increased expression levels of the pro-apoptotic gene BAD in K562-R [160] |
| Bothrops brazili | Acute T-cell leukemia–JURKAT | MTX-I e MTX-II | Likely induction of apoptosis | Independent of catalytic activity [160] |
| Bothrops moojeni | Acute T-cell leukemia–JURKAT | MjTX-II | X | Independent of catalytic activity, induction of apoptosis [161] |
| Crotalus oreganus helleri | Chronic myeloid leukemia–K-562 | CLP | Reduction in cell viability | Induction of apoptosis and necrosis resulting from increased lysosomal membrane permeability, mitochondrial swelling [162] |
| Bothrops pauloensis | Acute T-cell leukemia–JURKAT TIB-152™ | Bp-LAAO | Cell death | Dose-dependent cytotoxicity, inhibition of tumor growth [163] |
| Crotalus atrox | Promyelocytic leukemia–HL-60 | Apoxin I | X | Morphological cellular changes, induction of chromatin condensation and segregation, induction of apoptosis [164] |
| Crotalus atrox | T-cell lymphoma–S-49 | X | X | X [164] |
| Crotalus durissus terrificus | Murine erythroleukemia and chronic myeloid leukemia–K-562 | CTX | Reduction in cell viability | Cell death and lysis (40%), collapse of mitochondrial membrane potential, autophagy, apoptosis, vacuolization and mitochondrial swelling, nuclear condensation, pyknosis, organelle loss, significant reductions in cytochrome c levels in the cytosol, cell membrane rupture [165] |
| Bothrops jararaca | Promyelocytic leukemia–HL-60 | BJ-PLA 2 -I | Reduction in cell viability | Low cytotoxicity (70% to 80%) [166] |
| Bothrops jararacussu | Acute T-cell leukemia–JURKAT | BthA-I-PLA 2 | Cell death | Induction of apoptosis [166]. |
| Bothrops atrox | HL-60 (APL), Jurkat (T-ALL) | BatroxLAAO | H2O2 induces cytotoxicity through oxidative stress, activates apoptosis via caspases-3 and -9, and causes cell cycle arrest at the G0/G1 phase, inhibiting cell proliferation | Its main activity is pro-oxidant, acting as a generator of reactive oxygen species (ROS), which trigger these cellular responses [167] |
| Calloselasma rhodostoma | Jurkat (T-ALL), Bcr-Abl+ CML cells | CR-LAAO | H2O2 induces the transition from necrosis to apoptosis and modulates apoptomiRs and apoptosis-regulating proteins, such as Bcl-2, in chronic myeloid leukemia (CML) cells | It acts as a pro-oxidant, inducing oxidative stress and modulating apoptotic pathways, including microRNAs and apoptosis-regulating proteins [168,169] |
| Androctonus aeneas (Scorpion–North American) | JURKAT | Bmk AGAP | X | Blocks the action of lymphoma and glioma CCL-86 lineage and T-lymphocytes derived from adult T-cell leukemia/lymphoma [170] |
| H. bengalensis Kochveneno | Leukemic cells U937 and K562. | x | Inhibition of cell proliferation in U937 and K562 occurred through apoptosis, evidenced by damaged nuclei and cell cycle arrest in the sub G1 phase | Increased DNA fragmentation and also reduced telomerase activity [171] |
| Leiurus quinquestriatus | B-cell lymphoma-2 | x | x | Immunohistochemical results showed a decrease in the expression of molecular markers such as Ki-67, nuclear factor kappa-B, cyclooxygenase-2, B-cell lymphoma-2, and vascular endothelial growth factor in animals treated with venom [172] |
| Jordanian honeybee (JCBV) | Leukemic K562 | Melittin | Cell death | Late apoptotic cell death with moderate cell cycle arrest [173] |
| Bufo melanostictus | Leukemic K562, U937, ML1 e HL60 | Bufalina | Cell differentiation | Exhibited a potent differentiation-inducing activity [159] |
| Bufo melanostictus | Leukemic THP-1 and MOLT-3 | Bufalina | Cell death | Induced apoptosis [159] |
| Aetobatus narinari | Leukemic Jurkat E6-1 | SRV | Significant growth inhibitory effects in cells | Induced apoptosis and necrosis [174] |
| Micrurus mipartitus | Jurkat (T-ALL) | MipLAAO | Induces apoptosis via caspase-3, p53, and PUMA | Pro-oxidant and apoptosis inducer [175] |
| Heterometrus bengalensis | U937, K562 (CML) | Bengalin | Induces mitochondrial apoptosis with caspase-3/9 activation and PARP cleavage | Pro-apoptotic/mitochondrial apoptosis inducer [176] |
| Buthus martensii Karsch | THP-1 (Monocytic Leukemia), Jurkat (T Lymphoma) | SVCIII | Causes G1 cell cycle arrest (by downregulating cyclin D1) and inhibits the NF-κB pathway (by reducing IκBα degradation and p65 nuclear translocation) | Antiproliferative and NF-κB signaling inhibitor [177] |
| Maurus palmatus | KG1-a (AML), CCRF-CEM (ALL) | Smp24 | Induces cytotoxicity through membrane disruption and mitochondrial dysfunction, leading to apoptosis, cell cycle arrest, and autophagy | Cytotoxic, pro-apoptotic, and cell stress inducer [178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malachias-Pires, G.M.; Filardi, E.T.M.; Romanazzi, M.; Lopes-de-Oliveira, J.; Santos, I.C.d.; Melo-dos-Santos, G.; Rossi, A.B.; Procópio Machado, M.; Silva, T.A.d.; Pucca, M.B. Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma. Cancers 2025, 17, 2331. https://doi.org/10.3390/cancers17142331
Malachias-Pires GM, Filardi ETM, Romanazzi M, Lopes-de-Oliveira J, Santos ICd, Melo-dos-Santos G, Rossi AB, Procópio Machado M, Silva TAd, Pucca MB. Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma. Cancers. 2025; 17(14):2331. https://doi.org/10.3390/cancers17142331
Chicago/Turabian StyleMalachias-Pires, Geovanna M., Eloise T. M. Filardi, Marcela Romanazzi, Julia Lopes-de-Oliveira, Isabela C. dos Santos, Guilherme Melo-dos-Santos, Ana Beatriz Rossi, Michele Procópio Machado, Thiago A. da Silva, and Manuela B. Pucca. 2025. "Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma" Cancers 17, no. 14: 2331. https://doi.org/10.3390/cancers17142331
APA StyleMalachias-Pires, G. M., Filardi, E. T. M., Romanazzi, M., Lopes-de-Oliveira, J., Santos, I. C. d., Melo-dos-Santos, G., Rossi, A. B., Procópio Machado, M., Silva, T. A. d., & Pucca, M. B. (2025). Animal Venoms as Potential Antitumor Agents Against Leukemia and Lymphoma. Cancers, 17(14), 2331. https://doi.org/10.3390/cancers17142331









