The Added Benefits of Performing Liver Tumor Ablation in the Angiography Suite: A Pictorial Essay of Combining C-Arm CT Guidance with Hepatic Arteriography for Liver Tumor Ablation
Simple Summary
Abstract
1. Introduction
2. Technical Aspects
2.1. HepACAGA Procedure
2.2. Thermal Segmentectomy
3. Key Domains of Additional Benefits of HepACAGA
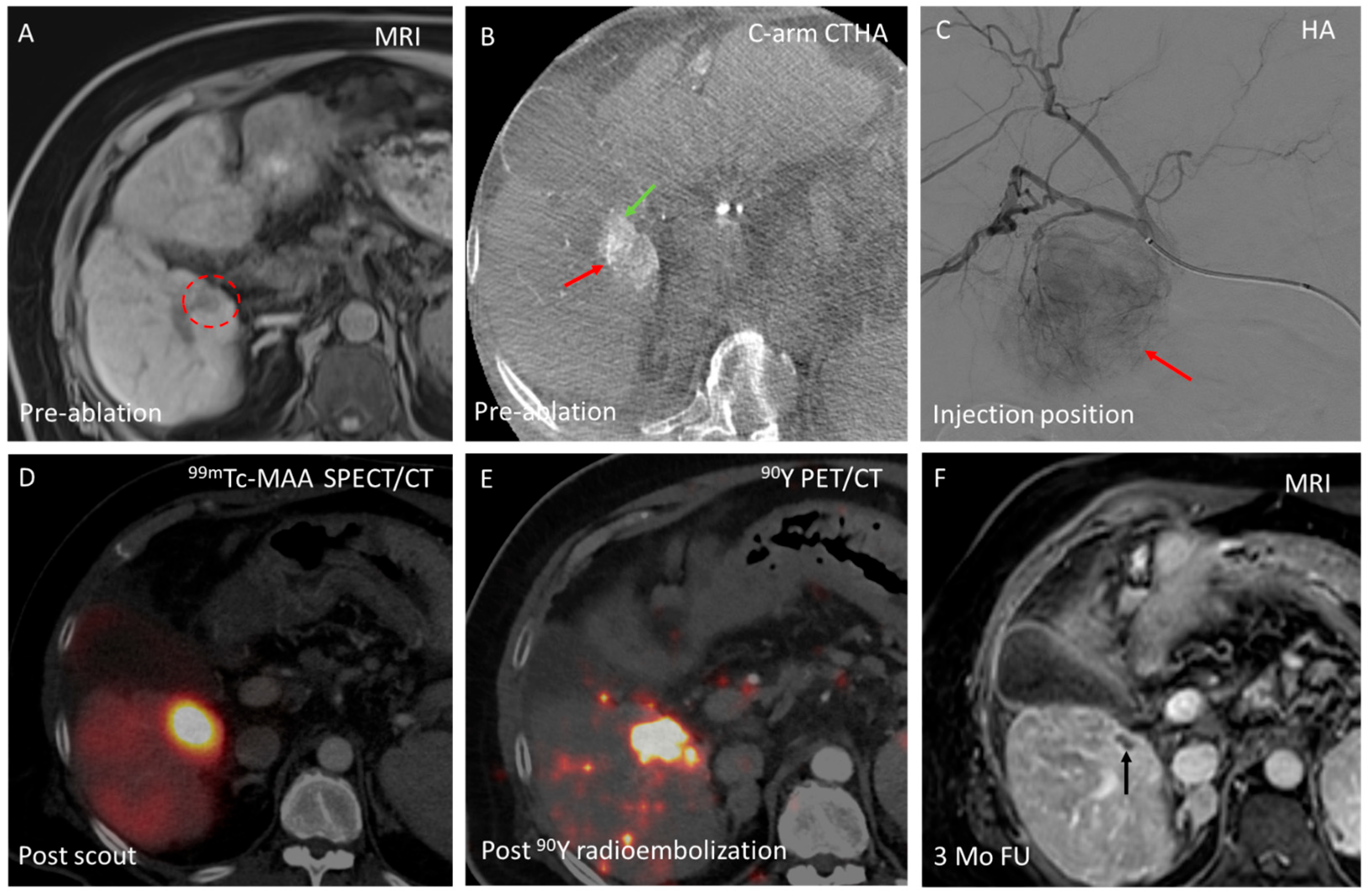
3.1. Domain A—Conversion from Ablation to Intra-Arterial Treatment
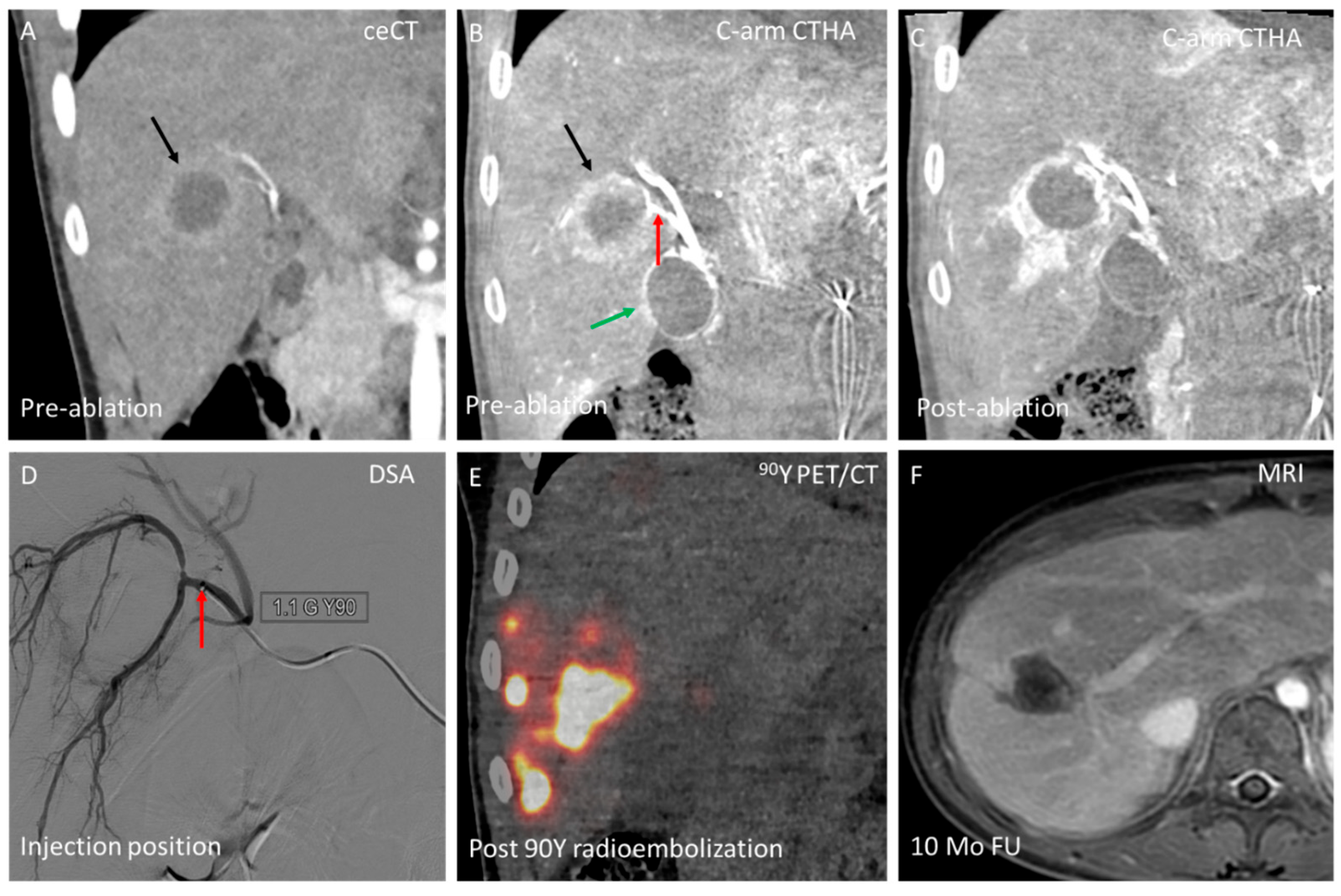
3.2. Domain B—Single-Session Combination of Ablation with Endovascular Treatment
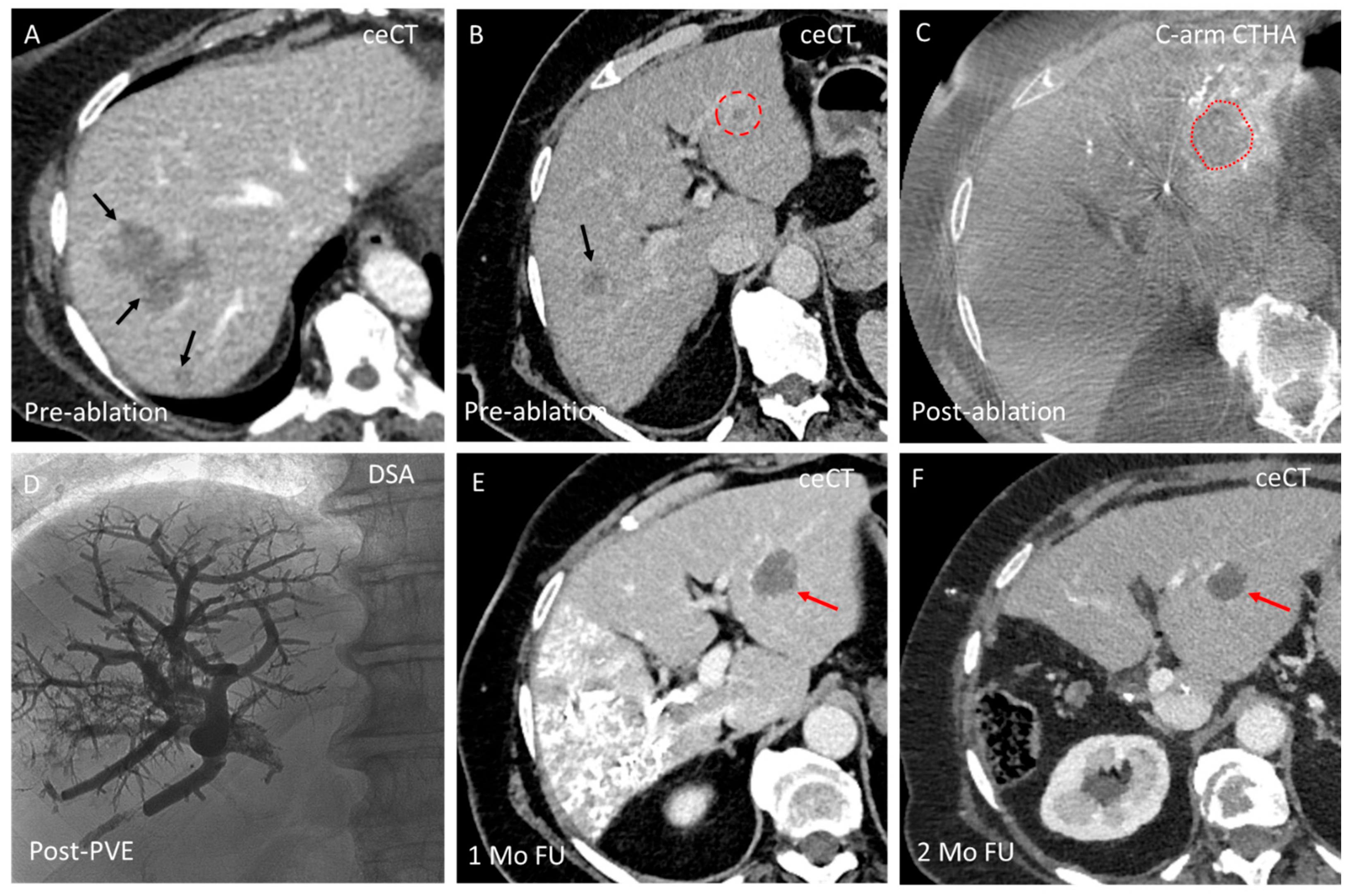
3.3. Domain C—Enhancing Ablation Effect
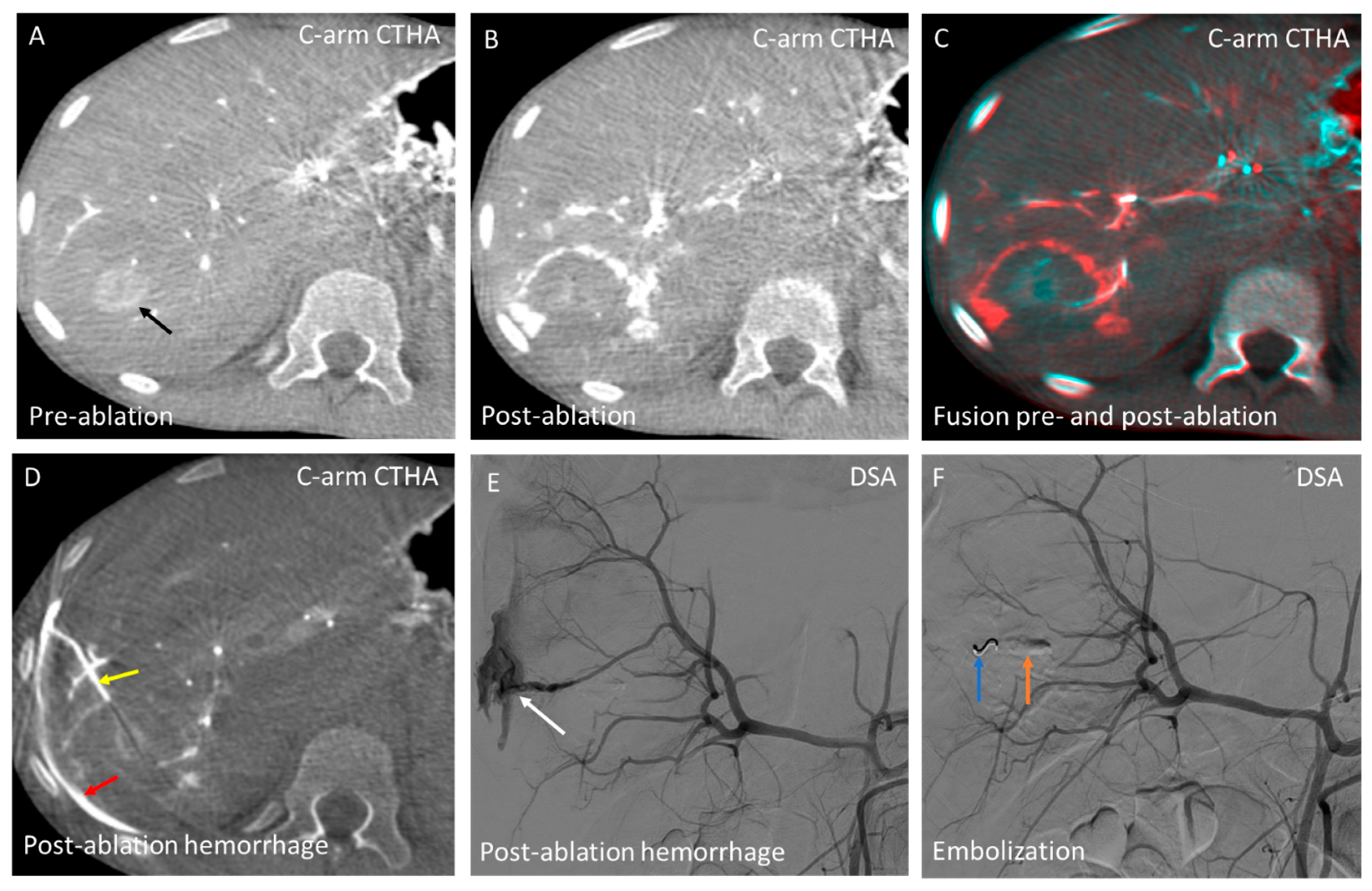
3.4. Domain D—Hemorrhage Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
References
- Campbell, W.A., IV; Makary, M.S. Advances in Image-Guided Ablation Therapies for Solid Tumors. Cancers 2024, 16, 2560. [Google Scholar] [CrossRef] [PubMed]
- Zensen, S.; Bücker, A.; Meetschen, M.; Haubold, J.; Opitz, M.; Theysohn, J.M.; Schramm, S.; Jochheim, L.; Kasper, S.; Forsting, M.; et al. Current use of percutaneous image-guided tumor ablation for the therapy of liver tumors: Lessons learned from the registry of the German Society for Interventional Radiology and Minimally Invasive Therapy (DeGIR) 2018–2022. Eur. Radiol. 2023, 34, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; van Waesberghe, J.H.T.M.; Comans, E.F.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Transcatheter CT arterial portography and CT hepatic arteriography for liver tumor visualization during percutaneous ablation. J. Vasc. Interv. Radiol. 2014, 25, 1101–1111.e4. [Google Scholar] [CrossRef] [PubMed]
- van der Lei, S.; Opperman, J.; Dijkstra, M.; Kors, N.; Boon, R.; van den Bemd, B.A.T.; Timmer, F.E.F.; Nota, I.M.G.C.; van den Bergh, J.E.; de Vries, J.J.J.; et al. The Added Diagnostic Value of Transcatheter CT Hepatic Arteriography for Intraprocedural Detection of Previously Unknown Colorectal Liver Metastases During Percutaneous Ablation and Impact on the Definitive Treatment Plan. Cardiovasc. Intervent. Radiol. 2023, 46, 1257–1266. [Google Scholar] [CrossRef]
- van Tilborg, A.A.J.M.; Scheffer, H.J.; van der Meijs, B.B.; van Werkum, M.H.; Melenhorst, M.C.A.M.; van den Tol, P.M.; Meijerink, M.R. Transcatheter CT hepatic arteriography-guided percutaneous ablation to treat ablation site recurrences of colorectal liver metastases: The incomplete ring sign. J. Vasc. Interv. Radiol. 2015, 26, 583–587.e1. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Scheffer, H.J.; Vroomen, L.G.P.H.; van Tilborg, A.A.J.M.; de Vries, J.J.J.; Berger, F.H.; van den Tol, P.M.P.; Meijerink, M.R. Percutaneous liver tumour ablation: Image guidance, endpoint assessment, and quality control. Can. Assoc. Radiol. J. 2018, 69, 51–62. [Google Scholar] [CrossRef]
- Smits, M.L.J.; Bruijnen, R.C.G.; Tetteroo, P.; Vonken, E.-J.P.A.; Meijerink, M.R.; Hagendoorn, J.; de Bruijne, J.; Prevoo, W. Hepatic arteriography and C-arm CT-guided ablation (HepACAGA) to improve tumor visualization, navigation and margin confirmation in percutaneous liver tumor ablation. Cardiovasc. Intervent. Radiol. 2023, 46, 1365–1374. [Google Scholar] [CrossRef]
- Wijnen, N.; Bruijnen, R.C.G.; Vonken, E.-J.P.A.; de Jong, H.W.A.M.; de Bruijne, J.; Bol, G.M.; Hagendoorn, J.; Intven, M.P.W.; Smits, M.L.J. Conventional versus hepatic arteriography and C-arm CT-guided ablation of liver tumors (HepACAGA): A comparative analysis. Cancers 2024, 16, 1925. [Google Scholar] [CrossRef]
- Wijnen, N.; Bruijnen, R.C.G.; Thelissen, A.A.B.; de Jong, H.W.A.M.; van Leeuwaarde, R.S.; Hagendoorn, J.; Bol, G.M.; Smits, M.L.J. Ablation of small liver metastases presenting as foci of diffusion restriction on MRI-results from the prospective Minimally Invasive Thermal Ablation (MITA) study. Cancers 2024, 16, 2409. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Crocetti, L.; Rocco, B.; Bozzi, E.; Gasparrini, F.; Tanzilli, A.; Catalano, C.; Iezzi, R. Percutaneous thermal segmentectomy: Proof of concept. Cardiovasc. Intervent. Radiol. 2022, 45, 665–676. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine 2015, 94, e580. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-Y.; Li, G.-L.; Chen, J.; Chen, Z.-W.; Chen, Y.-P.; Lin, S.-Z. Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J. Cancer Res. Ther. 2016, 12, C153–C158. [Google Scholar] [CrossRef] [PubMed]
- Adwan, H.; Adwan, M.; Vogl, T.J. Combination therapy of bland transarterial embolization and microwave ablation for hepatocellular carcinoma within the Milan criteria leads to significantly higher overall survival. Cancers 2023, 15, 5076. [Google Scholar] [CrossRef]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular carcinoma tumour volume doubling time: A systematic review and meta-analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef]
- Lucatelli, P.; Argirò, R.; Bascetta, S.; Saba, L.; Catalano, C.; Bezzi, M.; Levi Sandri, G.B. Single injection dual phase CBCT technique ameliorates results of trans-arterial chemoembolization for hepatocellular cancer. Transl. Gastroenterol. Hepatol. 2017, 2, 83. [Google Scholar] [CrossRef]
- Lucatelli, P.; De Rubeis, G.; Ginnani Corradini, L.; Basilico, F.; Di Martino, M.; Lai, Q.; Ginanni Corradini, S.; Cannavale, A.; Nardis, P.G.; Corona, M.; et al. Intra-procedural dual phase cone beam computed tomography has a better diagnostic accuracy over pre-procedural MRI and MDCT in detection and characterization of HCC in cirrhotic patients undergoing TACE procedure. Eur. J. Radiol. 2020, 124, 108806. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, J.; Wang, B.; Zhu, X.; Shi, X.; Ding, Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: A population-based study. Cancer Manag. Res. 2018, 10, 4401–4410. [Google Scholar] [CrossRef]
- Sheta, E.; El-Kalla, F.; El-Gharib, M.; Kobtan, A.; Elhendawy, M.; Abd-Elsalam, S.; Mansour, L.; Amer, I. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: A randomized-controlled study. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1198–1203. [Google Scholar] [CrossRef]
- Liu, C.; Li, T.; He, J.-t.; Shao, H. TACE combined with microwave ablation therapy vs. TACE alone for treatment of early- and intermediate-stage hepatocellular carcinomas larger than 5 cm: A meta-analysis. Diagn. Interv. Radiol. 2020, 26, 575–583. [Google Scholar] [CrossRef]
- Fei, J.; Qi, L.-W.; Liu, Y.; Shu, M.; Mo, W.-Q. Comparing transarterial chemoembolization alone to combined transarterial chemoembolization and radiofrequency ablation in primary hepatocellular carcinoma treatment. World J. Gastrointest. Oncol. 2025, 17, 102038. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, P.; Rietbergen, D.D.D.; van Erkel, A.R.; Coenraad, M.J.; Arntz, M.J.; Bennink, R.J.; Braat, A.E.; Crobach, S.; van Delden, O.M.; Dibbets-Schneider, P.; et al. Adjuvant holmium-166 radioembolization after radiofrequency ablation in early-stage hepatocellular carcinoma patients: A dose-finding study (HORA EST HCC trial). Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2085–2097. [Google Scholar] [CrossRef]
- Pamecha, V.; Levene, A.; Grillo, F.; Woodward, N.; Dhillon, A.; Davidson, B.R. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br. J. Cancer 2009, 100, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Garlipp, B.; de Baere, T.; Damm, R.; Irmscher, R.; van Buskirk, M.; Stübs, P.; Deschamps, F.; Meyer, F.; Seidensticker, R.; Mohnike, K.; et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology 2014, 59, 1864–1873. [Google Scholar] [CrossRef]
- Gillams, A.; Goldberg, N.; Ahmed, M.; Bale, R.; Breen, D.; Callstrom, M.; Chen, M.H.; Choi, B.I.; de Baere, T.; Dupuy, D.; et al. Thermal ablation of colorectal liver metastases: A position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontières meeting 2013. Eur. Radiol. 2015, 25, 3438–3454. [Google Scholar] [CrossRef]
- Chlorogiannis, D.-D.; Sotirchos, V.S.; Georgiades, C.; Filippiadis, D.; Arellano, R.S.; Gonen, M.; Makris, G.C.; Garg, T.; Sofocleous, C.T. The importance of optimal thermal ablation margins in colorectal liver metastases: A systematic review and meta-analysis of 21 studies. Cancers 2023, 15, 5806. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Rompianesi, G.; Mora-Guzmán, I.; Martín-Pérez, E.; Montalti, R.; Troisi, R.I. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur. J. Surg. Oncol. 2020, 46, 772–781. [Google Scholar] [CrossRef]
- Chinn, S.B.; Lee Jr, F.T.; Kennedy, G.D.; Chinn, C.; Johnson, C.D.; Winter, T.C., 3rd; Warner, T.F.; Mahvi, D.M. Effect of vascular occlusion on radiofrequency ablation of the liver: Results in a porcine model. AJR Am. J. Roentgenol. 2001, 176, 789–795. [Google Scholar] [CrossRef]
- Howenstein, M.J.; Sato, K.T. Complications of radiofrequency ablation of hepatic, pulmonary, and renal neoplasms. Semin. Interv. Radiol. 2010, 27, 285–295. [Google Scholar] [CrossRef]
- Lahat, E.; Eshkenazy, R.; Zendel, A.; Bar Zakai, B.B.; Maor, M.; Dreznik, Y.; Ariche, A. Complications after percutaneous ablation of liver tumors: A systematic review. Hepatobiliary Surg. Nutr. 2014, 3, 317–323. [Google Scholar] [CrossRef]
- Kim, K.R.; Thomas, S. Complications of image-guided thermal ablation of liver and kidney neoplasms. Semin. Interv. Radiol. 2014, 31, 138–148. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Sato, M.; Tateishi, R.; Yoshida, H.; Koike, K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: A systematic review. Eur. Radiol. 2011, 21, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Tarantino, L.; de Stefano, G.; Perrotta, A.; Aloisio, V.; del Viscovo, L.; Alaia, A.; Lettieri, G. Ultrasound-guided percutaneous ethanol injection under general anesthesia for the treatment of hepatocellular carcinoma on cirrhosis: Long-term results in 268 patients. Eur. J. Ultrasound 2000, 12, 145–154. [Google Scholar] [CrossRef]
- Livraghi, T.; Solbiati, L.; Meloni, M.F.; Gazelle, G.S.; Halpern, E.F.; Goldberg, S.N. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003, 226, 441–451. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijnen, N.; Ramdhani, K.; Bruijnen, R.C.G.; de Jong, H.W.A.M.; Lucatelli, P.; Smits, M.L.J. The Added Benefits of Performing Liver Tumor Ablation in the Angiography Suite: A Pictorial Essay of Combining C-Arm CT Guidance with Hepatic Arteriography for Liver Tumor Ablation. Cancers 2025, 17, 2330. https://doi.org/10.3390/cancers17142330
Wijnen N, Ramdhani K, Bruijnen RCG, de Jong HWAM, Lucatelli P, Smits MLJ. The Added Benefits of Performing Liver Tumor Ablation in the Angiography Suite: A Pictorial Essay of Combining C-Arm CT Guidance with Hepatic Arteriography for Liver Tumor Ablation. Cancers. 2025; 17(14):2330. https://doi.org/10.3390/cancers17142330
Chicago/Turabian StyleWijnen, Niek, Khalil Ramdhani, Rutger C. G. Bruijnen, Hugo W. A. M. de Jong, Pierleone Lucatelli, and Maarten L. J. Smits. 2025. "The Added Benefits of Performing Liver Tumor Ablation in the Angiography Suite: A Pictorial Essay of Combining C-Arm CT Guidance with Hepatic Arteriography for Liver Tumor Ablation" Cancers 17, no. 14: 2330. https://doi.org/10.3390/cancers17142330
APA StyleWijnen, N., Ramdhani, K., Bruijnen, R. C. G., de Jong, H. W. A. M., Lucatelli, P., & Smits, M. L. J. (2025). The Added Benefits of Performing Liver Tumor Ablation in the Angiography Suite: A Pictorial Essay of Combining C-Arm CT Guidance with Hepatic Arteriography for Liver Tumor Ablation. Cancers, 17(14), 2330. https://doi.org/10.3390/cancers17142330





