Early Predictive Markers and Histopathological Response to Neoadjuvant Endocrine Therapy in Postmenopausal Patients with HR+/HER2− Early Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Treatment Regimen
2.3. Pathological and Biomarker Assessment
2.4. Statistical Analysis
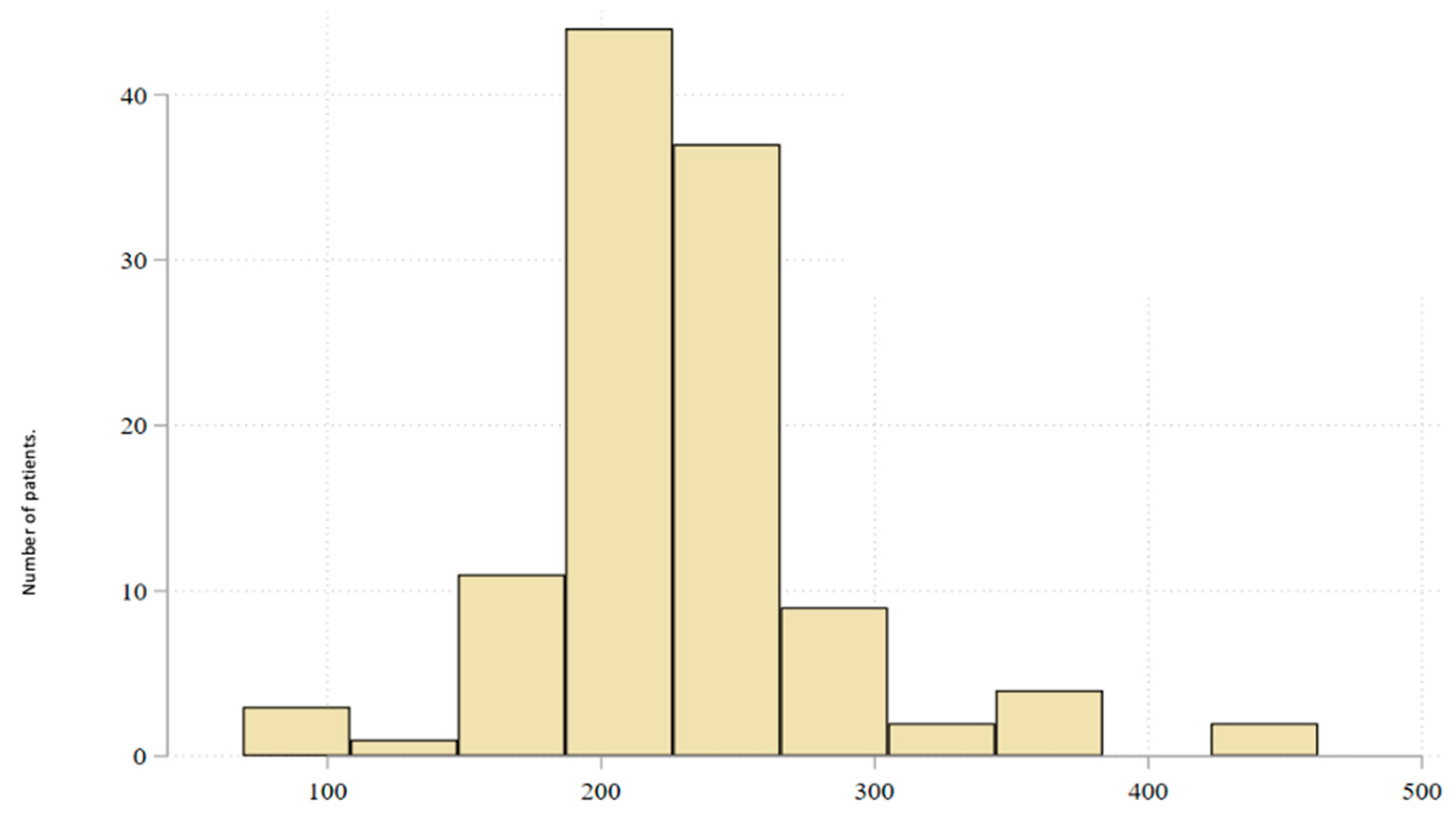
3. Results
3.1. Baseline Characteristics of the Study Population
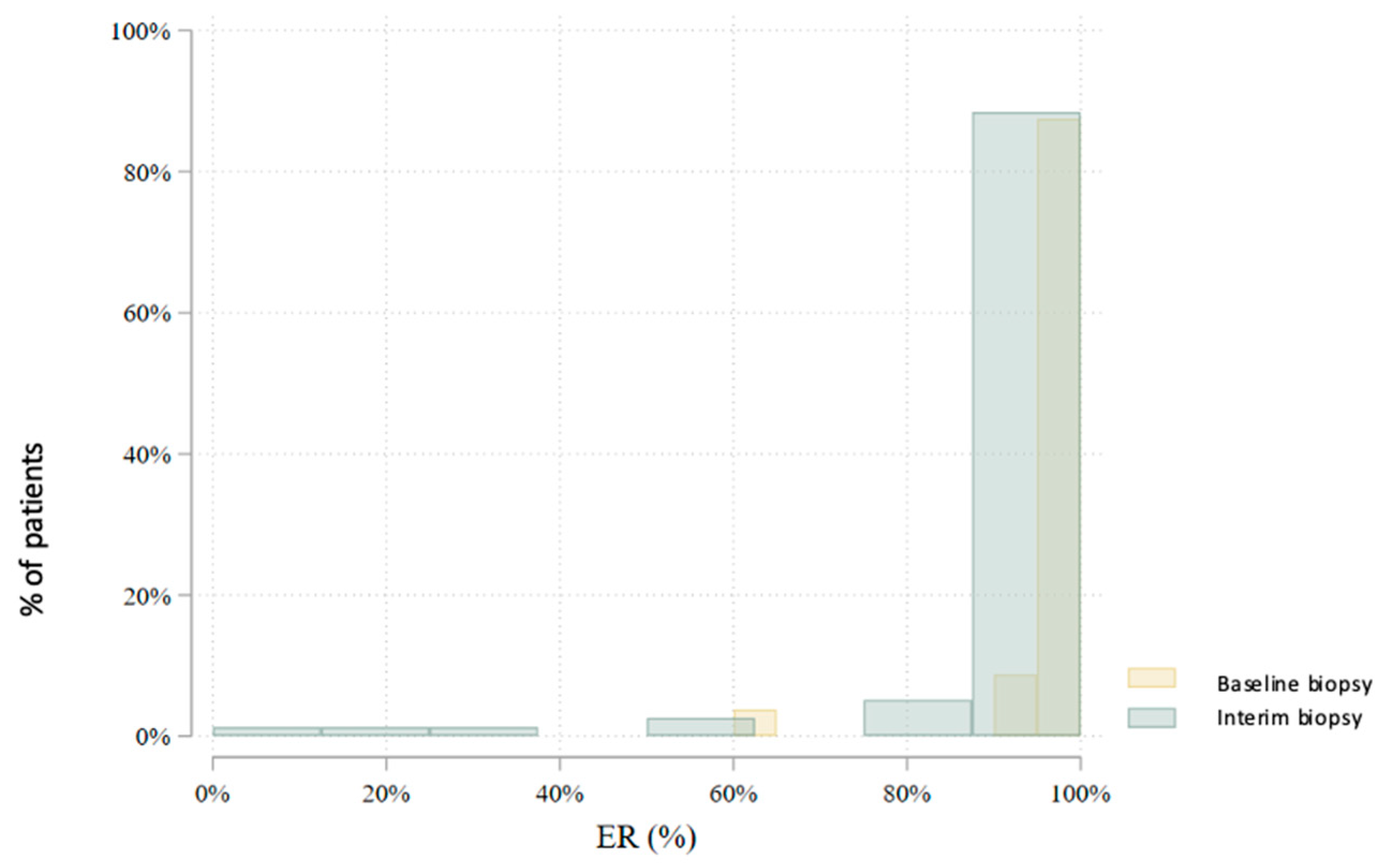
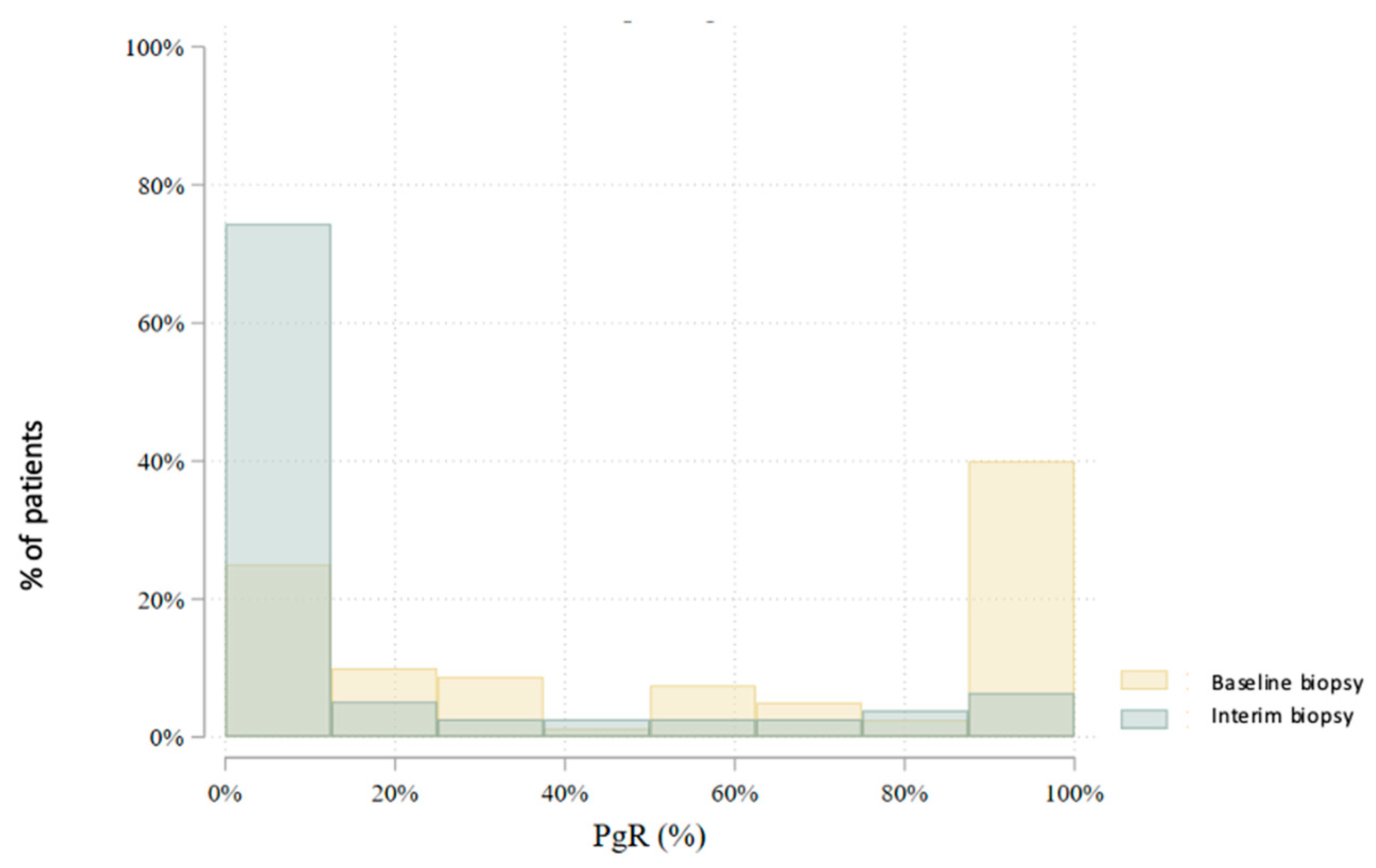
3.2. Interim Biopsy During Neoadjuvant Endocrine Therapy
3.3. Postoperative Results
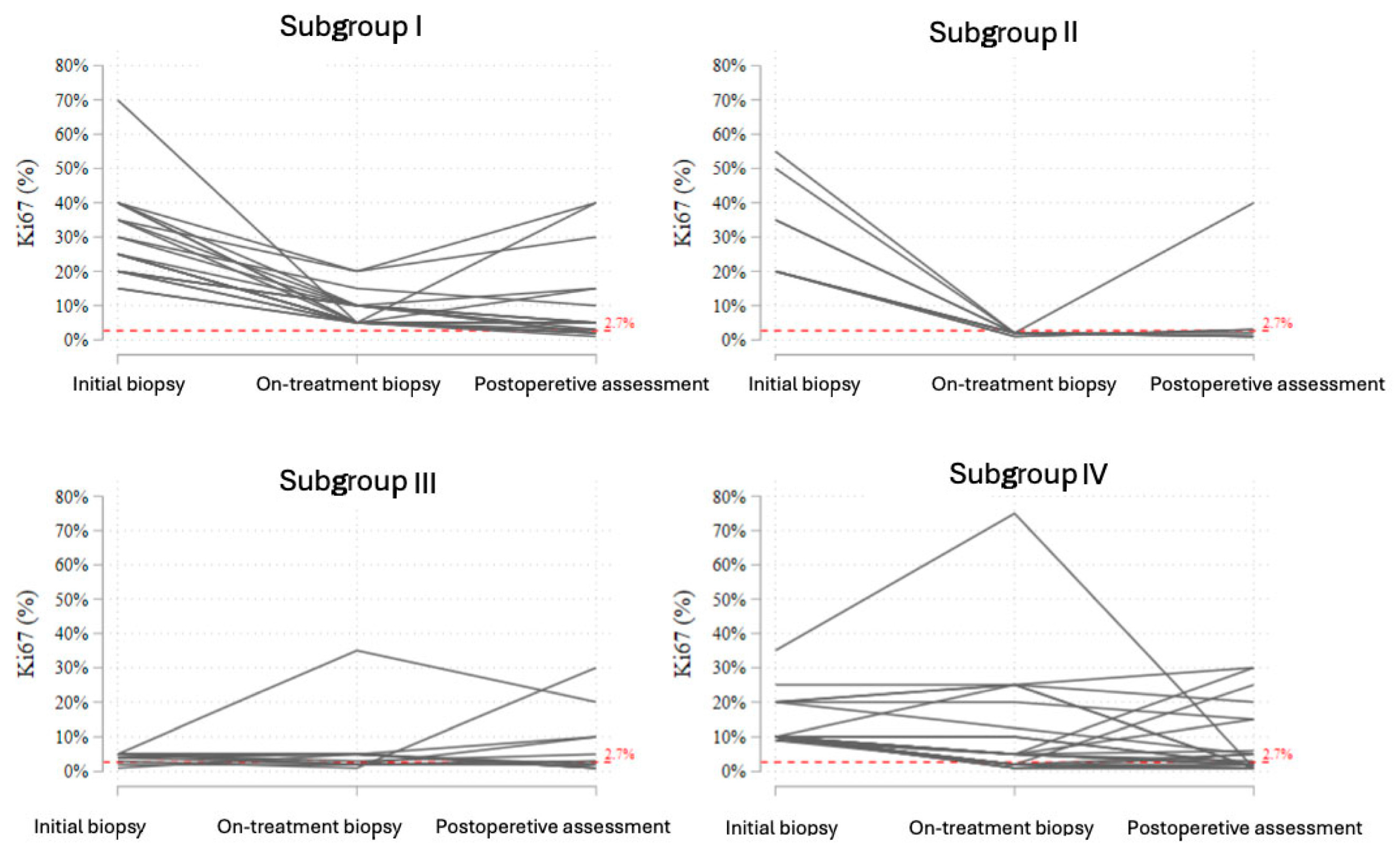
3.4. Analysis of Ki-67 Trajectories in Response to NET
- •
- Subgroup I included patients with initially elevated Ki-67 who showed a reduction during treatment, but the on-treatment value remained ≥2.7%. In this subgroup, most patients experienced further stabilization or a modest decline postoperatively, although complete suppression of proliferation (<2.7%) was not achieved.
- •
- Subgroup II consisted of patients who showed a profound early decline in Ki-67 to <2.7% at the on-treatment biopsy. In nearly all cases, this suppression persisted in the postoperative samples, reflecting a strong endocrine response and sustained biological effect.
- •
- Subgroup III comprised patients with initially low Ki-67 (<10%), who demonstrated stable or slightly increased values during treatment. The majority remained within the low-proliferative range throughout therapy.
- •
- Subgroup IV represented patients with elevated baseline Ki-67 (≥10%) who did not achieve significant suppression during treatment. Most of these tumors maintained a Ki-67 above 2.7%, although isolated cases exhibited paradoxical fluctuations or late declines postoperatively.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up †. Ann. Oncol. 2019, 30, 1194–1220, Erratum in: Ann. Oncol. 2021, 32, 284. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Höller, A.; Nguyen-Sträuli, B.D.; Frauchiger-Heuer, H.; Ring, A. Diagnostic and prognostic biomarkers of luminal breast cancer: Where are we now? Breast Cancer (Dove Med. Press) 2023, 15, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Gupta, A.; Reynolds, K.L.; Gadd, M.A.; Ellisen, L.W.; Isakoff, S.J.; Moy, B.; Bardia, A. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Panel Members. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Lin, L.; Snider, J.; Prat, A.; Parker, J.S.; Luo, J.; DeSchryver, K.; Allred, D.C.; et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 2011, 29, 2342–2349. [Google Scholar] [CrossRef]
- Dowsett, M.; Smith, I.E.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Griffith, C.; Boeddinghaus, I.; Salter, J.; Detre, S.; Hills, M.; et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin. Cancer Res. 2005, 11 Pt 2, 951s–958s. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, X.; Fan, C.; Mao, X. The role of Ki67 in evaluating neoadjuvant endocrine therapy of hormone receptor-positive breast cancer. Front. Endocrinol. 2021, 12, 687244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef]
- Morgan, J.L.; Reed, M.W.; Wyld, L. Primary endocrine therapy as a treatment for older women with operable breast cancer: A comparison of randomised controlled trial and cohort study findings. Eur. J. Surg. Oncol. 2014, 40, 676–684. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 102–132. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Barańska, K.; Miklewska, M.; Didkowska, J.A. Cancer incidence and mortality in Poland in 2020. Nowotw. J. Oncol. 2023, 73, 129–145. [Google Scholar] [CrossRef]
- Smith, I.E.; Dowsett, M.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Blohmer, J.U.; Ashley, S.E.; Francis, S.; Boeddinghaus, I.; Walsh, G. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J. Clin. Oncol. 2005, 23, 5108–5116. [Google Scholar] [CrossRef]
- Cataliotti, L.; Buzdar, A.U.; Noguchi, S.; Bines, J.; Takatsuka, Y.; Petrakova, K.; Dube, P.; de Oliveira, C.T. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The pre-operative “arimidex” compared to tamoxifen (PROACT) trial. Cancer 2006, 106, 2095–2103. [Google Scholar] [CrossRef]
- Dixon, J.; Love, C.; Bellamy, C.; Cameron, D.; Leonard, R.; Smith, H.; Miller, W. Letrozole as primary medical therapy for locally advanced and large operable breast cancer. Breast Cancer Res. Treat. 2001, 66, 191–199. [Google Scholar] [CrossRef]
- Martí, C.; Sánchez-Méndez, J.I. The present and future of neoadjuvant endocrine therapy for breast cancer treatment. Cancers 2021, 13, 2538. [Google Scholar] [CrossRef]
- Krainick-Strobel, U.E.; Lichtenegger, W.; Wallwiener, D.; Tulusan, A.H.; Jänicke, F.; Bastert, G.; Kiesel, L.; Wackwitz, B.; Paepke, S. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: A phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 2008, 8, 62. [Google Scholar] [CrossRef]
- Rusz, O.; Vörös, A.; Varga, Z.; Kelemen, G.; Uhercsák, G.; Nikolényi, A.; Ormándi, K.; Simonka, Z.; Kahán, Z. One-year neoadjuvant endocrine therapy in breast cancer. Pathol. Oncol. Res. 2015, 21, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A., Jr.; Budd, G.T.; Carey, L.A.; Harris, L.A.; Esserman, L.J.; Fleming, G.F.; Marcom, P.K.; Leight Jr, G.S.; Giuntoli, T.; Commean, P.; et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: Results from a multicenter phase II trial. J. Am. Coll. Surg. 2009, 208, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Thomssen, C.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2023: Optimization of treatment for patients with primary breast cancer—a brief summary of the consensus discussion. Breast Care 2023, 18, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef]
- Martí, C.; Yébenes, L.; Oliver, J.M.; Moreno, E.; Frías, L.; Berjón, A.; Loayza, A.; Meléndez, M.; Roca, M.J.; Córdoba, V.; et al. The clinical impact of neoadjuvant endocrine treatment on luminal-like breast cancers and its prognostic significance: Results from a single-institution prospective cohort study. Curr. Oncol. 2022, 29, 2199–2210. [Google Scholar] [CrossRef]
- Bo, J.; Yu, B.; Bi, R.; Xu, X.; Cheng, Y.; Tu, X.; Bai, Q.; Yang, W.; Shui, R. Conversion of ER and HER2 status after neoadjuvant therapy in Chinese breast cancer patients. Clin. Breast Cancer 2023, 23, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Montagna, G.; Sevilimedu, V.; Fornier, M.; Jhaveri, K.; Morrow, M.; Pilewskie, M.L. How effective is neoadjuvant endocrine therapy (NET) in downstaging the axilla and achieving breast-conserving surgery? Ann. Surg. Oncol. 2020, 27, 4702–4710. [Google Scholar] [CrossRef]
- Stafford, A.; Williams, A.; Edmiston, K.; Cocilovo, C.; Cohen, R.; Bruce, S.; Yoon-Flannery, K.; De La Cruz, L. Axillary response in patients undergoing neoadjuvant endocrine treatment for node-positive breast cancer: Systematic literature review and NCDB analysis. Ann. Surg. Oncol. 2020, 27, 4669–4677. [Google Scholar] [CrossRef]
- Dubsky, P.C.; Singer, C.F.; Egle, D.; Wette, V.; Petru, E.; Balic, M.; Pichler, A.; Greil, R.; Petzer, A.L.; Bago-Horvath, Z.; et al. The EndoPredict score predicts response to neoadjuvant chemotherapy and neoendocrine therapy in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients from the ABCSG-34 trial. Eur. J. Cancer 2020, 134, 99–106. [Google Scholar] [CrossRef]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Muñoz, M.; Paré, L.; Farré, B.G.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
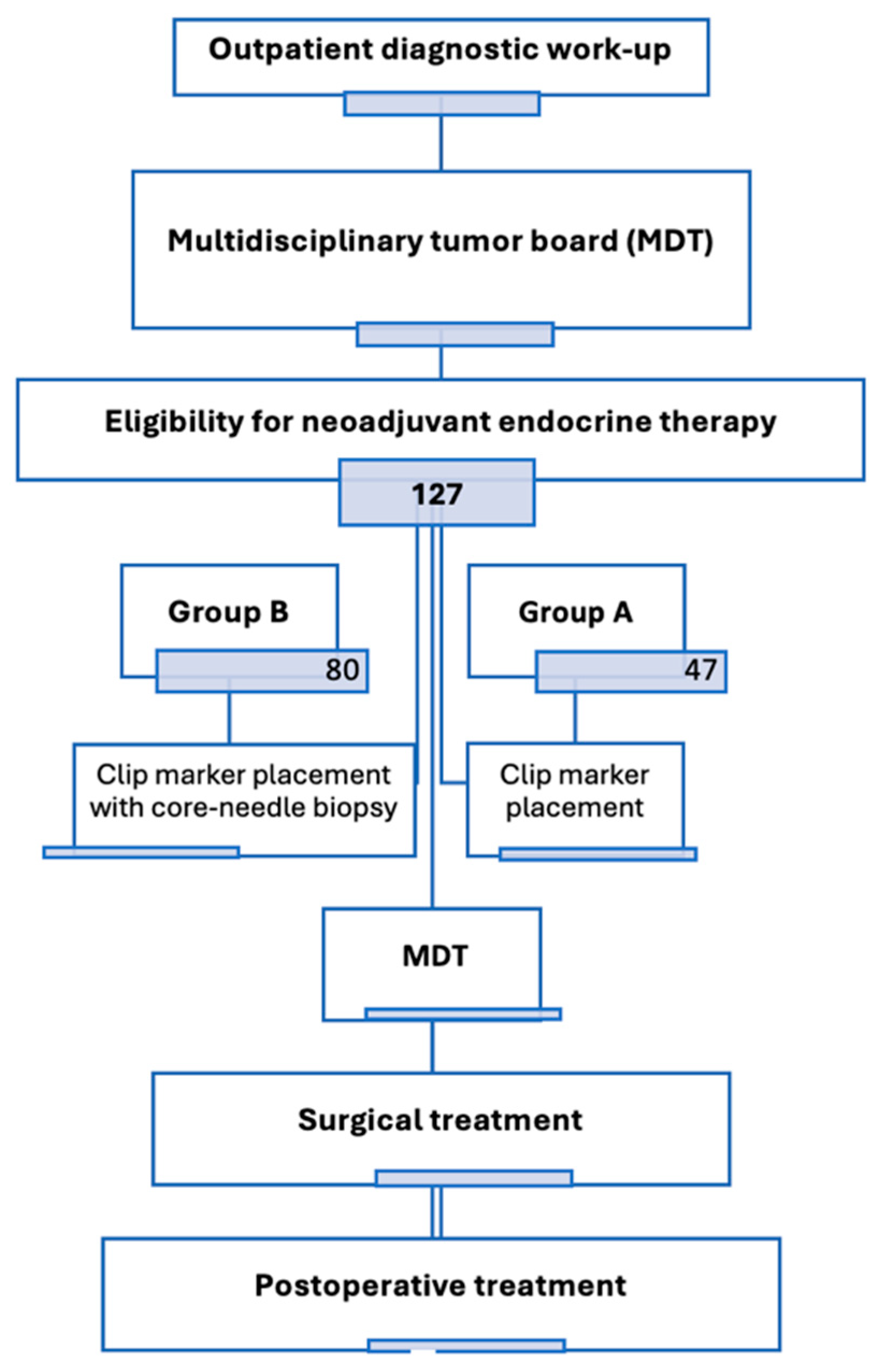


| Clinical Characteristics at Baseline | Group A (No Interim Biopsy) | Group B (with Interim Biopsy) | Number of Patients | p-Value | |
|---|---|---|---|---|---|
| Age | 50–59 | 5 (10.6%) | 11 (13.8%) | 16 (12.6%) | 0.734 |
| 60–69 | 26 (55.3%) | 38 (47.5%) | 64 (50.4%) | ||
| 70–79 | 12 (25.5%) | 26 (32.5%) | 38 (29.9%) | ||
| 80+ | 4 (8.5%) | 5 (6.3%) | 9 (7.1%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| cT | T1 | 4 (8.5%) | 5 (6.3%) | 9 (7.1%) | 0.738 |
| T2 | 22 (46.8%) | 45 (57.0%) | 67 (53.2%) | ||
| T3 | 17 (36.2%) | 23 (29.1%) | 40 (31.7%) | ||
| T4 | 4 (8.5%) | 6 (7.6%) | 10 (7.9%) | ||
| Total | 47 (100.0%) | 79 (100.0%) | 126 (100.0%) | ||
| cN | N0 | 31 (66.0%) | 33 (41.3%) | 64 (50.4%) | 0.026 |
| N1 | 11 (23.4%) | 34 (42.5%) | 45 (35.4%) | ||
| N2 | 5 (10.6%) | 13 (16.3%) | 18 (14.2%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Histological subtype of the primary tumor | Lobular | 7 (14.9%) | 18 (22.5%) | 25 (19.7%) | 0.298 |
| NOS | 40 (85.1%) | 62 (77.5%) | 102 (80.3%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Histological grade (G) | G1 | 15 (31.9%) | 17 (21.3%) | 32 (25.2%) | 0.138 |
| G2 | 31 (66.0%) | 55 (68.8%) | 86 (67.7%) | ||
| G3 | 1 (2.1%) | 8 (10.0%) | 9 (7.1%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| ER | Yes | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | |
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| PGR | <=20% | 11 (23.4%) | 28 (35.0%) | 39 (30.7%) | 0.171 |
| >20% | 36 (76.6%) | 52 (65.0%) | 88 (69.3%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| HER2 | No | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | |
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Ki67 (%) | <=20% | 36 (76.6%) | 55 (68.8%) | 91 (71.7%) | 0.344 |
| >20% | 11 (23.4%) | 25 (31.3%) | 36 (28.3%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Ki67 (%) | <2.7% | 1 (2.1%) | 2 (2.5%) | 3 (2.4%) | 0.894 |
| >=2.7% | 46 (97.9%) | 78 (97.5%) | 124 (97.6%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Drug | Letrozole | 47 (100.0%) | 77 (96.3%) | 124 (97.6%) | 0.406 |
| Anastrozole | 0 (0.0%) | 1 (1.3%) | 1 (0.8%) | ||
| Tamoxifen | 0 (0.0%) | 2 (2.5%) | 2 (1.6%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) | ||
| Primary surgical qualification | MRM | 9 (19.1%) | 29 (36.3%) | 38 (29.9%) | 0.145 |
| MST+SLNB | 13 (27.7%) | 13 (16.3%) | 26 (20.5%) | ||
| BCS/TU+SLNB | 21 (44.7%) | 34 (42.5%) | 55 (43.3%) | ||
| BCS/TU+ALND | 4 (8.5%) | 4 (5.0%) | 8 (6.3%) | ||
| Total | 47 (100.0%) | 80 (100.0%) | 127 (100.0%) |
| Baseline Biopsy | Interim Biopsy | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Median (IQR) | Range | N | Mean (SD) | Median (IQR) | Range | ||
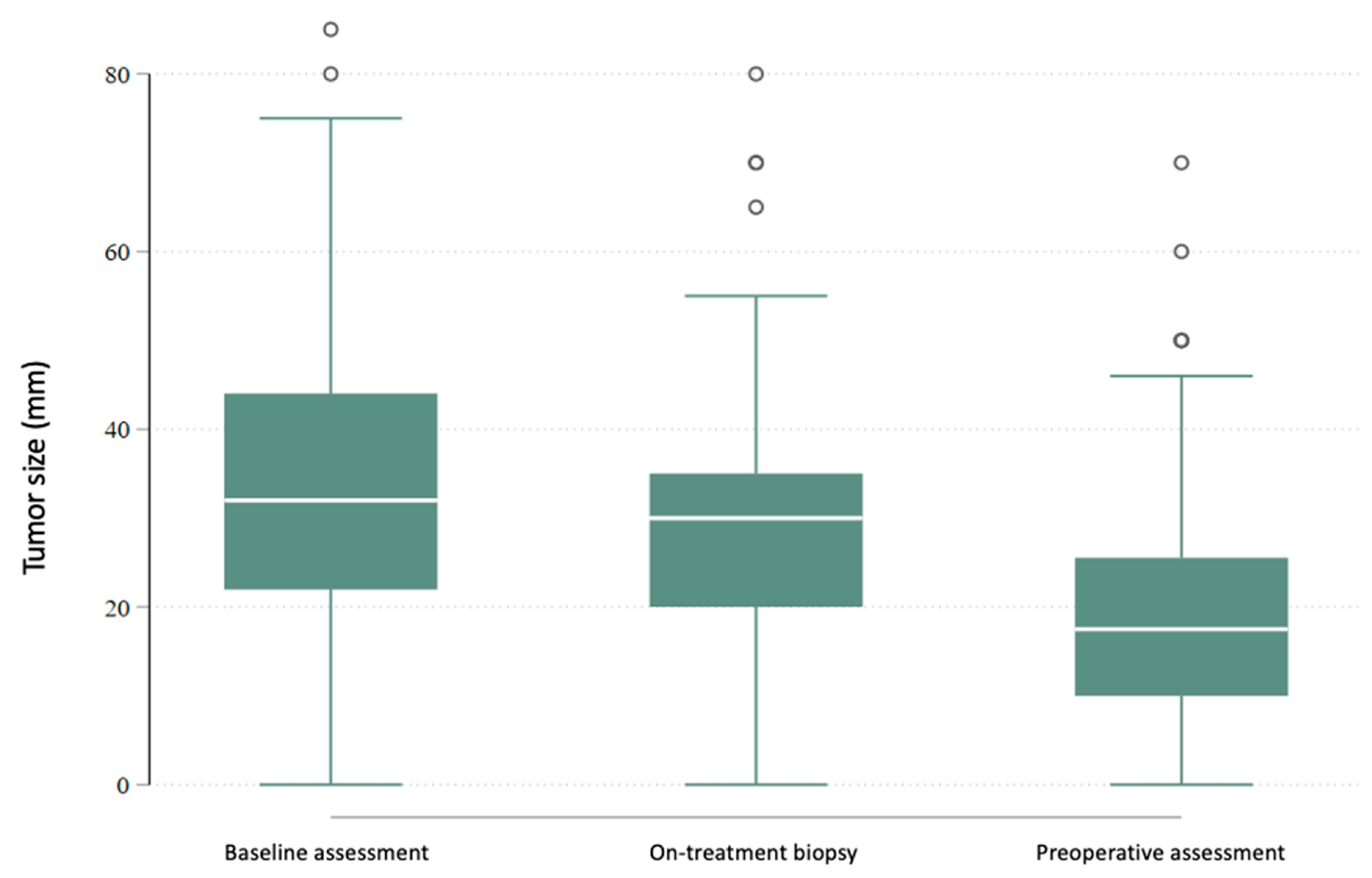
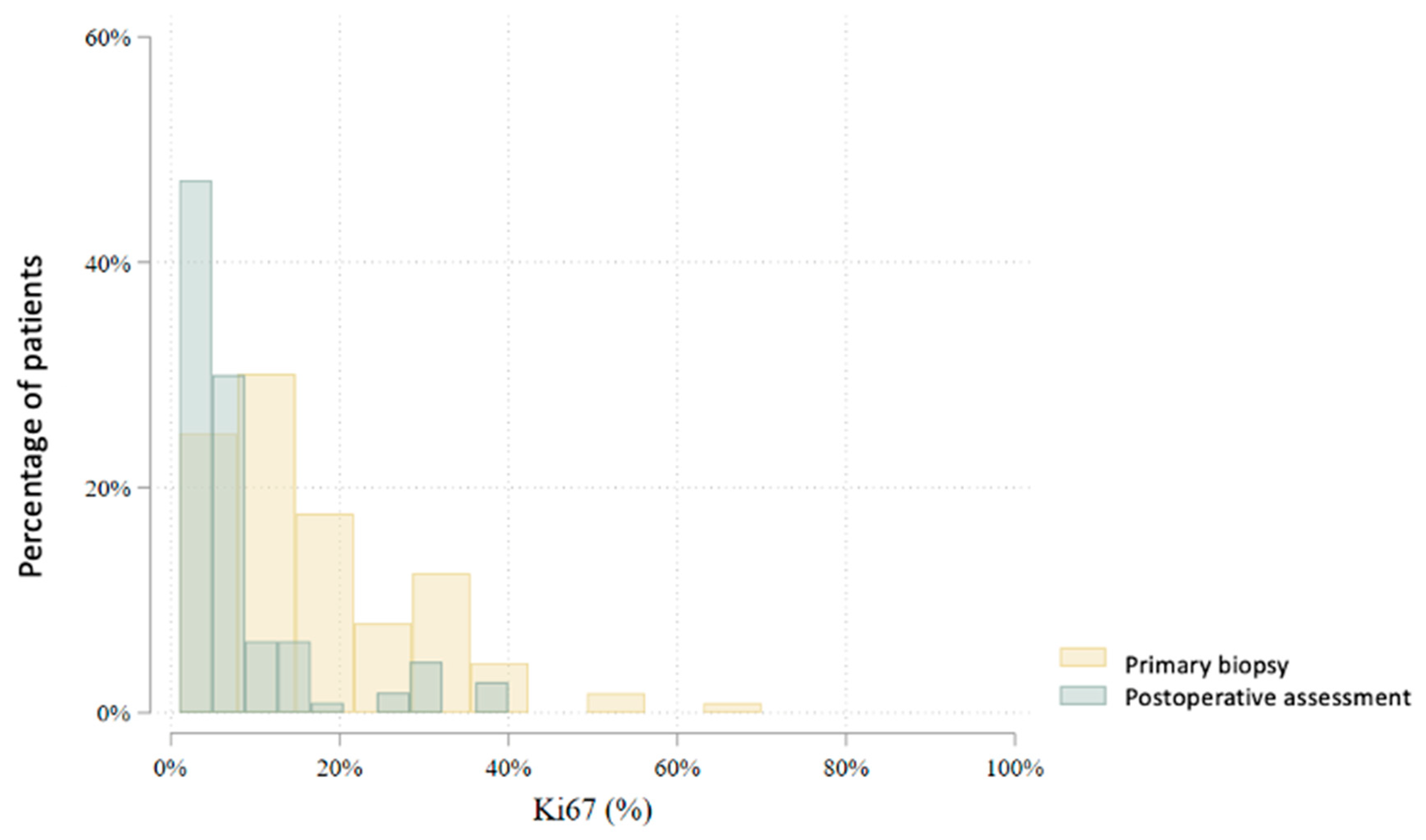
| Ki67 (%) | 80 | 19.1 (13.3) | 20.0 (10.0–25.0) | 1–70 | 78 | 8.0 (10.8) | 5.0 (2.0–10.0) | 1–75 | 0.000 |
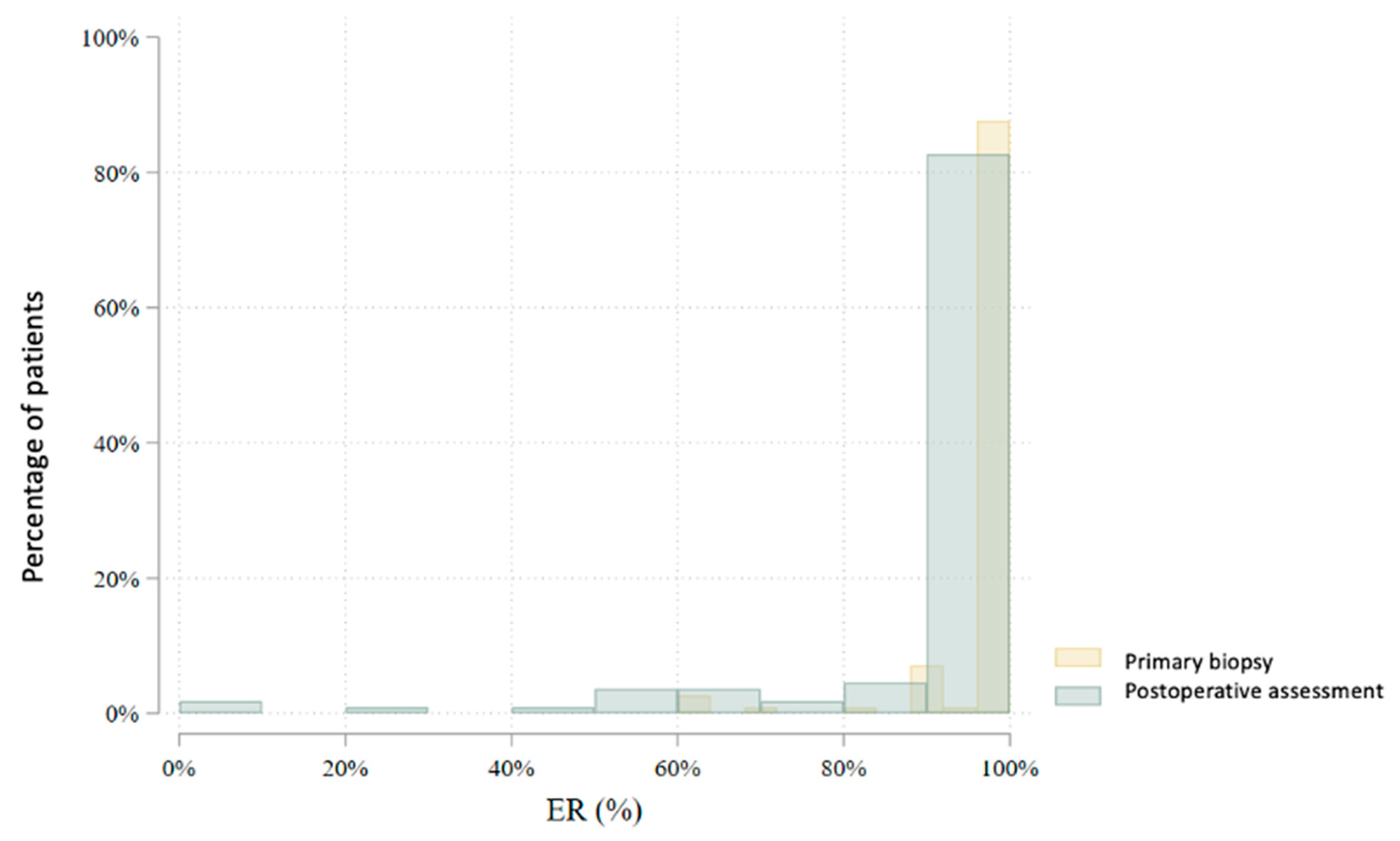
| ER (%) | 80 | 97.7 (8.0) | 100.0 (100.0–100.0) | 60–100 | 78 | 93.8 (18.2) | 100.0 (100.0–100.0) | 0–100 | 0.004 |
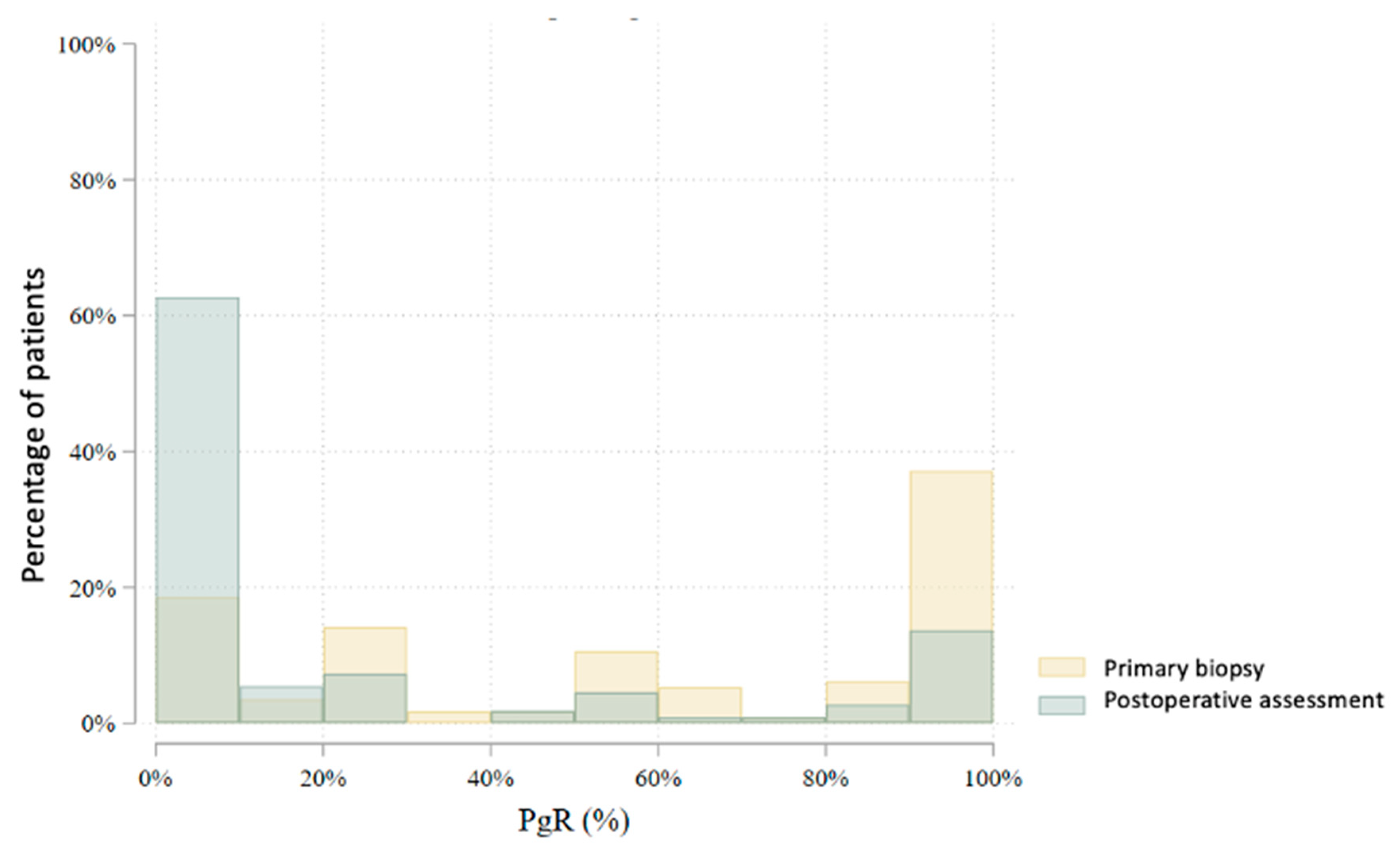
| PgR (%) | 80 | 55.5 (40.8) | 60.0 (15.0–100.0) | 0–100 | 78 | 16.4 (29.2) | 0.0 (0.0–15.0) | 0–100 | 0.000 |
| Surgical Procedure Performed | Initial Qualification | Total | |||
|---|---|---|---|---|---|
| N (%) | MRM | MST+SLNB | BCS/TU+SLNB | BCS/TU+ALND | |
| No surgery performed | 5 (14.3%) | 1 (3.8%) | 1 (2.0%) | 0 (0.0%) | 7 (5.8%) |
| MRM | 15 (42.9%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) | 17 (14.2%) |
| MST+SLNB | 5 (14.3%) | 18 (69.2%) | 5 (9.8%) | 0 (0.0%) | 28 (23.3%) |
| MST+ALND | 5 (14.3%) | 1 (3.8%) | 0 (0.0%) | 1 (12.5%) | 7 (5.8%) |
| BCS/TU+SLNB | 2 (5.7%) | 3 (11.5%) | 38 (74.5%) | 3 (37.5%) | 46 (38.3%) |
| BCS/TU+ALND | 3 (8.6%) | 0 (0.0%) | 7 (13.7%) | 3 (37.5%) | 13 (10.8%) |
| Other | 0 (0.0%) | 1 (3.8%) | 0 (0.0%) | 1 (12.5%) | 2 (1.7%) |
| Total | 35 (100.0%) | 26 (100.0%) | 51 (100.0%) | 8 (100.0%) | 120 (100.0%) |
| Baseline Biopsy | Postoperative Assessment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Mediana (IQR) | Range | N | Mean (SD) | Mediana (IQR) | Range | ||
| Ki67 (%) | 115 | 17.0 (12.7) | 10.0 (9.0–25.0) | 1–70 | 111 | 7.1 (9.0) | 5.0 (2.0–6.0) | 1–40 | 0.000 |
| ER (%) | 115 | 97.8 (7.5) | 100.0 (100.0–100.0) | 60–100 | 111 | 90.9 (18.5) | 100.0 (90.0–100.0) | 0–100 | 0.000 |
| PgR (%) | 115 | 57.5 (38.7) | 60.0 (20.0–100.0) | 0–100 | 111 | 22.9 (35.9) | 0.0 (0.0–30.0) | 0–100 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczna, A.; Rosinska, M. Early Predictive Markers and Histopathological Response to Neoadjuvant Endocrine Therapy in Postmenopausal Patients with HR+/HER2− Early Breast Cancer. Cancers 2025, 17, 2319. https://doi.org/10.3390/cancers17142319
Konieczna A, Rosinska M. Early Predictive Markers and Histopathological Response to Neoadjuvant Endocrine Therapy in Postmenopausal Patients with HR+/HER2− Early Breast Cancer. Cancers. 2025; 17(14):2319. https://doi.org/10.3390/cancers17142319
Chicago/Turabian StyleKonieczna, Aleksandra, and Magdalena Rosinska. 2025. "Early Predictive Markers and Histopathological Response to Neoadjuvant Endocrine Therapy in Postmenopausal Patients with HR+/HER2− Early Breast Cancer" Cancers 17, no. 14: 2319. https://doi.org/10.3390/cancers17142319
APA StyleKonieczna, A., & Rosinska, M. (2025). Early Predictive Markers and Histopathological Response to Neoadjuvant Endocrine Therapy in Postmenopausal Patients with HR+/HER2− Early Breast Cancer. Cancers, 17(14), 2319. https://doi.org/10.3390/cancers17142319






