Simple Summary
Many patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer experience recurrence after the initial treatment, and the effectiveness of chemotherapy decreases with each recurrence. Therefore, new strategies are urgently needed to improve outcomes in patients who have already undergone multiple lines of treatment. Hormonal therapy has been used for ovarian cancer, but its role when combined with chemotherapy and tailored to hormone receptor expression is unclear. This study examined whether combining chemotherapy with hormonal therapy could improve clinical outcomes in patients with heavily pretreated cancer. Patients whose tumors had more estrogen receptors received tamoxifen, while those with more progesterone receptors received megestrol acetate, each combined with chemotherapy. These findings suggest that the combination of tamoxifen and chemotherapy may provide potential clinical benefit and manageable safety in selected patients. These exploratory results support a further investigation of a personalized approach based on hormone receptor status in late-line ovarian cancer therapy.
Abstract
Background/Objectives: The effects of combining chemotherapy with hormonal therapy based on hormone receptor (HR) expression in epithelial ovarian, fallopian tube, or primary peritoneal (EOC) remain unclear. This study evaluated the efficacy and safety of physician-chosen chemotherapy combined with hormonal therapy in patients with heavily pretreated advanced EOC, stratified by HR expression. Methods: This phase II, multicenter, pilot study included patients with heavily pretreated advanced EOC, allocated to estrogen receptor (ER)-dominant or progesterone receptor (PR)-dominant arms. Patients in the ER-dominant arm received tamoxifen plus physician-selected chemotherapy, while those in the PR-dominant arm received megestrol acetate (MA) plus chemotherapy. The primary outcome was the best objective response rate (ORR) for six months, assessed using an optimal two-stage Simon design. Results: Among 33 ER-dominant patients with high-grade serous carcinoma (HGSC), the six-month best ORR was 27.3% (3% complete response, 24.2% partial response). The six-month ORR and clinical benefit rate (CBR) were 18.8% and 37.5%, respectively, with 62.5% experiencing progressive disease (PD). Among three PR-dominant patients (two clear cell carcinoma and one HGSC), the six-month best ORR was 0%. The six-month ORR and CBR were also 0%, and all experienced PD within six months. No unacceptable toxicity related to tamoxifen or MA was encountered. Conclusions: In heavily pretreated advanced HGSC patients with ER-dominant expression, chemotherapy combined with tamoxifen showed encouraging clinical activity with favorable safety. While limited by the study design, these findings suggest a potential role for tailored hormonal therapy combined with chemotherapy based on HR expression in heavily pretreated advanced EOC. Clinical Trial Registration: KCT0004571
1. Introduction
Approximately 75% of patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer (EOC) present with advanced-stage disease at diagnosis [1]. The standard therapy for EOC is staging surgery, including complete debulking surgery and platinum-based chemotherapy. Although the response rate to initial treatment is high, up to 85% of patients who achieve complete remission experience recurrence following first-line chemotherapy [2]. The efficacy of second- or later-line chemotherapy remains unsatisfactory [2,3]. Resistance to chemotherapy increases whenever EOC recurs [2,3]. The lack of methods to overcome chemotherapy resistance is the main reason for the poor prognosis in EOC.
Many studies have reported using hormonal receptors (HR) as prognostic biomarkers for EOC, despite results being inconsistent because of small sample sizes, discrepancies in antibodies used for immunohistochemistry, and inconsistent analysis methods [4,5,6,7]. Previous studies reported that the estrogen receptor (ER) and progesterone receptor (PRa) were expressed in 32–100% and 19–91% of EOC cases, respectively [8,9]. In addition, ER-α and ER-β are encoded by different genes, which have proliferative and anti-proliferative effects [9,10]. Previous studies reported that PRa mediates the growth inhibitory effect of progesterone and induces apoptosis [9,10].
Some studies with and without HR status evaluations have shown that a combination of tamoxifen or medroxyprogesterone acetate (MPA) and platinum-based chemotherapy in advanced or recurrent EOC is safe and may improve the chemotherapy response through a synergistic interaction [11,12]. In contrast, randomized controlled trials (RCTs) using tamoxifen or MPA combined with platinum-based chemotherapy as the first-line therapy for advanced EOC found that combination therapies were safe but failed to improve survival compared to platinum-based chemotherapy alone [13,14]. Nevertheless, in these studies, tamoxifen or MPA was not used based on HR expression [13,14]. Moreover, phase II, single-arm, prospective studies have shown that LHRH agonists linked to doxorubicin and androgen receptor (AR) inhibitors improve survival in recurrent EOC that expresses the LHRH receptor and AR, respectively [15,16].
EOC encompasses various types of cancers based on the histology and genetic findings [1]. Therefore, tailored therapy based on the cancer type is needed instead of the current uniform treatment approach. HR expression differs according to histological type in EOC [4]. A previous study reported that ER and PRa are expressed in 60.7% and 31.2% of high-grade serous ovarian cancers (HGSCs), respectively [4].
No studies have analyzed the efficacy and safety of chemotherapy combined with hormonal therapy according to HR expression in various histologic types of EOC. In heavily pretreated advanced EOC, this study hypothesized that chemotherapy combined with the tailored targeting of hormonal therapies according to HR expression could be a novel strategy to improve the chemotherapy response through synergistic interactions. Therefore, this study examined the efficacy and safety of physician-chosen chemotherapy with hormonal therapy according to HR expression in patients with heavily pretreated advanced EOC.
2. Materials and Methods
2.1. Study Design and Participants
ELSA, the Korean Gynecologic Oncology Group (KGOG) 3049, was a phase II, multicenter pilot study conducted at nine centers in South Korea. All participating centers were secondary or tertiary hospitals that regularly performed surgical care for ovarian cancer and had multidisciplinary teams, including specialized gynecologic oncologists, radiologists, and pathologists.
The eligible criteria were as follows: age 19 years or older, EOC histologically confirmed from cytoreductive surgery (upfront or interval), (two previous chemotherapy regimens and just previous progression-free interval < six months) or (≥ three previous chemotherapy regimens), Eastern Cooperative Oncology Group performance status of 0–2, measurable lesion on imaging (CT or MRI) or increase in CA125, an estimated life expectancy of at least six months, and adequate hematologic and end-organ function. The following patients were excluded: those diagnosed with or treated for other types of primary cancer within the past five years and patients undergoing anticancer therapy that included immunotherapy or other targeted therapies. Initially, the study protocol required patients to undergo surgery or biopsy immediately after the last recurrence to assess the current HR status. Nevertheless, the eligibility criteria were amended to permit enrollment based on previously available surgical or biopsy tissue because of the extremely low enrollment rate over the first year.
The study protocol was approved by the ethics committees at all participating institutions. All patients provided written informed consent. This study was conducted in accordance with the Bioethics and Safety Act of the Republic of Korea and was registered with the Clinical Research Information Service (CRIS; registration number KCT0004571) on 20 December 2019.
2.2. Procedures
HR expression in ovarian cancer tissue was assessed by conducting immunohistochemistry in the central laboratory using ‘primary surgery tissue’ or ‘tissue obtained from surgery or biopsy after recurrence’ if primary surgery tissues are unavailable (Figure 1). In patients with the primary surgery tissue and tissues obtained after recurrence, the last acquired tissue was used to assess HR expression. Two pathologists performed the central review using the Allred scoring system to assess HR expression. Allred scoring [17] uses the sum of intensity score (on a scale of 0–3) and proportion score (on a scale of 0–5). A total score of 0–2 was considered negative HR expression, while a score of 3–8 was considered positive.
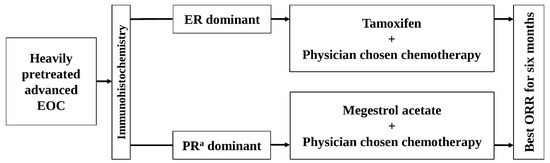
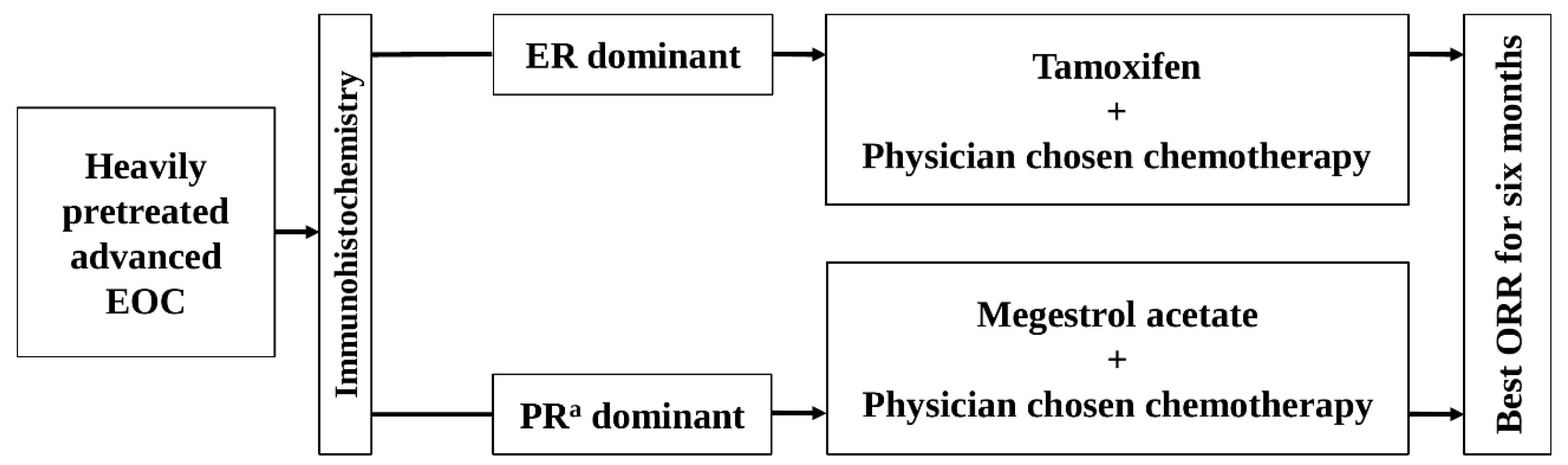
Figure 1.
The schema of this study. EOC, epithelial ovarian cancer; ER, estrogen receptor; ORR, objective response rate; PRa, progesterone receptor.
The HR status in each patient was categorized as ER-dominant, PRa-dominant, or no receptor expression, based on the relatively higher Allred score after comparing ER and PRa expression (Figure 1). In cases where the ER and PRa Allred scores were equal and positive, the patients were alternatively allocated to one of the dominant groups. Patients with no positive expression for either receptor were excluded from the study.
The ER-dominant patients (who had ER expression alone or a relatively higher ER expression rate) received oral tamoxifen (20 mg twice daily) and physician-chosen chemotherapy (Figure 1). The PRa-dominant patients (who had PRa expression alone or a relatively higher PRa expression rate) received oral megestrol acetate (MA) (160 mg once daily) and physician-chosen chemotherapy (Figure 1). The physician-chosen chemotherapy included belotecan, docetaxel, etoposide, gemcitabine, paclitaxel, pegylated liposomal doxorubicin, topotecan, and vinorelbine. Radiologists in the participating centers assessed tumors on CT or MRI, and individual investigators assessed the response of combined therapies according to “Revised RECIST Guideline (Version 1.1) [18].” The investigators evaluated the adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [19]. The association between adverse events and treatment drugs was assessed as unknown, unrelated, possible, probable, or definite.
The patients continuously received hormonal therapies from day 1 of the first cycle of each physician-chosen chemotherapy until progressive disease (PD) or the development of unacceptable toxicity, or for at least six months. Based on the response assessment performed at six months, patients showing a complete response (CR), partial response (PRb), or stable disease (SD) received continuous hormonal therapies until PD or the development of unacceptable toxicity, regardless of the continuation of physician-chosen chemotherapy if the patients wanted to take them. After a six-month response assessment, physician-chosen chemotherapy may be performed according to the investigators’ discretion.
The study was conducted independently in two arms: the ER-dominant group and the PRa-dominant group (Figure 1).
The dose modifications and treatment discontinuation for tamoxifen, MA, or physician-chosen chemotherapy were permitted to manage adverse events. The doses were reduced by 25%, and up to two reductions were allowed. The patient had a dose reduction twice, but was excluded from the study if the dose had to be reduced again. Within three weeks after discontinuing treatment, the patients could restart the treatment drugs at the investigators’ discretion, but these patients were excluded from the study if the treatment drugs could not be restarted.
The patients were followed up every eight weeks (±two weeks) during the combined therapies for six months. After a six-month response assessment, the patients were followed up every three months until PD, development of unacceptable toxicity, or the end of this study. This study ended three months after the final enrolled patient received combined therapies for six months. CT or MRI imaging and laboratory evaluations were performed at each follow-up visit, and adverse events were monitored. These evaluations, including imaging studies, laboratory data, and adverse event profiles, were assessed according to the RECIST and CTCAE criteria. All patients were followed up to finally assess the adverse events related to hormonal therapy at three months after discontinuing tamoxifen or MA.
2.3. Outcomes
In the ER-dominant arm, the effects of the combined therapy of tamoxifen and physician-chosen chemotherapy were evaluated. In the PRa-dominant arm, the effects of combined therapy of MA and physician-chosen chemotherapy were evaluated. The outcomes of the two arms were not compared (Figure 1).
The best objective response rate (ORR) for six months was assessed as the primary outcome (Figure 1). The best response among the three response assessments performed at two-month intervals was selected. CR or PRb was confirmed when those were repeated at the response assessment after two months. The secondary outcomes were as follows: best ORR according to the histologic types of EOC, time to progression (TTP), and adverse events. TTP was defined as the duration from the initiation of combination therapy to the date of radiologic or clinical disease progression.
2.4. Sample Size
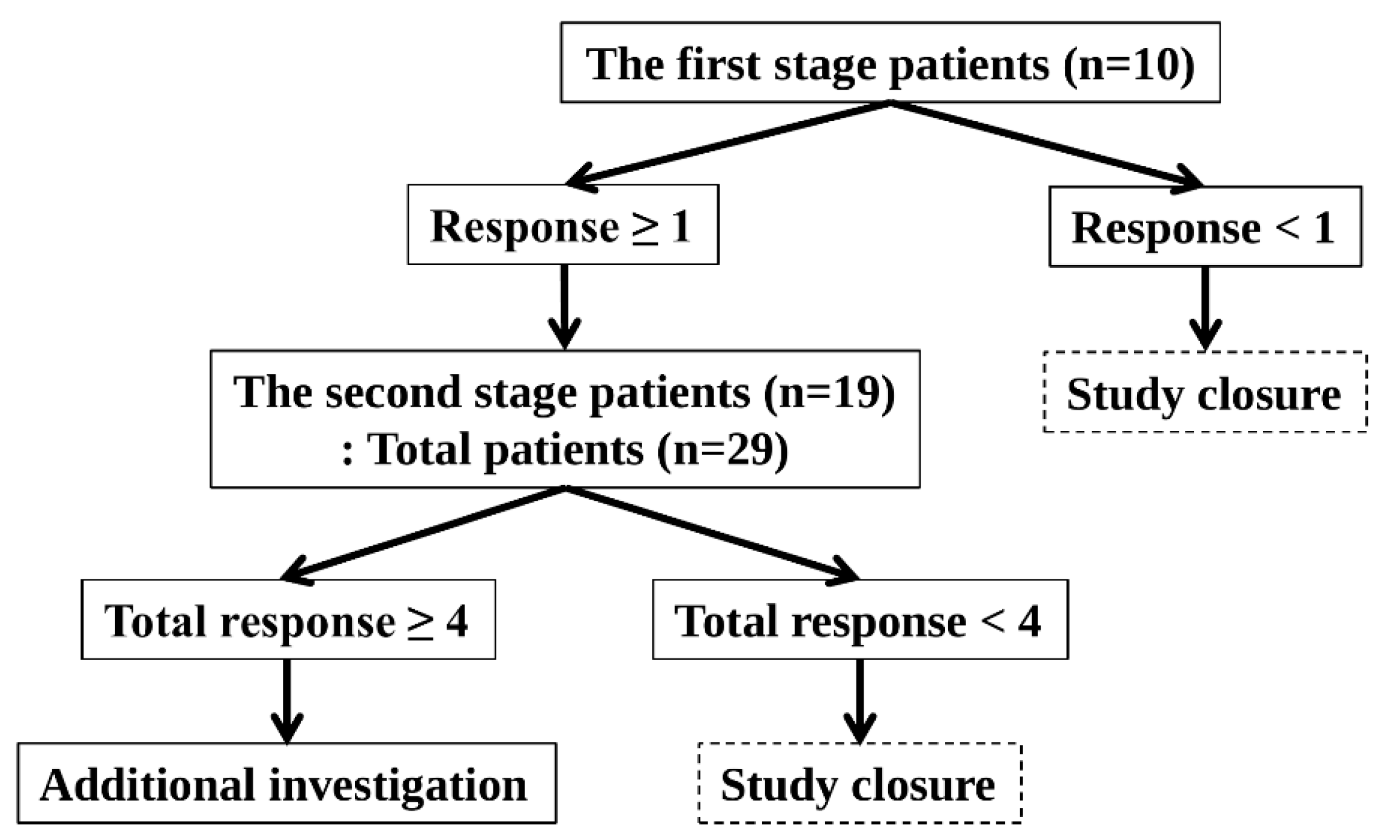
The optimal two-stage Simon design was used (Figure 2).
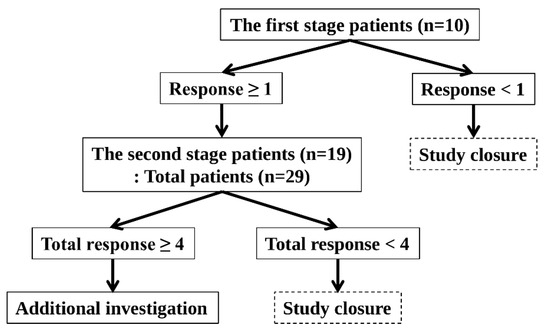
Figure 2.
Optimal Two-Stage Simon Design.
The response probabilities for the poor and good drugs were 0.05 and 0.20, respectively. The one-sided Type I error rate and power were 5% and 80%, respectively.
This study was conducted in accordance with the following principles. In the first stage, 10 patients were enrolled. The study was terminated if no patient demonstrated a response. The study proceeded to the second stage if more than one patient responded. In the second stage, an additional 19 patients were enrolled, bringing the total to 29 patients. The study was terminated if fewer than four patients showed a response. If four or more patients responded, the study was considered successful, and the null hypothesis was rejected. Hence, further investigation was warranted.
The best objective response for six months was used to assess the response. The development of unacceptable toxicity from tamoxifen or MA was considered a non-response. The study was performed in the ER-dominant and PRa-dominant arms. The planned number of patients was 58 (29 patients in the ER-dominant arm and 29 patients in the PRa-dominant arm).
The dropout rate was not initially accounted for in the study design, which planned to enroll patients continuously until the predefined number in each arm (29 patients) had either experienced PD, developed unacceptable toxicity, or completed the six-month response assessment. A high dropout rate was observed as the study neared completion, prompting the study team to enroll additional patients even before the planned number of patients in each arm had reached a study endpoint. This decision was made to prevent prolonged delays in study completion. The main reasons for dropout included withdrawal of consent, deterioration of the subjects’ general condition, allergic reactions to chemotherapy, and refusal to take oral medication due to ileus or other treatment-limiting conditions. As a result, four more patients than initially planned were enrolled in the ER-dominant arm and included in the final analysis. The final proportion of patients who reached a study endpoint (PD, unacceptable toxicity, or completion of the six-month response assessment) was 66.7% (36 out of 54).
Although the planned number of patients was 29 per arm, accrual in the PRa-dominant arm remained extremely low throughout the study. Because enrollment in the ER-dominant arm proceeded as planned, the data and safety monitoring committee (DSMC) permitted the study to continue without protocol amendment. As a result, only three patients were ultimately enrolled in the PRa-dominant arm.
2.5. Statistics
Descriptive statistics were used to summarize the treatment outcomes. The categorical variables were reported as frequencies and percentages. The best ORR, ORR, clinical benefit rate (CBR), and PD were calculated with exact binomial 95% confidence intervals (CIs). The Kaplan–Meier method was used to estimate the probability of progression over time. The results are presented as TTP probability curves. The 95% CIs for the Kaplan–Meier estimates were calculated using the log–log transformation method. Patients without progression at the time of analysis were censored at the date of the last follow-up. All statistical analyses were conducted using R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria), using the ′survival′ and ′survminer′ packages.
3. Results
The study was conducted between 1 September 2019 and 15 August 2024. In the ER-dominant arm, three patients showed responses among the first 10 enrolled patients in the first stage, and nine patients showed responses among the total 33 patients during the second stage. During the second stage, four additional patients were enrolled before the planned number of 19 patients had either experienced PD, developed unacceptable toxicity, or completed the six-month response assessment—the planned number required for the evaluation. One patient was allocated to the ER-dominant arm because the Allred scores for ER and PRa were equal. Consequently, 33 patients were included in the ER-dominant arm, and the efficacy and safety outcomes were analyzed. In the PRa-dominant arm, only three patients were enrolled during the entire study period of the ER-dominant arm. Therefore, this study in the PRa-dominant arm ended without additional patient enrollments. Four patients who had mucinous carcinoma (one patient), clear cell carcinoma (CCC) (two patients), and HGSC (one patient) dropped out of the study because no ER and PRa expression was detected.
3.1. Baseline Characteristics
Table 1 lists the baseline characteristics of the 36 study subjects. Thirty-three patients showed ER-dominant expression, and three showed PRa-dominant expression.

Table 1.
Baseline characteristics.
All patients with ER-dominant expression had high-grade serous ovarian cancer (HGSC) and received combination therapy with tamoxifen at the third to ninth lines of chemotherapy. The lines of chemotherapy were as follows: seven patients in the third line, eight patients in the fourth line, five patients in the fifth line, five patients in the sixth line, two patients in the seventh line, four patients in the eighth line, and two patients in the ninth line. Sixteen different chemotherapeutic agents were used among the 33 patients with ER-dominant expression. Each agent was used in one to five patients, and the distribution of agents was as follows: cyclophosphamide (five patients); pegylated liposomal doxorubicin, topotecan, and vinorelbine (four patients each); belotecan, topotecan/carboplatin, topotecan/cisplatin, and weekly paclitaxel (two patients each); and eight other agents were used in single patients. Moreover, the tissue samples for testing HR expression were obtained from primary surgery (29 patients), surgery at the first recurrence (one patient), biopsy at the second recurrence (one patient), and surgery at the fifth recurrence (two patients).
Patients with PRa-dominant expression had CCC (two patients) and HGSC (one patient). They received combination therapy with MA at the third line (one patient), fourth line (one patient), and seventh line (one patient) of chemotherapy. Three different chemotherapy agents were used across three patients: docetaxel/carboplatin, topotecan, and vinorelbine. The tissue for testing HR expression was obtained from primary surgery (two patients) and surgery at the second recurrence (one patient).
3.2. Response Assessment in Patients with ER-Dominant Expression
The best ORR for six months was (9/33) 27.3%. It consisted of (1/33) 3.0% of CR and (8/33) 24.2% of PRb (Table 2).

Table 2.
Response assessment in patients with ER-dominant expression.
The patient with CR received combination therapy with tamoxifen at the fourth line of chemotherapy (case 23). The patient showed CR in the two- and four-month response assessments and dropped out after a four-month response assessment. Therefore, the patient response was included in the best ORR for six months but not in the ORR at six months (Table 1, Table 2 and Table 3). The rates of PRb decreased according to the increase in the chemotherapeutic lines (Table 3).

Table 3.
Response assessment by line of chemotherapy in patients with ER-dominant expression.
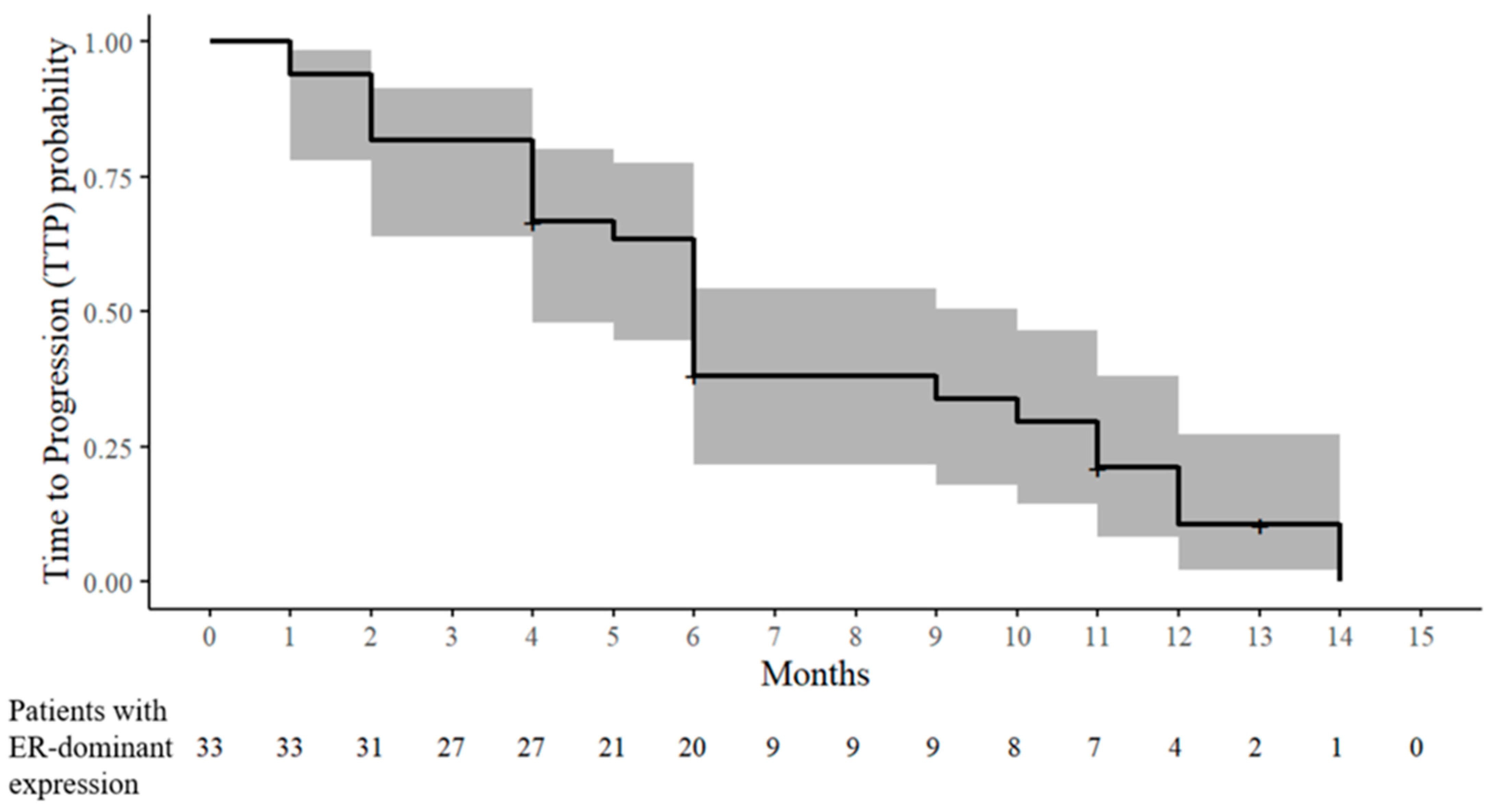
At six months, the CR, PRb, and SD were 0% (0/32), 18.8% (6/32), and 18.8% (6/32), respectively. Therefore, at six months, the ORR and CBR were 18.8% (6/32) and 37.5% (12/32), respectively. PD occurred in 62.5% (20/32) within six months. The TTP was distributed as follows: one month (two patients), two months (four patients), four months (five patients), five months (one patient), and six months (eight patients) (Table 2 and Figure 3). According to the increase in chemotherapeutic lines, the PRb and SD rates decreased, and the PD rates increased (Table 3).
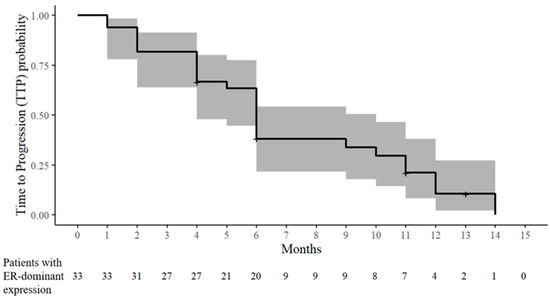
Figure 3.
Kaplan–Meier estimate of the time to progression (TTP) probability in patients with ER-dominant expression. The solid line denotes the estimated probability of remaining progression-free, and the shaded band represents the 95% CIs. The tick marks indicate censored observations. The numbers at risk are displayed below the x-axis.
After a six-month response assessment, nine patients received tamoxifen maintenance therapy with or without chemotherapy until PD (seven patients) or dropout (two patients) occurred. The maximum time until PD occurred was 14 months. Moreover, the maximum times when the patients were assessed as CR, PRb, and SD were nine, 10, and 13 months, respectively. In three patients with PRb at the six-month response assessment, the CR or PRb responses during tamoxifen maintenance therapy were as follows: CR at nine months and PD at 12 months after combined therapy at the third line of chemotherapy (case 20), PRb at 10 months and PD at 14 months after combined therapy at the fourth line of chemotherapy (case 2), and PRb at eight months and PD at 11 months after combined therapy at the third line of chemotherapy (case 28) (Table 1 and Table 2, and Figure 3).
3.3. Response Assessment in Patients with PRa-Dominant Expression
The best ORR for six months was 0% (0/3). At six months, ORR and CBR were 0% (0/3), and PD occurred in 100% (3/3) of cases until six months (Table 4). Two patients with CCC who received combination therapy with MA at the fourth or seventh line of chemotherapy showed PD at two months (cases 34 and 35). A patient with HGSC who received combination therapy with MA at the third line of chemotherapy showed PD at six months (case 36) (Table 1 and Table 4).

Table 4.
Response assessment in patients with PRa-dominant expression.
3.4. Adverse Events
No unacceptable toxicity related to tamoxifen or MA was shown. Of the 36 patients with ER-dominant expression, only one patient showed a possible adverse event (grade two nausea) within one month after she received a combination therapy of tamoxifen with paclitaxel/cisplatin at the fourth line of chemotherapy (case 31). No adverse events occurred in three patients with PRa-dominant expression (Table 1 and Table S1).
4. Discussion
This study was performed on patients with heavily pretreated advanced EOC. The ER-dominant patients received tamoxifen and physician-chosen chemotherapy, and the PRa-dominant patients received MA and physician-chosen chemotherapy. The best ORR for six months was assessed as the primary outcome. All 33 patients with ER-dominant expression had only HGSC. A favorable best ORR was observed in ER-dominant patients, with no unacceptable toxicity related to tamoxifen. By contrast, three patients with PRa-dominant expression had CCC and HGSC histology and progressed until six months. Unacceptable toxicity related to MA was not shown.
Several small studies examined the effects of the combination therapy of chemotherapy and tamoxifen or MPA in EOC. In these studies, however, hormonal therapy was not performed according to HR expression [11,12,13,14]. In a retrospective study without an evaluation of the HR status (n = 50), the platinum-based chemotherapy and tamoxifen combination induced an ORR of 50% in relapsed or progressive advanced EOC after platinum-based chemotherapy failed [11]. In an RCT, however, where primary adjuvant chemotherapy for advanced EOC was performed, and ER and PRa were evaluated in 72% of patients, a combination of platinum-based chemotherapy and tamoxifen (n = 49) showed similar survival rates to those of platinum-based chemotherapy (n = 51) without a correlation between therapy and HR [13]. A retrospective study of primary adjuvant chemotherapy for advanced EOC showed that a combination of platinum-based chemotherapy and MPA (n = 22) induced higher 10-year survival rates than platinum-based chemotherapy alone (n = 28), suggesting better survival with higher PRa expression [12]. By contrast, in another RCT (n = 71) where primary adjuvant chemotherapy for advanced EOC was performed, and HR was not evaluated, the overall remission and survival rates were similar in the groups given platinum-based chemotherapy alone or platinum-based chemotherapy in combination with either MPA or 5-fluorouracil [14]. In this study, where tailored hormonal therapy was conducted according to ER- or PRa-dominant expression, the combination therapy of physician-chosen chemotherapy and tamoxifen showed encouraging responses in patients with ER-dominant expression. Nevertheless, the findings are limited because of the lack of a comparator and the small sample size. Conversely, it was difficult to obtain valuable information from the combination therapy of physician-chosen chemotherapy and MA because of the extremely small number of patients.
The ORR of chemotherapy in patients with recurrent EOC ranges from 3% to 53% [2,20]. Moreover, advanced lines of chemotherapy beyond second-line chemotherapy in EOC are associated with low response [20]. A retrospective study reported the following ORRs in patients with recurrent EOC (n = 156): 51.6%, 11.9%, 2.9%, 4.5%, and 0% for second-, third-, fourth-, fifth-, and ≥ sixth-line chemotherapy, respectively [20]. A drastic decline in the clinical response rates of chemotherapy was observed in those who received advanced lines of chemotherapy. Hence, various therapeutic strategies are needed to overcome these in patients with heavily pretreated advanced EOC. Targeted therapies may be useful therapeutic strategies in heavily pretreated advanced EOC. Recent studies reported that the ORR of targeted therapy ranged from 32.4% to 63.6% in patients with advanced or recurrent EOC with high folate receptor α or human epidermal growth factor 2 expression [21,22,23]. This study evaluated the effects of chemotherapy combined with targeted hormonal therapies according to ER or PRa expression in patients with heavily pretreated advanced EOC. The combination of chemotherapy and tamoxifen, administered as third- to ninth-line therapy, improved the clinical response of patients with ER-dominant expression, suggesting a synergistic effect of the combined treatment. According to the increase in the chemotherapeutic lines of patients with ER-dominant expression, the number of patients enrolled decreased, and the responses decreased, showing low responses in advanced lines of chemotherapy beyond the fourth-line chemotherapy. Moreover, in this study, a few patients who received combined therapy at the third or fourth line of chemotherapy and received tamoxifen maintenance therapy after PRb at the six-month response assessment showed a good response and delay of disease progression, suggesting a prolonged synergistic effect of combined therapy and a beneficial effect of tamoxifen maintenance therapy in relatively early chemotherapeutic lines.
HGSC, the most common ovarian cancer subtype, accounts for 63.4% of EOC [24,25]. A large-scale ovarian tumor tissue analysis consortium study revealed the following HR expression: HGSC (n = 1610) (81% of ER, 31% of PRa, and 16% of no ER and PRa); CCC (n = 354) (19.2% of ER, 7.9% of PRa, and 79% of no ER and PRa); and mucinous carcinoma (n = 185) (20.5% of ER, 15.7% of PRa, and 77% of no ER and PRa) [4]. In this study, the participants were enrolled prospectively according to the eligibility criteria regardless of the histologic subtype of EOC. Nevertheless, all patients with ER-dominant expression had HGSC (n = 33), and patients with PRa-dominant expression had CCC (n = 2) and HGSC histology (n = 1). Moreover, patients without ER and PRa expression who dropped out had mucinous carcinoma (n = 1), CCC (n = 2), and HGSC (n = 1). Corresponding to previous studies [4,24,25], the results showed that HGSC was the most common histologic subtype of EOC and was associated with relatively high ER expression. In contrast, CCC and mucinous carcinoma did not show ER and PRa expression at high frequency. Moreover, CCC was associated with relatively high PRa expression. These findings revealed the beneficial effects of the chemotherapy combined with tamoxifen in 33 HGSC patients with ER-dominant expression and the poor effect (PD at two months) of chemotherapy combined with MA in two CCC patients with PRa-dominant expression.
Many studies have reported that tamoxifen and progestin therapy (MA and MPA) are associated with a low incidence of general and severe toxicity in gynecologic cancers [13,26,27,28,29,30]. Corresponding to previous studies [13,26,27,28,29,30], in the present study, no toxicity attributable to tamoxifen or MA was observed in patients with ER- or PRa-dominant expression, except for one case of mild toxicity.
Previous studies comparing ER and PRa expression in primary and recurrent HGSC patients reported controversial discordance rates [31,32]. One study (n = 107) reported non-significant discordance rates of 34% and 12.4% in ER and PRa, respectively, with higher ER and lower PRa expression in recurrent cancers compared to primary cancers [31]. Another study (n = 80) showed a non-significant discordance rate of 8.2% in ER (lower ER expression in recurrent cancers) and a significant discordance rate of 15.7% in PRa (lower PRa expression in recurrent platinum-sensitive cancers) [32]. HR expression assessments using the tissue of recurrent cancers are suitable for targeted hormonal therapy because HR expression may differ between primary and recurrent cancers. Nevertheless, in this study, only three patients underwent surgery or biopsy immediately before enrollment, and most did not receive surgery or biopsy after recurrence. Therefore, most HR expression assessments were performed using the tissues from primary surgery.
In this study, although physician-chosen chemotherapy regimens were used in combination with tamoxifen or MA, the risk of selection bias was limited. In the ER-dominant arm, 16 different chemotherapeutic agents were administered across 33 patients. Each agent was used in only one to five patients, and among the nine patients with the best ORR and six patients with ORR at six months, no specific agent was disproportionately represented. Similarly, each of the three patients in the PRa-dominant arm received a different chemotherapeutic regimen. This distribution suggests that there was no clustering of responses to specific agents. Moreover, as this was a multicenter pilot study involving heavily pretreated patients, treatment flexibility was essential to reflect real-world clinical practice and facilitate adequate accrual. Therefore, although treatment heterogeneity exists, its impact on the treatment efficacy appears minimal.
This study was the first to investigate the efficacy and safety of chemotherapy combined with hormonal therapy based on HR expression and the histologic types in EOC. This study had the following limitations. First, in the PRa-dominant arm, only three patients were enrolled throughout the study period, precluding a valuable assessment of the treatment efficacy. Although all three patients showed PD after combination therapy with MA, it remains unclear whether this reflects the true resistance or is simply due to insufficient data. Future studies with larger PRa-dominant cohorts are needed. Second, HR expression was primarily assessed using tissue from primary surgery because most patients did not undergo surgery or a biopsy after recurrence. This may not accurately reflect the receptor status at the time of treatment. Third, the ER-dominant arm lacked a control group and had a relatively small sample size, limiting the generalizability of the findings. The use of physician-chosen chemotherapy regimens also introduced treatment heterogeneity. Nevertheless, this heterogeneity had a limited impact on the outcomes, considering the broad distribution of agents and absence of clustering among responders. Therefore, these findings should be interpreted with caution and validated in larger, controlled studies with standardized treatment protocols.
5. Conclusions
This multicenter pilot study examined the efficacy and safety of physician-chosen chemotherapy combined with tamoxifen or MA based on ER- or PRa-dominant expression in patients with heavily pretreated advanced EOC. In patients with ER-dominant expression, chemotherapy combined with tamoxifen showed encouraging clinical activity and favorable safety in heavily pretreated advanced HGSC patients. Nevertheless, these findings require validation in larger, controlled trials given the exploratory nature, small sample size, and lack of a control group in this study. In contrast, the extremely small number of patients with PRa-dominant expression precluded valuable assessments of the treatment efficacy. Future large-scale studies are warranted to determine the clinical utility of combining chemotherapy with hormonal therapy based on HR expression in this setting.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17142320/s1. Table S1: Adverse events.
Author Contributions
Conceptualization, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L., S.A. and B.L.; methodology, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L., S.A. and B.L.; software, S.A. and B.L.; validation, B.L.; formal analysis, S.A. and B.L.; investigation, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L., S.A. and B.L.; resources, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L. and B.L.; data curation, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L. and B.L.; writing—original draft preparation, B.L.; writing—review and editing, K.K., C.H.C., S.-Y.P., M.K.K., K.H.L., E.-J.L., M.C.L., Y.H.P., M.S.K., J.H.N., D.H.S., J.-W.L., S.A. and B.L.; funding acquisition, B.L.; visualization, B.L.; supervision, S.-Y.P. and B.L.; project administration, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Dalim Biotech. However, this agency had no involvement in the study design, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication. Furthermore, this research was funded by a grant from the National Cancer Center of the Republic of Korea (No. RS-2024-00360954).
Institutional Review Board Statement
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the following Institutional Review Board at each participating institution: Inha University Hospital (No. 2019-11-027) on 16 December 2019; Seoul National University Bundang Hospital (No. B-1907/555-002) on September 16, 2019; Samsung Medical Center (No. 2020-12-006) on 18 January 2021; National Cancer Center (No. NCC2020-0069) on 10 March 2020; Samsung Changwon Hospital (No. 2019-07-007) on 28 August 2019; Seoul St. Mary’s Hospital (No. KC19MEDS0579) on 13 April 2020; Chung-Ang University Hospital (No. 2151-002-462) on 14 July 2021; Hallym University Sacred Heart Hospital (No. 2019-10-015) on 8 January 2020; Hallym University Dongtan Sacred Heart Hospital (No. 2019-07-015) on 15 January 2020.
Informed Consent Statement
All patients provided written informed consent.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest or financial ties to disclose.
Abbreviations
AR: androgen receptor; CBR: clinical benefit rate; CCC: clear cell carcinoma; CI: confidence interval; CR: complete response; CTCAE: Common Terminology Criteria for Adverse Events; DSMC: data and safety monitoring committee; EOC: epithelial ovarian, primary peritoneal, or fallopian tube cancer; ER: estrogen receptor; HGSC: high-grade serous ovarian cancer; HR: hormonal receptor; KGOG: Korean Gynecologic Oncology Group; MA: megestrol acetate; MPA: medroxyprogesterone acetate; ORR: objective response rate; PD: progressive disease; PRa: progesterone receptor; PRb: partial response; RCTs: randomized controlled trials; SD: stable disease; TTP: time to progression.
References
- Hennessy, B.T.; Coleman, R.L.; Markman, M. Ovarian cancer. Lancet 2009, 374, 1371–1382. [Google Scholar] [CrossRef]
- Foley, O.W.; Rauh-Hain, J.A.; Del Carmen, M.G. Recurrent epithelial ovarian cancer: An update on treatment. Oncology (Williston Park) 2013, 27, 288–294, 298. [Google Scholar] [PubMed]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; Defazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [PubMed]
- van Kruchten, M.; van der Marel, P.; de Munck, L.; Hollema, H.; Arts, H.; Timmer-Bosscha, H.; de Vries, E.; Hospers, G.; Reyners, A. Hormone receptors as a marker of poor survival in epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 634–639. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016, 6, 25408. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.X.; Wang, J.J.; Wang, Y.; Leung, T.H.Y.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S. Differential expression of estrogen receptor subtypes and variants in ovarian cancer: Effects on cell invasion, proliferation and prognosis. BMC Cancer 2017, 17, 606. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Hormone Receptors in Serous Ovarian Carcinoma: Prognosis, Pathogenesis, and Treatment Considerations. Clin. Med. Insights Oncol. 2016, 10, 17–25. [Google Scholar] [CrossRef]
- Zheng, H.; Kavanagh, J.; Hu, W.; Liao, Q.; Fu, S. Hormonal therapy in ovarian cancer. Int. J. Gynecol. Cancer 2006, 17, 325–338. [Google Scholar] [CrossRef]
- Modugno, F.; Laskey, R.; Smith, A.L.; Andersen, C.L.; Haluska, P.; Oesterreich, S. Hormone response in ovarian cancer: Time to reconsider as a clinical target? Endocr. Relat. Cancer 2012, 19, R255–R279. [Google Scholar] [CrossRef]
- Panici, P.B.; Greggi, S.; Amoroso, M.; Scambia, G.; Battaglia, F.A.; Gebbia, V.; Salerno, G.; Paratore, M.P.; Mancuso, S. A combination of platinum and tamoxifen in advanced ovarian cancer failing platinum-based chemotherapy: Results of a Phase II study. Int. J. Gynecol. Cancer 2001, 11, 438–444. [Google Scholar] [CrossRef]
- Niwa, K.; Onogi, K.; Wu, Y.; Mori, H.; Harrigan, R.C.; Tamaya, T. Clinical implication of medroxyprogesterone acetate against advanced ovarian carcinoma: A pilot study. Eur. J. Gynaecol. Oncol. 2008, 29, 252–255. [Google Scholar] [PubMed]
- Schwartz, P.E.; Chambers, J.T.; Kohorn, E.I.; Chambers, S.K.; Weitzman, H.; Voynick, I.M.; MacLusky, N.; Naftolin, F. Tamoxifen in combination with cytotoxic chemotherapy in advanced epithelial ovarian cancer. A prospective randomized trial. Cancer 1989, 63, 1074–1078. [Google Scholar] [CrossRef]
- Senn, H.J.; Lei, D.; Castaño-Almendral, A.; Brunner, K.W.; Martz, G.; Obrecht, P.; Melchert, F.; Rhomberg, W. Chemo-(hormonal)-therapy of advanced ovarian neoplasms in FIGO stages III and IV. Prospective SAKK-study 20/71. Schweiz. Med. Wochenschr. 1980, 110, 1202–1208. [Google Scholar] [PubMed]
- Emons, G.; Gorchev, G.; Sehouli, J.; Wimberger, P.; Stähle, A.; Hanker, L.; Hilpert, F.; Sindermann, H.; Gründker, C.; Harter, P. Efficacy and safety of AEZS-108 (INN: Zoptarelin Doxorubicin Acetate) an LHRH agonist linked to doxorubicin in women with platinum refractory or resistant ovarian cancer expressing LHRH receptors: A multicenter Phase II trial of the ago-study group (AGO GYN 5). Gynecol. Oncol. 2014, 133, 427–432. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Gordhandas, S.B.; Giri, D.D.; Iasonos, A.; Zhou, Q.; Girshman, J.; O’CEarbhaill, R.E.; Zamarin, D.; Lichtman, S.M.; Sabbatini, P.J.; et al. Phase II study of enzalutamide in androgen receptor positive, recurrent, high- and low-grade serous ovarian cancer. Gynecol. Oncol. 2021, 164, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Pervez, S. Allred scoring for ER reporting and it’s impact in clearly distinguishing ER negative from ER positive breast cancers. J. Pak. Med. Assoc. 2010, 60, 350–353. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). 2021. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 1 December 2019).
- Bruchim, I.; Jarchowsky-Dolberg, O.; Fishman, A. Advanced (>second) line chemotherapy in the treatment of patients with recurrent epithelial ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 94–98. [Google Scholar] [CrossRef]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study. J. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Morphological subtypes of ovarian carcinoma: A review with emphasis on new developments and pathogenesis. Pathology 2011, 43, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Markman, M.; Webster, K.; Zanotti, K.; Peterson, G.; Kulp, B.; Belinson, J. Phase 2 trial of carboplatin plus tamoxifen in platinum-resistant ovarian cancer and primary carcinoma of the peritoneum. Gynecol. Oncol. 2004, 94, 404–408. [Google Scholar] [CrossRef]
- Velden, J.; Gitsch, G.; Wain, G.; Friedlander, M.; Hacker, N. Tamoxifen in patients with advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 1995, 5, 301–305. [Google Scholar] [CrossRef]
- Mangioni, C.; Franceschi, S.; Vecchia, C.; D’Incalci, M. High-dose medroxyprogesterone acetate (MPA) in advanced epithelial ovarian cancer resistant to first- or second-line chemotherapy. Gynecol. Oncol. 1981, 12, 314–318. [Google Scholar] [CrossRef]
- Malfetano, J.; Beecham, J.B.; Bundy, B.N.; Hatch, K.D. A Phase II Trial of Medroxyprogesterone Acetate in Epithelial Ovarian Cancers. Am. J. Clin. oncology 1993, 16, 149–151. [Google Scholar] [CrossRef]
- van Weelden, W.J.; Birkendahl, P.B.; Lalisang, R.I.; IntHout, J.; Kruitwagen, R.F.P.M.; Romano, A.; Pijnenborg, J.M.A. The effect of progestin therapy in advanced and recurrent endometrial cancer: A systematic review and meta-analysis. Int. J. Obstet. Gynaecol. 2022, 130, 143–152. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Ju, X.; Bi, R.; Chen, X.; Yang, W.; Wu, X. Hormone receptor expression profiles differ between primary and recurrent high-grade serous ovarian cancers. Oncotarget 2017, 8, 32848–32855. [Google Scholar] [CrossRef]
- Vetter, M.; Stadlmann, S.; Bischof, E.; Margarint, E.L.G.; Schötzau, A.; Singer, G.; Heinzelmann-Schwarz, V.; Montavon, C. Hormone Receptor Expression in Primary and Recurrent High-Grade Serous Ovarian Cancer and Its Implications in Early Maintenance Treatment. Int. J. Mol. Sci. 2022, 23, 14242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).