Quality of Life in Rectal Cancer Treatments: An Updated Systematic Review of Randomized Controlled Trials (2013–2023)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection and Analysis
2.5. Quality Assessment
3. Results
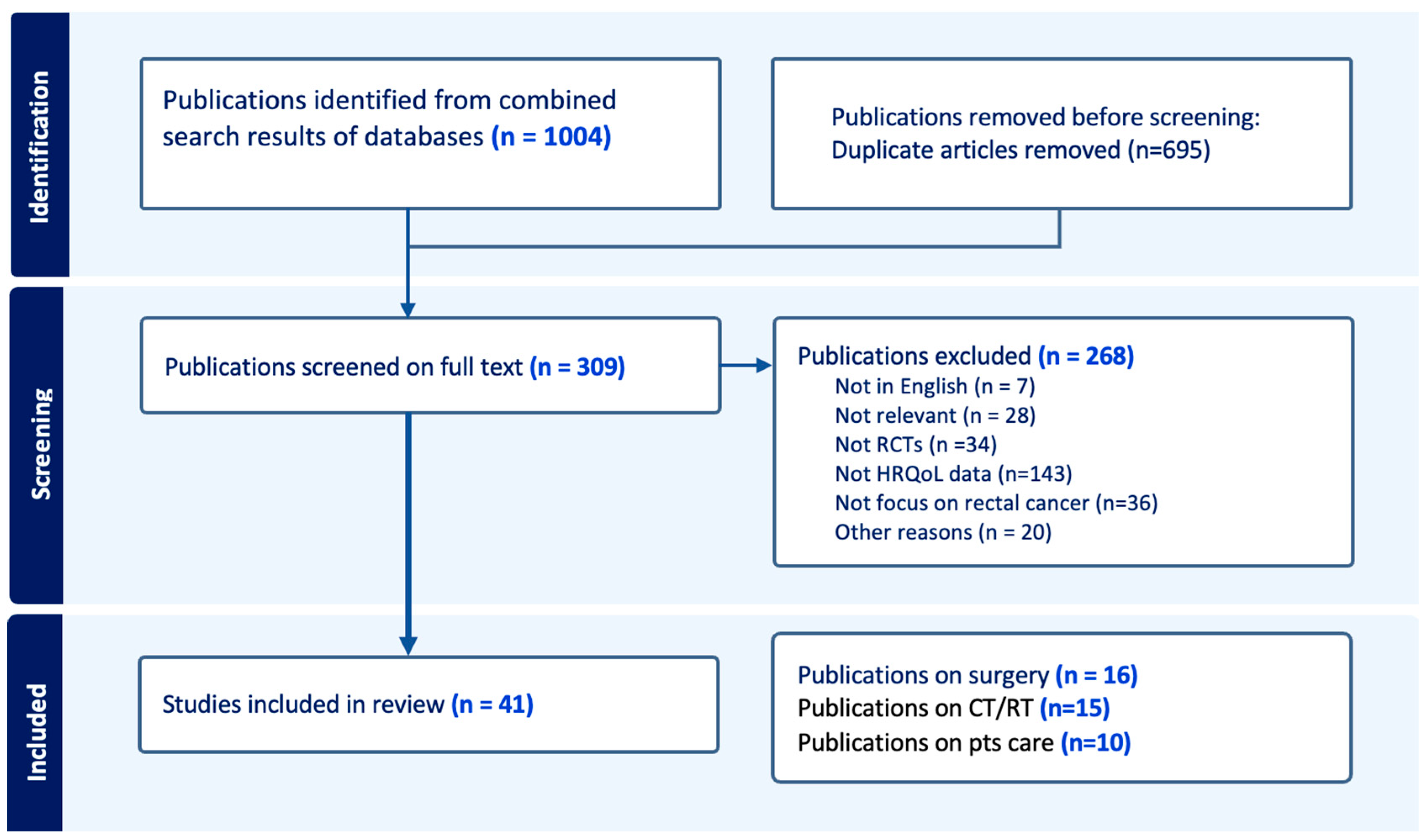
3.1. Study Selection
3.2. Synthesis of Clinicopathological Data
3.2.1. Surgical-Intervention Studies
3.2.2. Pre- and/or Post-CT and/or RT Studies
3.2.3. Patient Care Strategies Studies
| Author, Year [Ref.] | Duration of Study | Population | Intervention | Number of Patients | Questionnaire(s) | Times of Assessment | Key Results |
|---|---|---|---|---|---|---|---|
| Andersson et al., 2013 [16] | 2004–2010 | To compare HRQoL after laparoscopic vs. open surgery in patients undergoing LAR | Laparoscopic (n = 260) vs. Open Surgery (n = 125) | 385 | EORTC-QLQ CR38 EORTC-QLQ-C30 | 4 w, 6, 12 and 24 mo after operation | No difference |
| Russell et al., 2015 [17] | Jul 2004–Aug 2010 | To compare HRQoL after SS surgery vs. APR | SS surgery (n = 615) vs. APR (n = 372) SS: Sphincter Saving Surgery APR: Abdominoperineal Resection | 987 | FACT-C, EORTC QLQ CR38 | 12 mo after operation | Worse body image, sexual activity (only for males), micturition symptoms and GI tract symptoms for the APR group |
| Okkabaz et al., 2017 [18] | Jun 2009–N/A | To compare HRQoL after colonic j-pouch vs. side-to-end anastomosis | CJP (n = 29) vs. SCA (n = 28) CJP: Colon J-Pouch anastomosis SCA: Straight Colorectal anastomosis | 57 | SF-36 | 4, 8, 12 mo after stoma reversal | No difference |
| Musters et al., 2017 [19] | Feb 2013–Sep 2014 | To compare HRQoL after mesh closure vs. primary closure after APR | Mesh closure (n = 48) vs. Primary closure (n = 53) | 101 | EORTC-QLQ CR29 EORTC-QLQ-C30 SF36 | 3, 6, 9, 12 mo after the operation | No difference |
| Gadan et al., 2017 [20] | Dec 1999–June 2005 | To compare HRQoL after temporary ileostomy vs. no ileostomy in patients undergoing LAR | Temporary ileostomy (n = 41) vs. no ileostomy (n = 46) | 87 | EQ-5D-3L | 12 y after the operation | Worse self-reported HRQoL in those with major LARS |
| Jayne et al., 2017 [21] | Jan 2011–Sept 2014 | To compare HRQoL after robotic vs. laparoscopic surgery in patients undergoing LAR | Robotic Surgery (n = 237) vs. Laparoscopic surgery (n = 234) | 471 | SF 36 | 30 days, 6 mo after the operation | No difference |
| Kim et al., 2018 [22] | Feb 2012–March 2015 | To compare HRQoL after robotic vs. laparoscopic surgery in patients undergoing LAR | Robotic Surgery (n = 66) vs. Laparoscopic surgery (n = 73) | 139 | SF 36 | 3 we, 3 mo, 12 mo after the operation | No difference |
| Park et al., 2018 [23] | Feb 2011–Nov 2015 | To compare HRQoL after early vs. late ileostomy closure after LAR | Early ileostomy closure (8–13 days after surgery) (n = 55) vs. late ileostomy closure (>12 we after surgery) (n = 57) | 112 | EORTC-QLQ CR29 EORTC-QLQ-C30 SF36 | 3, 6, 12 mo after the operation | No difference |
| Parc et al., 2019 [24] | 2007–2009 | To compare HRQoL after colonic j-pouch vs. side-to-end anastomosis | CJP (n = 80) vs. SEA (n = 87) SEA: Side-to-End anastomosis CJP: Colon J-Pouch anastomosis | 167 | SF12, FACT-C | 6, 12 and 24 mo after surgery | No difference |
| Ribi et al., 2019 [25] | Sep 2005–May 2014 | To compare HRQoL after colonic j-pouch vs. side-to-end vs. straight colorectal anastomosis | CJP (n = 63) vs. SEA (n = 95) vs. SCA (n = 99) SEA: Side-to-End anastomosis CJP: Colon J-Pouch anastomosis SCA: Straight Colorectal anastomosis | 257 | FACT-C | 6, 12, 28 and 24 mo after surgery | No difference |
| Gavaruzzi et al., 2020 [26] | Oct 2009–Feb 2016 | To compare HRQoL after colonic j-pouch vs. straight colorectal anastomosis | CJP (n = 161) vs. SCA (n = 158) CJP: Colon J-Pouch anastomosis SCA: Straight Colorectal anastomosis | 319 | EORTC QLC C30 EORTC QLC C38 | 6, 12, 24 mo after the operation | No difference |
| Bach et al., 2020 [27] | Feb 2012–Dec 2014 | To compare HRQoL after organ preservation (LE) vs. radical surgery in cT2, or lower RC, N0, M0, who underwent short course RT | Organ preservation (LE) (n = 27) vs. radical surgery (n = 28) | 56 | EORTC QLC C30 EORTC QLC CR29 | 3, 6, 12, 24 and 36 mo after the operation | Worse QoL in the areas of health anxiety, role, and social function for the organ-preservation group |
| Elsner et al., 2021 [28] | Nov 2007–Mar 2014 | To compare HRQoL after early vs. late ileostomy closure after LAR | Early ileostomy closure (2 we) (n = 37) vs. late ileostomy closure (12 we) (n = 34) | 71 | EORTC- QLQ-C30 | 6 we and 4 mo after surgery | No difference |
| Dulskas et al., 2021 [29] | Dec 2011–Dec 2017 | To compare HRQoL after early vs. late ileostomy closure after LAR | Early ileostomy closure (30 days) (n = 26) vs. late ileostomy closure (90 days) (n = 25) | 51 | EORTC- QLQ-C30 | 36 mo | No difference |
| Ellebaek et al., 2023 [30] | Apr 2011–Sep 2018 | To compare HRQoL after early vs. late ileostomy closure after LAR | Early ileostomy closure (8-12 days) (n = 77) vs. late ileostomy closure (>3 mo) (n = 69) | 146 | GIQLI | 6 mo, 12 mo after the operation | No difference |
| Ahmadi-Amoli et al., 2023 [31] | 2016–2020 | To compare HRQoL after early vs. late ileostomy closure after LAR | Early ileostomy closure (2–3 we after the first two courses of adjuvant chemotherapy) (n = 50) vs. late ileostomy closure (2–3 we after the last course of adjuvant chemotherapy) (n = 54) | 104 | SF 36 | 3, 12 mo after the operation | No difference |
| Author, Year [Ref.] | Duration of Study | Population | Intervention | Number of Patients | Questionnaire(s) | Times of Assessment | Key Results |
|---|---|---|---|---|---|---|---|
| McLachlan et al., 2016 [32] | 2001–2006 | To compare HRQoL after short-course RT vs. long-course CRT in patients with cT3N0-2M0 | Short-course RT (n = 143) vs. Long-course CRT (n = 154) | 297 | EORTC QLQ C30—QLQC38 | 1, 2, 3, 6, 9, 12 mo after treatment | No difference |
| Wiltink et al., 2016 [33] | Jan 1996–Dec 1999 | To compare HRQoL after short course RT followed by TME vs. TME alone in patients with LARC planned for surgery | Short-course RT (study group, 5 × 5 Gy) followed by TME (n = 241) vs. TME alone (control group, n = 237) | 478 | EORTC QLQ C30—QLQCR29 | 3, 6, 12, 18, 24 mo, 5 y and 14 y | More bowel disfunction in the RT group |
| Araujo et al., 2018 [34] | Jan 2011—Feb 2013 | To compare HRQoL after nCRT (capecitabine) vs. nCRT (5-FU and leucovorin) in patients with stage II and III RC planned for surgery | nCRT (Group 1, capecitabine, n = 31) vs. nCRT (Group 2, 5-FU and leucovorin, n = 30) | 61 | EORTC QLQ C30—QLQCR38 | 6–8 we after nCRT, 30 days after surgery, after adjuvant CT, 1 year after the end of the treatment or stoma closure | No difference |
| Sang Hong et al., 2019 [35] | Nov 2008–Jun 2012 | To compare HRQoL after adjuvant CT with Fl vs. adjuvant CT with FOLFOX in patients with ypStage II or III RC | Adjuvant CT with FL (fluorouracil and leucovorin) (study group, n = 161) vs. adjuvant CT with FOLFOX (control group, n = 160) | 321 | EORTC QLQ C30—QLQCR38 | 2 mo, and at the end of treatment | No difference |
| Van der Valk et al., 2019 [36] | 2004–2013 | To compare HRQoL after adjuvant CT in patients with ypStage II or III RC who underwent preoperative (CT)RT | Adjuvant CT (Capecitabine, 8 courses) (study group, n = 115) vs. observation (Control group, n = 111) | 226 | EORTC QLQ C30—QLQCR38 | 1, 3, 6 and 12 mo after surgery | No difference |
| Sun et al., 2019 [37] | 2010–2015 | To compare HRQoL after tailored RCT before surgery | 5FU + RT/mFOLFOX6+ RT (nCRT group, n = 132) vs. mFOLFOX6 alone (nCT group, n = 88) | 220 | EORTC-QLQ C30/CR29 | 40 mo (median fu after the end of the treatment) | Better global health status, role functioning, and social functioning for the nCT group. Worse stool frequency, flatulence, fecal incontinence, sore skin, and embarrassment for the RT group. |
| Verweij et al., 2021 [38] | N/A | To compare HRQoL after tailored RCT before surgery | Dose-escalated CRT (5 × 3 Gy boost + CRT) (study group, n = 51) vs. CRT alone (control group, n = 64) | 115 | EORTC-QLQ C30/CR29 | 3, 6, 12, 18, 24 mo after start treatment | No difference |
| Erlandsson et al., 2021 [39] | 1998–2013 | To compare HRQoL after tailored RCT before surgery | 5 × 5 Gy RT plus surgery within 1 we (SRT, n = 51) vs. 5 × 5 Gy RT plus surgery after 4-8 we (SRT-delay, n = 57) vs. 25 × 2 Gy RT with surgery after 4-8 we (LRT-delay, n = 61) | 169 | EORTC QLC-C30 | 4–6 y | No difference |
| Kosmala et al., 2021 [40] | 2006–2010 | To compare HRQoL after tailored RCT before surgery | Preoperative CRT followed by TME and postoperative CT (5FU/OX) (n = 513) vs. preoperative CRT followed by TME and postoperative CT (5FU) (n = 512) | 1025 | EORTC QLQ C30 EORTC QLQ CR38 | After postoperative CT and during fu (1 and 3 y) | No difference |
| Fokas et al., 2022 [41] | 3 y | To compare HRQoL after tailored RCT before surgery | 5FU, leucovorin and oxaliplatin before 5FU/OXA CRT (Group A, n = 156) vs. CRT before CT (Group B, n = 150) | 306 | EORTC QLQ C30 | 1 and 3 y | No difference |
| Ganz et al., 2022 [42] | 2004–2010 | To compare HRQoL after tailored RCT before surgery | 5-FU + RT (n = 277) vs. 5-FU + OXA + RT (n = 266) vs. CAPE + RT (n = 283) vs. CAPE + OXA + RT(n = 286) | 1112 | SF36, FACT-C | 1, 5 y after surgery | No difference |
| Rouanet et al., 2022 [43] | May 2011–Oct 2014 | To compare HRQoL after tailored RCT and induction high-dose chemotherapy | Good responders after induction CT (n = 30): surgery (Group A, n = 11) or standard RCT plus surgery (Group B, n = 19) vs. poor responders after induction CT (n = 103): capecitabine (Group C, n = 52) or standard RCT (Group D, n = 51) before surgery | 133 | EORTC QLQ-C30 | 1, 4, 8, 12, 24, 36, 48, and 60 mo | No difference |
| Dijkstra et al., 2022 [44] | 2011–2026 | To compare HRQoL after short course RT, CT, TME vs. CRT, TME, and optional adjuvant CT in patients with LARC planned for surgery | Short course RT, CT, TME (study group, n = 243) vs. CRT, TME, and optional adjuvant CT (experimental group, n = 210) | 453 | EORTC QLQ C30 EORTC QLQ CR29 | 36 mo | No difference |
| Basch et al., 2023 [45] | Jun 2012–Dec 2018 | To compare HRQoL after preoperative FOLFOX vs. preoperative 5FUCRT in patients with LARC planned for surgery | Preoperative FOLFOX (n = 493) vs. preoperative 5FUCRT (n = 447) | 940 | EuroQoL EQ-5L | 12 mo after surgery | Lower rates of fatigue and neuropathy and better sexual function in FOLFOX group |
| Bascoul-Mollevi et al., 2023 [46] | 2012–2017 | To compare HRQoL after nCT then CRT, TME, and adjuvant CT vs. CRT, TME, and adjuvant CT in patients with LARC planned for surgery | Neoadjuvant CT (mFOLFIRINOX) then CRT, TME, and adjuvant CT (Study group, n = 183) vs. CRT, TME and adjuvant CT (experimental group, n = 187) | 370 | EORTC QLQ C30 EORTC QLQ CR29 | 1 y, 2 y after treatment | No difference |
| Author, Year [Ref.] | Duration of Study | Population | Intervention | Number of Patients | Questionnaire(s) | Times of Assessment | Results |
|---|---|---|---|---|---|---|---|
| Lee et al., 2013 [47] | Jul 2007–Sep 2011 | To compare HRQoL after rehabilitation vs. conventional care in patients who had undergone laparoscopic LAR with defunctioning ileostomy | Rehabilitation (n = 52) vs. conventional care (n = 46) | 98 | SF 36 | 30 days after the operation | No difference |
| Moug et al., 2019 [48] | Aug 2014–Mar 2016 | To compare HRQoL after prehabilitation vs. conventional care in patients planned for nCRT followed by potentially curative surgery | Prehabilitation (n = 24) vs. conventional care (n = 24) | 48 | EORTC-QLQ CR29 | 1-2 w before the operation | No difference |
| Cuicchi et al., 2020 [49] | Jan 2015–Oct 2015 | To compare HRQoL after percutaneous posterior tibial nerve stimulation vs. medical therapy alone in patients who underwent nCRT and LAR for cancer with LARS score ≥ 21 and ileostomy closed at least 18 mo earlier | Percutaneous posterior tibial nerve stimulation (study group, n = 6) vs. medical therapy alone (control group, n = 6) | 12 | EORTC-QLQ-C30 | 1 y | No difference |
| Yoon et al., 2020 [50] | Jun 2016–Mar 2018 | To compare HRQoL after probiotic vs. conventional care in patients undergoing ileostomy closure after LAR for RC | Probiotic (Lactobacillus plantarum) (study group, n = 17) vs. conventional care (control group, n = 19) | 36 | EORTC-QLQ CR29 EORTC-QLQ-C30 | 1 w and 3 w following ileostomy reversal | No difference |
| Morielli et al., 2021 [51] | N/A | To compare HRQoL after exercise vs. conventional care in patients undergoing nCRT | Exercise (study group, n = 16) vs. conventional care (control group, n = 16) | 32 | EORTC-QLQ CR29 EORTC-QLQ-C30 | Post nCRT, pre-surgery | Worst QoL in the study group (diarrhea p = 0.03 and social embarrassment p = 0.003) |
| Su et al., 2021 [52] | Jan 2015–Dec 2015 | To compare HRQoL after continuing care bundle vs. conventional care in patients with temporary ileostomy after LAR | Continuing care bundle (study group, n = 50) vs. conventional care (control group, n = 57) | 107 | Stoma-QOL | 4 and 12 weeks after surgery | Better QoL in the study group (p < 0.001) |
| Asnong et al., 2022 [53] | N/A | To compare HRQoL after PFMT vs. conventional care in patients after LAR and a minimal LARS score of 21/42 at 1 mo after surgery | PFMT (study group, n = 50) vs. conventional care (control group, n = 54) PFMT: pelvic floor muscle training | 104 | SF-12 | 1, 4, 6 and 12 mo after the operation | No difference |
| Van der Heijden et al., 2022 [54] | Oct 2017–Mar 2020 | To compare HRQoL after pelvic floor rehabilitation vs. conventional care in patients after LAR | Pelvic floor rehabilitation (study group, n = 44) vs. conventional care (control group, n = 51) | 95 | EORTC-QLQ-CR29 | 3 mo after surgery without temporary stoma or 6 we after stoma closure | No difference (only better HRQoL in patients suffering from fecal incontinence) |
| Pieniowskui et al., 2023 [55] | May 2016–Nov 2019 | To compare HRQoL after TAI vs. conventional care in patients after LAR and a LARS score of 21/42 at 6 mo after surgery | TAI (study group, n = 22) vs. conservative treatment (control group, n = 23) TAI: transanal irrigation | 45 | EORTC-QLQ-C30 | 12 mo | Better QoL in the intervention group |
| Kim et al., 2023 [56] | N/A | To compare HRQoL after bowel function improvement program vs. conventional care | Bowel function improvement program (study group, n = 21) vs. conventional care (control group, n = 21) | 42 | EORTC-QLQ-CR29 | 3 mo after surgery | No difference |
3.3. Quality of Included Studies and Risk of Bias
3.4. Review of Published Studies
3.4.1. Surgical Interventions Studies
3.4.2. Pre- and/or Post-CT and/or RT Studies
3.4.3. Patient-Care-Strategies Studies
4. Discussion
4.1. Surgical Interventions Studies and HRQoL
4.2. Pre- and/or Post-CT and/or RT Studies and HRQoL
4.3. Patient-Care-Strategies Studies and HRQoL
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Downing, A.; Morris, E.J.A.; Richards, M.; Corner, J.; Wright, P.; Sebag-Montefiore, D.; Finan, P.; Kind, P.; Wood, C.; Lawton, S.; et al. Health-Related Quality of Life After Colorectal Cancer in England: A Patient-Reported Outcomes Study of Individuals 12 to 36 Months After Diagnosis. J. Clin. Oncol. 2015, 33, 616–624. [Google Scholar] [CrossRef]
- Engel, J.; Kerr, J.; Schlesinger-Raab, A.; Eckel, R.; Sauer, H.; Hölzel, D. Quality of Life in Rectal Cancer Patients. Ann. Surg. 2003, 238, 203–213. [Google Scholar] [CrossRef]
- Camilleri-Brennan, J.; Steele, R.J.C. Quality of life after treatment for rectal cancer. J. Br. Surg. 1998, 85, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Gavaruzzi, T.; Giandomenico, F.; Del Bianco, P.; Lotto, L.; Perin, A.; Pucciarelli, S. Quality of Life After Surgery for Rectal Cancer. In Early Gastrointestinal Cancers II: Rectal Cancer, Proceedings of the 2nd St.Gallen EORTC Gastrointestinal Cancer Conference, Saint Gall, Switzerland, 6–8 March 2014; Springer: Cham, Switzerland, 2014; pp. 117–149. [Google Scholar]
- Paun, B.C.; Cassie, S.; MacLean, A.R.; Dixon, E.; Buie, W.D. Postoperative Complications Following Surgery for Rectal Cancer. Ann. Surg. 2010, 251, 807–818. [Google Scholar] [CrossRef]
- Vonk-Klaassen, S.M.; de Vocht, H.M.; den Ouden, M.E.M.; Eddes, E.H.; Schuurmans, M.J. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: A systematic review. Qual. Life Res. 2016, 25, 125–133. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Marijnen, C.A.M.; van de Velde, C.J.H.; Putter, H.; van den Brink, M.; Maas, C.P.; Martijn, H.; Rutten, H.J.; Wiggers, T.; Kranenbarg, E.K.; Leer, J.W.; et al. Impact of Short-Term Preoperative Radiotherapy on Health-Related Quality of Life and Sexual Functioning in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J. Clin. Oncol. 2005, 23, 1847–1858. [Google Scholar] [CrossRef]
- Dahlberg, M.; Glimelius, B.; Graf, W.; Påhlman, L. Preoperative irradiation affects functional results after surgery for rectal cancer. Dis. Colon Rectum 1998, 41, 543–549. [Google Scholar] [CrossRef]
- Riesco-Martinez, M.C.; Fernandez-Martos, C.; Gravalos-Castro, C.; Espinosa-Olarte, P.; La Salvia, A.; Robles-Diaz, L.; Modrego-Sanchez, A.; Garcia-Carbonero, R. Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers 2020, 12, 3655. [Google Scholar] [CrossRef] [PubMed]
- Brundage, M.; Blazeby, J.; Revicki, D.; Bass, B.; de Vet, H.; Duffy, H.; Efficace, F.; King, M.; Lam, C.L.; Moher, D.; et al. Patient-reported outcomes in randomized clinical trials: Development of ISOQOL reporting standards. Qual. Life Res. 2013, 22, 1161–1175. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Andersson, J.; Angenete, E.; Gellerstedt, M.; Angerås, U.; Jess, P.; Rosenberg, J.; Fürst, A.; Bonjer, J.; Haglind, E. Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br. J. Surg. 2013, 100, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.M.; Ganz, P.A.; Lopa, S.; Yothers, G.; Ko, C.Y.; Arora, A.; Atkins, J.N.; Bahary, N.; Soori, G.S.; Robertson, J.M.; et al. Comparative Effectiveness of Sphincter-Sparing Surgery Versus Abdominoperineal Resection in Rectal Cancer. Ann. Surg. 2015, 261, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Okkabaz, N.; Haksal, M.; Atici, A.E.; Altuntas, Y.E.; Gundogan, E.; Gezen, F.C.; Oncel, M. J-pouch vs. side-to-end anastomosis after hand-assisted laparoscopic low anterior resection for rectal cancer: A prospective randomized trial on short and long term outcomes including life quality and functional results. Int. J. Surg. 2017, 47, 4–12. [Google Scholar] [CrossRef]
- Musters, G.D.; Klaver, C.E.L.; Bosker, R.J.I.; Burger, J.W.A.; Van Duijvendijk, P.; Van Etten, B.; van Geloven, A.A.W.; de Graaf, E.J.R.; Hoff, C.; Leijtens, J.W.A.; et al. Biological mesh closure of the pelvic floor after extralevator abdominoperineal resection for rectal cancer: A multicenter randomized controlled trial (the BIOPEX-study). Ann. Surg. 2017, 265, 1074–1081. [Google Scholar] [CrossRef]
- Gadan, S.; Floodeen, H.; Lindgren, R.; Matthiessen, P. Does a Defunctioning Stoma Impair Anorectal Function After Low Anterior Resection of the Rectum for Cancer? A 12-Year Follow-up of a Randomized Multicenter Trial. Dis. Colon Rectum 2017, 60, 800–806. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs. Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer. JAMA 2017, 318, 1569. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.C.; Park, J.W.; Chang, H.J.; Kim, D.Y.; Nam, B.H.; Sohn, D.K.; Oh, J.H. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann. Surg. 2018, 267, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Danielsen, A.K.; Angenete, E.; Bock, D.; Marinez, A.C.; Haglind, E.; Jansen, J.E.; Skullman, S.; Wedin, A.; Rosenberg, J. Quality of life in a randomized trial of early closure of temporary ileostomy after rectal resection for cancer (EASY trial). J. Br. Surg. 2018, 105, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Parc, Y.; Ruppert, R.; Fuerst, A.; Golcher, H.; Zutshi, M.; Hull, T.; Tiret, E.; Hemminger, F.; Galandiuk, S.; Fender, S.; et al. Better Function with a Colonic J-Pouch or a Side-to-end Anastomosis?: A Randomized Controlled Trial to Compare the Complications, Functional Outcome, and Quality of Life in Patients with Low Rectal Cancer after a J-Pouch or a Side-to-end Anastomosis. Ann. Surg. 2019, 269, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ribi, K.; Marti, W.R.; Bernhard, J.; Grieder, F.; Graf, M.; Gloor, B.; Curti, G.; Zuber, M.; Demartines, N.; Andrieu, C.; et al. Quality of Life After Total Mesorectal Excision and Rectal Replacement: Comparing Side-to-End, Colon J-Pouch and Straight Colorectal Reconstruction in a Randomized, Phase III Trial (SAKK 40/04). Ann. Surg. Oncol. 2019, 26, 3568–3576. [Google Scholar] [CrossRef]
- Gavaruzzi, T.; Pace, U.; Giandomenico, F.; Pucciarelli, S.; Bianco, F.; Selvaggi, F.; Restivo, A.; Asteria, C.R.; Morpurgo, E.; Cuicchi, D.; et al. Colonic J-Pouch or Straight Colorectal Reconstruction after Low Anterior Resection for Rectal Cancer: Impact on Quality of Life and Bowel Function: A Multicenter Prospective Randomized Study. Dis. Colon Rectum 2020, 63, 1511–1523. [Google Scholar] [CrossRef]
- Bach, S.P.; Gilbert, A.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.G.; Khan, J.; et al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): A randomised, open-label feasibility study. Lancet Gastroenterol. Hepatol. 2021, 6, 92–105. [Google Scholar] [CrossRef]
- Elsner, A.T.; Brosi, P.; Walensi, M.; Uhlmann, M.; Egger, B.; Glaser, C.; Maurer, C.A. Closure of Temporary Ileostomy 2 Versus 12 Weeks After Rectal Resection for Cancer: A Word of Caution From a Prospective, Randomized Controlled Multicenter Trial. Dis. Colon Rectum 2021, 64, 1398–1406. [Google Scholar] [CrossRef]
- Dulskas, A.; Petrauskas, V.; Kuliavas, J.; Bickaite, K.; Kairys, M.; Pauza, K.; Kilius, A.; Sangaila, E.; Bausys, R.; Stratilatovas, E. Quality of life and bowel function following early closure of a temporary ileostomy in patients with rectal cancer: A report from a single-center randomized controlled trial. J. Clin. Med. 2021, 10, 768. [Google Scholar] [CrossRef]
- Ellebæk, M.B.; Perdawood, S.K.; Steenstrup, S.; Khalaf, S.; Kundal, J.; Möller, S.; Bang, J.C.; Støvring, J.; Qvist, N. Early versus late reversal of diverting loop ileostomy in rectal cancer surgery: A multicentre randomized controlled trial. Sci. Rep. 2023, 13, 5818. [Google Scholar] [CrossRef]
- Ahmadi-Amoli, H.; Rahimi, M.; Abedi-kichi, R.; Ebrahimian, N.; Hosseiniasl, S.M.; Hajebi, R.; Rahimpour, E. Early closure compared to late closure of temporary ileostomy in rectal cancer: A randomized controlled trial study. Langenbeck’s Arch. Surg. 2023, 408, 234. [Google Scholar] [CrossRef]
- McLachlan, S.A.; Fisher, R.J.; Zalcberg, J.; Solomon, M.; Burmeister, B.; Goldstein, D.; Leong, T.; Ackland, S.P.; McKendrick, J.; McClure, B.; et al. The impact on health-related quality of life in the first 12 months: A randomised comparison of preoperative short-course radiation versus long-course chemoradiation for T3 rectal cancer (Trans-Tasman Radiation Oncology Group Trial 01.04). Eur. J. Cancer 2016, 55, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wiltink, L.M.; Marijnen, C.A.M.; Kranenbarg, E.M.K.; Van De Velde, C.J.H.; Nout, R.A. A comprehensive longitudinal overview of health-related quality of life and symptoms after treatment for rectal cancer in the TME trial. Acta Oncol. 2016, 55, 502–508. [Google Scholar] [CrossRef]
- Araujo, R.O.; Vieira, F.M.; Victorino, A.P.; Torres, C.; Martins, I.; Guaraldi, S.; Valadão, M.; Linhares, E.; Ferreira, C.G.; Thuler, L.C. Quality of life in a randomized trial comparing two neoadjuvant regimens for locally advanced rectal cancer—INCAGI004. Support. Care Cancer 2022, 30, 6557–6572. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, S.Y.; Lee, J.S.; Nam, B.H.; Kim, K.P.; Kim, J.E.; Park, Y.S.; Park, J.O.; Baek, J.Y.; Kim, T.Y.; et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, M.J.M.; Hilling, D.E.; Meershoek-Klein Kranenbarg, E.; Peeters, K.C.M.J.; Kapiteijn, E.; Tsonaka, R.; van de Velde, C.J.H.; Marang-Van de Mheen, P.J. Quality of Life after Curative Resection for Rectal Cancer in Patients Treated with Adjuvant Chemotherapy Compared with Observation: Results of the Randomized Phase III SCRIPT Trial. Dis. Colon Rectum 2019, 62, 711–720. [Google Scholar] [CrossRef]
- Sun, W.; Dou, R.; Chen, J.; Lai, S.; Zhang, C.; Ruan, L.; Kang, L.; Deng, Y.; Lan, P.; Wang, L.; et al. Impact of Long-Course Neoadjuvant Radiation on Postoperative Low Anterior Resection Syndrome and Quality of Life in Rectal Cancer: Post Hoc Analysis of a Randomized Controlled Trial. Ann. Surg. Oncol. 2019, 26, 746–755. [Google Scholar] [CrossRef]
- Verweij, M.E.; Hoendervangers, S.; Couwenberg, A.M.; Burbach, J.P.M.; Berbee, M.; Buijsen, J.; Roodhart, J.; Reerink, O.; Pronk, A.; Consten, E.C.J.; et al. Impact of Dose-Escalated Chemoradiation on Quality of Life in Patients With Locally Advanced Rectal Cancer: 2-Year Follow-Up of the Randomized RECTAL-BOOST Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 694–703. [Google Scholar] [CrossRef]
- Erlandsson, J.; Fuentes, S.; Radu, C.; Frödin, J.E.; Johansson, H.; Brandberg, Y.; Holm, T.; Glimelius, B.; Martling, A. Radiotherapy regimens for rectal cancer: Long-term outcomes and health-related quality of life in the Stockholm III trial. BJS Open 2021, 5, zrab137. [Google Scholar] [CrossRef]
- Kosmala, R.; Fokas, E.; Flentje, M.; Sauer, R.; Liersch, T.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Arnold, D.; Hofheinz, R.D.; et al. Quality of life in rectal cancer patients with or without oxaliplatin in the randomised CAO/ARO/AIO-04 phase 3 trial. Eur. J. Cancer 2021, 144, 281–290. [Google Scholar] [CrossRef]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef]
- Ganz, P.A.; Hays, R.D.; Spritzer, K.L.; Rogatko, A.; Ko, C.Y.; Colangelo, L.H.; Arora, A.; Hopkins, J.O.; Evans, T.L.; Yothers, G. Health-related quality of life outcomes after neoadjuvant chemoradiotherapy for rectal cancer in NRG Oncology/NSABP R-04. Cancer 2022, 128, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Rullier, E.; Lelong, B.; Maingon, P.; Tuech, J.J.; Pezet, D.; Castan, F.; Nougaret, S.; GRECCAR Study Group. Tailored Strategy for Locally Advanced Rectal Carcinoma (GRECCAR 4): Long-term Results from a Multicenter, Randomized, Open-Label, Phase II Trial. Dis. Colon Rectum 2022, 65, 986–995. [Google Scholar] [CrossRef]
- Dijkstra, E.A.; Hospers, G.A.P.; Kranenbarg, E.M.K.; Fleer, J.; Roodvoets, A.G.H.; Bahadoer, R.R.; Guren, M.G.; Tjalma, J.J.J.; Putter, H.; Crolla, R.M.P.H.; et al. Quality of life and late toxicity after short-course radiotherapy followed by chemotherapy or chemoradiotherapy for locally advanced rectal cancer—The RAPIDO trial. Radiother. Oncol. 2022, 171, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Dueck, A.C.; Mitchell, S.A.; Mamon, H.; Weiser, M.; Saltz, L.; Gollub, M.; Rogak, L.; Ginos, B.; Mazza, G.L.; et al. Patient-Reported Outcomes during and after Treatment for Locally Advanced Rectal Cancer in the PROSPECT Trial (Alliance N1048). J. Clin. Oncol. 2023, 41, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Bascoul-Mollevi, C.; Gourgou, S.; Borg, C.; Etienne, P.L.; Rio, E.; Rullier, E.; Juzyna, B.; Castan, F.; Conroy, T. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER PRODIGE 23): Health-related quality of life longitudinal analysis. Eur. J. Cancer 2023, 186, 151–165. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, S.B.; Jang, J.H.; Park, J.S.; Hong, S.; Lee, T.G.; Ahn, S. Early rehabilitation versus conventional care after laparoscopic rectal surgery: A prospective, randomized, controlled trial. Surg. Endosc. 2013, 27, 3902–3909. [Google Scholar] [CrossRef]
- Moug, S.J.; Mutrie, N.; Barry, S.J.E.; Mackay, G.; Steele, R.J.C.; Boachie, C.; Buchan, C.; Anderson, A.S. Prehabilitation is Feasible in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy and may Minimize Physical Deterioration: Results from the REx trial. Colorectal. Dis. 2019, 21, 548–562. [Google Scholar]
- Cuicchi, D.; Di Fabio, F.; Guido, A.; Llimpe, F.L.R.; Morganti, A.G.; Ardizzoni, A.; Coscia, M.; Poggioli, G. Randomized Pilot Trial of Percutaneous Posterior Tibial Nerve Stimulation Versus Medical Therapy for the Treatment of Low Anterior Resection Syndrome: One-Year Follow-up. Dis. Colon Rectum 2020, 63, 1602–1609. [Google Scholar] [CrossRef]
- Yoon, B.J.; Oh, H.K.; Lee, J.; Cho, J.R.; Kim, M.J.; Kim, D.W.; Kang, S.B. Effects of probiotics on bowel function restoration following ileostomy closure in rectal cancer patients: A randomized controlled trial. Color. Dis. 2021, 23, 901–910. [Google Scholar] [CrossRef]
- Morielli, A.R.; Boulé, N.G.; Usmani, N.; Tankel, K.; Joseph, K.; Severin, D.; Fairchild, A.; Nijjar, T.; Courneya, K.S. Effects of exercise during and after neoadjuvant chemoradiation on symptom burden and quality of life in rectal cancer patients: A phase II randomized controlled trial. J. Cancer Surviv. 2023, 17, 1171–1183. [Google Scholar] [CrossRef]
- Su, X.; Zhong, M.H.; Ye, X.M.; Zhen, L.; Yin, X.X.; Qin, F.; Zhu, M.L.; Kuang, Y.Y.; Wang, H.Z. Effects of Evidence-Based Continuing Care Bundle on Health Outcomes in Rectal Cancer Patients With Temporary Stomas: A Multicenter Randomized Controlled Trial. Cancer Nurs. 2021, 44, 223–234. [Google Scholar] [CrossRef]
- Asnong, A.; D’Hoore, A.; van Kampen, M.; Wolthuis, A.; van Molhem, Y.; van Geluwe, B.; Devoogdt, N.; De Groef, A.; Guler Caamano Fajardo, I.; Geraerts, I. The role of pelvic floor muscle training on low anterior resection syndrome a multicenter randomized controlled trial. Ann. Surg. 2022, 276, 761–768. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, J.A.G.; Kalkdijk-Dijkstra, A.J.; Pierie, J.P.E.N.; van Westreenen, H.L.; Broens, P.M.A.; Klarenbeek, B.R. Pelvic Floor Rehabilitation After Rectal Cancer Surgery. Ann. Surg. 2022, 276, 38–45. [Google Scholar] [CrossRef]
- Pieniowski, E.H.A.; Bergström, C.M.; Nordenvall, C.A.M.; Westberg, K.S.; Johar, A.M.; Tumlin Ekelund, S.F.; Larsson, K.R.; Pekkari, K.J.; Jansson Palmer, G.C.; Lagergren, P.; et al. A Randomized Controlled Clinical Trial of Transanal Irrigation Versus Conservative Treatment in Patients with Low Anterior Resection Syndrome after Rectal Cancer Surgery. Ann. Surg. 2023, 277, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Oh, E.G.; Chu, S.H.; Park, J.; Lee, Y.J.; Kim, N.K. Effects of a bowel function improvement program for patients with rectal cancer surgery: A randomized controlled trial. Eur. J. Oncol. Nurs. 2023, 66, 102382. [Google Scholar] [CrossRef]
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu (accessed on 1 December 2024).
- Maguire, B.; Clancy, C.; Connelly, T.M.; Mehigan, B.J.; McCormick, P.; Altomare, D.F.; Gosselink, M.P.; Larkin, J.O. Quality of life meta-analysis following coloanal anastomosis versus abdominoperineal resection for low rectal cancer. Color. Dis. 2022, 24, 811–820. [Google Scholar] [CrossRef]
- Pucciarelli, S.; Del Bianco, P.; Toppan, P.; Serpentini, S.; Efficace, F.; Pasetto, L.M.; Friso, M.L.; De Salvo, G.L.; Nitti, D. Health-Related Quality of Life Outcomes in Disease-Free Survivors of Mid-Low Rectal Cancer After Curative Surgery. Ann. Surg. Oncol. 2008, 15, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.M.; Omer, D.M.; Lin, S.; Kim, J.K.; Yuval, J.B.; Verheij, F.S.; Qin, L.X.; Gollub, M.J.; Wu, A.J.; Lee, M.; et al. Organ Preservation and Survival by Clinical Response Grade in Patients With Rectal Cancer Treated With Total Neoadjuvant Therapy: A Secondary Analysis of the OPRA Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2350903, Erratum in: JAMA Netw. Open 2024, 7, e242456. Erratum in: JAMA Netw. Open 2025, 8, e256079. [Google Scholar] [CrossRef]
- van Oostendorp, S.E.; Smits, L.J.H.; Vroom, Y.; Detering, R.; Heymans, M.W.; Moons, L.M.G.; Tanis, P.J.; de Graaf, E.J.R.; Cunningham, C.; Denost, Q.; et al. Local recurrence after local excision of early rectal cancer: A meta-analysis of completion TME, adjuvant (chemo)radiation, or no additional treatment. Br. J. Surg. 2020, 107, 1719–1730. [Google Scholar] [CrossRef]
- Borstlap, W.A.; Coeymans, T.J.; Tanis, P.J.; Marijnen, C.A.; Cunningham, C.; Bemelman, W.A.; Tuynman, J.B. Meta-analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br. J. Surg. 2016, 103, 1105–1116. [Google Scholar] [CrossRef]
- Chude, G.G.; Rayate, N.V.; Patris, V.; Koshariya, M.; Jagad, R.; Kawamoto, J.; Lygidakis, N.J. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: Should we make an ileostomy as a routine procedure? A prospective randomized study. Hepato-Gastroenterol. 2008, 55, 1562–1567. [Google Scholar]
- Hanna, M.H.; Vinci, A.; Pigazzi, A. Diverting ileostomy in colorectal surgery: When is it necessary? Langenbeck’s Arch. Surg. 2015, 400, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Blok, R.D.; Stam, R.; Westerduin, E.; Borstlap, W.A.A.; Hompes, R.; Bemelman, W.A.; Tanis, P.J. Impact of an institutional change from routine to highly selective diversion of a low anastomosis after TME for rectal cancer. Eur. J. Surg. Oncol. 2018, 44, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Khan, S.M.; Garoufalia, Z.; Silva-Alvarenga, E.; Gefen, R.; Horesh, N.; Freund, M.R.; Wexner, S.D. When Is a Diverting Stoma Indicated after Low Anterior Resection? A Meta-analysis of Randomized Trials and Meta-Regression of the Risk Factors of Leakage and Complications in Non-Diverted Patients. J. Gastrointest. Surg. 2022, 26, 2368–2379. [Google Scholar] [CrossRef]
- Ahmad, N.Z.; Abbas, M.H.; Khan, S.U.; Parvaiz, A. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int. J. Color. Dis. 2021, 36, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Son, D.N.; Choi, D.J.; Woo, S.U.; Kim, J.; Keom, B.R.; Kim, C.H.; Baek, S.J.; Kim, S.H. Relationship between diversion colitis and quality of life in rectal cancer. World J. Gastroenterol. 2013, 19, 542–549. [Google Scholar] [CrossRef]
- Barina, A.; De Paoli, A.; Delrio, P.; Guerrieri, M.; Muratore, A.; Bianco, F.; Vespa, D.; Asteria, C.; Morpurgo, E.; Restivo, A.; et al. Rectal sparing approach after preoperative radio- and/or chemotherapy (RESARCH) in patients with rectal cancer: A multicentre observational study. Tech. Coloproctology 2017, 21, 633–640. [Google Scholar] [CrossRef]
- Downing, A.; Glaser, A.W.; Finan, P.J.; Wright, P.; Thomas, J.D.; Gilbert, A.; Corner, J.; Richards, M.; Morris, E.J.A.; Sebag-Montefiore, D. Functional Outcomes and Health-Related Quality of Life After Curative Treatment for Rectal Cancer: A Population-Level Study in England. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1132–1142. [Google Scholar] [CrossRef]
- Croese, A.D.; Lonie, J.M.; Trollope, A.F.; Vangaveti, V.N.; Ho, Y.H. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int. J. Surg. 2018, 56, 234–241. [Google Scholar] [CrossRef]
- Chen, T.Y.T.; Wiltink, L.M.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Laurberg, S.; Marijnen, C.A.M.; van de Velde, C.J. Bowel Function 14 Years After Preoperative Short-Course Radiotherapy and Total Mesorectal Excision for Rectal Cancer: Report of a Multicenter Randomized Trial. Clin. Color. Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef]
- Yeoh, E.K.; Holloway, R.H.; Fraser, R.J.; Botten, R.J.; Di Matteo, A.C.; Butters, J. Pathophysiology and natural history of anorectal sequelae following radiation therapy for carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e593–e599. [Google Scholar] [CrossRef]
- Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; Fyles, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309, Erratum in: Lancet Oncol. 2018, 19, e184. [Google Scholar]
- Skolka, M.; Shelly, S.; Pinto, M.V.; Dubey, D.; Oishi, T.; Uhm, J.H.; Santilli, A.; Staff, N.P.; Spinner, R.J.; Dyck, P.J.B.; et al. Clinical, Neurophysiologic, and Pathologic Features in Patients With Early-Onset Postradiation Neuropathy. Neurology 2023, 101, e1455–e1460. [Google Scholar] [CrossRef]
- Schrag, D.; Shi, Q.; Weiser, M.R.; Gollub, M.J.; Saltz, L.B.; Musher, B.L.; Goldberg, J.; Al Baghdadi, T.; Goodman, K.A.; McWilliams, R.R.; et al. Preoperative Treatment of Locally Advanced Rectal Cancer. N. Engl. J. Med. 2023, 389, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Keane, C.; Fearnhead, N.S.; Bordeianou, L.; Christensen, P.; Espin Basany, E.; Laurberg, S.; Mellgren, A.; Messick, C.; Orangio, G.R.; Verjee, A.; et al. International consensus definition of low anterior resection syndrome. Color. Dis. 2020, 22, 331–341. [Google Scholar] [CrossRef]
- McCutchan, G.M.; Hughes, D.; Davies, Z.; Torkington, J.; Morris, C.; Cornish, J.A. Acceptability and benefit of rectal irrigation in patients with low anterior resection syndrome: A qualitative study. Color. Dis. 2018, 20, O76–O84. [Google Scholar] [CrossRef]
- Rosen, H.R.; Boedecker, C.; Fürst, A.; Krämer, G.; Hebenstreit, J.; Kneist, W. “Prophylactic” transanal irrigation (TAI) to prevent symptoms of low anterior resection syndrome (LARS) after rectal resection: Results at 12-month follow-up of a controlled randomized multicenter trial. Tech. Coloproctology 2020, 24, 1247–1253. [Google Scholar] [CrossRef]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, 2012, CD007566. [Google Scholar]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Thompson, C.; Peterson, S.; Mandrola, J.; Beg, M.S. The Future of Wearable Technologies and Remote Monitoring in Health Care. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 115–121. [Google Scholar] [CrossRef]
- Sirintrapun, S.J.; Lopez, A.M. Telemedicine in Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Gillespie, E.F.; Cha, E.; Kim, S.Y.; Benvengo, S.; Chino, F.; Kang, J.J.; Li, Y.; Atkinson, T.M.; Lee, N.; et al. Impact of Telemedicine on Patient Satisfaction and Perceptions of Care Quality in Radiation Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Training. Assessing Risk of Bias in a Randomized Trial. Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 1 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negro, S.; Bergamo, F.; Dell’Atti, L.; Prete, A.A.; Galuppo, S.; Scarpa, M.; Bao, Q.R.; Ferrari, S.; Lonardi, S.; Spolverato, G.; et al. Quality of Life in Rectal Cancer Treatments: An Updated Systematic Review of Randomized Controlled Trials (2013–2023). Cancers 2025, 17, 2310. https://doi.org/10.3390/cancers17142310
Negro S, Bergamo F, Dell’Atti L, Prete AA, Galuppo S, Scarpa M, Bao QR, Ferrari S, Lonardi S, Spolverato G, et al. Quality of Life in Rectal Cancer Treatments: An Updated Systematic Review of Randomized Controlled Trials (2013–2023). Cancers. 2025; 17(14):2310. https://doi.org/10.3390/cancers17142310
Chicago/Turabian StyleNegro, Silvia, Francesca Bergamo, Lorenzo Dell’Atti, Alessandra Anna Prete, Sara Galuppo, Marco Scarpa, Quoc Riccardo Bao, Stefania Ferrari, Sara Lonardi, Gaya Spolverato, and et al. 2025. "Quality of Life in Rectal Cancer Treatments: An Updated Systematic Review of Randomized Controlled Trials (2013–2023)" Cancers 17, no. 14: 2310. https://doi.org/10.3390/cancers17142310
APA StyleNegro, S., Bergamo, F., Dell’Atti, L., Prete, A. A., Galuppo, S., Scarpa, M., Bao, Q. R., Ferrari, S., Lonardi, S., Spolverato, G., & Urso, E. D. L. (2025). Quality of Life in Rectal Cancer Treatments: An Updated Systematic Review of Randomized Controlled Trials (2013–2023). Cancers, 17(14), 2310. https://doi.org/10.3390/cancers17142310









