Cell Carriers for Oncolytic Virus Delivery: Prospects for Systemic Administration
Simple Summary
Abstract
1. Introduction
2. What Makes a Perfect Cell Carrier?
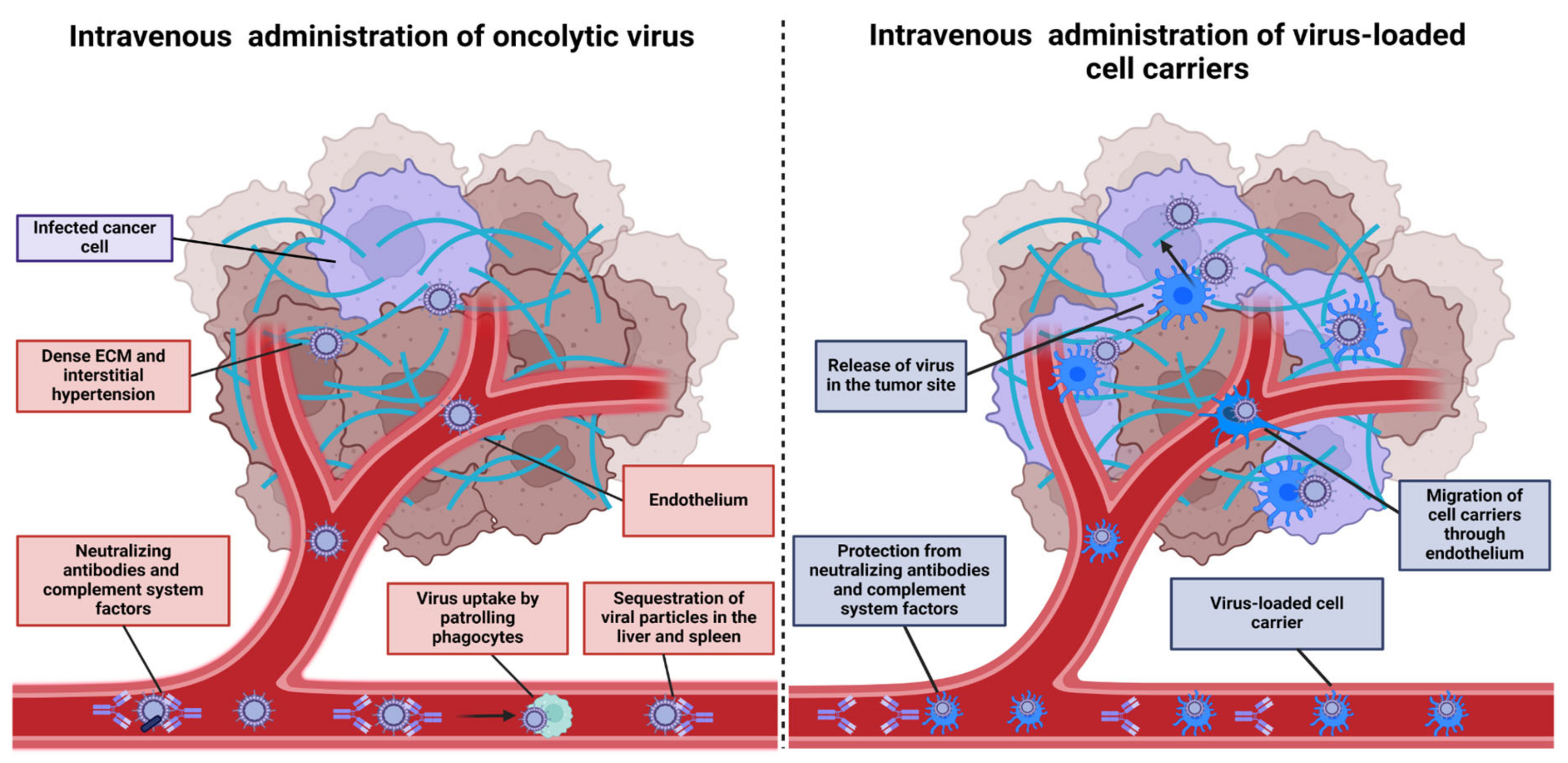
3. Cell-Based Carriers for OV Delivery
3.1. Mesenchymal Stem Cells (MSCs)
3.2. Neural Stem Cells (NSCs)
3.3. Blood Outgrowth Endothelial Cells (BOECs)
3.4. Cancer Cell Lines and Other Immortalized Cell Lines
4. Immune Cells as OV Carriers
4.1. T-Cells and Cytokine-Induced Killer (CIK) Cells
4.2. Monocytes and Macrophages
4.3. Myeloid-Derived Suppressor Cells (MDSCs)
4.4. Dendritic Cells (DCs)
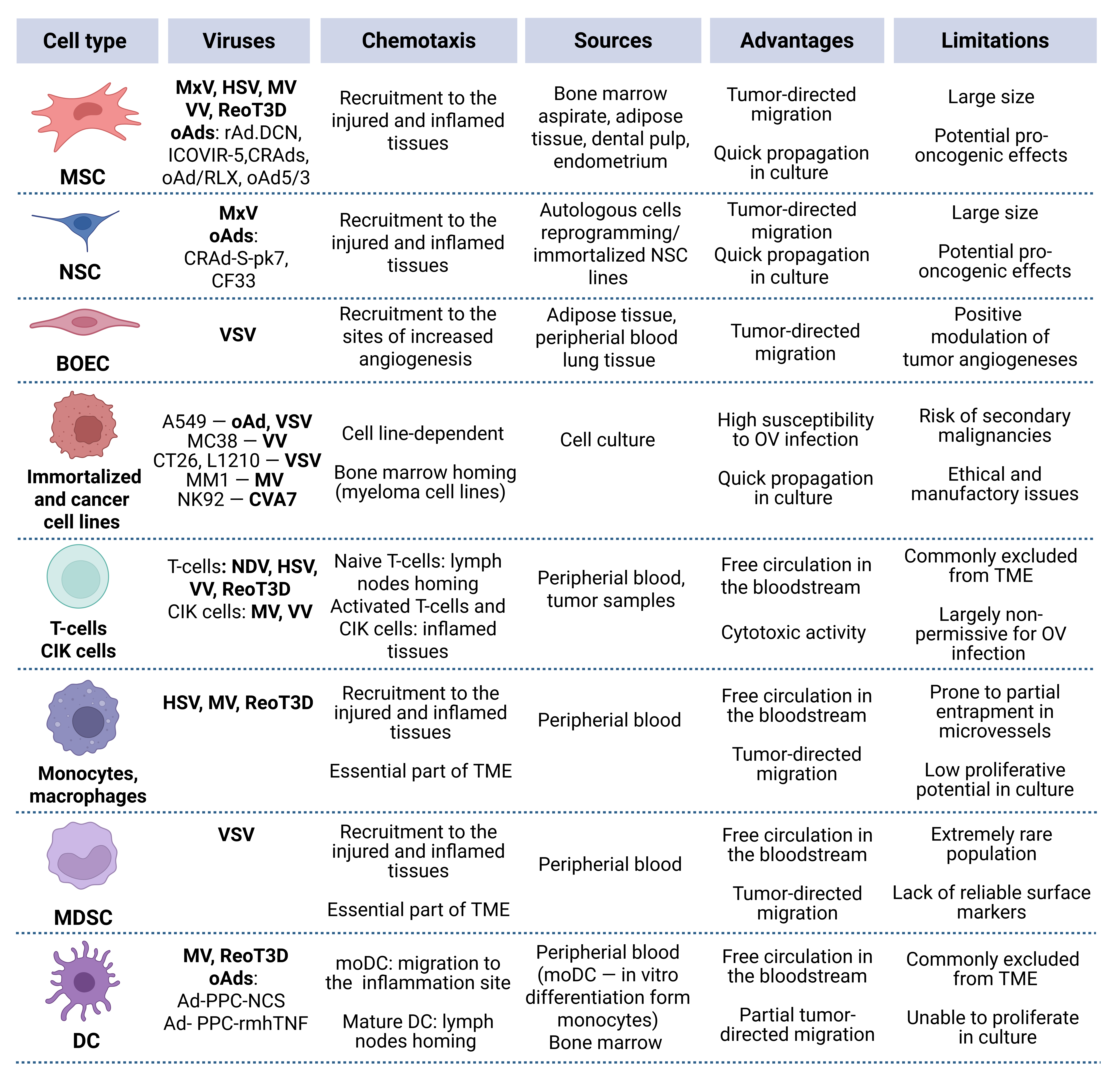
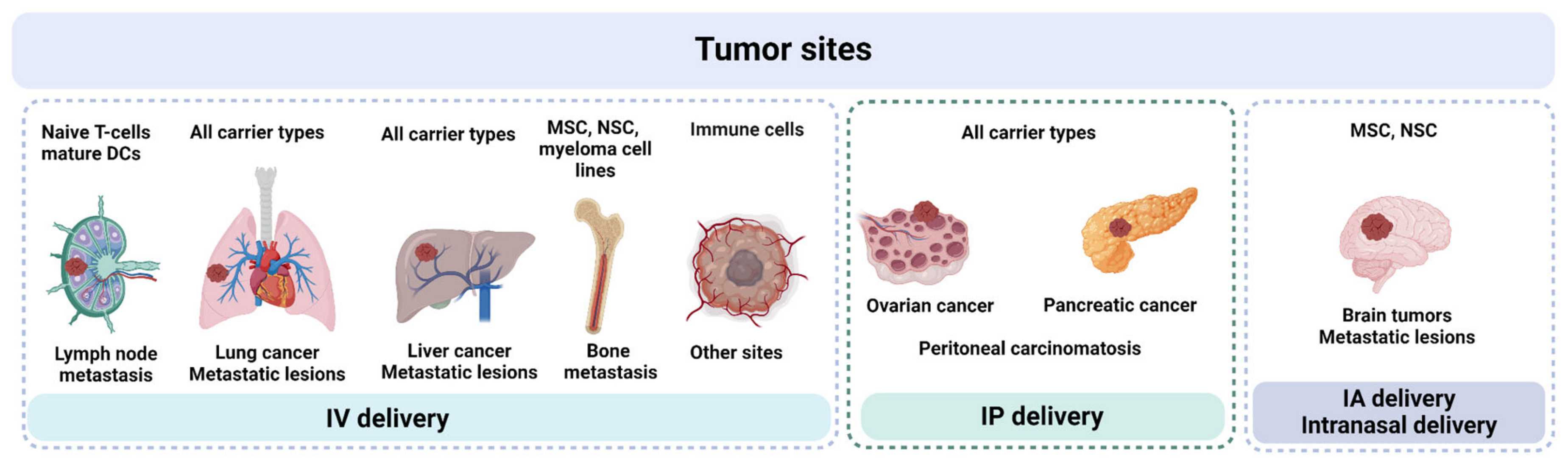
5. Comparative Analysis and Selection of Cell Carriers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.L.; Lee, P.W. Reovirus as a novel oncolytic agent. J. Clin. Investig. 2000, 105, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J. Live viruses in cancer treatment. Oncology 2002, 16, 1483–1492. [Google Scholar] [PubMed]
- Heise, C.; Kirn, D.H. Replication-selective adenoviruses as oncolytic agents. J. Clin. Investig. 2000, 105, 847–851. [Google Scholar]
- Msaouel, P.; Iankov, I.D.; Dispenzieri, A.; Galanis, E. Attenuated oncolytic measles virus strains as cancer therapeutics. Curr. Pharm. Biotechnol. 2012, 13, 1732–1741. [Google Scholar] [CrossRef]
- Kirn, D. Replication-selective oncolytic adenoviruses: Virotherapy aimed at genetic targets in cancer. Oncogene 2000, 19, 6660–6669. [Google Scholar]
- Takano, G.; Esaki, S.; Goshima, F.; Enomoto, A.; Hatano, Y.; Ozaki, H.; Watanabe, T.; Sato, Y.; Kawakita, D.; Murakami, S.; et al. Oncolytic activity of naturally attenuated herpes-simplex virus HF10 against an immunocompetent model of oral carcinoma. Mol. Ther. Oncolytics 2021, 20, 220–227. [Google Scholar] [CrossRef]
- Hosseini, M.; Farassati, F.S.; Farassati, F. Targeting Cancer Stem Cells by Oncolytic Viruses and Nano-Mediated Delivery. OncoTargets Ther. 2020, 13, 9349–9350. [Google Scholar] [CrossRef]
- Eriksson, M.; Guse, K.; Bauerschmitz, G.; Virkkunen, P.; Tarkkanen, M.; Tanner, M.; Hakkarainen, T.; Kanerva, A.; Desmond, R.A.; Pesonen, S.; et al. Oncolytic adenoviruses kill breast cancer initiating CD44+CD24-/low cells. Mol. Ther. 2007, 15, 2088–2093. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Chen, N.G.; Warner, S.G. Oncolytic Virotherapy versus Cancer Stem Cells: A Review of Approaches and Mechanisms. Cancers 2018, 10, 124. [Google Scholar] [CrossRef]
- Jazowiecka-Rakus, J.; Sochanik, A.; Hadryś, A.; Fidyk, W.; Chmielik, E.; Rahman, M.M.; McFadden, G. Combination of LIGHT (TNFSF14)-Armed Myxoma Virus Pre-Loaded into ADSCs and Gemcitabine in the Treatment of Experimental Orthotopic Murine Pancreatic Adenocarcinoma. Cancers 2022, 14, 2022. [Google Scholar] [CrossRef]
- Hwang, J.K.; Hong, J.; Yun, C.O. Oncolytic Viruses and Immune Checkpoint Inhibitors: Preclinical Developments to Clinical Trials. Int. J. Mol. Sci. 2020, 21, 8627. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Chi, H.; Liu, Y.; Yu, G. The combination therapy of oncolytic virotherapy. Front. Pharmacol. 2024, 15, 1380313. [Google Scholar] [CrossRef]
- El-Sayes, N.; Walsh, S.; Vito, A.; Reihani, A.; Ask, K.; Wan, Y.; Mossman, K. IFNAR blockade synergizes with oncolytic VSV to prevent virus-mediated PD-L1 expression and promote antitumor T cell activity. Mol. Ther. Oncolytics 2022, 25, 16–30. [Google Scholar] [CrossRef]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017, 8, 14754. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Fong, Y.; Warner, S.G. Oncolytic Virotherapy for Cancer: Clinical Experience. Biomedicines 2021, 9, 419. [Google Scholar] [CrossRef]
- Bradbury, P.A.; Morris, D.G.; Nicholas, G.; Tu, D.; Tehfe, M.; Goffin, J.R.; Shepherd, F.A.; Gregg, R.W.; Rothenstein, J.; Lee, C.; et al. Canadian Cancer Trials Group (CCTG) IND211: A randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer 2018, 120, 142–148. [Google Scholar] [CrossRef]
- Cohn, D.E.; Sill, M.W.; Walker, J.L.; O’Malley, D.; Nagel, C.I.; Rutledge, T.L.; Bradley, W.; Richardson, D.L.; Moxley, K.M.; Aghajanian, C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin(R)) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 146, 477–483. [Google Scholar] [CrossRef]
- Monga, V.; Miller, B.J.; Tanas, M.; Boukhar, S.; Allen, B.; Anderson, C.; Stephens, L.; Hartwig, S.; Varga, S.; Houtman, J.; et al. Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: Phase IB/II trial. J. Immunother. Cancer 2021, 9, e003119. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Yoon, A.R.; Rivera-Cruz, C.; Gimble, J.M.; Yun, C.O.; Figueiredo, M.L. Immunotherapy by mesenchymal stromal cell delivery of oncolytic viruses for treating metastatic tumors. Mol. Ther. Oncolytics 2022, 25, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Milhem, M.M.; Sacco, J.J.; Michels, J.; In, G.K.; Muñoz-Couselo, E.; Schadendorf, D.; Beasley, G.M.; Niu, J.J.; Chmielowski, B.; et al. LBA46 Primary efficacy, safety, and survival data from the registration-intended cohort of patients with anti–PD-1–failed melanoma from the IGNYTE clinical trial with RP1 combined with nivolumab. Ann. Oncol. 2024, 35, S1236–S1237. [Google Scholar] [CrossRef]
- Tyson, M.D.; Uchio, E.M.; Nam, J.-K.; Joshi, S.S.; Bivalacqua, T.J.; Steinberg, G.D.; Kitamura, H.; Tran, B.; Li, R. Final results: Bond-003 cohort c- phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for high-risk bcg-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 2025, 213. [Google Scholar] [CrossRef]
- Willmon, C.; Harrington, K.; Kottke, T.; Prestwich, R.; Melcher, A.; Vile, R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 2009, 17, 1667–1676. [Google Scholar]
- Masurier, C.; Salomon, B.; Guettari, N.; Pioche, C.; Lachapelle, F.; Guigon, M.; Klatzmann, D. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 1998, 72, 7822–7829. [Google Scholar] [CrossRef]
- Engering, A.; Van Vliet, S.J.; Geijtenbeek, T.B.; Van Kooyk, Y. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood 2002, 100, 1780–1786. [Google Scholar] [CrossRef]
- Groeneveldt, C.; Kinderman, P.; Griffioen, L.; Rensing, O.; Labrie, C.; van den Wollenberg, D.J.M.; Hoeben, R.C.; Coffey, M.; Loghmani, H.; Verdegaal, E.M.E.; et al. Neutralizing Antibodies Impair the Oncolytic Efficacy of Reovirus but Permit Effective Combination with T cell-Based Immunotherapies. Cancer Immunol. Res. 2024, 12, 334–349. [Google Scholar] [CrossRef]
- Power, A.T.; Wang, J.; Falls, T.J.; Paterson, J.M.; Parato, K.A.; Lichty, B.D.; Stojdl, D.F.; Forsyth, P.A.; Atkins, H.; Bell, J.C. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 2007, 15, 123–130. [Google Scholar] [CrossRef]
- Groeneveldt, C.; van den Ende, J.; van Montfoort, N. Preexisting immunity: Barrier or bridge to effective oncolytic virus therapy? Cytokine Growth Factor. Rev. 2023, 70, 1–12. [Google Scholar] [CrossRef]
- Hill, C.; Carlisle, R. Achieving systemic delivery of oncolytic viruses. Expert. Opin. Drug Deliv. 2019, 16, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Errington, F.; Diaz, R.M.; Pandha, H.S.; Harrington, K.J.; Melcher, A.A.; Vile, R.G. The case of oncolytic viruses versus the immune system: Waiting on the judgment of Solomon. Hum. Gene Ther. 2009, 20, 1119–1132. [Google Scholar] [PubMed]
- Fulci, G.; Breymann, L.; Gianni, D.; Kurozomi, K.; Rhee, S.S.; Yu, J.; Kaur, B.; Louis, D.N.; Weissleder, R.; Caligiuri, M.A.; et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12873–12878. [Google Scholar]
- Lun, X.Q.; Jang, J.H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 2009, 15, 2777–2788. [Google Scholar] [CrossRef]
- Kambara, H.; Saeki, Y.; Chiocca, E.A. Cyclophosphamide allows for in vivo dose reduction of a potent oncolytic virus. Cancer Res. 2005, 65, 11255–11258. [Google Scholar]
- Guo, Z.S.; Parimi, V.; O’Malley, M.E.; Thirunavukarasu, P.; Sathaiah, M.; Austin, F.; Bartlett, D.L. The combination of immunosuppression and carrier cells significantly enhances the efficacy of oncolytic poxvirus in the pre-immunized host. Gene Ther. 2010, 17, 1465–1475. [Google Scholar] [CrossRef]
- Davola, M.E.; Mossman, K.L. Oncolytic viruses: How “lytic” must they be for therapeutic efficacy? Oncoimmunology 2019, 8, e1581528. [Google Scholar] [CrossRef]
- Ricca, J.M.; Oseledchyk, A.; Walther, T.; Liu, C.; Mangarin, L.; Merghoub, T.; Wolchok, J.D.; Zamarin, D. Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy. Mol. Ther. 2018, 26, 1008–1019. [Google Scholar] [CrossRef]
- Cyrelle Ornella, M.S.; Kim, J.J.; Cho, E.; Cho, M.; Hwang, T.H. Dose Considerations for Vaccinia Oncolytic Virus Based on Retrospective Reanalysis of Early and Late Clinical Trials. Vaccines 2024, 12, 1010. [Google Scholar] [CrossRef]
- Chen, L.; Ma, Z.; Xu, C.; Xie, Y.; Ouyang, D.; Song, S.; Zhao, X.; Liu, F. Progress in oncolytic viruses modified with nanomaterials for intravenous application. Cancer Biol. Med. 2023, 20, 830–855. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kottke, T.; Willmon, C.; Galivo, F.; Wongthida, P.; Diaz, R.M.; Thompson, J.; Ryno, P.; Barber, G.N.; Chester, J.; et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ilett, E.J.; Prestwich, R.J.; Kottke, T.; Errington, F.; Thompson, J.M.; Harrington, K.J.; Pandha, H.S.; Coffey, M.; Selby, P.J.; Vile, R.G.; et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009, 16, 689–699. [Google Scholar] [CrossRef]
- Ong, H.T.; Hasegawa, K.; Dietz, A.B.; Russell, S.J.; Peng, K.W. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007, 14, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Berkeley, R.A.; Steele, L.P.; Mulder, A.A.; van den Wollenberg, D.J.M.; Kottke, T.J.; Thompson, J.; Coffey, M.; Hoeben, R.C.; Vile, R.G.; Melcher, A.; et al. Antibody-Neutralized Reovirus Is Effective in Oncolytic Virotherapy. Cancer Immunol. Res. 2018, 6, 1161–1173. [Google Scholar] [CrossRef]
- Reagan, M.R.; Kaplan, D.L. Concise review: Mesenchymal stem cell tumor-homing: Detection methods in disease model systems. Stem Cells 2011, 29, 920–927. [Google Scholar] [CrossRef]
- Bunuales, M.; Garcia-Aragoncillo, E.; Casado, R.; Quetglas, J.I.; Hervas-Stubbs, S.; Bortolanza, S.; Benavides-Vallve, C.; Ortiz-de-Solorzano, C.; Prieto, J.; Hernandez-Alcoceba, R. Evaluation of monocytes as carriers for armed oncolytic adenoviruses in murine and Syrian hamster models of cancer. Hum. Gene Ther. 2012, 23, 1258–1268. [Google Scholar] [CrossRef]
- Hamada, K.; Zhang, T.; Desaki, J.; Nakashiro, K.; Itoh, H.; Tani, K.; Koyama, Y.; Hamakawa, H. Carrier cell-mediated cell lysis of squamous cell carcinoma cells by squamous cell carcinoma antigen 1 promoter-driven oncolytic adenovirus. J. Gene Med. 2010, 12, 545–554. [Google Scholar] [CrossRef]
- Ghasemi Darestani, N.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal stem cell-released oncolytic virus: An innovative strategy for cancer treatment. Cell Commun. Signal 2023, 21, 43. [Google Scholar] [CrossRef]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2014, 158, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; He, Z. Mesenchymal stem cell carriers enhance anti-tumor efficacy of oncolytic virotherapy. Oncol. Lett. 2021, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.K.; Butler, G.; Dowdy, S.C.; Mariani, A.; Knutson, K.L.; Federspiel, M.J.; Russell, S.J.; Galanis, E.; Dietz, A.B.; Peng, K.W. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J. Transl. Med. 2013, 11, 1479–5876. [Google Scholar]
- Wang, Y.; Huso, D.L.; Harrington, J.; Kellner, J.; Jeong, D.K.; Turney, J.; McNiece, I.K. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy 2005, 7, 509–519. [Google Scholar] [CrossRef]
- Borgonovo, T.; Solarewicz, M.M.; Vaz, I.M.; Daga, D.; Rebelatto, C.L.; Senegaglia, A.C.; Ribeiro, E.; Cavalli, I.J.; Brofman, P.S. Emergence of clonal chromosomal alterations during the mesenchymal stromal cell cultivation. Mol. Cytogenet. 2015, 8, 94. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Afkhami, H.; Mahmoudvand, G.; Fakouri, A.; Shadab, A.; Mahjoor, M.; Komeili Movahhed, T. New insights in application of mesenchymal stem cells therapy in tumor microenvironment: Pros and cons. Front. Cell Dev. Biol. 2023, 11, 1255697. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Kim, B.-S.; Ryu, D.-B.; Kim, T.W.; Park, G.; Min, C.-K. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-γ and poly(I:C) priming through promoting the expression of indoleamine 2,3-dioxygenase. Stem Cell Res. Ther. 2021, 12, 37. [Google Scholar] [CrossRef]
- Hass, R. Role of MSC in the tumor microenvironment. Cancers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Gupta, S.; Mohanty, S. Mesenchymal stem cells modulate the immune system in developing therapeutic interventions. In Immune Response Activation and Immunomodulation; Tyagi, R.K., Bisen, P.S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Blanc, K.L.; Dazzi, F.; English, K.; Farge, D.; Galipeau, J.; Horwitz, E.M.; Kadri, N.; Krampera, M.; Lalu, M.M.; Nolta, J.; et al. ISCT MSC committee statement on the US FDA approval of allogenic bone-marrow mesenchymal stromal cells. Cytotherapy 2025, 27, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.R.; Hong, J.; Li, Y.; Shin, H.C.; Lee, H.; Kim, H.S.; Yun, C.O. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4503–4514. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Wang, T.; Kong, F.; Zhang, H.; Yi, J.; Dong, X.; Duan, H.; Tao, N.; Yang, Y.; et al. Therapeutic effects of mesenchymal stem cells loaded with oncolytic adenovirus carrying decorin on a breast cancer lung metastatic mouse model. Mol. Ther. Oncolytics 2022, 24, 486–496. [Google Scholar] [CrossRef]
- Hoyos, V.; Del Bufalo, F.; Yagyu, S.; Ando, M.; Dotti, G.; Suzuki, M.; Bouchier-Hayes, L.; Alemany, R.; Brenner, M.K. Mesenchymal Stromal Cells for Linked Delivery of Oncolytic and Apoptotic Adenoviruses to Non-small-cell Lung Cancers. Mol. Ther. 2015, 23, 1497–1506. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Mathis, J.M.; Banerjee, N.S.; Moon, A.S.; Hess, A.; Rocconi, R.P.; Numnum, T.M.; Everts, M.; Chow, L.T.; et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res. Treat. 2007, 105, 157–167. [Google Scholar] [CrossRef]
- Jazowiecka-Rakus, J.; Sochanik, A.; Rusin, A.; Hadryś, A.; Fidyk, W.; Villa, N.; Rahman, M.M.; Chmielik, E.; Franco, L.S.; McFadden, G. Myxoma Virus-Loaded Mesenchymal Stem Cells in Experimental Oncolytic Therapy of Murine Pulmonary Melanoma. Mol. Ther. Oncolytics 2020, 18, 335–350. [Google Scholar] [CrossRef]
- Leoni, V.; Gatta, V.; Palladini, A.; Nicoletti, G.; Ranieri, D.; Dall’Ora, M.; Grosso, V.; Rossi, M.; Alviano, F.; Bonsi, L.; et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget 2015, 6, 34774–34787. [Google Scholar] [CrossRef]
- Pereboeva, L.; Komarova, S.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells 2003, 21, 389–404. [Google Scholar] [CrossRef]
- Conget, P.A.; Minguell, J.J. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp. Hematol. 2000, 28, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Nam, J.P.; Hong, J.; Oh, E.; Shin, H.C.; Kim, H.S.; Kim, S.W.; Yun, C.O. Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. J. Control. Release Off. J. Control. Release Soc. 2019, 305, 75–88. [Google Scholar] [CrossRef]
- Ong, H.T.; Federspiel, M.J.; Guo, C.M.; Ooi, L.L.; Russell, S.J.; Peng, K.W.; Hui, K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013, 59, 999–1006. [Google Scholar]
- Garcia-Castro, J.; Alemany, R.; Cascallo, M.; Martinez-Quintanilla, J.; Arriero Mdel, M.; Lassaletta, A.; Madero, L.; Ramirez, M. Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: An exploratory study. Cancer Gene Ther. 2010, 17, 476–483. [Google Scholar] [CrossRef]
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940. [Google Scholar] [CrossRef]
- Cui, L.L.; Kerkelä, E.; Bakreen, A.; Nitzsche, F.; Andrzejewska, A.; Nowakowski, A.; Janowski, M.; Walczak, P.; Boltze, J.; Lukomska, B.; et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 2015, 6, 11. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria de la Torre, R.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Na Kim, H.; Yeol Kim, D.; Hee Oh, S.; Sook Kim, H.; Suk Kim, K.; Hyu Lee, P. Feasibility and Efficacy of Intra-Arterial Administration of Mesenchymal Stem Cells in an Animal Model of Double Toxin-Induced Multiple System Atrophy. Stem Cells Transl. Med. 2017, 6, 1424–1433. [Google Scholar] [CrossRef]
- Du, W.; Seah, I.; Bougazzoul, O.; Choi, G.; Meeth, K.; Bosenberg, M.W.; Wakimoto, H.; Fisher, D.; Shah, K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc. Natl. Acad. Sci. USA 2017, 114, E6157–E6165. [Google Scholar] [CrossRef]
- Duebgen, M.; Martinez-Quintanilla, J.; Tamura, K.; Hingtgen, S.; Redjal, N.; Wakimoto, H.; Shah, K. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. J. Natl. Cancer Inst. 2014, 106, dju090. [Google Scholar] [CrossRef]
- Komarova, S.; Kawakami, Y.; Stoff-Khalili, M.A.; Curiel, D.T.; Pereboeva, L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol. Cancer Ther. 2006, 5, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.K.; Maeyama, Y.; Lin, Y.; Butler, G.W.; Russell, H.M.; Galanis, E.; Russell, S.J.; Dietz, A.B.; Peng, K.W. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin. Cancer Res. 2009, 15, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Jazowiecka-Rakus, J.; Hadrys, A.; Rahman, M.M.; McFadden, G.; Fidyk, W.; Chmielik, E.; Pazdzior, M.; Grajek, M.; Kozik, V.; Sochanik, A. Myxoma Virus Expressing LIGHT (TNFSF14) Pre-Loaded into Adipose-Derived Mesenchymal Stem Cells Is Effective Treatment for Murine Pancreatic Adenocarcinoma. Cancers 2021, 13, 1394. [Google Scholar] [CrossRef]
- Babaei, A.; Bannazadeh Baghi, H.; Nezhadi, A.; Jamalpoor, Z. In Vitro Anti-cancer Activity of Adipose-Derived Mesenchymal Stem Cells Increased after Infection with Oncolytic Reovirus. Adv. Pharm. Bull. 2021, 11, 361–370. [Google Scholar] [CrossRef]
- Jazowiecka-Rakus, J.; Pogoda-Mieszczak, K.; Rahman, M.M.; McFadden, G.; Sochanik, A. Adipose-Derived Stem Cells as Carrier of Pro-Apoptotic Oncolytic Myxoma Virus: To Cross the Blood-Brain Barrier and Treat Murine Glioma. Int. J. Mol. Sci. 2024, 25, 11225. [Google Scholar] [CrossRef]
- Josiah, D.T.; Zhu, D.; Dreher, F.; Olson, J.; McFadden, G.; Caldas, H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol. Ther. 2010, 18, 377–385. [Google Scholar] [CrossRef]
- Draganov, D.D.; Santidrian, A.F.; Minev, I.; Nguyen, D.; Kilinc, M.O.; Petrov, I.; Vyalkova, A.; Lander, E.; Berman, M.; Minev, B.; et al. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J. Transl. Med. 2019, 17, 100. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, L.X.; Shao, J.Z.; Pan, R.L.; Wang, Y.X.; Dong, X.J.; Zhang, G.R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell Mol. Med. 2010, 14, 1494–1508. [Google Scholar] [CrossRef]
- Chapel, A.; Semont, A.; Francois, S.; Mouiseddine, M.; Thierry, D. Human Mesenchymal Stem Cells (MSC) Home at Injured Sites after Local Irradiation and Contribute To Reduce Radiation-Induced Intestinal Lesion. Blood 2005, 106, 1691. [Google Scholar] [CrossRef]
- Xuan, X.; Tian, C.; Zhao, M.; Sun, Y.; Huang, C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021, 21, 595. [Google Scholar] [CrossRef]
- Teo, G.S.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.; Wu, Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef]
- Prinyakupt, J.; Pluempitiwiriyawej, C. Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. Biomed. Eng. Online 2015, 14, 63. [Google Scholar] [CrossRef]
- Moll, G.; Le Blanc, K. Engineering more efficient multipotent mesenchymal stromal (stem) cells for systemic delivery as cellular therapy. ISBT Sci. Ser. 2015, 10, 357–365. [Google Scholar] [CrossRef]
- Gao, J.; Dennis, J.E.; Muzic, R.F.; Lundberg, M.; Caplan, A.I. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 2001, 169, 12–20. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transpl. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Lee, E.S.; Im, H.J.; Kim, H.S.; Youn, H.; Lee, H.J.; Kim, S.U.; Hwang, D.W.; Lee, D.S. In vivo brain delivery of v-myc overproduced human neural stem cells via the intranasal pathway: Tumor characteristics in the lung of a nude mouse. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Li, Z.; Oganesyan, D.; Mooney, R.; Rong, X.; Christensen, M.J.; Shahmanyan, D.; Perrigue, P.M.; Benetatos, J.; Tsaturyan, L.; Aramburo, S.; et al. L-MYC Expression Maintains Self-Renewal and Prolongs Multipotency of Primary Human Neural Stem Cells. Stem Cell Rep. 2016, 7, 483–495. [Google Scholar] [CrossRef]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D’Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T.; et al. Neural Stem Cell-Based Anticancer Gene Therapy: A First-in-Human Study in Recurrent High-Grade Glioma Patients. Clin. Cancer Res. 2017, 23, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Thaci, B.; Ahmed, A.U.; Ulasov, I.V.; Tobias, A.L.; Han, Y.; Aboody, K.S.; Lesniak, M.S. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: Targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012, 19, 431–442. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Thaci, B.; Alexiades, N.G.; Han, Y.; Qian, S.; Liu, F.; Balyasnikova, I.V.; Ulasov, I.Y.; Aboody, K.S.; Lesniak, M.S. Neural stem cell-based cell carriers enhance therapeutic efficacy of an oncolytic adenovirus in an orthotopic mouse model of human glioblastoma. Mol. Ther. 2011, 19, 1714–1726. [Google Scholar] [CrossRef]
- Morshed, R.A.; Gutova, M.; Juliano, J.; Barish, M.E.; Hawkins-Daarud, A.; Oganesyan, D.; Vazgen, K.; Yang, T.; Annala, A.; Ahmed, A.U.; et al. Analysis of glioblastoma tumor coverage by oncolytic virus-loaded neural stem cells using MRI-based tracking and histological reconstruction. Cancer Gene Ther. 2015, 22, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- Mooney, R.; Majid, A.A.; Batalla-Covello, J.; Machado, D.; Liu, X.; Gonzaga, J.; Tirughana, R.; Hammad, M.; Lesniak, M.S.; Curiel, D.T.; et al. Enhanced Delivery of Oncolytic Adenovirus by Neural Stem Cells for Treatment of Metastatic Ovarian Cancer. Mol. Ther. Oncolytics 2019, 12, 79–92. [Google Scholar] [CrossRef]
- Hammad, M.; Cornejo, Y.R.; Batalla-Covello, J.; Majid, A.A.; Burke, C.; Liu, Z.; Yuan, Y.C.; Li, M.; Dellinger, T.H.; Lu, J.; et al. Neural Stem Cells Improve the Delivery of Oncolytic Chimeric Orthopoxvirus in a Metastatic Ovarian Cancer Model. Mol. Ther. Oncolytics 2020, 18, 326–334. [Google Scholar] [CrossRef]
- Cornejo, Y.; Li, M.; Dellinger, T.H.; Mooney, R.; Rahman, M.M.; McFadden, G.; Aboody, K.S.; Hammad, M. NSCs are permissive to oncolytic Myxoma virus and provide a delivery method for targeted ovarian cancer therapy. Oncotarget 2020, 11, 4693–4698. [Google Scholar] [CrossRef]
- Glass, R.; Synowitz, M.; Kronenberg, G.; Walzlein, J.H.; Markovic, D.S.; Wang, L.P.; Gast, D.; Kiwit, J.; Kempermann, G.; Kettenmann, H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 2005, 25, 2637–2646. [Google Scholar] [CrossRef]
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851. [Google Scholar] [CrossRef]
- Imitola, J.; Raddassi, K.; Park, K.I.; Mueller, F.J.; Nieto, M.; Teng, Y.D.; Frenkel, D.; Li, J.; Sidman, R.L.; Walsh, C.A.; et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 18117–18122. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Yu, D.; Kanojia, D.; Li, G.; Sukhanova, M.; Spencer, D.A.; Pituch, K.C.; Zhang, L.; Han, Y.; Ahmed, A.U.; et al. Intranasal Oncolytic Virotherapy with CXCR4-Enhanced Stem Cells Extends Survival in Mouse Model of Glioma. Stem Cell Rep. 2016, 7, 471–482. [Google Scholar] [CrossRef]
- Aboody, K.S.; Bush, R.A.; Garcia, E.; Metz, M.Z.; Najbauer, J.; Justus, K.A.; Phelps, D.A.; Remack, J.S.; Yoon, K.J.; Gillespie, S.; et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS ONE 2006, 1, e23. [Google Scholar] [CrossRef]
- Danks, M.K.; Yoon, K.J.; Bush, R.A.; Remack, J.S.; Wierdl, M.; Tsurkan, L.; Kim, S.U.; Garcia, E.; Metz, M.Z.; Najbauer, J.; et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007, 67, 22–25. [Google Scholar] [CrossRef]
- Frank, R.T.; Edmiston, M.; Kendall, S.E.; Najbauer, J.; Cheung, C.W.; Kassa, T.; Metz, M.Z.; Kim, S.U.; Glackin, C.A.; Wu, A.M.; et al. Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS ONE 2009, 4, e8314. [Google Scholar] [CrossRef]
- Cao, P.; Mooney, R.; Tirughana, R.; Abidi, W.; Aramburo, S.; Flores, L.; Gilchrist, M.; Nwokafor, U.; Haber, T.; Tiet, P.; et al. Intraperitoneal Administration of Neural Stem Cell-Nanoparticle Conjugates Targets Chemotherapy to Ovarian Tumors. Bioconjug Chem. 2017, 28, 1767–1776. [Google Scholar] [CrossRef]
- Lan, X.; Sun, Z.; Chu, C.; Boltze, J.; Li, S. Dental Pulp Stem Cells: An Attractive Alternative for Cell Therapy in Ischemic Stroke. Front. Neurol. 2019, 10, 824. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- van Beem, R.T.; Verloop, R.E.; Kleijer, M.; Noort, W.A.; Loof, N.; Koolwijk, P.; van der Schoot, C.E.; van Hinsbergh, V.W.; Zwaginga, J.J. Blood outgrowth endothelial cells from cord blood and peripheral blood: Angiogenesis-related characteristics in vitro. J. Thromb. Haemost. 2009, 7, 217–226. [Google Scholar] [CrossRef]
- Lin, R.Z.; Moreno-Luna, R.; Muñoz-Hernandez, R.; Li, D.; Jaminet, S.C.; Greene, A.K.; Melero-Martin, J.M. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis 2013, 16, 735–744. [Google Scholar] [CrossRef]
- Alphonse, R.S.; Vadivel, A.; Zhong, S.; McConaghy, S.; Ohls, R.; Yoder, M.C.; Thébaud, B. The isolation and culture of endothelial colony-forming cells from human and rat lungs. Nat. Protoc. 2015, 10, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Cheung, C. Origins and functional differences of blood endothelial cells. Semin. Cell Dev. Biol. 2024, 155, 23–29. [Google Scholar] [CrossRef]
- Liu, Y.; Lyons, C.J.; Ayu, C.; O’Brien, T. Recent advances in endothelial colony-forming cells: From the transcriptomic perspective. J. Transl. Med. 2024, 22, 313. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Jacobson, B.A.; Ji, Y.; Hebbel, R.P.; Kratzke, R.A. Blood Outgrowth Endothelial Cells as a Cellular Carrier for Oncolytic Vesicular Stomatitis Virus Expressing Interferon-β in Preclinical Models of Non-Small Cell Lung Cancer. Transl. Oncol. 2020, 13, 100782. [Google Scholar] [CrossRef] [PubMed]
- Pagan, J.; Przybyla, B.; Jamshidi-Parsian, A.; Gupta, K.; Griffin, R.J. Blood outgrowth endothelial cells increase tumor growth rates and modify tumor physiology: Relevance for therapeutic targeting. Cancers 2013, 5, 205–217. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Bodempudi, V.; Welsh, B.W.; Jasinski, P.; Griffin, R.J.; Milbauer, L.; Hebbel, R.P. Systemic inhibition of tumour angiogenesis by endothelial cell-based gene therapy. Br. J. Cancer 2007, 97, 513–522. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, G.; Dai, T.; Huang, H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007, 50, 274–280. [Google Scholar] [CrossRef]
- Asahara, T.; Takahashi, T.; Masuda, H.; Kalka, C.; Chen, D.; Iwaguro, H.; Inai, Y.; Silver, M.; Isner, J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef]
- Raykov, Z.; Balboni, G.; Aprahamian, M.; Rommelaere, J. Carrier cell-mediated delivery of oncolytic parvoviruses for targeting metastases. Int. J. Cancer 2004, 109, 742–749. [Google Scholar] [CrossRef]
- Iankov, I.D.; Blechacz, B.; Liu, C.; Schmeckpeper, J.D.; Tarara, J.E.; Federspiel, M.J.; Caplice, N.; Russell, S.J. Infected cell carriers: A new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 2007, 15, 114–122. [Google Scholar]
- Colombo, M.; Mirandola, L.; Platonova, N.; Apicella, L.; Berta, D.G.; Lancellotti, M.; Lazzari, E.; Cobos, E.; Chiriva-Internati, M.; Chiaramonte, R. Notch signaling drives myeloma cells homing to the bone marrow by regulating the CXCR4/CXCL12 axis. Clin. Lymphoma Myeloma Leuk. 2015, 15, e227–e228. [Google Scholar] [CrossRef]
- Liu, C.; Russell, S.J.; Peng, K.W. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 2010, 18, 1155–1164. [Google Scholar]
- Munguia, A.; Ota, T.; Miest, T.; Russell, S.J. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008, 15, 797–806. [Google Scholar] [PubMed]
- Podshivalova, E.S.; Semkina, A.S.; Kravchenko, D.S.; Frolova, E.I.; Chumakov, S.P. Efficient delivery of oncolytic enterovirus by carrier cell line NK-92. Mol. Ther. Oncolytics 2021, 21, 110–118. [Google Scholar] [CrossRef]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef]
- Pfirschke, C.; Schirrmacher, V. Cross-infection of tumor cells by contact with T lymphocytes loaded with Newcastle disease virus. Int. J. Oncol. 2009, 34, 951–962. [Google Scholar] [PubMed][Green Version]
- Cole, C.; Qiao, J.; Kottke, T.; Diaz, R.M.; Ahmed, A.; Sanchez-Perez, L.; Brunn, G.; Thompson, J.; Chester, J.; Vile, R.G. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat. Med. 2005, 11, 1073–1081. [Google Scholar] [CrossRef]
- Kanzaki, A.; Kasuya, H.; Yamamura, K.; Sahin, T.T.; Nomura, N.; Shikano, T.; Shirota, T.; Tan, G.; Fukuda, S.; Misawa, M.; et al. Antitumor efficacy of oncolytic herpes simplex virus adsorbed onto antigen-specific lymphocytes. Cancer Gene Ther. 2012, 19, 292–298. [Google Scholar] [CrossRef]
- Thorne, S.H.; Negrin, R.S.; Contag, C.H. Synergistic antitumor effects of immune cell-viral biotherapy. Science 2006, 311, 1780–1784. [Google Scholar] [CrossRef]
- Ilett, E.J.; Barcena, M.; Errington-Mais, F.; Griffin, S.; Harrington, K.J.; Pandha, H.S.; Coffey, M.; Selby, P.J.; Limpens, R.W.; Mommaas, M.; et al. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 2011, 17, 2767–2776. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, H.; Kottke, T.; Diaz, R.M.; Willmon, C.; Hudacek, A.; Thompson, J.; Parato, K.; Bell, J.; Naik, J.; et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008, 15, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, N.; Xu, L.; Lu, H.; Chen, Y.; Wang, Z.; Lu, Q.; Zhong, K.; Zhu, Z.; Wang, G.; et al. Systemic delivery of oncolytic herpes virus using CAR-T cells enhances targeting of antitumor immuno-virotherapy. Cancer Immunol. Immunother. 2024, 73, 173. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J. Mechanisms of CD8(+) T cell exclusion and dysfunction in cancer resistance to anti-PD-(L)1. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 163, 114824. [Google Scholar] [CrossRef]
- Meng, Y.; Yu, Z.; Wu, Y.; Du, T.; Chen, S.; Meng, F.; Su, N.; Ma, Y.; Li, X.; Sun, S.; et al. Cell-based immunotherapy with cytokine-induced killer (CIK) cells: From preparation and testing to clinical application. Hum. Vaccin. Immunother. 2017, 13, 1379–1387. [Google Scholar] [CrossRef]
- Liu, C.; Suksanpaisan, L.; Chen, Y.W.; Russell, S.J.; Peng, K.W. Enhancing cytokine-induced killer cell therapy of multiple myeloma. Exp. Hematol. 2013, 41, 508–517. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Reale, A.; Krutzke, L.; Cadamuro, M.; Vitiello, A.; von Einem, J.; Kochanek, S.; Palù, G.; Parolin, C.; Calistri, A. Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer. Int. J. Mol. Sci. 2023, 24, 9255. [Google Scholar] [CrossRef]
- Iscaro, A.; Jones, C.; Forbes, N.; Mughal, A.; Howard, F.N.; Janabi, H.A.; Demiral, S.; Perrie, Y.; Essand, M.; Weglarz, A.; et al. Targeting circulating monocytes with CCL2-loaded liposomes armed with an oncolytic adenovirus. Nanomedicine 2022, 40, 102506. [Google Scholar] [CrossRef]
- Peng, K.W.; Dogan, A.; Vrana, J.; Liu, C.; Ong, H.T.; Kumar, S.; Dispenzieri, A.; Dietz, A.B.; Russell, S.J. Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am. J. Hematol. 2009, 84, 401–407. [Google Scholar] [CrossRef]
- Muthana, M.; Rodrigues, S.; Chen, Y.Y.; Welford, A.; Hughes, R.; Tazzyman, S.; Essand, M.; Morrow, F.; Lewis, C.E. Macrophage delivery of an oncolytic virus abolishes tumor regrowth and metastasis after chemotherapy or irradiation. Cancer Res. 2013, 73, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Nutter Howard, F.; Winder, N.; Atkinson, E.; Jailani, A.; Patel, P.; Allen, R.; Ottewell, P.; Shaw, G.; Conner, J.; et al. Macrophage Delivered HSV1716 Is Active against Triple Negative Breast Cancer. Future Pharmacol. 2022, 2, 444–459. [Google Scholar] [CrossRef]
- Combes, F.; Mc Cafferty, S.; Meyer, E.; Sanders, N.N. Off-Target and Tumor-Specific Accumulation of Monocytes, Macrophages and Myeloid-Derived Suppressor Cells after Systemic Injection. Neoplasia 2018, 20, 848–856. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.L.; Davis, J.M.; Steiner, J.L.; Enos, R.T.; Jung, S.H.; Carson, J.A.; Pena, M.M.; Carnevale, K.A.; Berger, F.G.; Murphy, E.A. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: Role of MCP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1087–G1095. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Arwert, E.N.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018, 23, 1239–1248. [Google Scholar] [CrossRef]
- Kranjc, M.K.; Novak, M.; Pestell, R.G.; Lah, T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol. Oncol. 2019, 53, 397–406. [Google Scholar] [CrossRef]
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872. [Google Scholar] [CrossRef]
- Lang, S.; Bruderek, K.; Kaspar, C.; Höing, B.; Kanaan, O.; Dominas, N.; Hussain, T.; Droege, F.; Eyth, C.; Hadaschik, B.; et al. Clinical Relevance and Suppressive Capacity of Human Myeloid-Derived Suppressor Cell Subsets. Clin. Cancer Res. 2018, 24, 4834–4844. [Google Scholar] [CrossRef]
- Apodaca, M.C.; Wright, A.E.; Riggins, A.M.; Harris, W.P.; Yeung, R.S.; Yu, L.; Morishima, C. Characterization of a whole blood assay for quantifying myeloid-derived suppressor cells. J. Immunother. Cancer 2019, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Fleming, V.; Hu, X.; Weller, C.; Weber, R.; Groth, C.; Riester, Z.; Hüser, L.; Sun, Q.; Nagibin, V.; Kirschning, C.; et al. Melanoma Extracellular Vesicles Generate Immunosuppressive Myeloid Cells by Upregulating PD-L1 via TLR4 Signaling. Cancer Res. 2019, 79, 4715–4728. [Google Scholar] [CrossRef] [PubMed]
- Valenti, R.; Huber, V.; Iero, M.; Filipazzi, P.; Parmiani, G.; Rivoltini, L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007, 67, 2912–2915. [Google Scholar] [CrossRef]
- Arkhypov, I.; Özbay Kurt, F.G.; Bitsch, R.; Novak, D.; Petrova, V.; Lasser, S.; Hielscher, T.; Groth, C.; Lepper, A.; Hu, X.; et al. HSP90α induces immunosuppressive myeloid cells in melanoma via TLR4 signaling. J. Immunother. Cancer 2022, 10, e005551. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, S.; Coakley, B.A.; Briley-Saebo, K.; Ma, G.; Chen, H.M.; Meseck, M.; Ward, S.; Divino, C.; Woo, S.; Chen, S.H.; et al. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013, 73, 5003–5015. [Google Scholar] [CrossRef]
- Hawila, E.; Razon, H.; Wildbaum, G.; Blattner, C.; Sapir, Y.; Shaked, Y.; Umansky, V.; Karin, N. CCR5 Directs the Mobilization of CD11b(+)Gr1(+)Ly6C(low) Polymorphonuclear Myeloid Cells from the Bone Marrow to the Blood to Support Tumor Development. Cell Rep. 2017, 21, 2212–2222. [Google Scholar] [CrossRef]
- Jeong, J.; Suh, Y.; Jung, K. Context Drives Diversification of Monocytes and Neutrophils in Orchestrating the Tumor Microenvironment. Front. Immunol. 2019, 10, 1817. [Google Scholar] [CrossRef]
- Trellakis, S.; Bruderek, K.; Hütte, J.; Elian, M.; Hoffmann, T.K.; Lang, S.; Brandau, S. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. 2013, 19, 328–336. [Google Scholar] [CrossRef]
- Cassetta, L.; Bruderek, K.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Hu, X.; Rundgren, I.M.; Lin, A.; Santegoets, K.; Horzum, U.; Godinho-Santos, A.; et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 2020, 8, e001223. [Google Scholar] [CrossRef]
- Marigo, I.; Bosio, E.; Solito, S.; Mesa, C.; Fernandez, A.; Dolcetti, L.; Ugel, S.; Sonda, N.; Bicciato, S.; Falisi, E.; et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 2010, 32, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Solito, S.; Falisi, E.; Diaz-Montero, C.M.; Doni, A.; Pinton, L.; Rosato, A.; Francescato, S.; Basso, G.; Zanovello, P.; Onicescu, G.; et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 2011, 118, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Dauer, M.; Schad, K.; Herten, J.; Junkmann, J.; Bauer, C.; Kiefl, R.; Endres, S.; Eigler, A. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J. Immunol. Methods 2005, 302, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Iankov, I.D.; Msaouel, P.; Allen, C.; Federspiel, M.J.; Bulur, P.A.; Dietz, A.B.; Gastineau, D.; Ikeda, Y.; Ingle, J.N.; Russell, S.J.; et al. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res. Treat. 2009, 122, 745. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Liang, X.; Li, H.C.; Wang, Z.M.; Chong, T. Dendritic cells serve as a “Trojan horse” for oncolytic adenovirus delivery in the treatment of mouse prostate cancer. Acta Pharmacol. Sin. 2016, 37, 1121–1128. [Google Scholar] [CrossRef]
- Na, Y.R.; Jung, D.; Gu, G.J.; Seok, S.H. GM-CSF Grown Bone Marrow Derived Cells Are Composed of Phenotypically Different Dendritic Cells and Macrophages. Mol. Cells 2016, 39, 734–741. [Google Scholar] [CrossRef]
- Brasel, K.; De Smedt, T.; Smith, J.L.; Maliszewski, C.R. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 2000, 96, 3029–3039. [Google Scholar]
- In, H.; Park, J.S.; Shin, H.S.; Ryu, S.H.; Sohn, M.; Choi, W.; Park, S.; Hwang, S.; Park, J.; Che, L.; et al. Identification of dendritic cell precursor from the CD11c(+) cells expressing high levels of MHC class II molecules in the culture of bone marrow with FLT3 ligand. Front. Immunol. 2023, 14, 1179981. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, J. Properties of immature and mature dendritic cells: Phenotype, morphology, phagocytosis, and migration. RSC Adv. 2019, 9, 11230–11238. [Google Scholar] [CrossRef]
- Crespo, H.J.; Lau, J.T.; Videira, P.A. Dendritic cells: A spot on sialic Acid. Front. Immunol. 2013, 4, 491. [Google Scholar] [CrossRef]
- Zhu, K.; Shen, Q.; Ulrich, M.; Zheng, M. Human monocyte-derived dendritic cells expressing both chemotactic cytokines IL-8, MCP-1, RANTES and their receptors, and their selective migration to these chemokines. Chin. Med. J. 2000, 113, 1124–1128. [Google Scholar]
- Ritter, U.; Wiede, F.; Mielenz, D.; Kiafard, Z.; Zwirner, J.; Körner, H. Analysis of the CCR7 expression on murine bone marrow-derived and spleen dendritic cells. J. Leukoc. Biol. 2004, 76, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Creusot, R.J.; Yaghoubi, S.S.; Chang, P.; Chia, J.; Contag, C.H.; Gambhir, S.S.; Fathman, C.G. Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood 2009, 113, 6638–6647. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Scandella, E.; Men, Y.; Gillessen, S.; Förster, R.; Groettrup, M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 2002, 100, 1354–1361. [Google Scholar] [CrossRef]
- Le Nouën, C.; Hillyer, P.; Winter, C.C.; McCarty, T.; Rabin, R.L.; Collins, P.L.; Buchholz, U.J. Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human respiratory syncytial virus and human metapneumovirus. PLoS Pathog. 2011, 7, e1002105. [Google Scholar] [CrossRef]
- Moran, T.P.; Nakano, H.; Kondilis-Mangum, H.D.; Wade, P.A.; Cook, D.N. Epigenetic control of Ccr7 expression in distinct lineages of lung dendritic cells. J. Immunol. 2014, 193, 4904–4913. [Google Scholar] [CrossRef]
- Langlet, C.; Tamoutounour, S.; Henri, S.; Luche, H.; Ardouin, L.; Grégoire, C.; Malissen, B.; Guilliams, M. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J. Immunol. 2012, 188, 1751–1760. [Google Scholar] [CrossRef]
- Nakano, H.; Burgents, J.E.; Nakano, K.; Whitehead, G.S.; Cheong, C.; Bortner, C.D.; Cook, D.N. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal Immunol. 2013, 6, 678–691. [Google Scholar] [CrossRef]
- Nakano, H.; Lin, K.L.; Yanagita, M.; Charbonneau, C.; Cook, D.N.; Kakiuchi, T.; Gunn, M.D. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat. Immunol. 2009, 10, 394–402. [Google Scholar] [CrossRef]
- Schmidt, N.O.; Dührsen, L.; Reitz, M.; Henze, M.; Sedlacik, J.; Riecken, K.; Fehse, B.; Westphal, M. Repeated intranasal application of neural stem cell-mediated enzym/prodrug therapy using a novel HSV-thimidine kinase variant improves therapeutic efficiency in an intracranial glioblastoma model. Neuro Oncol. 2014, 16, iii50. [Google Scholar] [CrossRef]
- Li, G.; Bonamici, N.; Dey, M.; Lesniak, M.S.; Balyasnikova, I.V. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert. Opin. Drug Deliv. 2018, 15, 163–172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkisova, V.A.; Dalina, A.A.; Neymysheva, D.O.; Zenov, M.A.; Ilyinskaya, G.V.; Chumakov, P.M. Cell Carriers for Oncolytic Virus Delivery: Prospects for Systemic Administration. Cancers 2025, 17, 2296. https://doi.org/10.3390/cancers17142296
Sarkisova VA, Dalina AA, Neymysheva DO, Zenov MA, Ilyinskaya GV, Chumakov PM. Cell Carriers for Oncolytic Virus Delivery: Prospects for Systemic Administration. Cancers. 2025; 17(14):2296. https://doi.org/10.3390/cancers17142296
Chicago/Turabian StyleSarkisova, Viktoria A., Alexandra A. Dalina, Daria O. Neymysheva, Martin A. Zenov, Galina V. Ilyinskaya, and Peter M. Chumakov. 2025. "Cell Carriers for Oncolytic Virus Delivery: Prospects for Systemic Administration" Cancers 17, no. 14: 2296. https://doi.org/10.3390/cancers17142296
APA StyleSarkisova, V. A., Dalina, A. A., Neymysheva, D. O., Zenov, M. A., Ilyinskaya, G. V., & Chumakov, P. M. (2025). Cell Carriers for Oncolytic Virus Delivery: Prospects for Systemic Administration. Cancers, 17(14), 2296. https://doi.org/10.3390/cancers17142296





