A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the ACT WONDER2S Study
2.2. Recruitment
2.3. Interviews
2.4. Quantitative Analysis
2.5. Qualitative Analysis
3. Results
3.1. Perceived Barriers, Facilitators, and Needs for Minority CCT Referral and Enrollment
3.1.1. Community Factors
3.1.2. Institutional Factors
3.1.3. Interpersonal Factors
3.1.4. Intrapersonal Factors
3.2. ACT WONDER2S Intervention Feedback
3.2.1. Community
Community Health Educators
3.2.2. Institutional
3.2.3. Institutional and Interpersonal
3.2.4. Individual
4. Discussion
4.1. Community Outreach and Engagement Strategies
4.2. Institutional and Interpersonal Approaches
4.3. Patient-Level CCT Decision-Making
4.4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCTs | Clinical Trials |
| MLIs | Multilevel Interventions |
| MCC | Moffitt Cancer Center |
| CRCs | Clinical Research Coordinators |
| ACT | Advancing Clinical Trials: Working Through Outreach, Navigation, and Digitally |
| WONDER2S | Enabled Referral and Recruitment Strategies |
Appendix A
| MCC Physician Clinical Characteristics | Statistic |
|---|---|
| Years at MCC; M (SD) range | 8.8 (5.79) 3–23 |
| Oncology specialty; n (%) | |
| Medical oncology | 9 (90%) |
| Radiology | 1 (10%) |
| Clinical Program; n (%) | |
| Blood and Marrow Transplant and Cellular Immunotherapy | 3 (30%) |
| Breast Oncology | 1 (10%) |
| Cutaneous Oncology | 1 (10%) |
| Genitourinary Oncology | 2 (20%) |
| Malignant Hematology | 1 (10%) |
| Radiation Oncology | 1 (10%) |
| Thoracic Oncology | 1 (10%) |
| Track; n (%) | |
| Clinical investigator | 4 (40%) |
| Physician educator | 5 (50%) |
| Physician scientist | 1 (10%) |
| Rank; n (%) | |
| Assistant member | 1 (10%) |
| Associate member | 9 (90%) |
Appendix B
| MCC CRC Clinical Characteristics | Statistic |
|---|---|
| Years at MCC; M (SD) range | 1.4 (0.54) 1–2.5 |
| Department; n (%) | |
| Malignant Hematology and Myeloid | 3 (30%) |
| Bone Marrow Transplant | 2 (20%) |
| Thoracic | 2 (20%) |
| Head and Neck | 2 (20%) |
| Phase 1 Clinical Trials | 1 (10%) |
Appendix C
| Participant Characteristic | Statistic |
|---|---|
| Age; M (SD) range (in years) | 45.7 (8.68) 33–58 |
| Practice Type; n (%) | |
| Private practice | 1 (20%) |
| Group practice | 4 (40%) |
| Medical center | 3 (30%) |
| Number of patients seen per month; M (SD) range | 451 (576.78) 160–2000 |
| Volume of cancer patients seen and/or diagnosed per month (nononcologists only, N = 6); M (SD) range | 3.88 (3.59) 0.3–10 |
| Health care system offers cancer clinical trials; n (%) | 3 (30%) |
| Has led a therapeutic cancer trial in the past 5 years; n (%) | 4 (40%) |
| Has referred a patient outside of their health system for cancer care; n (%) | 9 (90%) |
| Has referred a patient to MCC for cancer care; n (%) | 8 (80%) |
| Has referred a patient outside of their health system to a therapeutic cancer trial; n (%) | 6 (60%) |
| Has referred a patient to MCC for a therapeutic clinical trial; n (%) | 4 (40%) |
| Has attended a MCC physician CME activity; n (%) | 6 (60%) |
| Has engaged with a member of the MCC physician liaison team; n (%) | 5 (50%) |
References
- Hamel, L.M.; Penner, L.A.; Albrecht, T.L.; Heath, E.; Gwede, C.K.; Eggly, S. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients with Cancer. Cancer Control. 2016, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Wilson, R.E.; King, L.M.; Marty, P.J.; Whiteman, V.E. Socio-ecological Model as a Framework for Overcoming Barriers and Challenges in Randomized Control Trials in Minority and Underserved Communities. Int. J. MCH AIDS 2015, 3, 85–95. [Google Scholar] [PubMed]
- Allison, K.; Patel, D.; Kaur, R. Assessing Multiple Factors Affecting Minority Participation in Clinical Trials: Development of the Clinical Trials Participation Barriers Survey. Cureus 2022, 14, e24424. [Google Scholar] [CrossRef]
- Rivers, D.; August, E.M.; Sehovic, I.; Green, B.L.; Quinn, G.P. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp. Clin. Trials 2013, 35, 13–32. [Google Scholar] [CrossRef]
- Salman, A.; Nguyen, C.; Lee, Y.-H.; Cooksey-James, T. A Review of Barriers to Minorities’ Participation in Cancer Clinical Trials: Implications for Future Cancer Research. J. Immigr. Minor. Health 2015, 18, 447–453. [Google Scholar] [CrossRef]
- Gopal, D.P.; Chetty, U.; O’DOnnell, P.; Gajria, C.; Blackadder-Weinstein, J. Implicit bias in healthcare: Clinical practice, research and decision making. Futur. Health J. 2021, 8, 40–48. [Google Scholar] [CrossRef]
- Nipp, R.D.; Lee, H.; Gorton, E.; Lichtenstein, M.; Kuchukhidze, S.; Park, E.; Chabner, B.A.; Moy, B. Addressing the Financial Burden of Cancer Clinical Trial Participation: Longitudinal Effects of an Equity Intervention. Oncologist 2019, 24, 1048–1055. [Google Scholar] [CrossRef]
- Awidi, M.; Al Hadidi, S. Participation of Black Americans in Cancer Clinical Trials: Current Challenges and Proposed Solutions. JCO Oncol. Pract. 2021, 17, 265–271. [Google Scholar] [CrossRef]
- Ford, J.G.; Howerton, M.W.; Lai, G.Y.; Gary, T.L.; Bolen, S.; Gibbons, M.C.; Tilburt, J.; Baffi, C.; Tanpitukpongse, T.P.; Wilson, R.F.; et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer 2007, 112, 228–242. [Google Scholar] [CrossRef]
- Kanarek, N.F.; Tsai, H.-L.; Metzger-Gaud, S.; Damron, D.; Guseynova, A.; Klamerus, J.F.; Rudin, C.M. Geographic Proximity and Racial Disparities in Cancer Clinical Trial Participation. J. Natl. Compr. Cancer Netw. 2010, 8, 1343–1351. [Google Scholar] [CrossRef]
- Sabin, J.A.; Nosek, B.A.; Greenwald, A.G.; Rivara, F.P. Physicians’ implicit and explicit attitudes about race by MD race, ethnicity, and gender. J. Health Care Poor Underserved 2009, 20, 896–913. [Google Scholar]
- Niranjan, S.J.; Wenzel, J.A.; Martin, M.Y.; Fouad, M.N.; Vickers, S.M.; Konety, B.R.; Durant, R.W. Perceived Institutional Barriers Among Clinical and Research Professionals: Minority Participation in Oncology Clinical Trials. JCO Oncol. Pract. 2021, 17, e666–e675. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.B.; Burris, J.L.; Borger, T.N.; Acree, T.; Abufarsakh, B.M.; Arnold, S.M. Perspectives of facilitators and barriers to cancer clinical trial participation: A mixed-methods study. J. Clin. Oncol. 2024, 42, e23206. [Google Scholar] [CrossRef]
- Kumar, G.; Chaudhary, P.; Quinn, A.; Su, D. Barriers for cancer clinical trial enrollment: A qualitative study of the perspectives of healthcare providers. Contemp. Clin. Trials Commun. 2022, 28, 100939. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.A.; McCaskill-Stevens, W.; Wolfe, P.; Marcus, A.C. Physician Perspectives on Increasing Minorities in Cancer Clinical Trials: An Eastern Cooperative Oncology Group (ECOG) Initiative. Ann. Epidemiol. 2000, 10, S78–S84. [Google Scholar] [PubMed]
- Niranjan, S.J.; Martin, M.Y.; Fouad, M.N.; Vickers, S.M.; Wenzel, J.A.; Cook, E.D.; Konety, B.R.; Durant, R.W. Bias and stereotyping among research and clinical professionals: Perspectives on minority recruitment for oncology clinical trials. Cancer 2020, 126, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Durant, R.W.; Wenzel, J.A.; Scarinci, I.C.; Paterniti, D.A.; Fouad, M.N.; Hurd, T.C.; Martin, M.Y. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: Enhancing minority participation in clinical trials (EMPaCT). Cancer 2014, 120 (Suppl. 7), 1097–1105. [Google Scholar]
- Perez, G.K.; Oberoi, A.R.; Finkelstein-Fox, L.; Park, E.R.; Nipp, R.D.; Moy, B. Qualitative study of Oncology Clinicians’ Perceptions of Barriers to Offering Clinical Trials to Underserved Populations. Cancer Control 2023, 30, 10732748231187829. [Google Scholar] [CrossRef]
- Michaels, M.; D’aGostino, T.A.; Blakeney, N.; Weiss, E.S.; Binz-Scharf, M.C.; Golant, M.; Bylund, C.L. Impact of Primary Care Provider Knowledge, Attitudes, and Beliefs about Cancer Clinical Trials: Implications for Referral, Education and Advocacy. J. Cancer Educ. 2014, 30, 152–157. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Megally, S.; Plotkin, E.; Shivakumar, L.; Salgia, N.J.; Zengin, Z.B.; Meza, L.; Chawla, N.; Castro, D.V.; Dizman, N.; et al. Barriers to Clinical Trial Implementation Among Community Care Centers. JAMA Netw. Open 2024, 7, e248739-e. [Google Scholar]
- Wong, A.R.; Sun, V.; George, K.; Liu, J.; Padam, S.; Chen, B.A.; George, T.; Amini, A.; Li, D.; Sedrak, M.S. Barriers to Participation in Therapeutic Clinical Trials as Perceived by Community Oncologists. JCO Oncol. Pract. 2020, 16, e849–e858. [Google Scholar] [CrossRef]
- Cunningham-Erves, J.; Barajas, C.; Mayo-Gamble, T.L.; McAfee, C.R.; Hull, P.C.; Sanderson, M.; Canedo, J.; Beard, K.; Wilkins, C.H. Formative research to design a culturally-appropriate cancer clinical trial education program to increase participation of African American and Latino communities. BMC Public Health 2020, 20, 840. [Google Scholar] [CrossRef]
- An, J.; Ferrante, J.M.; Macenat, M.; Ganesan, S.; Hudson, S.V.; Omene, C.; Garcia, H.; Kinney, A.Y. Promoting informed approaches in precision oncology and clinical trial participation for Black patients with cancer: Community-engaged development and pilot testing of a digital intervention. Cancer 2023, 130, 3561–3577. [Google Scholar] [CrossRef]
- Langford, A.T.; Hawley, S.T.; Stableford, S.; Studts, J.L.; Byrne, M.M. Development of a Plain Language Decision Support Tool for Cancer Clinical Trials: Blending Health Literacy, Academic Research, and Minority Patient Perspectives. J. Cancer Educ. 2019, 35, 454–461. [Google Scholar] [CrossRef]
- Torres, S.; de la Riva, E.E.; Tom, L.S.; Clayman, M.L.; Taylor, C.; Dong, X.; Simon, M.A. The Development of a Communication Tool to Facilitate the Cancer Trial Recruitment Process and Increase Research Literacy among Underrepresented Populations. J. Cancer Educ. 2015, 30, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Barrett, N.J.; Boehmer, L.; Schrag, J.; Benson, A.B.; Green, S.; Hamroun-Yazid, L.; Howson, A.; Matin, K.; Oyer, R.A.; Pierce, L.; et al. An Assessment of the Feasibility and Utility of an ACCC-ASCO Implicit Bias Training Program to Enhance Racial and Ethnic Diversity in Cancer Clinical Trials. JCO Oncol. Pract. 2023, 19, e570–e580. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.; Pressman, A.; Hurley, P.; Garrett-Mayer, E.; Bruinooge, S.S.; Howson, A.; Kaltenbaugh, M.; Williams, J.H.; Boehmer, L.; Bernick, L.A.; et al. Increasing Racial and Ethnic Equity, Diversity, and Inclusion in Cancer Treatment Trials: Evaluation of an ASCO-Association of Community Cancer Centers Site Self-Assessment. JCO Oncol. Pract. 2023, 19, e581–e588. [Google Scholar] [CrossRef] [PubMed]
- Michaels, M.; Weiss, E.S.; Sae-Hau, M.; Illei, D.; Lilly, B.; Szumita, L.; Connell, B.; Lee, M.; Cooks, E.; McPheeters, M. Strategies for increasing accrual in cancer clinical trials: What is the evidence? Cancer Med. 2024, 13, e7298. [Google Scholar] [CrossRef]
- Gerido, L.H.; He, Z. Improving Patient Participation in Cancer Clinical Trials: A Qualitative Analysis of HSRProj & RePORTER. Stud. Health Technol. Inform. 2019, 264, 1925–1926. [Google Scholar]
- Oyer, R.A.; Hurley, P.; Boehmer, L.; Bruinooge, S.S.; Levit, K.; Barrett, N.; Benson, A.; Bernick, L.A.; Byatt, L.; Charlot, M.; et al. Increasing Racial and Ethnic Diversity in Cancer Clinical Trials: An American Society of Clinical Oncology and Association of Community Cancer Centers Joint Research Statement. J. Clin. Oncol. 2022, 40, 2163–2171. [Google Scholar] [CrossRef]
- Heller, C.; Balls-Berry, J.E.; Nery, J.D.; Erwin, P.J.; Littleton, D.; Kim, M.; Kuo, W.P. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemp. Clin. Trials 2014, 39, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.P.; Fair, A.M.; Joosten, Y.A.; Dolor, R.J.; Williams, N.A.; Sherden, L.; Stallings, S.; Smoot, D.T.; Wilkins, C.H. A Multilevel Approach to Stakeholder Engagement in the Formulation of a Clinical Data Research Network. Med. Care 2018, 56, S22–S26. [Google Scholar] [CrossRef] [PubMed]
- de Lange, A.H.; Teoh, K.; Fleuren, B.; Christensen, M.; Medisauskaite, A.; Løvseth, L.T.; Solms, L.; Reig-Botella, A.; Brulin, E.; Innstrand, S.T.; et al. Opportunities and challenges in designing and evaluating complex multilevel, multi-stakeholder occupational health interventions in practice. Work Stress 2024, 38, 352–372. [Google Scholar] [CrossRef]
- Racine, E.; Mahony, L.O.; Riordan, F.; Flynn, G.; Kearney, P.M.; McHugh, S.M. What and how do different stakeholders contribute to intervention development? A mixed methods study. HRB Open Res. 2022, 5, 35. [Google Scholar] [PubMed]
- Laird, Y.; Manner, J.; Baldwin, L.; Hunter, R.; McAteer, J.; Rodgers, S.; Williamson, C.; Jepson, R. Stakeholders’ experiences of the public health research process: Time to change the system? Health Res. Policy Syst. 2020, 18, 83. [Google Scholar]
- Morton, K.L.; Atkin, A.J.; Corder, K.; Suhrcke, M.; Turner, D.; van Sluijs, E.M.F. Engaging stakeholders and target groups in prioritising a public health intervention: The Creating Active School Environments (CASE) online Delphi study. BMJ Open 2017, 7, e013340. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.S.; Thompson, V.L.S. The science of stakeholder engagement in research: Classification, implementation, and evaluation. Transl. Behav. Med. 2017, 7, 486–491. [Google Scholar] [CrossRef]
- Davis, T.C.; Arnold, C.L.; Mills, G.; Miele, L. A Qualitative Study Exploring Barriers and Facilitators of Enrolling Underrepresented Populations in Clinical Trials and Biobanking. Front. Cell Dev. Biol. 2019, 7, 74. [Google Scholar] [CrossRef]
- Wenzel, J.A.; Mbah, O.; Xu, J.; Moscou-Jackson, G.; Saleem, H.; Sakyi, K.; Ford, J.G. A Model of Cancer Clinical Trial Decision-making Informed by African-American Cancer Patients. J. Racial Ethn. Health Disparities 2014, 2, 192–199. [Google Scholar] [CrossRef]
- Sprague, M.L.; Freeman, E.R.; Winkfield, K.M. Perceptions of Cancer Care and Clinical Trials in the Black Community: Implications for Care Coordination Between Oncology and Primary Care Teams. Oncologist 2017, 22, 1094–1101. [Google Scholar]
- Shields, M.; Rivelli, A.; Molina, Y.; Ozoani-Lohrer, O.; Lefaiver, C.; Ingle, M.; Fitzpatrick, V. Trial staff and community member perceptions of barriers and solutions to improving racial and ethnic diversity in clinical trial participation; a mixed method study. Contemp. Clin. Trials Commun. 2024, 38, 101262. [Google Scholar] [CrossRef]
- Arevalo, M.; Heredia, N.I.; Krasny, S.; Rangel, M.L.; Gatus, L.A.; McNeill, L.H.; Fernandez, M.E. Mexican-American perspectives on participation in clinical trials: A qualitative study. Contemp. Clin. Trials Commun. 2016, 4, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Naderbagi, A.; Loblay, V.; Zahed, I.U.M.; Ekambareshwar, M.; Poulsen, A.; Song, Y.J.C.; Ospina-Pinillos, L.; Krausz, M.; Kamel, M.M.; Hickie, I.B.; et al. Cultural and Contextual Adaptation of Digital Health Interventions: Narrative Review. J. Med. Internet Res. 2024, 26, e55130. [Google Scholar] [CrossRef] [PubMed]
- Kvale, S. Interviews: An Introduction to Qualiitative Research Interviewing; Sage: London, UK, 1996. [Google Scholar]
- Guest, G.; Bunce, A.; Johnson, L. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field Methods 2006, 18, 59–82. [Google Scholar]
- Garcia, K.K.S.; Abrahão, A.A. Research Development Using REDCap Software. Health Inform. Res. 2021, 27, 341–349. [Google Scholar] [CrossRef]
- Guest, G.; MacQueen, K.; Namey, E. Applied Thematic Analysis; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2012. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Vuong, I.; Wright, J.; Nolan, M.B.; Eggen, A.; Bailey, E.; Strickland, R.; Traynor, A.; Downs, T. Overcoming Barriers: Evidence-Based Strategies to Increase Enrollment of Underrepresented Populations in Cancer Therapeutic Clinical Trials—A Narrative Review. J. Cancer Educ. 2019, 35, 841–849. [Google Scholar] [CrossRef]
- Hendren, S.; Chin, N.; Fisher, S.; Winters, P.; Griggs, J.; Mohile, S.; Fiscella, K. Patients’ Barriers to Receipt of Cancer Care, and Factors Associated With Needing More Assistance From a Patient Navigator. J. Natl. Med. Assoc. 2011, 103, 701–710. [Google Scholar] [CrossRef]
- Kwon, D.H.; Tisnado, D.M.; Keating, N.L.; Klabunde, C.N.; Adams, J.L.; Rastegar, A.; Hornbrook, M.C.; Kahn, K.L. Physician-reported barriers to referring cancer patients to specialists: Prevalence, factors, and association with career satisfaction. Cancer 2015, 121, 113–122. [Google Scholar]
- Odedina, F.T.; Wieland, M.L.; Barbel-Johnson, K.; Crook, J.M. Community Engagement Strategies for Underrepresented Racial and Ethnic Populations. Mayo Clin. Proc. 2024, 99, 159–171. [Google Scholar] [CrossRef]
- Kelsey, M.D.; Patrick-Lake, B.; Abdulai, R.; Broedl, U.C.; Brown, A.; Cohn, E.; Curtis, L.H.; Komelasky, C.; Mbagwu, M.; Mensah, G.A.; et al. Inclusion and diversity in clinical trials: Actionable steps to drive lasting change. Contemp. Clin. Trials 2022, 116, 106740. [Google Scholar] [CrossRef] [PubMed]
- Pohl, S.A.; Nelson, B.A.; Patwary, T.R.; Amanuel, S.; Benz, E.J., Jr.; Lathan, C.S. Evolution of community outreach and engagement at National Cancer Institute-Designated Cancer Centers, an evolving journey. CA Cancer J. Clin. 2024, 74, 383–396. [Google Scholar] [PubMed]
- Cunningham-Erves, J.; Mayo-Gamble, T.L.; Hull, P.C.; Lu, T.; Barajas, C.; McAfee, C.R.; Sanderson, M.; Canedo, J.R.; Beard, K.; Wilkins, C.H. A pilot study of a culturally-appropriate, educational intervention to increase participation in cancer clinical trials among African Americans and Latinos. Cancer Causes Control. 2021, 32, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.F.; Jackson, S.A.; Camacho, F.; Hall, M.A. Attitudes of African American and Low Socioeconomic Status White Women toward Medical Research. J. Health Care Poor Underserved 2007, 18, 85–99. [Google Scholar] [CrossRef]
- Leader, A.E.; Brandt, H.M.; Briant, K.J.; Curry, G.; Ellis, K.; Gonzalez, E.T.; Guerra, C.E.; Harding, G.; Hull, P.C.; Israel, A.; et al. Community Outreach and Engagement at U.S. Cancer Centers: Notes from the Third Cancer Center Community Impact Forum. Cancer Epidemiol. Biomark. Prev. 2023, 32, 1777–1782. [Google Scholar] [CrossRef]
- Gray, D.M.; Nolan, T.S.; Gregory, J.; Joseph, J.J. Diversity in clinical trials: An opportunity and imperative for community engagement. Lancet Gastroenterol. Hepatol. 2021, 6, 605–607. [Google Scholar] [CrossRef]
- Monreal, I.; Chappell, H.; Kiss, R.; Friedman, D.R.; Akesson, J.; Sae-Hau, M.; Szumita, L.; Halwani, A.; Weiss, E.S. Understanding the Barriers to Clinical Trial Referral and Enrollment Among Oncology Providers Within the Veterans Health Administration. Mil. Med. 2024, 190, e891–e898. [Google Scholar] [CrossRef]
- Fouad, M.N.; Acemgil, A.; Bae, S.; Forero, A.; Lisovicz, N.; Martin, M.Y.; Oates, G.R.; Partridge, E.E.; Vickers, S.M. Patient Navigation As a Model to Increase Participation of African Americans in Cancer Clinical Trials. J. Oncol. Pract. 2016, 12, 556–563. [Google Scholar] [CrossRef]
- Nouvini, R.; Parker, P.A.; Malling, C.D.; Godwin, K.; Costas-Muñiz, R. Interventions to increase racial and ethnic minority accrual into cancer clinical trials: A systematic review. Cancer 2022, 128, 3860–3869. [Google Scholar] [CrossRef]
- Pelto, D.J.; Sadler, G.R.; Njoku, O.; Rodriguez, M.C.; Villagra, C.; Malcarne, V.L.; Riley, N.E.; Behar, A.I.; Jandorf, L. Adaptation of a Cancer Clinical Trials Education Program for African American and Latina/o Community Members. Health Educ. Behav. 2015, 43, 381–388. [Google Scholar] [CrossRef]
- Nolan, T.S.; Bell, A.M.; Chan, Y.; Bryant, A.L.; Bissram, J.S.; Hirschey, R. Use of Video Education Interventions to Increase Racial and Ethnic Diversity in Cancer Clinical Trials: A Systematic Review. Worldviews Evid.-Based Nurs. 2021, 18, 302–309. [Google Scholar] [CrossRef]
- Barrios, C.H.; Werutsky, G.; Martinez-Mesa, J. The Global Conduct of Cancer Clinical Trials: Challenges and Opportunities. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e132–e139. [Google Scholar] [CrossRef]
- Izarn, F.; Henry, J.; Besle, S.; Ray-Coquard, I.; Blay, J.-Y.; Allignet, B. Globalization of clinical trials in oncology: A worldwide quantitative analysis. ESMO Open 2024, 10, 104086. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, E.A.; Christy, S.M.; Simmons, V.N.; Vadaparampil, S.T.; Gwede, C.K.; Meade, C.D. Learner Verification: A Methodology to Create Suitable Education Materials. HLRP Health Lit. Res. Pract. 2021, 5, e49–e59. [Google Scholar] [CrossRef] [PubMed]
- Walden, A.; Garvin, L.; Smerek, M.; Johnson, C. User-centered design principles in the development of clinical research tools. Clin. Trials 2020, 17, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Dabbs, A.D.V.R.; Myers, B.A.; MC Curry, K.R.; Dunbar-Jacob, J.R.; Hawkins, R.P.; Begey, A.B.; Dew, M.A. User-Centered Design and Interactive Health Technologies for Patients. CIN Comput. Inform. Nurs. 2009, 27, 175–183. [Google Scholar] [CrossRef]
- Rollison, D.E.; Amorrortu, R.P.; Fuzzell, L.N.; Garcia, M.A.; Tapia-Kwan, E.S.; Zhao, Y.; Eschrich, S.A.; Gore, B.R.; Mittman, B.S.; Stanley, N.B.; et al. Abstract 4824: Multi-level intervention to increase minority cancer patient enrollment to clinical treatment trials—Study design considerations and baseline characteristics from the ACTWONDER2S Study. Cancer Res. 2024, 84, 4824. [Google Scholar] [CrossRef]
| Community Outreach and Education Interventions | Description | End User |
| Enhancing Patient Support | CHE patient support, including support in the new patient process, connecting patients to social or financial resources, relaying questions to clinical care teams, and answering questions about the new patient process via phone or email. | MCC patients, community residents |
| Clinical Trial Education | Clinical trial education sessions for the broader community. | Community residents |
| Continuing Medical Education | Education sessions on advances in cancer treatment, information on referral and enrollment into clinical trials, and implicit bias training. | Community physicians, MCC physicians |
| Digital interventions | Description | End user |
| Precision Engagement Tool | To identify geographic areas for Black/African American (AA) and Hispanic patient and physician cancer clinical trial (CCT) outreach. | MCC physicians |
| Portfolio Profiler | To view open trials at MCC and to explore potential gaps in the CCT portfolio relative to the cancer burden of MCC patients. | MCC physicians |
| Recruitment Dashboard | To display CCT enrollment rates and demographic characteristics compared to the overall MCC patient population. | MCC physicians, CRCs |
| Eligibility Criteria Calculator | To explore the impact of CCT criteria on patient eligibility. | MCC physicians |
| Trial Connect Portal | To facilitate rapid referral of patients to CCTs by (1) assisting with trial identification and (2) embedding communication between MCC clinical teams and physicians in the community. | MCC physicians, CRCs, community physicians |
| CHOICES Decision Aid (DA) | A self-guided interactive website to improve patient decision-making related to CCT participation. | MCC physicians |
| MCC Participants | Community Participants | ||||
|---|---|---|---|---|---|
| MCC Physicians (n = 10) | MCC CRCs (n = 10) | MCC Patients (n = 10) | Community Physicians (n = 10) | Community Residents (n = 10) | |
| Age; M (SD) a range (in years) | 43.3 (8.04) 36–62 | 28.4 (3.29) 22–34 | 57.8 (14.05) 29–79 | 45.7 (8.68) 33–58 | 55.8 (16.41) 25–70 |
| Sex; n (%) | |||||
| Female | 3 (30%) | 5 (50%) | 5 (50%) | 5 (50%) | 4 (40%) |
| Male | 7 (70%) | 5 (50%) | 5 (50%) | 5 (50%) | 5 (50%) |
| Race; n (%) a | |||||
| White | 3 (30%) | 4 (40%) | 3 (30%) | 5 (50%) | 4 (40%) |
| Black or African American | 2 (20%) | 2 (20%) | 5 (50%) | 3 (30%) | 5 (50%) |
| Asian | 4 (40%) | 2 (20%) | 1 (10%) | 1 (10%) | 0 (0%) |
| More than one race | 1 (10%) | 2 (20%) | 1 (10%) | 1 (10%) | 1 (10%) |
| Ethnicity; n (%) a | |||||
| Non-Hispanic | 9 (90%) | 8 (80%) | 5 (50%) | 6 (60%) | 6 (60%) |
| Hispanic | 1 (10%) | 2 (20%) | 5 (50%) | 4 (40%) | 4 (40%) |
| Themes | Exemplary Illustrative Quotes |
|---|---|
| Community | |
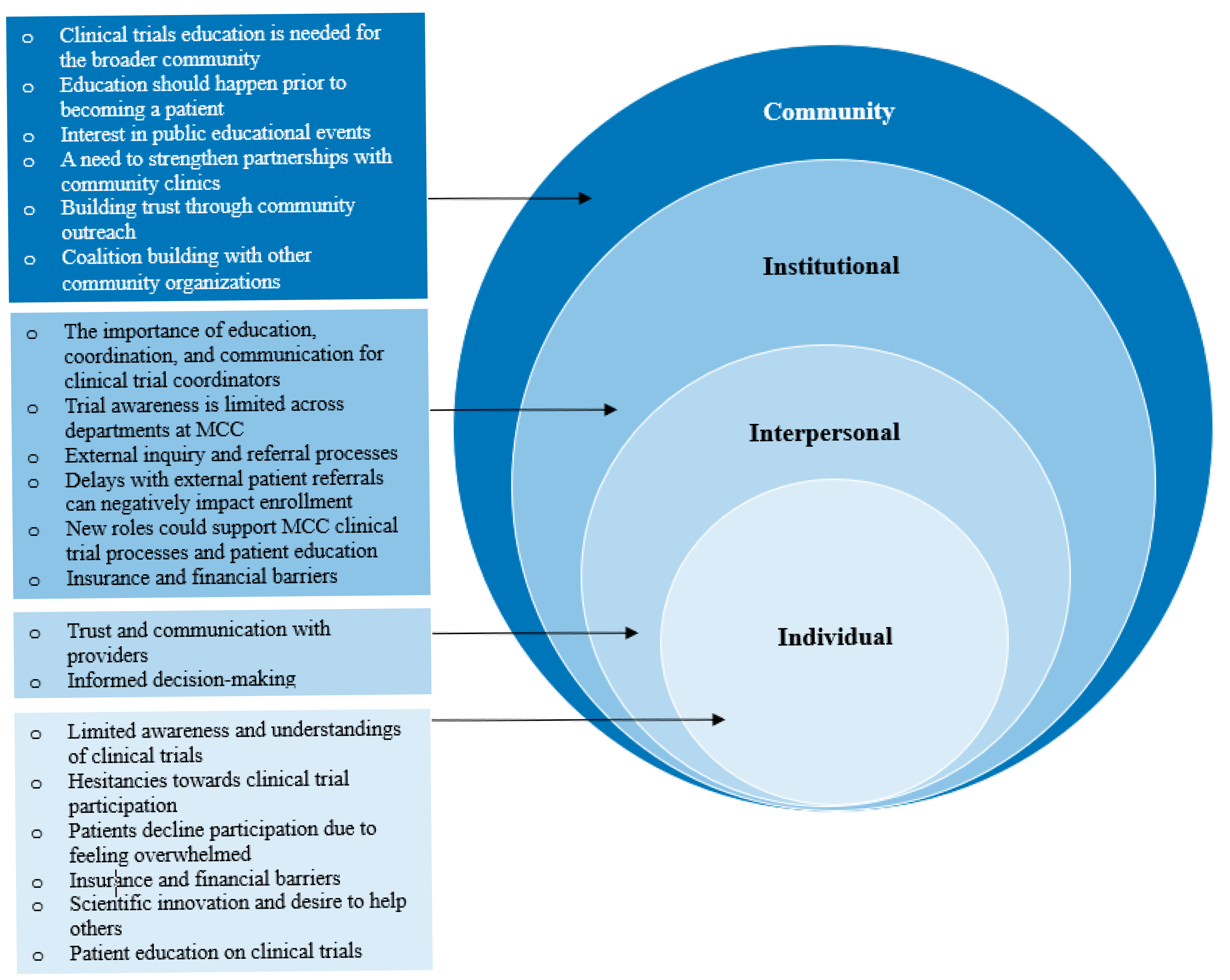
| Clinical trial education is needed for the broader community | “Even though Moffitt does, I think, do a decent job of physician liaisons and outreach for patient referrals in general, I don’t think we do nearly as good a job of disseminating these clinical trial ideas out to the community. And that sometimes has to be done in the form of conferences and things like that that are put on by the various departments, too…incorporating the clinical trial piece to the outreach process I think would help tremendously.” (MCC Physician 1047) |
| Education should happen prior to becoming a patient | “…so there’s the community education, and then there’s educating the specific patient. I think part of the problem is that you don’t have time to educate someone when they’re coming through the door, so that’s where the community piece is important.” (MCC Physician 1044) |
| Interest in public educational events | “…I’ve gone through with my churches. They have health fairs. We just had one October 7th were we had a variety of everything that you could think of, breast cancer. They had a gynecologist there. They talked about prostate cancer, cholesterol. They took people’s blood pressure, cholesterol, anything you could think of…And the churches in my community cater to that as well.” (MCC Patient 1042) |
| Building trust through community outreach | “Well, for many years that population have not really trusted the medical community for many reasons as you may know or may not…And I think that’s getting better as time goes on with the new generation. But at the same time, it’s not. The more people that have a better experience with it moving forward, then they can spread the word of why it’s important. So, just a historical thing that have occurred.” (Community Physician 1050). |
| A need to strengthen partnerships with community clinics | “Because I think oftentimes, whether we like it or not, these minority patients are being seen at places like the VA [Veterans Affairs] hospital. They’re county hospitals. So, oftentimes at those hospitals, the last thing on their provider’s minds is to send these patients in for a clinical trial because they’re probably not aware that the trial is ongoing. And really, they may think that it’s going to be impossible for those patients to get into Moffitt. So, again, I think it comes down to education and maybe even directed education at those sites. Although you are also going to have to look at the return on your investment.” (MCC Physician 1047) |
| Coalition building with other community organizations | “I believe that there was a better collaboration with the American Cancer Society liaisons in the past. I don’t even know if we have an ACS liaison in this area anymore. But we used to literally have people come around to the office and talk about, well, you know, Moffitt’s doing this or whatever it may be. And so I don’t know if Moffitt maybe has some community liaisons that would in-person kind of making that connection and get people engaged, but I think that there is some value in that.” (Community Physician 1013) |
| Institutional | |
| The importance of coordination, communication, and ongoing education for clinical trial coordinators | “…the treating physicians will contact either myself if they are within Moffitt, or they’ll contact the PI of the study if they are outside of Moffitt. So, they connect with one of our doctors at Moffitt…If the patient is already a Moffitt patient, it’s easier because all that happens is that a treating physician will reach out to me…So, they ask me to do my work, and then I go do my research on this patient, their history, their medical records, and match that to the study, and see if they are actually eligible or not eligible… So, the work that has kind of a connection is not easy, because there’s a web that you’re at the center of as a coordinator…What we try to do is to be trained on each other’s studies. Because you have to know that someone there is backing you up if you have an emergency, if you’re on PTO [paid time off], etc. So, this is something important.” (CRC 1012) |
| Trial awareness is limited across departments at MCC | “So, when I was at Moffitt, it’s such a big place, it’s got a lot of different departments. Very hard to keep up with what’s happening everywhere without talking to someone in that department… And then also, the clinical trials, coordinators a lot of times, there is a lot of turnover, even among the staff that run these things. So, ultimately a lot of times you kinda have to talk to the doctors in the department to know what’s going on research-wise there.” (Community Physician 1002). |
| External inquiry and referral processes | “…..most of the doctors that know about us are from specific hospitals or areas that do not have a lot of patients of color. We have to reach out to more areas that we know that these have majority of patients from Hispanic or from African American or from Middle Eastern or from Asian ethnicities…. Because I think the way our connection works is just with specific doctors that we know, or people at Moffitt. And we limit ourselves to the patients of those areas that mainly might not have a lot of people of color.” (CRC 1012) |
| Delays with external patient referrals can negatively impact enrollment | “And if we’re talking about Moffitt in particular this has become a bigger issue recently because there is a big process on getting an appointment which can be very slow. So, you tell a patient they have malignancy, and this is what you offer, and you offer them a second opinion. And then it takes three weeks or four weeks to even hear about an appointment. And sometimes the appointments are out a month and a half or something like that. Nobody wants to wait that long. I don’t want to wait that long.” (Community Physician 1051) |
| Insurance and financial barriers | “Okay, this is a big issue. If clinical trials are covered but our patients can’t get in until they get a referral with insurance that Moffitt takes, that’s another reason why they never get into clinical trials. Because then when I call the doctors and I say, “I have this patient with this disease I don’t know how to treat.” And then I say to them, “do you take the patient’s insurance,” they don’t know. They never know. The patients can’t get in there unless they are referred, even if they might be on a clinical trial.” (Community Physician 1037) |
| Interpersonal | |
| Trust and communication with providers | “Well, the reason I’m doing [the clinical trial participation] now is because of the doctor. I trust [name of doctor]. And when the screener called me and told me [name of doctor] recommended me, I trust him. So, I said, “Well, if he feels I’ll be a good candidate for it, I trust him enough that I would do this.” But normally, like I said, doing any type of trial situation, I usually don’t do it until I know a lot more about it. But that comfort and trust in him is why I’m doing this now. (MCC patient 1043)” |
| Informed decision-making | “Yeah. Given my history, I would want them to know if there’s any risks or any issue with me because of my cancer history doing this particular trial for this type of drug. I definitely would consult with them…So, I’m gonna read and do my due diligence before I step forward.” (MCC Patient 1043) |
| Individual | |
| Limited understanding and awareness of trials | “in movies or in TV shows people do clinical trials for extra money or things like that. (Community resident 1021)” |
| Hesitancies toward clinical trial participation | “They want people to join, so they know what this drug or whatever therapy they have will help, let’s say, age group, ethnicity…I’m a little wary of it because… Like at the meeting, I asked her if I had cancer, and I was directed to a clinical trial, could my using that or being in that program hurt me? And she said no. She said the doctors there would make sure that I was taken care of. And if anything went wrong, that they would take me off and steer me in the right direction. But for myself, the barrier was: What if this hurts me and makes me sicker?” (Community Resident 1040) |
| Patients decline trial participation due to feeling overwhelmed | “When I went in for my initial diagnosis for cancer, they asked if I wanted to participate in a trial. And I said no at that time…… that was the first time being approached…I just decided it was too soon. I was too much overwhelmed with the actual diagnosis of cancer and trying to absorb the treatment plan and all that. It was just not a good time for my mind to wrap around doing something like that.” (MCC Patient 1043) |
| Patient education on clinical trials needed | “I find that that can be a significant barrier for people sometimes, is there’s still a misconception and kind of negative perception about what clinical trials are and what it means to be on a trial. I still see people, when I mention a trial, who say, “I don’t wanna get a placebo.” And a majority of the trials I do are in the metastatic setting, and those patients wouldn’t be getting a placebo. Right? It’s not ethical for them to be able to be on a trial like that. So I have to kind of spend some time to backtrack and re-educate a lot of my patients.” (MCC Physician 1045) |
| A need for community physician education | “I’m not familiar because I’m a gynecologist. So, I don’t usually refer to medical oncology, it’s usually GYN oncology…Specifically not for a clinical trial, but I have referred them to other facilities for cancer treatment.” (Community Physician 1005) |
| Quantitative Feedback | Qualitative Feedback | |
|---|---|---|
| Community | ||
| Precision Engagement Tool a | ||
| Mean helpfulness scores | Suggestions for improvement g | |
| Overall helpfulness | 3.75 |
|
| Willingness to use the precision engagement tool | 3.0 | |
| Identify geographic areas for targeted community physician and patient outreach | 4.1 | |
| Access to a list of community physicians in priority zones and referral patterns | 4.4 | |
| Access to services provided by CHEs | 3.5 | |
| Institutional | ||
| Portfolio Profiler b | ||
| Mean helpfulness scores | Suggestions for improvement | |
| Overall helpfulness | 4.0 |
|
| Show the number of trials open by department and cancer characteristics | 4.1 | |
| Show the % of patients with specific cancer characteristics in your department | 4.0 | |
| Show the % of patients eligible for a trial by race, ethnicity, and cancer type relative to catchment area | 3.9 | |
| Recruitment Dashboard c | ||
| Mean helpfulness scores | Suggestions for improvement | |
| Overall helpfulness | 3.62 |
|
| Show demographic characteristics of MCC patients versus trial enrollees | 4.05 | |
| Show referral and enrollment rates by race and ethnicity | 4.0 | |
| Show patient referral and enrollment rates by geographic region (e.g., catchment area) | 3.6 | |
| Show patient enrollment rate by trial sponsor | 2.85 | |
| Eligibility Criteria Calculator d | ||
| Mean helpfulness scores | Suggestions for improvement | |
| Overall helpfulness | 3.6 |
|
| Willing to enter trial criteria | 3.4 | |
| Show the number and percent of eligible patients by race and ethnicity | 3.7 | |
| Output a score of generalizability of trial criteria | 3.4 | |
| Compare the proportions of Black Hispanic patients vs. non-Hispanic white that would be excluded based on criteria | 3.6 | |
| Alter the eligibility criteria to determine whether the change lessens the disparity | 3.9 | |
| Trial Connect Portal e | ||
| Mean helpfulness scores | Suggestions for improvement | |
| Overall helpfulness | 4.27 |
|
| Willing to use the precision engagement tool | 4.07 | |
| Ability to search for open trials at Moffitt (by cancer characteristics) | 4.57 | |
| Ability for community physicians to send electronic patient referrals | 4.6 | |
| Ability for community physicians to communicate with MCC physicians about referrals | 4.53 | |
| Ability to assess patient eligibility | 3.57 | |
| Individual | ||
| CHOICES DA f | ||
| Mean helpfulness scores | Suggestions for improvement | |
| Overall helpfulness | 4.53 |
|
| Integrate the tool into Moffitt patient registration | 4.3 | |
| Help to generate a list of questions related to clinical trial participation | 4.3 | |
| Send questions electronically before visit | 4.6 | |
| Share with care team the specific barriers when receiving care | 4.9 | |
| Mean (M) Helpfulness Score | Suggestions for Additional Patient Support a | |||
|---|---|---|---|---|
| MCC Patients | Community Residents | MCC Patients | Community Residents | |
| Provide support to you during the new patient process | 4.8 | 4.8 |
|
|
| Connect you to social or financial resources | 5.0 | 5.0 | ||
| Relay your questions to your care team | 4.8 | 4.3 | ||
| Answer questions about participating in clinical trials via phone or email | 4.3 | 4.5 | ||
| Answer questions about the new patient portal process via phone or email | 4.5 | 4.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, M.; Geiss, C.; Blackwell, R.; Maconi, M.L.; Amorrortu, R.P.; Tapia-Kwan, E.; Turner, K.; Fuzzell, L.; Zhao, Y.; Eschrich, S.A.; et al. A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model. Cancers 2025, 17, 2282. https://doi.org/10.3390/cancers17142282
Garcia M, Geiss C, Blackwell R, Maconi ML, Amorrortu RP, Tapia-Kwan E, Turner K, Fuzzell L, Zhao Y, Eschrich SA, et al. A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model. Cancers. 2025; 17(14):2282. https://doi.org/10.3390/cancers17142282
Chicago/Turabian StyleGarcia, Melany, Carley Geiss, Rebecca Blackwell, Melinda L. Maconi, Rossybelle P. Amorrortu, Elliott Tapia-Kwan, Kea Turner, Lindsay Fuzzell, Yayi Zhao, Steven A. Eschrich, and et al. 2025. "A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model" Cancers 17, no. 14: 2282. https://doi.org/10.3390/cancers17142282
APA StyleGarcia, M., Geiss, C., Blackwell, R., Maconi, M. L., Amorrortu, R. P., Tapia-Kwan, E., Turner, K., Fuzzell, L., Zhao, Y., Eschrich, S. A., Rollison, D. E., & Vadaparampil, S. T. (2025). A Formative Evaluation of Interventions to Enhance Clinical Trial Diversity Guided by the Socioecological Model. Cancers, 17(14), 2282. https://doi.org/10.3390/cancers17142282






