Characteristics and Treatment of Primary Hepatic Perivascular Epithelioid Cell Tumor (PEComa) in Adults: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
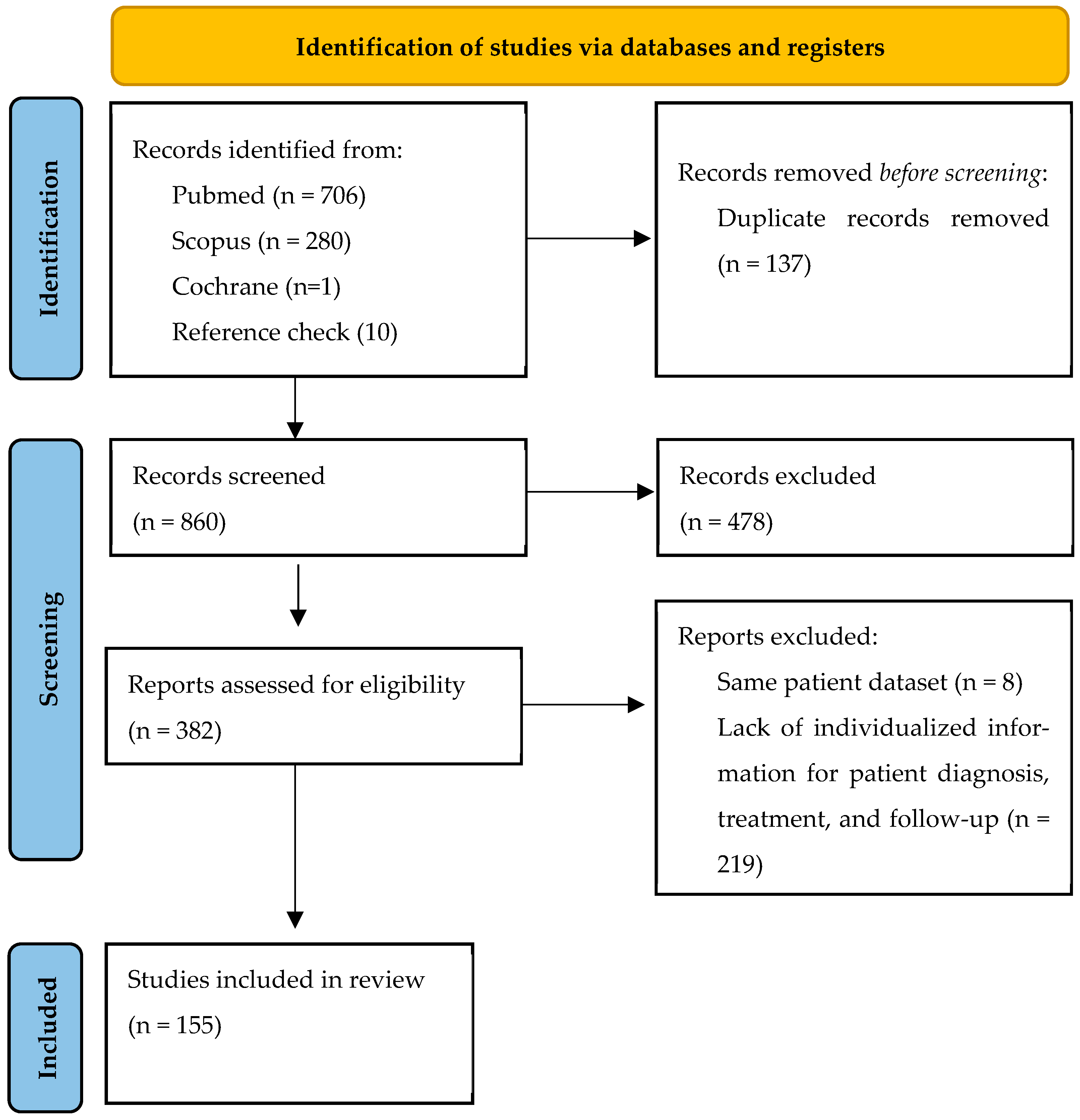
2.1. Search Strategy
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction and Synthesis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bonetti, F.; Pea, M.; Martignoni, G.; Zamboni, G. PEC and sugar. Am. J. Surg. Pathol. 1992, 16, 307–308. [Google Scholar] [CrossRef]
- Dong, B.N.; Zhan, H.; Luan, T.; Wang, J.S. Comprehensive Insights Into Renal Perivascular Epithelioid Cell Neoplasms: From Molecular Mechanisms to Clinical Practice. World J. Oncol. 2024, 15, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.; Parkash, V.; Yilmaz, Y.; Mariappan, M.R.; Ma, L.; Hileeto, D.; Qumsiyeh, M.B.; Hui, P. Perivascular epithelioid cell tumor (PEComa) of the uterine cervix associated with intraabdominal “PEComatosis”: A clinicopathological study with comparative genomic hybridization analysis. World J. Surg. Oncol. 2004, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Teng, H.; Zhao, R.; Ding, W.; Yu, K.; Zhu, L.; Zhang, J.; Han, Y. Malignant perivascular epithelioid cell tumor of the lung synchronous with a primary adenocarcinoma: One case report and review of the literature. BMC Cancer 2019, 19, 235. [Google Scholar] [CrossRef]
- Caliskan, S.; Akar, O.S.; Gun, S.; Kefeli, M. Malignant Perivascular Epithelioid Cell Tumor (PEComa) of the Uterus as Part of the Hereditary Cancer Syndrome: A Case Diagnosed with Multiple Malignancies. Turk. Patoloji. Derg. 2023, 39, 212–217. [Google Scholar] [CrossRef]
- Haiges, D.; Kurz, P.; Laaff, H.; Meiss, F.; Kutzner, H.; Technau-Hafsi, K. Malignant PEComa. J. Cutan. Pathol. 2018, 45, 84–89. [Google Scholar] [CrossRef]
- Dashraath, P.; Sidek, N.A.; Kalaichelvan, V.; Makmur, A.; Lim, D.G.S.; Low, J.J.H.; Ng, J.S.Y. Malignant perivascular epithelioid cell tumor (PEComa) of uterus. Ultrasound Obstet. Gynecol. 2022, 59, 826–828. [Google Scholar] [CrossRef]
- Algashaamy, K.; Montgomery, E.A.; Garcia-Buitrago, M. Liver mesenchymal neoplasms: Something old, something new. Pathology 2022, 54, 225–235. [Google Scholar] [CrossRef]
- Yan, S.; Lu, J.J.; Chen, L.; Cai, W.H.; Wu, J.Z. Hepatic perivascular epithelioid cell tumors: The importance of preoperative diagnosis. World J. Gastroenterol. 2024, 30, 1926–1933. [Google Scholar] [CrossRef]
- Khan, H.M.; Katz, S.C.; Libbey, N.P.; Somasundar, P.S. Hepatic PEComa: A potential pitfall in the evaluation of hepatic neoplasms. BMJ Case Rep. 2014, 2014, bcr2014204122. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.H.; Hwang, S.; Hong, S.M.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Alshahrani, A.A.; Lee, S.G. Clinico-pathological correlation of hepatic angiomyolipoma: A series of 23 resection cases. ANZ J. Surg. 2018, 88, E60–E65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Hu, Y.; Wang, J.; Li, W. Fat-Poor Hepatic Angiomyolipoma in Noncirrhotic Livers: Imaging Features, Pathology, and Differential Diagnosis. J. Comput. Assist. Tomogr. 2024, 48, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, F.; Jiang, W.; Meng, Q.; Wang, F. Hepatic epithelioid angiomyolipoma with an unusual pathologic appearance: Expanding the morphologic spectrum. Int. J. Clin. Exp. Pathol. 2014, 7, 6364–6369. [Google Scholar]
- Son, H.J.; Kang, D.W.; Kim, J.H.; Han, H.Y.; Lee, M.K. Hepatic perivascular epithelioid cell tumor (PEComa): A case report with a review of literatures. Clin. Mol. Hepatol. 2017, 23, 80–86. [Google Scholar] [CrossRef]
- Kvietkauskas, M.; Samuolyte, A.; Rackauskas, R.; Luksaite-Lukste, R.; Karaliute, G.; Maskoliunaite, V.; Valkiuniene, R.B.; Sokolovas, V.; Strupas, K. Primary Liver Perivascular Epithelioid Cell Tumor (PEComa): Case Report and Literature Review. Medicina 2024, 60, 409. [Google Scholar] [CrossRef]
- Katsakhyan, L.; Shahi, M.; Eugene, H.C.; Nonogaki, H.; Gross, J.M.; Nucci, M.R.; Vang, R.; Xing, D. Uterine Leiomyosarcoma Associated With Perivascular Epithelioid Cell Tumor: A Phenomenon of Differentiation/Dedifferentiation and Evidence Suggesting Cell-of-Origin. Am. J. Surg. Pathol. 2024, 48, 761–772. [Google Scholar] [CrossRef]
- Nie, P.; Wu, J.; Wang, H.; Zhou, R.; Sun, L.; Chen, J.; Yang, G. Primary hepatic perivascular epithelioid cell tumors: Imaging findings with histopathological correlation. Cancer Imaging 2019, 19, 32. [Google Scholar] [CrossRef]
- Dymkowski, M.; Kalman, P.; Niecikowski, P.; Koperski, Ł.; Kosieradzki, M. Case report: Liver PEComa after kidney transplantation in recipient with tuberous sclerosis complex. Front. Oncol. 2024, 14, 1386569. [Google Scholar] [CrossRef]
- Henske, E.P.; Jóźwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016, 2, 16035. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K.; Antonescu, C.R.; Kwiatkowski, D.J.; Malinowska, I.A. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: Clinical and molecular correlates. Int. J. Cancer 2013, 132, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Izubuchi, Y.; Tanaka, T. PEComa—Its clinical features, histopathology, and current therapy. Jpn. J. Clin. Oncol. 2025, 55, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Aulmann, S.; Illei, P.B.; Netto, G.J.; Ro, J.; Cho, H.Y.; Dogan, S.; Ladanyi, M.; Martignoni, G.; Goldblum, J.R.; et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am. J. Surg. Pathol. 2010, 34, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Melamed, J.; Wu, J. PEComa with Transcription Factor E3 Overexpression: A Diagnostic and Therapeutic Challenge. Case Rep. Oncol. 2017, 10, 531–533. [Google Scholar] [CrossRef]
- Aedma, S.K.; Kasi, A. Li-Fraumeni Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Butz, H.; Lövey, J.; Szentkereszty, M.; Bozsik, A.; Tóth, E.; Patócs, A. Case Report: A Novel Pathomechanism in PEComa by the Loss of Heterozygosity of TP53. Front. Oncol. 2022, 12, 849004. [Google Scholar] [CrossRef]
- Jasim, S.; Tamboli, P.; Lee, S.-C.; Strong, L.C.; Elsayes, K.; Ayala-Ramirez, M.; Habra, M.A. Epithelioid Angiomyolipoma in a Patient With Li-Fraumeni Syndrome: Rare Pathologic Diagnosis. AACE Clin. Case Rep. 2016, 2, e251–e255. [Google Scholar] [CrossRef]
- Song, Z.; Xu, F.; Dai, C. Chills and fever as the first presentation of hepatic perivascular epithelioid cell tumor. Hepatobiliary Surg. Nutr. 2019, 8, 436–438. [Google Scholar] [CrossRef]
- Kou, Y.Q.; Yang, Y.P.; Ye, W.X.; Yuan, W.N.; Du, S.S.; Nie, B. Perivascular epithelioid cell tumors of the liver misdiagnosed as hepatocellular carcinoma: Three case reports. World J. Clin. Cases 2023, 11, 426–433. [Google Scholar] [CrossRef]
- Senne, M.; Sgourakis, G.; Molmenti, E.P.; Schroeder, T.; Beckebaum, S.; Nadalin, S.; Malagó, M.; Radtke, A. Portal and Hepatic Venous Territorial Mapping in Healthy Human Livers: Virtual Three-Dimensional Computed Tomography Size-Shape-Topography Study. Exp. Clin. Transpl. 2022, 20, 826–834. [Google Scholar] [CrossRef]
- Klompenhouwer, A.J.; Verver, D.; Janki, S.; Bramer, W.M.; Doukas, M.; Dwarkasing, R.S.; de Man, R.A.; IJzermans, J.N.M. Management of hepatic angiomyolipoma: A systematic review. Liver Int. 2017, 37, 1272–1280. [Google Scholar] [CrossRef]
- Nese, N.; Martignoni, G.; Fletcher, C.D.; Gupta, R.; Pan, C.C.; Kim, H.; Ro, J.Y.; Hwang, I.S.; Sato, K.; Bonetti, F.; et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: A clinicopathologic study of 41 cases: Detailed assessment of morphology and risk stratification. Am. J. Surg. Pathol. 2011, 35, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, Q.; Shang, M.; Li, S.; Hu, X.; Hu, X. Multimodal imaging study of hepatic perivascular epithelioid cell tumors: A case report. Front. Med. 2023, 10, 1322048. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, Y.; Liu, S.; Zhang, M.; Qiao, B. Hepatic perivascular epithelioid cell tumor: A retrospective analysis of 36 cases. Front. Oncol. 2024, 14, 1416254. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhou, X.; Zhou, H.; Jia, W.; Hu, C.; Chu, J.; Kong, L. Retrospective analysis of hepatic perivascular epithelioid cell tumour (PEComa) in a single centre for clinical diagnosis and treatment clinical diagnosis and treatment of hepatic PEComa. Medicine 2022, 101, e29506. [Google Scholar]
- Kacała, A.; Dorochowicz, M.; Matus, I.; Puła, M.; Korbecki, A.; Sobański, M.; Jacków-Nowicka, J.; Patrzałek, D.; Janczak, D.; Guziński, M. Hepatic Hemangioma: Review of Imaging and Therapeutic Strategies. Medicina 2024, 60, 449. [Google Scholar] [CrossRef]
- LeGout, J.D.; Bolan, C.W.; Bowman, A.W.; Caserta, M.P.; Chen, F.K.; Cox, K.L.; Sanyal, R.; Toskich, B.B.; Lewis, J.T.; Alexander, L.F. Focal Nodular Hyperplasia and Focal Nodular Hyperplasia-like Lesions. Radiographics 2022, 42, 1043–1061. [Google Scholar] [CrossRef]
- Guo, Y.L.; Dang, Y.; Zhang, H.; Lian, J. Contrast-Enhanced Ultrasound of a Hepatic Perivascular Epithelioid Cell Tumor: A Case Report and Literature Review. Cureus 2025, 17, e81579. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, H.; Xiao, E.H. Perivascular epithelioid cell tumour: Dynamic CT, MRI and clinicopathological characteristics--analysis of 32 cases and review of the literature. Clin. Radiol. 2013, 68, 555–561. [Google Scholar] [CrossRef]
- Ma, R.; Feng, S.-T.; Meicheng, C.; Wang, J.; Dong, Z.; Zhou, X. Hepatic Pecoma versus Hepatocellular Carcinoma In The Noncirrhotic Liver on Gd-EOB-DTPA-Enhanced MRI: A Diagnostic Challenge. Curr. Med. Imaging 2024, 20, e15734056269369. [Google Scholar] [CrossRef]
- Sun, L.; Sun, X.; Li, Y.; Xing, L. The role of (18)F-FDG PET/CT imaging in patient with malignant PEComa treated with mTOR inhibitor. Onco Targets Ther. 2015, 8, 1967–1970. [Google Scholar] [CrossRef]
- Streba, C.T.; Streba, L.A.M.; Dumitrescu, D.; Georgescu, E.F. Risks and Benefits of Liver Biopsy in Focal Liver Disease. In Liver Biopsy—Indications, Procedures, Results; Tagaya, N., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Bao, L.; Shi, Y.; Zhong, J.; Zhao, M.; Wu, J.; Hai, L.; Xu, X.; Du, H.; Shi, Y. Histopathologic characteristics and immunotypes of perivascular epithelioid cell tumors (PEComa). Int. J. Clin. Exp. Pathol. 2019, 12, 4380–4389. [Google Scholar]
- Kumagai, A.; Kondo, F.; Sano, K.; Inoue, M.; Fujii, T.; Hashimoto, M.; Watanabe, M.; Soejima, Y.; Ishida, T.; Tokairin, T.; et al. Immunohistochemical study of hepatocyte, cholangiocyte and stem cell markers of hepatocellular carcinoma: The second report: Relationship with tumor size and cell differentiation. J. Hepatobiliary Pancreat. Sci. 2016, 23, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Britt, A.; Mohyuddin, G.R.; Al-Rajabi, R. Maintenance of Stable Disease in Metastatic Perivascular Epithelioid Cell Tumor of the Liver With Single-Agent Sorafenib. Am. J. Ther. 2020, 29, e259–e261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Xu, L.; Ma, L.; Song, M.Q.; Wu, L.Q.; Zhou, X. Hepatic falciform ligament clear cell myomelanocytic tumor: A case report and a comprehensive review of the literature on perivascular epithelioid cell tumors. BMC Cancer 2015, 15, 1004. [Google Scholar] [CrossRef]
- Chao, C.H.; Lin, C.Y.; Chan, S.C.; Chen, K.S. Concurrent hepatic and ruptured renal angiomyolipoma in tuberous sclerosis complex. Chang. Gung. Med. J. 2004, 27, 696–700. [Google Scholar]
- Liu, J.L.; Lin, Y.M.; Lin, M.C.; Yeh, K.T.; Hsu, J.C.; Chin, C.J. Perivascular epithelioid cell tumor (PEComa) of the uterus with aggressive behavior at presentation. Hematol. Oncol. Stem Cell Ther. 2009, 2, 426–430. [Google Scholar] [CrossRef]
- Kirste, S.; Kayser, G.; Zipfel, A.; Grosu, A.L.; Brunner, T. Unresectable hepatic PEComa: A rare malignancy treated with stereotactic body radiation therapy (SBRT) followed by complete resection. Radiat. Oncol. 2018, 13, 28. [Google Scholar] [CrossRef]
- Wang, W.T.; Li, Z.Q.; Zhang, G.H.; Guo, Y.; Teng, M.J. Liver transplantation for recurrent posthepatectomy malignant hepatic angiomyolipoma: A case report. World J. Gastroenterol. 2015, 21, 3755–3758. [Google Scholar] [CrossRef]
- Liu, C.H.; Chao, W.T.; Lin, S.C.; Lau, H.Y.; Wu, H.H.; Wang, P.H. Malignant perivascular epithelioid cell tumor in the female genital tract: Preferred reporting items for systematic reviews and meta-analyses. Medicine 2019, 98, e14072. [Google Scholar] [CrossRef]
- Wagner, A.J.; Ravi, V.; Riedel, R.F.; Ganjoo, K.; Van Tine, B.A.; Chugh, R.; Cranmer, L.; Gordon, E.M.; Hornick, J.L.; Du, H.; et al. nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors. J. Clin. Oncol. 2021, 39, 3660–3670. [Google Scholar] [CrossRef]
- Bergamo, F.; Maruzzo, M.; Basso, U.; Montesco, M.C.; Zagonel, V.; Gringeri, E.; Cillo, U. Neoadjuvant sirolimus for a large hepatic perivascular epithelioid cell tumor (PEComa). World J. Surg. Oncol. 2014, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Abhirup, B.; Kaushal, K.; Sanket, M.; Ganesh, N. Malignant hepatic perivascular epithelioid cell tumor (PEComa)—Case report and a brief review. J. Egypt. Natl. Cancer Inst. 2015, 27, 239–242. [Google Scholar] [CrossRef]
- Fukuda, Y.; Omiya, H.; Takami, K.; Mori, K.; Kodama, Y.; Mano, M.; Nomura, Y.; Akiba, J.; Yano, H.; Nakashima, O.; et al. Malignant hepatic epithelioid angiomyolipoma with recurrence in the lung 7 years after hepatectomy: A case report and literature review. Surg. Case Rep. 2016, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Gennatas, C.; Michalaki, V.; Kairi, P.V.; Kondi-Paphiti, A.; Voros, D. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J. Surg. Oncol. 2012, 10, 181. [Google Scholar] [CrossRef]
- Świtaj, T.; Sobiborowicz, A.; Teterycz, P.; Klimczak, A.; Makuła, D.; Wągrodzki, M.; Szumera-Ciećkiewicz, A.; Rutkowski, P.; Czarnecka, A.M. Efficacy of Sirolimus Treatment in PEComa–10 Years of Practice Perspective. J. Clin. Med. 2021, 10, 3705. [Google Scholar]
- Folpe, A.L.; Mentzel, T.; Lehr, H.A.; Fisher, C.; Balzer, B.L.; Weiss, S.W. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am. J. Surg. Pathol. 2005, 29, 1558–1575. [Google Scholar] [CrossRef]
- Yoo, Y.; Kim, J.; Song, I.H. Risk prediction criteria for the primary hepatic perivascular epithelioid cell tumour family, including angiomyolipoma: Analysis of 132 cases with a literature review. Histopathology 2025, 86, 979–992. [Google Scholar] [CrossRef]
- Kudo, M.; Okuno, T.; Tomita, S.; Kajiwara, T.; Shirane, H.; Usuki, N.; Todo, A. Hepatic angiomyolipoma pre-operatively diagnosed by imaging. J. Gastroenterol. Hepatol. 1993, 8, 483–488. [Google Scholar] [CrossRef]
- Yamada, N.; Shinzawa, H.; Makino, N.; Matsuhashi, T.; Itasaka, S.; Takahashi, T.; Fuyama, S. Small angiomyolipoma of the liver diagnosed by fine-needle aspiration biopsy under ultrasound guidance. J. Gastroenterol. Hepatol. 1993, 8, 495–498. [Google Scholar] [CrossRef]
- Carmody, E.; Yeung, E.; McLoughlin, M. Angiomyolipomas of the liver in tuberous sclerosis. Abdom. Imaging 1994, 19, 537–539. [Google Scholar] [CrossRef]
- Kimura, N.; Kubota, M.; Nagura, H. A hepatic tumor associated with bilateral renal angiomyolipomas: A variant of angiomyolipoma? Pathol. Int. 1994, 44, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Peh, W.C.; Ngan, H.; Fan, S.T.; Ng, I.O. Case report: Variable imaging appearances of angiomyolipomas of the liver. Br. J. Radiol. 1995, 68, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.L.; Emre, S.; Verham, R.P.; Petrovic, L.M.; Eguchi, S.; Silverman, J.L.; Geller, S.A.; Schwartz, M.E.; Miller, C.M.; Makowka, L. Hepatic angiomyolipoma: Two case reports of caudate-based lesions and review of the literature. Liver Transplant. Surg. 1997, 3, 46–53. [Google Scholar] [CrossRef]
- Sawai, H.; Manabe, T.; Yamanaka, Y.; Kurahashi, S.; Kamiya, A. Angiomyolipoma of the liver: Case report and collective review of cases diagnosed from fine needle aspiration biopsy specimens. J. Hepatobiliary Pancreat. Surg. 1998, 5, 333–338. [Google Scholar] [CrossRef]
- Röcken, C.; Schneider-Stock, R.; Buhtz, P.; Manger, T.; Roessner, A. Hepatic angiomyolipoma in a 26-year-old Caucasian woman with a history of tibial osteosarcoma. Pathol. Res. Pract. 1999, 195, 765–772. [Google Scholar] [CrossRef]
- Sajima, S.; Kinoshita, H.; Okuda, K.; Saito, N.; Hashino, K.; Sugimoto, R.; Eriguchi, N.; Aoyagi, S. Angiomyolipoma of the liver--a case report and review of 48 cases reported in Japan. Kurume Med. J. 1999, 46, 127–131. [Google Scholar] [CrossRef]
- Dalle, I.; Sciot, R.; de Vos, R.; Aerts, R.; van Damme, B.; Desmet, V.; Roskams, T. Malignant angiomyolipoma of the liver: A hitherto unreported variant. Histopathology 2000, 36, 443–450. [Google Scholar] [CrossRef]
- Yamasaki, S.; Tanaka, S.; Fujii, H.; Matsumoto, T.; Okuda, C.; Watanabe, G.; Suda, K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology 2000, 36, 451–456. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, X.; Xu, J.; Zhou, J.; Tan, Y.; Wang, J.; Fan, J.; Zhou, Y. Hepatic angiomyolipoma: A clinicopathologic study of 10 cases. Chin. Med. J. 2001, 114, 280–285. [Google Scholar]
- Tang, L.H.; Hui, P.; Garcia-Tsao, G.; Salem, R.R.; Jain, D. Multiple angiomyolipomata of the liver: A case report. Mod. Pathol. 2002, 15, 167–171. [Google Scholar] [CrossRef]
- Rimola, J.; Martín, J.; Puig, J.; Darnell, A.; Gil, D. Hepatic angiomyolipoma: Progressive changes in size and tumor composition. Abdom. Imaging 2003, 28, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, T.; Sakamoto, M.; Shimazu, M.; Kitajima, M. Pedunculated angiomyolipoma of the liver with a predominant pelioid pattern. Virchows Arch. 2004, 444, 467–469. [Google Scholar] [CrossRef] [PubMed]
- De Bruecker, Y.; Ballaux, F.; Allewaert, S.; Vanbeckevoort, D.; Bielen, D.; Roskams, T.; Aerts, R.; Roex, L.; Simoens, M. A solitary hepatic lesion: MRI-pathological correlation of an hepatic angiomyolipoma (2004:4b). Eur. Radiol. 2004, 14, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Nakamura, S.; Ohno, Y.; Sugihara, S.; Sakata, N.; Masawa, N. Hepatic angiomyolipoma resembling an inflammatory pseudotumor of the liver. A case report. Pathol. Res. Pract. 2004, 200, 713–716. [Google Scholar] [CrossRef]
- Lin, K.J.; Eng, H.L.; Lu, S.N.; Chiu, K.W.; Kuo, F.Y. Hepatic angiomyolipoma: Report of two cases with emphasis on smear cytomorphology and the use of cell block with immunohistochemical stains. Diagn. Cytopathol. 2004, 31, 263–266. [Google Scholar] [CrossRef]
- Romano, F.; Franciosi, C.; Bovo, G.; Cesana, G.C.; Isella, G.; Colombo, G.; Uggeri, F. Case report of a hepatic angiomyolipoma. Tumori J. 2004, 90, 139–143. [Google Scholar] [CrossRef]
- Saito, M.; Tsukamoto, T.; Takahashi, T.; Sai, K.; Fujii, H.; Nagashima, K. Multifocal angiomyolipoma affecting the liver and lung without tuberous sclerosis. J. Clin. Pathol. 2004, 57, 221–224. [Google Scholar] [CrossRef]
- Sebastian, S.; Tuite, D.; Torreggiani, W.; Crotty, P.; Buckley, M. Angiomyolipoma of the liver causing Budd-Chiari syndrome. J. Gastroenterol. Hepatol. 2004, 19, 722–723. [Google Scholar] [CrossRef]
- Tryggvason, G.; Blöndal, S.; Goldin, R.D.; Albrechtsen, J.; Björnsson, J.; Jónasson, J.G. Epithelioid angiomyolipoma of the liver: Case report and review of the literature. Apmis 2004, 112, 612–616. [Google Scholar] [CrossRef]
- Takamura, K.; Miyake, H.; Fujii, M.; Nishi, M.; Tashiro, S.; Shimada, M. Multiple hepatic angiomyolipomas with a solitary omental angiomyolipoma. J. Med. Investig. 2005, 52, 218–222. [Google Scholar] [CrossRef]
- Yen, Y.H.; Wang, J.H.; Lu, S.N.; Changchien, C.S. Contrast-enhanced ultrasonography in hepatic angiomyolipoma. J. Ultrasound Med. 2005, 24, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Flor, N.; Sardanelli, F.; Serantoni, S.; Brovelli, F.; Cornalba, G.P. Low-fat angiomyolipoma of the liver studied with contrast-enhanced ultrasound and multidetector computed tomography. Acta Radiol. 2006, 47, 543–546. [Google Scholar] [CrossRef]
- Parfitt, J.R.; Bella, A.J.; Izawa, J.I.; Wehrli, B.M. Malignant neoplasm of perivascular epithelioid cells of the liver. Arch. Pathol. Lab. Med. 2006, 130, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Tsai, K.B.; Lee, K.T. Hepatic angiomyolipoma with trace amounts of fat: A case report and literature review. J. Clin. Pathol. 2006, 59, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.H.; Zhou, L.N.; Jin, M.; Hu, J.B. Perivascular epithelioid cell tumor of the liver: A report of two cases and review of the literature. World J. Gastroenterol. 2007, 13, 5537–5539. [Google Scholar] [CrossRef]
- Larbcharoensub, N.; Karnsombut, P.; Jatchavala, J.; Wasutit, Y.; Nitiyanant, P. Primary hepatic clear cell myomelanocytic tumor. Case report and review of the literature. Apmis 2007, 115, 1454–1459. [Google Scholar] [CrossRef]
- Yang, C.Y.; Ho, M.C.; Jeng, Y.M.; Hu, R.H.; Wu, Y.M.; Lee, P.H. Management of hepatic angiomyolipoma. J. Gastrointest. Surg. 2007, 11, 452–457. [Google Scholar] [CrossRef]
- Della Vigna, P.; Preda, L.; Monfardini, L.; Gorone, M.S.; Maffini, F.A.; Bellomi, M. Growing perivascular epithelioid cell tumor of the liver studied with contrast-enhanced ultrasonography and magnetic resonance imaging. J. Ultrasound Med. 2008, 27, 1781–1785. [Google Scholar] [CrossRef]
- Deng, Y.F.; Lin, Q.; Zhang, S.H.; Ling, Y.M.; He, J.K.; Chen, X.F. Malignant angiomyolipoma in the liver: A case report with pathological and molecular analysis. Pathol. Res. Pract. 2008, 204, 911–918. [Google Scholar] [CrossRef]
- Lenci, I.; Angelico, M.; Tisone, G.; Orlacchio, A.; Palmieri, G.; Pinci, M.; Bombardieri, R.; Curatolo, P. Massive hepatic angiomyolipoma in a young woman with tuberous sclerosis complex: Significant clinical improvement during tamoxifen treatment. J. Hepatol. 2008, 48, 1026–1029. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gorman, B.; Shields, D.; Goodman, Z. Malignant hepatic angiomyolipoma: Report of a case and review of literature. Am. J. Surg. Pathol. 2008, 32, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.E.; Moraes Neto, F.A.; Agaimy, A.; Custodio Domingues, M.A.; Rogatto, S.R. Perivascular epithelioid cell tumor of the liver coexisting with a gastrointestinal stromal tumor. World J. Gastroenterol. 2008, 14, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, W.H.; Shi, P.Z.; Xiang, J.J.; Xu, R.J.; Liu, J.H. Coincidence of hepatocelluar carcinoma and hepatic angiomyolipomas in tuberous sclerosis complex: A case report. World J. Gastroenterol. 2008, 14, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; von der Brelie, C.; Berger, B.; Kappeler, A.; Candinas, D. Primary perivascular epithelioid cell tumor of the liver not related to hepatic ligaments: Hepatic PEComa as an emerging entity. Histol. Histopathol. 2008, 23, 1185–1193. [Google Scholar] [CrossRef]
- Akitake, R.; Kimura, H.; Sekoguchi, S.; Nakamura, H.; Seno, H.; Chiba, T.; Fujimoto, S. Perivascular epithelioid cell tumor (PEComa) of the liver diagnosed by contrast-enhanced ultrasonography. Intern. Med. 2009, 48, 2083–2086. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, T.; Liu, H. Hepatic angiomyolipoma mimicking hepatic clear cell carcinoma. J. Int. Med. Res. 2009, 37, 257–263. [Google Scholar] [CrossRef]
- Priola, A.M.; Priola, S.M.; Cataldi, A.; Marci, V.; Fava, C. Acute abdomen as an unusual presentation of hepatic PEComa. A case report. Tumori 2009, 95, 123–128. [Google Scholar] [CrossRef]
- Strzelczyk, J.M.; Durczynski, A.; Szymanski, D.; Jablkowski, M.; Dworniak, D.; Sporny, S. Primary perivascular epithelioid cell tumor (PEComa) of the liver: Report of a case. Surg. Today 2009, 39, 916–921. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, Y.C.; Chien, C.C.; Chen, C.J.; Yang, R.N.; Fang, C.L. Diagnosis of hepatic angiomyolipomata using CT: Report of three cases and review of the literature. Clin. Radiol. 2009, 64, 329–334. [Google Scholar] [CrossRef]
- Kamimura, K.; Oosaki, A.; Sugahara, S.; Mori, S.; Moroda, T.; Satoh, O.; Morita, T.; Kimura, K.; Kamura, T.; Nomoto, M.; et al. Malignant potential of hepatic angiomyolipoma: Case report and literature review. Clin. J. Gastroenterol. 2010, 3, 104–110. [Google Scholar] [CrossRef]
- Shi, H.; Cao, D.; Wei, L.; Sun, L.; Guo, A. Inflammatory angiomyolipomas of the liver: A clinicopathologic and immunohistochemical analysis of 5 cases. Ann. Diagn. Pathol. 2010, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.P.; Dong, J.H.; Zhang, W.Z.; Wang, J.; Pang, X.P. Hepatic angiomyolipoma: A clinical experience in diagnosis and treatment. Dig. Dis. Sci. 2010, 55, 3235–3240. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Hur, B. Primary Perivascular Epithelioid Cell Tumor (PEComa) of the Liver: A Case Report and Review of the Literature. J. Pathol. Transl. Med. 2011, 45, S93–S97. [Google Scholar] [CrossRef]
- Mima, K.; Beppu, T.; Chikamoto, A.; Ishiko, T.; Horino, K.; Hayashi, N.; Watanabe, M.; Takamori, H.; Okabe, K.; Yamanaka, T.; et al. Laparoscopy-assisted resection of an undiagnosed liver tumor and ascending colon cancer via mini median laparotomy: Report of a case. Surg. Today 2011, 41, 1633–1638. [Google Scholar] [CrossRef]
- Selvaggi, F.; Risio, D.; Claudi, R.; Cianci, R.; Angelucci, D.; Pulcini, D.; D’Aulerio, A.; Legnini, M.; Cotellese, R.; Innocenti, P. Malignant PEComa: A case report with emphasis on clinical and morphological criteria. BMC Surg. 2011, 11, 3. [Google Scholar] [CrossRef]
- Tani, A.; Yoshida, H.; Mamada, Y.; Taniai, N.; Mineta, S.; Yoshioka, M.; Kawano, Y.; Ueda, J.; Naito, Z.; Uchida, E. Hepatic angiomyolipoma with a giant hemangioma. J. Nippon. Med. Sch. 2011, 78, 317–321. [Google Scholar] [CrossRef]
- Vagefi, P.A.; Eilers, H.; Hiniker, A.; Freise, C.E. Liver transplantation for giant hepatic angiomyolipoma. Liver Transplant. 2011, 17, 985–986. [Google Scholar] [CrossRef]
- Agaimy, A.; Vassos, N.; Croner, R.S.; Strobel, D.; Lell, M. Hepatic angiomyolipoma: A series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int. J. Clin. Exp. Pathol. 2012, 5, 512–521. [Google Scholar]
- Costa, S.; Tente, D.; Costa, A.; Maciel, J. Sporadic exophytic hepatic angiomyolipoma. BMJ Case Rep. 2012, 2012, 007224. [Google Scholar] [CrossRef]
- Durczyński, A.; Hogendorf, P.; Szymański, D.; Sporny, S.; Strzelczyk, J. Synchronous occurrence of multiple focal nodular hyperplasia and huge hepatic perivascular epithelioid cells tumor (PEComa) in young woman after oral contraceptive use--is there a common pathogenesis? Pol. Przegl. Chir. 2012, 84, 457–460. [Google Scholar]
- Liu, Y.; Wang, J.; Lin, X.Y.; Xu, H.T.; Qiu, X.S.; Wang, E.H. Inflammatory angiomyolipoma of the liver: A rare hepatic tumor. Diagn. Pathol. 2012, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Xiao, E.H. Hepatic perivascular epithelioid cell tumor (PEComa): Dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom. Imaging 2012, 37, 781–787. [Google Scholar] [CrossRef]
- Agaimy, A.; Märkl, B. Inflammatory angiomyolipoma of the liver: An unusual case suggesting relationship to IgG4-related pseudotumor. Int. J. Clin. Exp. Pathol. 2013, 6, 771–779. [Google Scholar]
- Cheung, T.T.; Trendell-Smith, N.; Poon, R.T. Primary perivascular epithelioid cell tumour (PEComa) of the liver. BMJ Case Rep. 2013, 2013, 008706. [Google Scholar] [CrossRef]
- Jafari, A.; Fischer, H.P.; von Websky, M.; Hong, G.S.; Kalff, J.C.; Manekeller, S. Primary perivascular epitheloid cell tumour (PEComa) of the liver: Case report and review of the literature. Z. Gastroenterol. 2013, 51, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Vij, M.; Kota, V.; Kancherla, R.; Rela, M. Pigmented perivascular epithelioid cell tumor of the liver: Report of a rare case with brief review of literature. J. Cancer Res. Ther. 2013, 9, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.Q.; Chen, D.F.; Sun, X.H.; Li, X.; Xu, J.; Hu, X.B.; Li, M.Q.; Wu, T.; Zhang, R.Y.; Li, K.Z. MRI diagnosis of perivascular epithelioid cell tumor (PEComa) of the liver. Rom. J. Morphol. Embryol. 2013, 54, 643–647. [Google Scholar]
- Shi, H.; Bai, Y.; Guo, A. Four cases of primary malignant perivascular epithelioid cell tumour of the liver. Pathology 2013, 45, 614–616. [Google Scholar] [CrossRef]
- Tay Sh, Y.; Lao, W.T.; Chen Ch, L.; Chan, W.P. Contrast-enhanced ct and angiographic findings in hepatic perivascular epithelioid cell tumor. J. Belg. Soc. Radiol. 2013, 96, 308–310. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Z.; Dong, R.; Fan, J.; Du, Y.; Zhang, Y.; Wang, X.; Cheng, X.; Guo, J. Is surgery necessary for patients with hepatic angiomyolipoma? Retrospective analysis from eight Chinese cases. J. Gastroenterol. Hepatol. 2013, 28, 1648–1653. [Google Scholar] [CrossRef]
- Yu, D.; Tang, S. Hepatic perivascular epithelioid cell tumor: A case report and review of the literature. Intern. Med. 2013, 52, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Yang, Y.J.; Wu, H.; Huang, S.M.; Liu, K. Perivascular epithelioid cell tumor of the liver: A case report and literature review. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1665–1668. [Google Scholar] [PubMed]
- Fang, S.; Zhou, L.; Jin, M.; Hu, J. Perivascular epithelioid cell tumour of the liver. Liver Int. 2007, 27, 1293–1294. [Google Scholar] [CrossRef] [PubMed]
- Ameurtesse, H.; Chbani, L.; Bennani, A.; Toughrai, I.; Beggui, N.; Kamaoui, I.; Elfatemi, H.; Harmouch, T.; Amarti, A. Primary perivascular epithelioid cell tumor of the liver: New case report and literature review. Diagn. Pathol. 2014, 9, 149. [Google Scholar] [CrossRef]
- Barbier, L.; Torrents, J.; Hardwigsen, J. Hepatic angiomyolipoma: What management? Acta Chir. Belg. 2014, 114, 139–142. [Google Scholar]
- Kechaou, I.; Cherif, E.; Ben Hassine, L.; Khalfallah, N. Liver involvement in tuberous sclerosis. BMJ Case Rep. 2014, 2014, 201650. [Google Scholar] [CrossRef]
- Kumasaka, S.; Arisaka, Y.; Tokue, A.; Higuchi, T.; Nakajima, T.; Tsushima, Y. A case of multiple hepatic angiomyolipomas with high (18) F-fluorodeoxyglucose uptake. BMC Med. Imaging 2014, 14, 17. [Google Scholar] [CrossRef]
- Liu, D.; Shi, D.; Xu, Y.; Cao, L. Management of perivascular epithelioid cell tumor of the liver: A case report and review of the literature. Oncol. Lett. 2014, 7, 148–152. [Google Scholar] [CrossRef]
- Solarana Ortíz, J.A.; Placencia Gilart, J.E.; Rodríguez Diéguez, M.; Miranda Moles, Z.; Pullés Labadié, M.; Lau Cuza, J.C.; Corpas Fuster, S. Primary tumour of the round ligament of the liver: A case presentation. Pathologica 2014, 106, 26–28. [Google Scholar]
- Sun, K.; Zhao, M.; Yao, H.; Wang, L.; Wei, J. Premelanosome-negative inflammatory angiomyolipoma of liver with expression of cathepsin K and TFE3. Int. J. Clin. Exp. Pathol. 2014, 7, 8170–8175. [Google Scholar]
- Tan, Y.; Zhang, H.; Wang, X.C. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres. Indian J. Pathol. Microbiol. 2014, 57, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Li, H.Y.; Wang, H.; Guo, X.D.; Liu, C.C.; Liu, S.H.; Gao, X.D.; Qu, J.H.; Liu, Z.; Chang, X.J.; et al. Hepatic angiomyolipoma mimicking hepatocellular carcinoma: Magnetic resonance imaging and clinical pathological characteristics in 9 cases. Medicine 2014, 93, e194. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ueda, Y.; Suzuki, T. Hepatic angiomyolipoma growing to cause epigastric discomfort: A case report. Clin. J. Gastroenterol. 2014, 7, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.W.; Zeng, H.Y.; Su-Jie, A.; Du, M.; Ji, Y.; Tan, Y.S.; Hou, Y.Y.; Xu, J.F. Hepatocellular carcinoma with concomitant hepatic angiomyolipoma and cavernous hemangioma in one patient. World J. Gastroenterol. 2015, 21, 3414–3419. [Google Scholar] [CrossRef]
- Maebayashi, T.; Abe, K.; Aizawa, T.; Sakaguchi, M.; Ishibashi, N.; Abe, O.; Takayama, T.; Nakayama, H.; Matsuoka, S.; Nirei, K.; et al. Improving recognition of hepatic perivascular epithelioid cell tumor: Case report and literature review. World J. Gastroenterol. 2015, 21, 5432–5441. [Google Scholar] [CrossRef]
- Neofytou, K.; Famularo, S.; Khan, A.Z. PEComa in a Young Patient with Known Li-Fraumeni Syndrome. Case Rep. Med. 2015, 2015, 906981. [Google Scholar] [CrossRef]
- Zhou, G.; Hu, W.; Bao, H.; Zhang, Q. A rare case of xanthogranulomatous pyelonepheritis with hepatic angiomyolipoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11819–11822. [Google Scholar]
- Hao, B.B.; Rao, J.H.; Fan, Y.; Zhang, C.Y.; Dai, X.Z.; Li, X.; Leng, Y.; Zhang, F. Hepatic perivascular epithelioid cell tumor in three patients. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 660–664. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Tsukamoto, T.; Mizoguchi, Y.; Ishihara, S.; Horiguchi, A.; Tokoro, T.; Kato, Y.; Sugioka, A.; Kuroda, M. Intrahepatic peribiliary perivascular epithelioid cell tumor (PEComa) associated with heterotopic pancreas: A case report. Diagn. Pathol. 2016, 11, 81. [Google Scholar] [CrossRef]
- Lan, Y.Z.; Hua, X.E. Hepatic multiple perivascular epithelioid cell neoplasm: A case report and literature review. Mol. Clin. Oncol. 2016, 4, 619–621. [Google Scholar] [CrossRef]
- Tang, D.; Wang, J.; Tian, Y.; Li, Q.; Yan, H.; Wang, B.; Xiong, L.; Li, Q. Hepatic perivascular epithelioid cell tumor: Case report and brief literature review. Medicine 2016, 95, e5572. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, H.; Silva, M.; Vilas-Boas, F.; Cunha, R.; Lopes, J.; Maia, J.C.; Macedo, G. Hepatic perivascular epithelioid tumor (PEComa). A case report. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e43–e46. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Garmpi, A.; Nonni, A.; Sakellariou, S.; Margonis, G.A.; Spartalis, E.; Schizas, D.; Andreatos, N.; Magkouti, E.; et al. Angiomyolipoma of the Liver: A Rare Benign Tumor Treated with a Laparoscopic Approach for the First Time. In Vivo 2017, 31, 1169–1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guan, H.; Zou, Y.; Lv, Y.; Wang, C. Hepatic perivascular epithelioid cell tumor treated by transarterial embolization plus radiofrequency ablation: A case report and literature review. Medicine 2017, 96, e6969. [Google Scholar] [CrossRef] [PubMed]
- Hekimoglu, K.; Haberal, M. Liver Perivascular Epithelioid Cell Tumor with an Unusual Location: Diagnostic Characteristics with Multidetector Computed Tomography and Magnetic Resonance Imaging. J. Clin. Imaging Sci. 2017, 7, 36. [Google Scholar] [CrossRef]
- Kubo, H.; Yamazaki, H.; Okada, T.; Takahashi, Y.; Nishi, Y.; Yokomori, H. Primary hepatic angiomyolipoma: Immunohistochemistry and electron microscopic observations: A case report. J. Med. Case. Rep. 2017, 11, 76. [Google Scholar] [CrossRef]
- Miyata, T.; Yamashita, Y.; Yamao, T.; Umezaki, N.; Tsukamoto, M.; Kitano, Y.; Yamamura, K.; Arima, K.; Kaida, T.; Nakagawa, S.; et al. Hepatobiliary and Pancreatic: Hepatocellular carcinoma developed with angiomyolipoma. J. Gastroenterol. Hepatol. 2017, 32, 547. [Google Scholar] [CrossRef]
- Kirnap, M.; Ozgun, G.; Moray, G.; Haberal, M. Perivascular epithelioid cell tumor outgrowth from the liver. Int. J. Surg. Case Rep. 2018, 53, 295–298. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, P.; Gao, H.; Zhai, W. Hepatic perivascular epithelioid cell tumor (PEComa): Analyses of 13 cases and review of the literature. Int. J. Clin. Exp. Pathol. 2018, 11, 2759–2767. [Google Scholar]
- Nell, R.J.; Wong, D.D.; van Vliet, C.; Parry, J.; Fermoyle, S. Inflammatory angiomyolipoma of the liver: A diagnostic pitfall. Pathology 2018, 50, 352–355. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Tatsi, V.; Milionis, H.J.; Goussia, A.; Xydis, V.; Glantzounis, G.K. Liver perivascular epithelioid cell tumor in a patient with systemic lupus erythematosus. Int. J. Surg. Case Rep. 2018, 53, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Galera López, M.D.M.; Márquez Rodas, I.; Agra Pujol, C.; García Pérez, Á.; Velasco Sánchez, E.; Álvarez Álvarez, R. Simultaneous diagnosis of liver PEComa in a family with known Li-Fraumeni syndrome: A case report. Clin. Sarcoma. Res. 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Xu, H.; Yang, H.; Du, S.; Mao, Y. Primary hepatic perivascular epithelioid cell neoplasm (PEComa) with fever in a 53-year-old man. Postgrad. Med. J. 2020, 96, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Attard, A.; Piscopo, N.; Schembri, J.; Buhagiar, T.; Cortis, K.; Ellul, P. A Rare Case of PEComa of the Liver. GE Port. J. Gastroenterol. 2021, 28, 217–221. [Google Scholar] [CrossRef]
- Ergün, S.; Akinci, O.; Çomunoğlu, N.; Kocael, A. Clear-Cell Myomelanocytic Tumor of Ligamentum Teres Hepatis. Cerrahpaşa Med. J. 2021, 45, 57–59. [Google Scholar] [CrossRef]
- He, F.; Xia, Y.; Ling, X. Diagnosis and Individualized Treatment of Three Primary Malignant Tumors: A Case Report. Breast Cancer (Dove. Med. Press) 2021, 13, 519–527. [Google Scholar] [CrossRef]
- Huang, Z.; Xin, J.Y.; Li, K.Y. Ultrasound contrast agent Sonazoid for the diagnosis of hepatic epithelioid angiomyolipoma: A case report. BMC Gastroenterol. 2021, 21, 487. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.Y.; Liu, Y. Sonazoid-enhanced ultrasonography and pathologic characters of CD68 positive cell in primary hepatic perivascular epithelioid cell tumors: A case report and literature review. Open Med. (Wars) 2021, 16, 737–741. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, L.; Xie, Y.J. Hepatic perivascular epithelioid cell tumor: A case report. World J. Clin. Cases 2022, 10, 4273–4279. [Google Scholar] [CrossRef]
- Wang, S.; Xia, H.; Liu, X.; Liu, Y.; Lou, C. Hepatic epithelioid angiomyolipoma mimicking hepatocellular carcinoma on MR and (18)F-FDG PET/CT imaging: A case report and literature review. Hell J. Nucl. Med. 2022, 25, 205–209. [Google Scholar]
- Zhang, X.; Chen, J.; Huang, B.; Wang, L. Case report: Hepatic epithelioid angiomyolipoma with elevated alpha-fetoprotein and a history of breast cancer. Front. Surg. 2022, 9, 991228. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, X.; Lin, X.; Li, X.; Tian, H.; Chang, B.; Wang, Y.; Tong, J.; Wang, N.; Li, D.; et al. Tuberous Sclerosis Complex With Multiple Organ Tumors: Case Report and Literature Review. Front. Oncol. 2022, 12, 916016. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, G.; Sun, G.; Xie, B.; Xiao, W.; Li, Y. Primary hepatic epithelioid angiomyolipoma: A small case series. ANZ J. Surg. 2022, 92, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Sun, S.; Deng, Y.; Liu, J.; Pan, S. Hepatic epithelioid angiomyolipoma is scattered and unsuitable for surgery: A case report. J. Int. Med. Res. 2023, 51, 3000605231154657. [Google Scholar] [CrossRef]
- Harwal, R.; Joseph Rosemary, L.J.; Raju, P.; Chidambaranathan, S.; Bharathi Vidya Jayanthi, J.; Obla Lakshmanamoorthy, N.B. Hepatic Perivascular Epithelioid Cell Tumor Mimicking Hepatocellular Carcinoma. ACG Case Rep. J. 2023, 10, e00962. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Yang, C. Hepatic caudate epithelioid angiomyolipoma mimicking hydatid cyst. Asian J. Surg. 2023, 46, 1700–1701. [Google Scholar] [CrossRef]
- Li, Z.; Su, D.; Zhou, S.; Wang, Y.; Chen, Y. Comparison of 18 F-FDG and 68 Ga-FAPI PET/CT in a Patient With Hepatic Perivascular Epithelioid Cell Neoplasm. Clin. Nucl. Med. 2023, 48, 1124–1126. [Google Scholar] [CrossRef]
- Marinho, B.M.; Canha, A.G.; Silva, D.S.; Rodrigues, A.P. Hepatic angiomyolipoma, misdiagnosed as hepatocellular carcinoma. J. Surg. Case Rep. 2023, 2023, rjad556. [Google Scholar] [CrossRef]
- Matrood, S.; Görg, C.; Safai Zadeh, E.; Alhyari, A. Hepatic perivascular epithelioid cell tumor (PEComa): Contrast-enhanced ultrasound (CEUS) characteristics—A case report and literature review. Clin. J. Gastroenterol. 2023, 16, 444–449. [Google Scholar] [CrossRef]
- Mochizuki, K.; Aoki, T.; Kusano, T.; Tomioka, K.; Tashiro, Y.; Koizumi, T.; Matsuda, K.; Enami, Y.; Yamochi, T.; Murakami, M. Laparoscopic Resection of a Hepatic Epithelioid Angiomyolipoma Revealed by Indocyanine Green Fluorescence Imaging. Am. Surg. 2023, 89, 2061–2063. [Google Scholar] [CrossRef]
- Wannasai, K.; Charoenchue, P.; Lapisatepun, W.; Wongsuriyathai, T.; Kongkarnka, S. Primary hepatic perivascular epithelioid cell tumor: A case report and review of literature. Hum. Pathol. Rep. 2023, 31, 300699. [Google Scholar] [CrossRef]
- Costa, C.J.; Nguyen, M.T.T.; Ibrahim, E.; Potashinsky, A. Perivascular Endothelial Carcinomas: An Uncommon Hepatic Tumor Requiring Resection. Ann. Intern. Med. Clin. Cases 2024, 3, 230. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y. A comprehensive study on the feasibility and diagnostic potential of fluctuation imaging in liver tumor assessment. Sci. Rep. 2024, 14, 30662. [Google Scholar] [CrossRef]
- Liu, X.L.; Cui, J.; Tian, H. Hepatic perivascular epithelioid cell neoplasm in a 58-year-old woman. Pol. Arch. Intern. Med. 2024, 134, 16615. [Google Scholar] [CrossRef]
- Tababi, R.; Medhioub, M.; Soussi, I.; Noomen, I.; Kallel, Y.; Yakoubi, M.; Mohamed, A.B.; Mahmoudi, M.; Gharbi, G.; Bouassida, M.; et al. Hepatic Angiomyolipoma: A Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2024, 12, 23247096241306542. [Google Scholar] [CrossRef]
- Takada, R.; Takahashi, M.; Hayashi, T.; Higashihara, T.; Morita, Y.; Inoue, D.; Okada, H.; Araki, J. Laparoscopic resection of liver PEComa associated with Li-Fraumeni syndrome: A case report. Biomed. Rep. 2024, 21, 154. [Google Scholar] [CrossRef]
- Tan, L.L.Y.; Lee, V.T.W.; Lim, T.K.H. A case of liver angiomyolipoma accompanied with multiple focal nodular hyperplasia. Pathology 2024, 56, 755–758. [Google Scholar] [CrossRef]
- Vijayanirmala, P.; Yadav, R.; Goyal, S.; Barwad, A.; Bhowmik, S.; Malik, R.; Pal, S.; Sharma, R.; Sakhuja, P.; Das, P. Hepatic and perihepatic PEComas: A study describing a series of five rare cases. Indian J. Pathol. Microbiol. 2024, 67, 355–361. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, F.R.; He, N.; She, Y.H.; Du, Y.Y.; Shi, W.G.; Yang, J.; Chen, G.; Zhang, S.Z.; Cui, F.; et al. Massive simultaneous hepatic and renal perivascular epithelioid cell tumor benefitted from surgery and everolimus treatment: A case report. World J. Gastrointest. Surg. 2024, 16, 3334–3342. [Google Scholar] [CrossRef]
- Yazıcı, C.; Gündoğdu, E. Very Rare Liver Tumor: PEComa Case Report with and a Review of Literature. Indian J. Radiol. Imaging 2024, 34, 172–176. [Google Scholar] [CrossRef]
- Zaidi, A.; Chatterjee, D.; Bhargav, V.; Gupta, V.; Das, A. Clear cell myomelanocytic tumor of ligamentum teres. Autops. Case Rep. 2024, 14, e2024503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Song, S.; Wang, D.; Kuang, D.; Cheng, S.; Zhou, J.; Zou, S. Hepatic perivascular epithelioid cell tumor resembling hepatic adenoma and hepatocellular carcinoma on preoperative imaging: A case report. Front. Oncol. 2024, 14, 1292313. [Google Scholar] [CrossRef]
- Abe, N.; Yamazaki, F.; Tsujikawa, H.; Kasuga, R.; Taniki, N.; Shimada, H. Case review of perivascular epithelioid cell tumor occurring in patients with Li-Fraumeni syndrome. Fam. Cancer 2025, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Marcellini, M.M.; Ruggeri, E.; Felicani, C.; Brighenti, A.; Loiacono, R.; Ercolani, G.; Serra, C. Ceus features of liver pecoma: A case report and literature review. J. Ultrasound 2025, 28, 261–268. [Google Scholar] [CrossRef]
- Paula, D.; Amaral, M.J.; Madeira, J.; Simões, J.; Lázaro, A.; Silva, N.; Tralhão, J.G. Rare Encounter: A Case Report of Hepatic Perivascular Epithelioid Cell Tumor—An Uncommon Mesenchymal Tumor in the Liver. Case Rep. Gastroenterol. 2025, 19, 43–51. [Google Scholar] [CrossRef]
- Saadoun, J.E.; Traversari, E.; Meillat, H.; Guiramand, J. Perivascular epithelioid cell tumour (PEComa) of the ligamentum teres hepatis, a rare and mobile tumour presentation. ANZ J. Surg. 2025, 95, 832–833. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.; Woo, C.G.; Lee, O.J.; Son, S.M. Epithelioid angiomyolipoma of the liver in a patient with Li-Fraumeni syndrome: A case report. Diagn. Pathol. 2024, 19, 16. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Study origin | |
| Asia | 97 studies (66.6%) |
| Europe | 35 studies (24.1%) |
| North America | 8 studies (5.5%) |
| South America | 3 studies (2.1%) |
| Africa | 1 study (0.7%) |
| Australia | 1 study (0.7%) |
| Age | 46 years (IQR: 35.25–53.75) |
| Females | 208 patients (74%) |
| Comorbidities | |
| Hepatitis B | 23 patients (8.2%) |
| Hepatitis C | 5 patients (1.8%) |
| History of extrahepatic malignancy | 30 patients (10.7%) |
| TSC | 9 patients (3.2%) |
| Li–Fraumeni syndrome | 6 patients (2.1%) |
| Presentation | |
| Asymptomatic | 157 patients (55.9%) |
| Abdominal pain | 84 patients (29.9%) |
| Abdominal discomfort | 24 patients (8.5%) |
| Fever | 15 patients (5.3%) |
| Weight loss | 10 patients (3.6%) |
| Fatigue | 7 patients (2.5%) |
| Vomiting | 5 patients (1.8%) |
| Nausea | 5 patients (1.8%). |
| Diagnostic workup | |
| U/S | 152 patients (54.1%) |
| CT | 221 patients (78.6%) |
| MRI | 156 patients (55.5%) |
| PET-CT | 31 patients (11%) |
| Tumor size | 5.15 cm (IQR: 3–9.3) |
| Tumor location | |
| Right lobe | 149 cases (53%) |
| Left lobe | 101 cases (34%) |
| Caudate lobe | 12 cases (4.3%) |
| Tumor nodules | |
| Single liver lesion | 249 patients (88.6%) |
| Multiple nodules | 22 patients (7.8%) |
| Positive immunohistochemical markers | |
| HMB-45 | 261/264 (98.9%) |
| MELAN-A | 124/132 (93.2%) |
| SMA | 179/200 (89.5%) |
| Vimentin | 62/79 (78.5%) |
| Negative immunohistochemical markers | |
| S-100 | 85/126 (67.5%) |
| Desmin | 45/83 (54.2%) |
| Cytokeratins | 123/125 (98.1%) |
| EMA | 26/28 (92.9%) |
| HepPar-1 | 43/44 (97.7%) |
| Final diagnosis | |
| Radiology | 29 cases (10.3%) |
| Biopsy | 44 cases (15.7%) |
| Post-surgical analysis | 208 cases (74%) |
| PEComa type | |
| AML | 197 patients (70.1%) |
| PEComa-NOS | 74 patients (26.3%) |
| CCMMT of the falciform ligament | 7 patients (2.5%) |
| Clear-cell sugar tumor | 3 cases (1.1%) |
| Malignancy | 30 cases (10.7%) |
| Surgery | 251 patients (89.3%) |
| Follow-up duration (months) | 24 months (IQR: 12–48) |
| Recurrence | 17 patients (6%) |
| Death due to PEComa | 8 patients (2.85%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantoniou, K.; Aggeletopoulou, I.; Kalafateli, M.; Triantos, C. Characteristics and Treatment of Primary Hepatic Perivascular Epithelioid Cell Tumor (PEComa) in Adults: A Systematic Review. Cancers 2025, 17, 2276. https://doi.org/10.3390/cancers17142276
Papantoniou K, Aggeletopoulou I, Kalafateli M, Triantos C. Characteristics and Treatment of Primary Hepatic Perivascular Epithelioid Cell Tumor (PEComa) in Adults: A Systematic Review. Cancers. 2025; 17(14):2276. https://doi.org/10.3390/cancers17142276
Chicago/Turabian StylePapantoniou, Konstantinos, Ioanna Aggeletopoulou, Maria Kalafateli, and Christos Triantos. 2025. "Characteristics and Treatment of Primary Hepatic Perivascular Epithelioid Cell Tumor (PEComa) in Adults: A Systematic Review" Cancers 17, no. 14: 2276. https://doi.org/10.3390/cancers17142276
APA StylePapantoniou, K., Aggeletopoulou, I., Kalafateli, M., & Triantos, C. (2025). Characteristics and Treatment of Primary Hepatic Perivascular Epithelioid Cell Tumor (PEComa) in Adults: A Systematic Review. Cancers, 17(14), 2276. https://doi.org/10.3390/cancers17142276









