Myeloid-Derived Suppressor Cells (MDSCs) at the Crossroad of Senescence and Cancer
Simple Summary
Abstract
1. Introduction
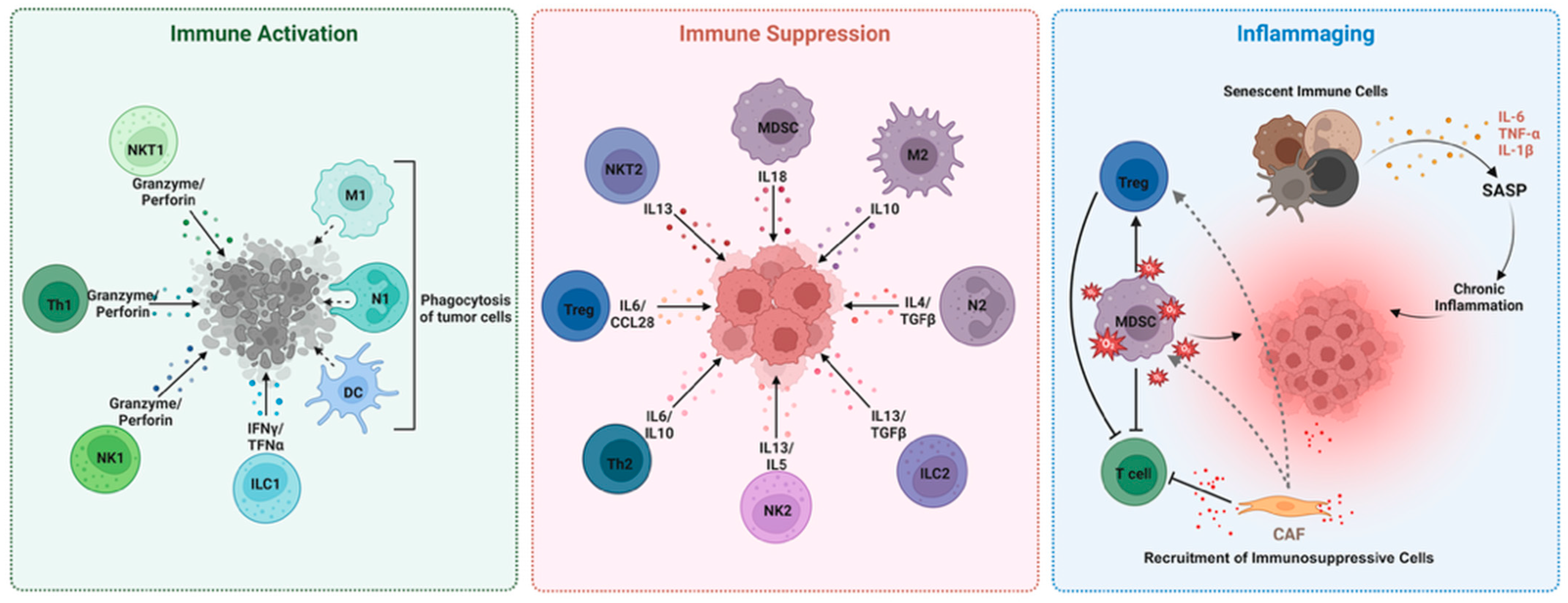
2. MDSCs in the Aged TME: Drivers of Tumor Progression and Immune Evasion
3. MDSCs-Related Evidence in Preclinical Models of Cancer and Other Diseases
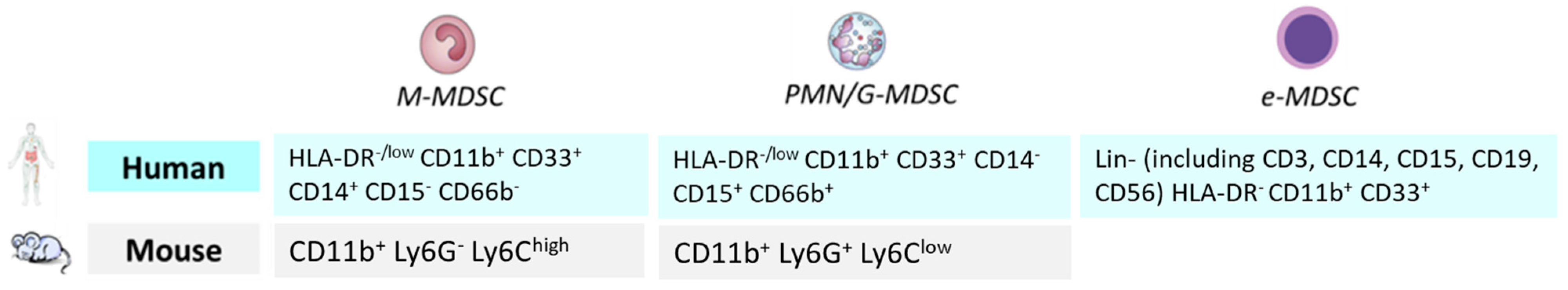
4. Myeloid-Derived Suppressor Cells in Elderly Cancer Patients
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| APC | Antigen-presenting cell |
| ARG1 | Arginase-1 |
| Breg | Regulatory B cell |
| CAF | Cancer-associated fibroblast |
| CAR | Chimeric antigen receptor |
| CCL2 | C-C motif chemokine ligand 2 |
| CHIP | Clonal hematopoiesis of indeterminate potential |
| CSF | Colony stimulating factor |
| DC | Dendritic cell |
| ECM | Extracellular matrix |
| eMDSC | Early-stage myeloid-derived suppressor cell |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| G-MDSC/PMN-MDSC | Granulocytic or polymorphonuclear myeloid-derived suppressor cell |
| HSC | Hematopoietic stem cell |
| HIF-1α | Hypoxia-inducible factor-1α |
| ICI | Immune checkpoint inhibitor |
| IDO | Indoleamine-2,3-dioxygenase |
| IFN-γ | Interferon gamma |
| IL | Interleukin (e.g., IL-6, IL-10, IL-13, IL-1β) |
| ILC1 | Type 1 innate lymphoid cell (tumor-inhibitory) |
| ILC2 | Type 2 innate lymphoid cell (tumor-promoting) |
| iNOS | Inducible nitric oxide synthase |
| M-MDSC | Monocytic myeloid-derived suppressor cell |
| M1 macrophage | Classically activated macrophage (pro-inflammatory) |
| M2 macrophage | Alternatively activated macrophage (anti-inflammatory) |
| MDSC | Myeloid-derived suppressor cell |
| MMP | Matrix metalloproteinase |
| N1 neutrophil | Anti-tumoral neutrophil |
| N2 neutrophil | Pro-tumoral neutrophil |
| NHL | Non-Hodgkin lymphoma |
| NK | Natural killer (cell) |
| NK1 | Type 1 natural killer cell (tumor-inhibitory) |
| NK2 | Type 2 natural killer cell (tumor-promoting) |
| NKT1 | Type 1 natural killer T cell (tumor-inhibitory) |
| NKT2 | Type 2 natural killer T cell (tumor-promoting) |
| NSCLC | Non-small cell lung cancer |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PSA | Prostate-specific antigen |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| scRNA-seq | Single-cell RNA sequencing |
| scT | Stem cell-like T cell |
| S100A8/A9 | S100 calcium-binding protein A8/A9 |
| SOX9 | SRY-box transcription factor 9 |
| TAM | Tumor-associated macrophage |
| TCR | T cell receptor |
| TGF-β | Transforming growth factor beta |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumor necrosis factor alpha |
| Th1 | Type 1 T helper cell (tumor-inhibitory) |
| Th2 | Type 2 T helper cell (tumor-promoting) |
| Treg | Regulatory T cell |
| VEGF | Vascular endothelial growth factor |
References
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23 (Suppl. S8), viii6–viii9. [Google Scholar] [CrossRef] [PubMed]
- Laconi, E.; Marongiu, F.; DeGregori, J. Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. Cancer 2020, 122, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Gui, J.; Mustachio, L.M.; Su, D.M.; Craig, R.W. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012, 3, 280–290. [Google Scholar] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Morrisette-Thomas, V.; Cohen, A.A.; Fulop, T.; Riesco, E.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Belanger, F.; Ferrucci, L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef]
- Pawelec, G.; Picard, E.; Bueno, V.; Verschoor, C.P.; Ostrand-Rosenberg, S. MDSCs, ageing and inflammageing. Cell Immunol. 2021, 362, 104297. [Google Scholar] [CrossRef]
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58. [Google Scholar] [CrossRef]
- Droz, J.P.; Aapro, M.; Balducci, L.; Boyle, H.; Van den Broeck, T.; Cathcart, P.; Dickinson, L.; Efstathiou, E.; Emberton, M.; Fitzpatrick, J.M.; et al. Management of prostate cancer in older patients: Updated recommendations of a working group of the International Society of Geriatric Oncology. Lancet Oncol. 2014, 15, e404–e414. [Google Scholar] [CrossRef]
- Higuera, O.; Ghanem, I.; Nasimi, R.; Prieto, I.; Koren, L.; Feliu, J. Management of pancreatic cancer in the elderly. World J. Gastroenterol. 2016, 22, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.J.; Reynolds, C.H.; Langer, C.J. Caring for the Older Population With Advanced Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razeq, H.; Abu Rous, F.; Abuhijla, F.; Abdel-Razeq, N.; Edaily, S. Breast Cancer in Geriatric Patients: Current Landscape and Future Prospects. Clin. Interv. Aging 2022, 17, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Soler-Gonzalez, G.; Sastre-Valera, J.; Viana-Alonso, A.; Aparicio-Urtasun, J.; Garcia-Escobar, I.; Gomez-Espana, M.A.; Guillen-Ponce, C.; Molina-Garrido, M.J.; Girones-Sarrio, R. Update on the management of elderly patients with colorectal cancer. Clin. Transl. Oncol. 2024, 26, 69–84. [Google Scholar] [CrossRef]
- Mogilenko, D.A.; Shchukina, I.; Artyomov, M.N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 2022, 22, 484–498. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, B.; Feng, N.; Zhang, X.; Zhang, X.; Wei, Y.; Zhang, W. Aging microenvironment and antitumor immunity for geriatric oncology: The landscape and future implications. J. Hematol. Oncol. 2023, 16, 28. [Google Scholar] [CrossRef]
- Sitnikova, S.I.; Walker, J.A.; Prickett, L.B.; Morrow, M.; Valge-Archer, V.E.; Robinson, M.J.; Wilkinson, R.W.; Dovedi, S.J. Age-induced changes in anti-tumor immunity alter the tumor immune infiltrate and impact response to immuno-oncology treatments. Front. Immunol. 2023, 14, 1258291. [Google Scholar] [CrossRef]
- Chen, A.C.Y.; Jaiswal, S.; Martinez, D.; Yerinde, C.; Ji, K.; Miranda, V.; Fung, M.E.; Weiss, S.A.; Zschummel, M.; Taguchi, K.; et al. The aged tumor microenvironment limits T cell control of cancer. Nat. Immunol. 2024, 25, 1033–1045. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Bromme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- Ding, Z.; Shi, R.; Hu, W.; Tian, L.; Sun, R.; Wu, Y.; Zhang, X. Cancer-associated fibroblasts in hematologic malignancies: Elucidating roles and spotlighting therapeutic targets. Front. Oncol. 2023, 13, 1193978. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Dewar, R.; Kieran, M.; Kalluri, R. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia 2009, 23, 2233–2241. [Google Scholar] [CrossRef]
- Shafat, M.S.; Gnaneswaran, B.; Bowles, K.M.; Rushworth, S.A. The bone marrow microenvironment—Home of the leukemic blasts. Blood Rev. 2017, 31, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel, C.H., 3rd; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H.; et al. Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mohamady Farouk Abdalsalam, N.; Liang, Z.; Kashaf Tariq, H.; Li, R.; Afolabi, L.O.; Rabiu, L.; Chen, X.; Xu, S.; Xu, Z.; et al. MDSC checkpoint blockade therapy: A new breakthrough point overcoming immunosuppression in cancer immunotherapy. Cancer Gene Ther. 2025, 32, 371–392. [Google Scholar] [CrossRef]
- Sui, H.; Dongye, S.; Liu, X.; Xu, X.; Wang, L.; Jin, C.Q.; Yao, M.; Gong, Z.; Jiang, D.; Zhang, K.; et al. Immunotherapy of targeting MDSCs in tumor microenvironment. Front. Immunol. 2022, 13, 990463. [Google Scholar] [CrossRef] [PubMed]
- Millrud, C.R.; Bergenfelz, C.; Leandersson, K. On the origin of myeloid-derived suppressor cells. Oncotarget 2017, 8, 3649–3665. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Fu, Z.; Mowday, A.M.; Smaill, J.B.; Hermans, I.F.; Patterson, A.V. Tumour Hypoxia-Mediated Immunosuppression: Mechanisms and Therapeutic Approaches to Improve Cancer Immunotherapy. Cells 2021, 10, 1006. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, F.; Qin, W.; Yang, Y.; Li, X.; Liu, R. Metabolic regulation of myeloid-derived suppressor cells in tumor immune microenvironment: Targets and therapeutic strategies. Theranostics 2025, 15, 2159–2184. [Google Scholar] [CrossRef]
- Chen, W.; Ning, X.; Liu, Y.; Shen, T.; Liu, M.; Yin, H.; Ding, Y.; Zhou, J.; Yin, R.; Cai, L.; et al. Myeloid-derived suppressor cells from tumour-bearing mice induce the population expansion of CD19(hi)FcgammaRIIb(hi) regulatory B cells via PD-L1. Immunology 2024, 172, 127–143. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Falvo, P.; Gruener, S.; Orecchioni, S.; Pisati, F.; Talarico, G.; Mitola, G.; Lombardi, D.; Bravetti, G.; Winkler, J.; Barozzi, I.; et al. Age-dependent differences in breast tumor microenvironment: Challenges and opportunities for efficacy studies in preclinical models. Cell Death Differ. 2025, 32, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; French, D.L.; Ma, G.; Eisenstein, S.; Chen, Y.; Divino, C.M.; Keller, G.; Chen, S.H.; Pan, P.Y. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells 2010, 28, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Bueno, V.; Sant’Anna, O.A.; Lord, J.M. Ageing and myeloid-derived suppressor cells: Possible involvement in immunosenescence and age-related disease. Age 2014, 36, 9729. [Google Scholar] [CrossRef]
- Sun, S.N.; Ni, S.H.; Li, Y.; Liu, X.; Deng, J.P.; Chen, Z.X.; Li, H.; Feng, W.J.; Huang, Y.S.; Li, D.N.; et al. G-MDSCs promote aging-related cardiac fibrosis by activating myofibroblasts and preventing senescence. Cell Death Dis. 2021, 12, 594. [Google Scholar] [CrossRef]
- Yan, H.; Kawano, T.; Kanki, H.; Nishiyama, K.; Shimamura, M.; Mochizuki, H.; Sasaki, T. Role of Polymorphonuclear Myeloid-Derived Suppressor Cells and Neutrophils in Ischemic Stroke. J. Am. Heart Assoc. 2023, 12, e028125. [Google Scholar] [CrossRef]
- Grizzle, W.E.; Xu, X.; Zhang, S.; Stockard, C.R.; Liu, C.; Yu, S.; Wang, J.; Mountz, J.D.; Zhang, H.G. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech. Ageing Dev. 2007, 128, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, H.; Su, N.; Zhang, G.; Wang, L. Myeloid-derived suppressor cells promote age-related increase of lung cancer growth via B7-H1. Exp. Gerontol. 2015, 61, 84–91. [Google Scholar] [CrossRef]
- Orecchioni, S.; Talarico, G.; Labanca, V.; Calleri, A.; Mancuso, P.; Bertolini, F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br. J. Cancer 2018, 118, 1329–1336. [Google Scholar] [CrossRef]
- Carpen, L.; Falvo, P.; Orecchioni, S.; Mitola, G.; Hillje, R.; Mazzara, S.; Mancuso, P.; Pileri, S.; Raveane, A.; Bertolini, F. A single-cell transcriptomic landscape of innate and adaptive intratumoral immunity in triple negative breast cancer during chemo- and immunotherapies. Cell Death Discov. 2022, 8, 106. [Google Scholar] [CrossRef]
- Falvo, P.; Orecchioni, S.; Hillje, R.; Raveane, A.; Mancuso, P.; Camisaschi, C.; Luzi, L.; Pelicci, P.; Bertolini, F. Cyclophosphamide and Vinorelbine Activate Stem-Like CD8(+) T Cells and Improve Anti-PD-1 Efficacy in Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 685–697. [Google Scholar] [CrossRef]
- Falvo, P.; Orecchioni, S.; Raveane, A.; Mitola, G.; Bertolini, F. A “two-hit” (chemo)therapy to improve checkpoint inhibition in cancer. Oncoscience 2021, 8, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, S.; Falvo, P.; Talarico, G.; Mitola, G.; Bravetti, G.; Mancuso, P.; Nicoli, P.; Bertolini, F. Vinorelbine and Intermittent Cyclophosphamide Sensitize an Aggressive Myc-Driven B-Cell Lymphoma to Anti-PD-1 by an Immunological Memory Effective against Tumor Re-Challenge. J. Clin. Med. 2023, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.D. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin. Immunol. 2012, 24, 321–330. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Montgomery, R.R.; Shaw, A.C. Paradoxical changes in innate immunity in aging: Recent progress and new directions. J. Leukoc. Biol. 2015, 98, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fulop, T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Dorshkind, K.; Hofer, T.; Montecino-Rodriguez, E.; Pioli, P.D.; Rodewald, H.R. Do haematopoietic stem cells age? Nat. Rev. Immunol. 2020, 20, 196–202. [Google Scholar] [CrossRef]
- Geiger, H.; Denkinger, M.; Schirmbeck, R. Hematopoietic stem cell aging. Curr. Opin. Immunol. 2014, 29, 86–92. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Xinyi, Y.; Vladimirovich, R.I.; Beeraka, N.M.; Satyavathi, A.; Kamble, D.; Nikolenko, V.N.; Lakshmi, A.N.; Basappa, B.; Reddy, Y.P.; Fan, R.; et al. Emerging insights into epigenetics and hematopoietic stem cell trafficking in age-related hematological malignancies. Stem Cell Res. Ther. 2024, 15, 401. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Schrier, S.L.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Alves, A.S.; Ishimura, M.E.; Duarte, Y.A.O.; Bueno, V. Parameters of the Immune System and Vitamin D Levels in Old Individuals. Front. Immunol. 2018, 9, 1122. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Johnstone, J.; Millar, J.; Dorrington, M.G.; Habibagahi, M.; Lelic, A.; Loeb, M.; Bramson, J.L.; Bowdish, D.M. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 2013, 93, 633–637. [Google Scholar] [CrossRef]
- Wang, M.; Han, Y.; Yao, X.; Duan, X.; Wan, J.; Lou, X.; Yan, Y.; Zheng, P.; Wang, F.; Zhu, L.; et al. Hyperexpression of tumor necrosis factor receptor 2 inhibits differentiation of myeloid-derived suppressor cells by instigating apolarity during ageing. MedComm (2020) 2024, 5, e605. [Google Scholar] [CrossRef]
- Passaro, A.; Mancuso, P.; Gandini, S.; Spitaleri, G.; Labanca, V.; Guerini-Rocco, E.; Barberis, M.; Catania, C.; Del Signore, E.; de Marinis, F.; et al. Gr-MDSC-linked asset as a potential immune biomarker in pretreated NSCLC receiving nivolumab as second-line therapy. Clin. Transl. Oncol. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Bonomi, M.; Ahmed, T.; Addo, S.; Kooshki, M.; Palmieri, D.; Levine, B.J.; Ruiz, J.; Grant, S.; Petty, W.J.; Triozzi, P.L. Circulating immune biomarkers as predictors of the response to pembrolizumab and weekly low dose carboplatin and paclitaxel in NSCLC and poor PS: An interim analysis. Oncol. Lett. 2019, 17, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martin, A.; Galiana, I.L.; Berenguer Frances, M.A.; Cacicedo, J.; Canas Cortes, R.; Comas Anton, S.; Padrones Sanchez, S.; Bolivar Cuevas, S.; Parry, R.; Guedea Edo, F. Preliminary Study of the Effect of Stereotactic Body Radiotherapy (SBRT) on the Immune System in Lung Cancer Patients Unfit for Surgery: Immunophenotyping Analysis. Int. J. Mol. Sci. 2018, 19, 3963. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khalaji, A.; Bahri Najafi, M.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Khaksary Mahabady, M.; Rahimian, N.; Mirzaei, H. NF-kappaB pathway and angiogenesis: Insights into colorectal cancer development and therapeutic targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Nair, V.; Saleh, R.; Toor, S.M.; Alajez, N.M.; Elkord, E. Transcriptomic Analyses of Myeloid-Derived Suppressor Cell Subsets in the Circulation of Colorectal Cancer Patients. Front. Oncol. 2020, 10, 1530. [Google Scholar] [CrossRef]
- Kimura, T.; McKolanis, J.R.; Dzubinski, L.A.; Islam, K.; Potter, D.M.; Salazar, A.M.; Schoen, R.E.; Finn, O.J. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev. Res. 2013, 6, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Spataro, C.; Zagato, E.; Di Mitri, D.; Gil, V.; Crespo, M.; De Bernardis, G.; Losa, M.; Mirenda, M.; Pasquini, E.; et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018, 559, 363–369. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagata, M.; Hachiya, T.; Wakita, H.; Ikehata, Y.; Takahashi, K.; China, T.; Shimizu, F.; Lu, J.; Jin, Y.; et al. Increased circulating polymorphonuclear myeloid-derived suppressor cells are associated with prognosis of metastatic castration-resistant prostate cancer. Front. Immunol. 2024, 15, 1372771. [Google Scholar] [CrossRef]
- van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Beer, T.M.; Gerritsen, W.; Oudard, S.; Wiechno, P.; Kukielka-Budny, B.; Samal, V.; Hajek, J.; Feyerabend, S.; Khoo, V.; et al. Efficacy and Safety of Autologous Dendritic Cell-Based Immunotherapy, Docetaxel, and Prednisone vs Placebo in Patients With Metastatic Castration-Resistant Prostate Cancer: The VIABLE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.M.; Roxa, A.; Leandersson, K.; Bergenfelz, C. Impact of systemic therapy on circulating leukocyte populations in patients with metastatic breast cancer. Sci. Rep. 2019, 9, 13451. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, R.; Duggan, M.C.; Stiff, A.; Markowitz, J.; Trikha, P.; Levine, K.M.; Schoenfield, L.; Abdel-Rasoul, M.; Layman, R.; Ramaswamy, B.; et al. Circulating myeloid-derived suppressor cells increase in patients undergoing neo-adjuvant chemotherapy for breast cancer. Cancer Immunol. Immunother. 2017, 66, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
| Feature/Observation | Details | Clinical Implication | Potential Strategy |
|---|---|---|---|
| MDSC expansion with age | Predominantly granulocytic MDSCs (G-MDSCs) increase in elderly; M-MDSCs remain stable | Associated with impaired anti-tumor immunity | Targeted depletion of G-MDSCs; monitoring as a biomarker |
| Functional enhancement | Increased suppressive capacity; altered differentiation; cytokine-driven polarization (e.g., via TNFR2/JNK pathway) | Promotes immune evasion and resistance to immunotherapy | Inhibition of suppressive signaling pathways (e.g., STAT3, TNFR2) |
| Inflammaging and emergency myelopoiesis | Chronic low-grade inflammation (IL-6, IL-1β, TNF-α) favors expansion of immature myeloid cells | Fuels MDSC accumulation and tumor-supportive microenvironment | Anti-inflammatory therapies; inhibitors of IL-6/STAT3 axis |
| Clonal hematopoiesis and myeloid bias | CHIP (e.g., TET2, DNMT3A mutations) and bone marrow remodeling drive myeloid skewing | Sustained MDSC output from progenitor pools | Targeting CHIP-related pathways; niche-modifying agents |
| Tumor-specific associations | High MDSCs correlate with poor outcomes in lung, colorectal, prostate, and breast cancers | Predictive of resistance to chemo- and immunotherapy | MDSC monitoring to stratify patients; combination therapies |
| Immunotherapy interactions | MDSC reduction linked to improved response to ICIs and cancer vaccines | MDSCs limit efficacy of T and NK cell-based therapies | Combine immunotherapies with MDSC-targeting agents |
| Age-related clinical gap | Elderly underrepresented in trials; limited age-specific data on MDSCs | Limits generalizability of immunotherapy outcomes | Inclusion of elderly in clinical trials; development of geriatric immuno-oncology models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarico, G.; Orecchioni, S.; Falvo, P.; Bertolini, F. Myeloid-Derived Suppressor Cells (MDSCs) at the Crossroad of Senescence and Cancer. Cancers 2025, 17, 2251. https://doi.org/10.3390/cancers17132251
Talarico G, Orecchioni S, Falvo P, Bertolini F. Myeloid-Derived Suppressor Cells (MDSCs) at the Crossroad of Senescence and Cancer. Cancers. 2025; 17(13):2251. https://doi.org/10.3390/cancers17132251
Chicago/Turabian StyleTalarico, Giovanna, Stefania Orecchioni, Paolo Falvo, and Francesco Bertolini. 2025. "Myeloid-Derived Suppressor Cells (MDSCs) at the Crossroad of Senescence and Cancer" Cancers 17, no. 13: 2251. https://doi.org/10.3390/cancers17132251
APA StyleTalarico, G., Orecchioni, S., Falvo, P., & Bertolini, F. (2025). Myeloid-Derived Suppressor Cells (MDSCs) at the Crossroad of Senescence and Cancer. Cancers, 17(13), 2251. https://doi.org/10.3390/cancers17132251







