Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
Aims
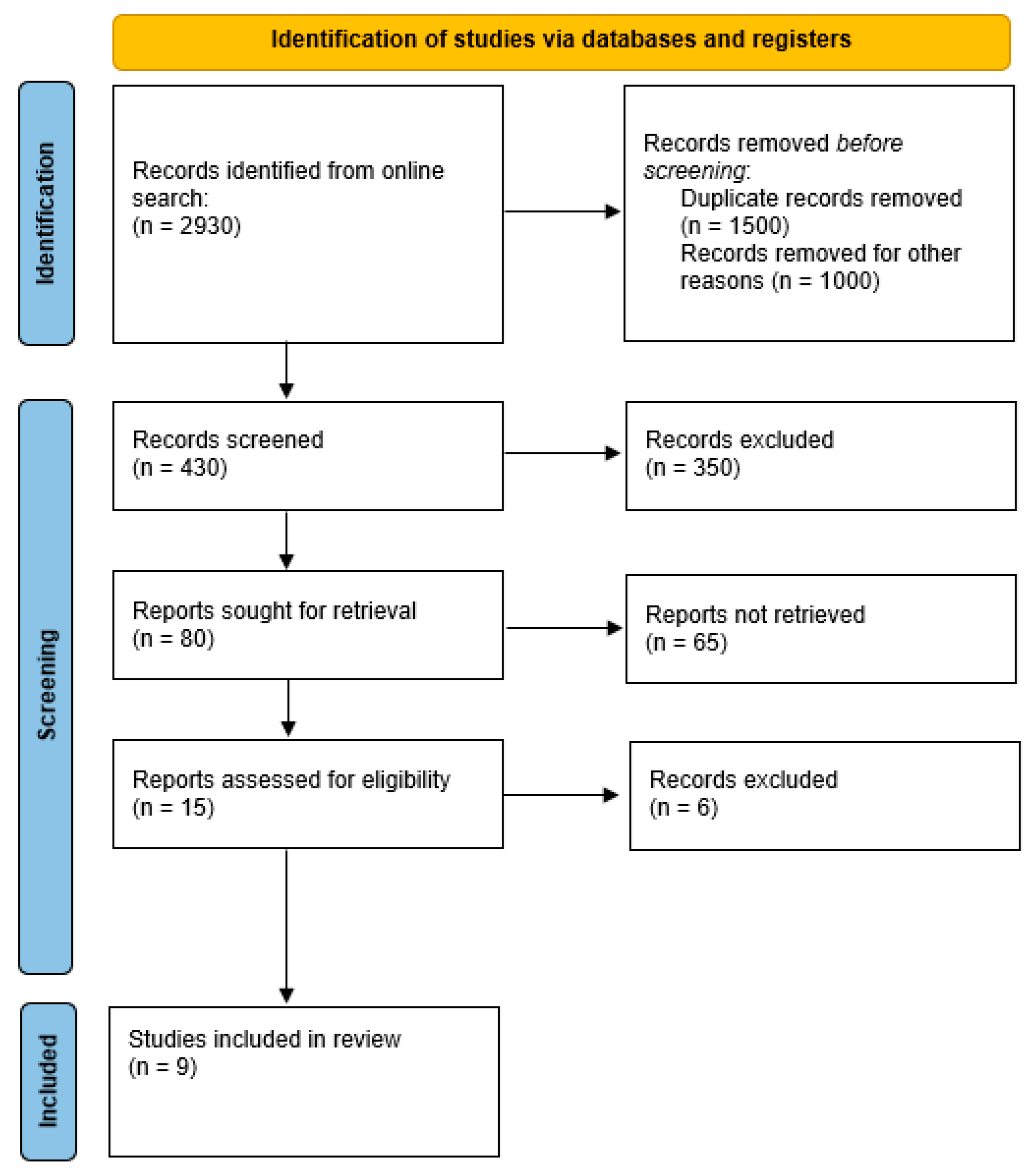
2. Materials and Methods
2.1. Identifying Target Population
2.2. Systematic Literature Search
3. Results
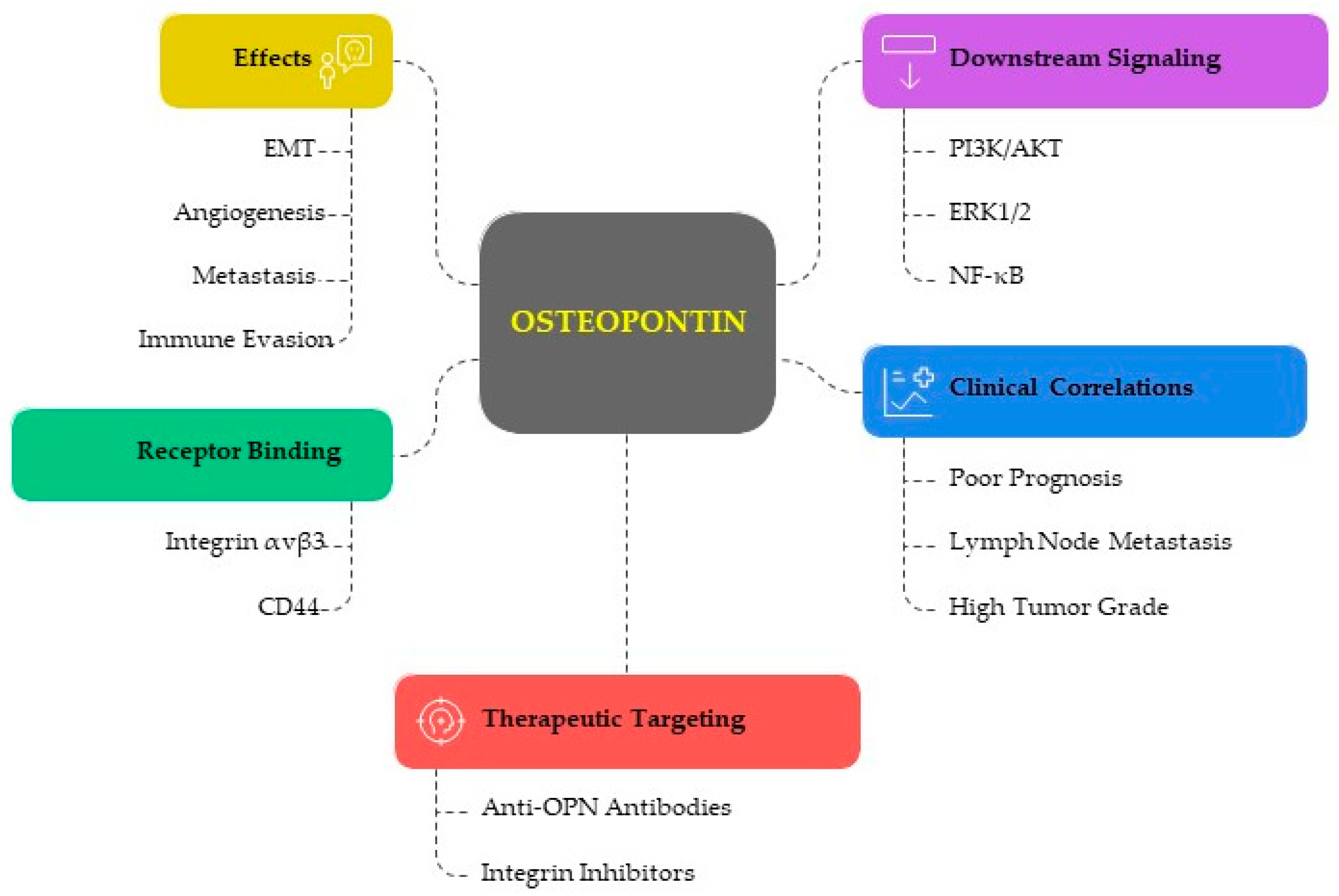
3.1. The Functional Role of Osteopontin in EC
3.1.1. OPN in Tumor Progression and Aggressiveness
3.1.2. OPN and Immune Modulation
- -
- Immune Evasion: OPN has been shown to interact with immune cells, promoting tumor immune evasion. It can affect the function of T cells, macrophages, and natural killer (NK) cells, thus enabling the tumor to escape immune surveillance.
- -
- Inflammation and Tumor Microenvironment: OPN contributes to the inflammatory tumor microenvironment by recruiting inflammatory cells and promoting the secretion of pro-inflammatory cytokines, which can enhance tumor growth and resistance to chemotherapy. It has been demonstrated that poor prognoses and survival rates in a variety of human cancers are linked to the expression of OPN as a tumor microenvironment component in cancer tissues, plasma, and serum. According to recent research, OPN uses a variety of mechanisms to promote tumor growth and aggressiveness [6].
3.1.3. Angiogenic Role of OPN in EC
3.1.4. Metastasis and Invasion
3.2. Clinical Implications and Prognostic Value
3.2.1. Diagnostic Marker and Serum and Tissue Biomarker
3.2.2. Prognostic Significance and Therapeutic Targeting
3.2.3. Involvement of OPN in Treatment Resistance
| Category | Key Findings | Mechanisms/Pathways | Clinical Implications | Citations |
|---|---|---|---|---|
| Tumor Progression & Aggressiveness | Overexpressed in EC tissues in serum vs. healthy controls, Correlations with advanced tumor grade/stage OPN knockdown reduces proliferation, increases apoptosis | PI3K/AKT, cell cycle regulation | Tumor marker, Therapeutic target potential | [8,11,25,27,29] |
| Immune Modulation | Promotion of EMT and immune escape, Regulation of T cells, NK cells, macrophages, DCs, Enhancement Th1/Th17, suppression of Th2 responses | Integrins, CD44 interactions post-translational modifications (phosphorylation, glycosylation) | Immune-based therapy target | [9,12,25,30,31,32,33,34,35,36] |
| Tumor Microenvironment | Promotion of inflammation, cytokine secretion Recruitment of immune/ inflammatory cells | NF-κB, integrin/PI3K/Src signaling pathways | Targeting OPN may disrupt tumor-supporting microenvironment | [6,30,31,32] |
| Angiogenesis | Stimulation of endothelial migration and tube formation, Increase in VEGF expression | OPN-VEGF axis ECM remodeling and angiogenic signaling | Anti-angiogenic therapy potential | [9,19,21] |
| Metastasis and Invasion | Enhancement of EMT, migration, invasion, Upregulation of MMP (particularly MMPs-2/9) | PI3K/AKT, ERK1/2 MMP-mediated ECM degradation | Linked to aggressive EC phenotype | [12,25,28,37,38] |
| Obesity & Hormonal Link | OPN is upregulated in obesity, OPN increases estradiol synthesis | OPN-MMP-aromatase axis TNFα, IL-1 | Explains obesity-related risk of estrogen-dependent EC | [25,28,38] |
| Prognostic Value | Higher OPN linked to poor prognostic factors (grade, invasion, lymph node metastasis) Opposite findings on survival correlation | Correlation with adiponectin/leptin | Prognostic marker (in specific subtypes/stages) | [13,24,25,41] |
| Diagnostic Utility | Higher serum/tissue OPN in EC vs. controls. It may outperform CA125 in early-stage EC detection | Non-invasive diagnostic biomarker | [13,25,41,42] | |
| Therapy Resistance | Contributes to radiotherapy resistance | PI3K/AKT, MAPK, NF-κB DNA damage repair involvement | Blocking OPN could sensitize EC cells to radiotherapy | [19,43] |
| Therapeutic Target | OPN inhibitors may block proliferation, invasion, immune evasion, Resetting gene networks linked to metastasis | Targeting integrins, CD44, ECM, downstream Signaling | Candidate for personalized therapy or combination regimens | [4,9,40] |
4. Discussion
5. Conclusions
Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Young, M.F.; Kerr, J.M.; Termine, J.D.; Wewer, U.M.; Wang, M.G.; McBride, O.W.; Fisher, L.W. cDNA Cloning, mRNA Distribution and Heterogeneity, Chromosomal Location, and RFLP Analysis of Human Osteopontin (OPN). Genomics 1990, 7, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Figula, J.; Baran, R.M.; Jach, R. Osteopontin as a Marker of Endometriosis—The Current State of Knowledge. Ginekol. Pol. 2024, 95, 152–155. [Google Scholar] [CrossRef]
- von Wolff, M.; Bohlmann, M.K.; Fiedler, C.; Ursel, S.; Strowitzki, T. Osteopontin is Up-Regulated in Human Decidual Stromal Cells. Fertil. Steril. 2004, 81 (Suppl. S1), 741–748. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Lin, S.W.; Lee, Y.R.; Tzeng, C.R.; Kao, S.H. Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 15328. [Google Scholar] [CrossRef]
- Fu, X.; Yao, M.; Ye, C.; Fang, T.; Wu, R. Osteopontin Regulates Endometrial Stromal Cell Migration in Endometriosis through the PI3K Pathway. Reprod. Sci. 2021, 28, 435–446. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediators Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The Multiple Functions and Mechanisms of Osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wen, G.; Sun, Y.; Shen, Y.; Zeng, Y.; Du, M.; Zhu, G.; Wang, G.; Meng, X. Osteopontin Promotes Colorectal Cancer Cell Invasion and the Stem Cell-Like Properties through the PI3K-AKT-GSK/3β-β/Catenin Pathway. Med. Sci. Monit. 2019, 25, 3014–3025. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Wang, H.; Liu, X.; Li, J.; Zhao, W.; Wu, Q.; Yang, Z.; Xu, Y.; Zhou, M.; et al. Osteopontin and Its Downstream Carcinogenic Molecules: Regulatory Mechanisms and Prognostic Value in Cancer Progression. Neoplasma 2022, 69, 1253–1269. [Google Scholar] [CrossRef]
- Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Transl. Med. 2022, 2, 419–447. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, J.; Wang, X.; Chen, L.; Li, Y.; Zhao, H.; Sun, Q.; Wu, R. Role of Osteopontin in Cancer Development and Treatment. Heliyon 2023, 9, e21055. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Xu, L.; Huang, L.; Zhang, Y.; Wang, W.; Shen, R.; Li, X.; Zhuang, C.; Zhao, Q.; Xu, J.; et al. Osteopontin—A Promising Biomarker for Cancer Therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Sun, L.; Liu, N.; Xu, L.; Wang, Q.; Zhang, J.; Zhao, P.; Wu, X. Osteopontin Promotes Invasion, Migration, and Epithelial-Mesenchymal Transition of Human Endometrial Carcinoma Cell HEC-1A Through AKT and ERK1/2 Signaling. Cell. Physiol. Biochem. 2015, 37, 1503–1512. [Google Scholar] [CrossRef]
- Cho, H.; Kang, E.S.; Kim, Y.T.; Kim, J.H. Diagnostic and prognostic impact of osteopontin expression in endometrial cancer. Cancer Investig. 2009, 27, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Brewer, M.A. Endometrial Cancer: Is This a New Disease? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.I.; Nicosia, A.; Ligori, A.; Volpicelli, A.I.; Surico, D. Microbiota Status and Endometrial Cancer: A Narrative Review About Possible Correlations in Affected Versus Healthy Patients. Sci 2024, 6, 75. [Google Scholar] [CrossRef]
- Aquino, C.I.; Troisi, J.; Antonio, A.; Giugliano, L.; Raffone, A.; Sarno, L.; Saccone, G.; Scala, G.; Guida, M. Endometrial Carcinoma and Bisphenol A: A Pilot Case-Control Study. Biomed. J. Sci. Tech. Res. 2019, 21, 16073–16079. [Google Scholar]
- Aquino, C.I.; Stampini, V.; Osella, E.; Troìa, L.; Rocca, C.; Guida, M.; Faggiano, F.; Remorgida, V.; Surico, D. Menopausal Hormone Therapy, an Ever-Present Topic: A Pilot Survey about Women’s Experience and Medical Doctors’ Approach. Medicina 2024, 60, 774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedenreich, C.M.; Neilson, H.K.; Lynch, B.M. Physical Activity, Obesity, and Sedentary Behavior in Cancer Etiology: Epidemiologic Evidence and Biologic Mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef]
- Hahne, J.C.; Meyer, S.R.; Kranke, P.; Dietl, J.; Guckenberger, M.; Polat, B.; Hönig, A. Studies on the Role of Osteopontin-1 in Endometrial Cancer Cell Lines. Strahlenther. Onkol. 2013, 189, 1040–1048. [Google Scholar] [CrossRef]
- Ramachandran, S.; Kwon, K.Y.; Shin, S.J.; Kwon, S.H.; Cha, S.D.; Lee, H.G.; Hong, Y.B.; Bae, I.; Lee, G.H.; Cho, C.H. Regulatory role of osteopontin in malignant transformation of endometrial cancer. Mol. Biol. Rep. 2013, 40, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Jiang, T.; Sheng, X.G.; Gao, R.; Li, Q.S. Inhibition of Osteopontin Suppresses In Vitro and In Vivo Angiogenesis in Endometrial Cancer. Gynecol. Oncol. 2009, 115, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Chiasserini, D.; Whetton, A.D.; Crosbie, E.J. Proteomic Biomarkers for the Detection of Endometrial Cancer. Cancers 2019, 11, 1572. [Google Scholar] [CrossRef]
- Bostan, I.-S.; Mihaila, M.; Roman, V.; Radu, N.; Neagu, M.T.; Bostan, M.; Mehedintu, C. Landscape of Endometrial Cancer: Molecular Mechanisms, Biomarkers, and Target Therapy. Cancers 2024, 16, 2027. [Google Scholar] [CrossRef] [PubMed]
- Kardelen, E.; Atakul, T.; Yüksel, H. In Vitro Effects of Osteopontin on Endometrial Cancer Cells and Signaling Pathways in Epithelial-Mesenchymal Transition. Eur. J. Gynaecol. Oncol. 2024, 45, 5. [Google Scholar]
- Al Maghrabi, H.; Gomaa, W.; Al-Maghrabi, J. Increased Osteopontin Expression in Endometrial Carcinoma Is Associated with Better Survival Outcome. Ginekol. Pol. 2020, 91, 73–78. [Google Scholar] [CrossRef]
- Briese, J.; Schulte, H.M.; Bamberger, C.M.; Löning, T.; Bamberger, A.M. Expression Pattern of Osteopontin in Endometrial Carcinoma: Correlation with Expression of the Adhesion Molecule CEACAM1. Int. J. Gynecol. Pathol. 2006, 25, 161–169. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Tsuda, H.; Bandera, C.A.; Nishimura, S.; Inoue, T.; Kawamura, N.; Berkowitz, R.S.; Mok, S.C. Comparison of Osteopontin Expression in Endometrioid Endometrial Cancer and Ovarian Endometrioid Cancer. Med. Oncol. 2006, 23, 205–212. [Google Scholar] [CrossRef]
- Bastos, A.C.S.F.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942. [Google Scholar] [CrossRef]
- Murry, C.E.; Giachelli, C.M.; Schwartz, S.M.; Vracko, R. Macrophages Express Osteopontin During Repair of Myocardial Necrosis. Am. J. Pathol. 1994, 145, 1450–1462. [Google Scholar]
- Kunii, Y.; Niwa, S.; Hagiwara, Y.; Maeda, M.; Seitoh, T.; Suzuki, T. The Immunohistochemical Expression Profile of Osteopontin in Normal Human Tissues Using Two Site-Specific Antibodies Reveals a Wide Distribution of Positive Cells and Extensive Expression in the Central and Peripheral Nervous Systems. Med. Mol. Morphol. 2009, 42, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone Sialoprotein and Osteopontin in Bone Metastasis of Osteotropic Cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A Novel Regulator at the Crossroads of Inflammation, Obesity and Diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef]
- Weiss, J.M.; Renkl, A.C.; Maier, C.S.; Kimmig, M.; Liaw, L.; Ahrens, T.; Kon, S.; Maeda, M.; Hotta, H.; Uede, T.; et al. Osteopontin Is Involved in the Initiation of Cutaneous Contact Hypersensitivity by Inducing Langerhans and Dendritic Cell Migration to Lymph Nodes. J. Exp. Med. 2001, 194, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; da Silva, A.P.; Bansal, A.K.; Bansal, M.; Sun, C.; Lee, H.; Glogauer, M.; Sodek, J.; Zohar, R. Role of Osteopontin in Neutrophil Function. Immunology 2007, 122, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Shek, K.; Wang, S.; Huang, X.; Lau, A.; Yin, Z.; Sun, H.; Liu, W.; Garcia, B.; Rittling, S.; et al. Osteopontin Expressed in Tubular Epithelial Cells Regulates NK Cell-Mediated Kidney Ischemia Reperfusion Injury. J. Immunol. 2010, 185, 967–973. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef]
- Leitner, L.; Jürets, A.; Itariu, B.K.; Keck, M.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Osteopontin Promotes Aromatase Expression and Estradiol Production in Human Adipocytes. Breast Cancer Res. Treat. 2015, 154, 63–69. [Google Scholar] [CrossRef]
- Weber, G.F. The Cancer Biomarker Osteopontin: Combination with Other Markers. Cancer Genom. Proteom. 2011, 8, 263–288. [Google Scholar]
- Zou, C.; Luo, Q.; Qin, J.; Shi, Y.; Yang, L.; Ju, B.; Song, G. Osteopontin Promotes Mesenchymal Stem Cell Migration and Lessens Cell Stiffness via Integrin β1, FAK, and ERK Pathways. Cell Biochem. Biophys. 2013, 65, 455–462. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Parisi, E.; Sorolla, A. Determinants of Sensitivity to Radiotherapy in Endometrial Cancer. Cancers 2020, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Cha, S.; Kwon, S.; Kwon, Y.; Kang, S.; Lee, J.; Kim, K.; Yea, J. Overexpression of the Osteopontin Correlates with the Aggressiveness of Endometrial Cancer. J. Clin. Oncol. 2007, 25 (Suppl. S18), 16048. [Google Scholar] [CrossRef]
- Dab, S.; Abdelhay, N.; Figueredo, C.A.; Ganatra, S.; Gibson, M.P. Characterization of SIBLING Proteins in the Mineralized Tissues. Dent. J. 2022, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S64–S74. [Google Scholar] [CrossRef]
- Mukherjee, T.; Mukherjee, S.; Dutta, R. Early Diagnosis of Gynecological Cancers in Ladies with Review of Literature. Open J. Obstet. Gynecol. 2017, 7, 469–481. [Google Scholar] [CrossRef]
- Omer, B.; Genc, S.; Takmaz, O.; Dirican, A.; Kusku-Kiraz, Z.; Berkman, S.; Gurdol, F. The Diagnostic Role of Human Epididymis Protein 4 and Serum Amyloid-A in Early-Stage Endometrial Cancer Patients. Tumour Biol. 2013, 34, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Abreu, H.; Cappellano, G. Osteopontin: A Versatile Biomarker—Insights and Innovations from Three Decades of Research. Biomedicines 2024, 12, 1647. [Google Scholar] [CrossRef]
| Authors/Year | Country | Sample | Main Results | Mechanisms | Clinical Significance |
|---|---|---|---|---|---|
| Kardelen et al., 2024 [25] | Aydın Adnan Menderes University Biochemistry Laboratory, Turkey | In vitro— HUVEC and Ishikawa human Endometrial Adenocarcinoma cells | With increasing OPN levels, cells showed increased proliferation and migration. At 400 ng/mL, OPN induced EMT | Involvement of PI3K and ERK1/2 pathways | OPN may serve as a prognostic indicator for EC progression |
| Li et al., 2015 [13] | Dalian Medical University, China | In vitro— HEC-1A cell line | OPN promotes EC cell proliferation, migration, invasion, and EMT | Activation of AKT/ERK1/2 signaling and increased MMP-2 expression | Potential for OPN-targeted therapies in EC treatment |
| Hahne et al., 2013 [20] | University of Würzburg, Germany | In vitro— Endometrial cell lines Ishikawa, Hec-1A, and An-3CA as well as M14/MDA-MB435 | Lower OPN expression reduced invasion/migration and increased sensitivity to radiation therapy. OPN increased apoptosis | Effects of invasion/ migration related to OPN level of expression. Reduction in PARP and caspase-3 | Targeting OPN could enhance radiosensitivity in EC |
| Ramachandran et al., 2013 [21] | Keimyung University School of Medicine, South Korea | In vitro — Hec1A and Ishikawa cells | Reduced OPN expression altered proliferation; recombinant OPN restored colony formation | Effects are mediated via PI3K, Src, and integrin signaling pathways | Highlights potential for novel targeted therapies involving OPN pathways |
| Du et al., 2009 [22] | Shandong Cancer Hospital; China | In vitro and in vivo— ISK cells and mouse xenografts | Silencing OPN decreased migration (67.4%), invasion (51.2%), and tumor angiogenesis | Effects on angiogenesis caused by binding to αVβ3 integrin and enhancement tube formation and invasiveness | OPN with a pro-tumorigenic role; possible anti-angiogenic strategies targeting OPN |
| Al Maghrabi et al., 2020 [26] | Department of Pathology, King Abdulaziz University, Jeddah, Saudi Arabia | Human tissue samples—71 EC vs. 30 non-neoplastic endometrial tissues | 100% of non-neoplastic tissues demonstrated high OPN immunostaining; 64.8% of EC cases showed an increase in OPN. Higher OPN seems to be linked to better overall survival. No association with clinicopathological features | OPN could serve as a diagnostic and prognostic biomarker in EC | |
| Cho et al., 2009 [14] | Yonsei University College of Medicine, South Korea | Clinical study— 56 EC patients vs. 154 benign controls | Plasma OPN was significantly higher in EC patients; detecting early-stage disease missed by CA125 | Elevated OPN is associated with immune/ inflammatory responses | OPN is an independent predictor of DFS and could complement CA125 in diagnosis |
| Briese et al., 2006 [27] | University Clinic Hamburg-Eppendorf, Germany | Tissue analysis—20 normal benign, 17 hyperplastic, 43 EC tissues | 67.4% of EC showed strong OPN expression; serous carcinomas had highest OPN. Loss of OPN correlated with higher malignancy grade | Effects are related to the creation of functional complexes with CEACAM1; post-translational effects possible | OPN contributes to biological diversity between EC subtypes; may aid in subtyping and prognosis |
| Hashiguchi et al., 2006 [28] | Osaka City General Hospital, Japan | Comparative study— 30 ovarian OEC and 33 EEC | 50% of both OEC and EEC showed OPN expression; no significant difference between groups. Strong correlation between OPN mRNA and protein levels | Gene expression patterns suggest transcriptional regulation of OPN | May help in understanding molecular similarities/differences between OEC and EEC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, C.I.; Venkatesan, S.; Ligori, A.; Tinelli, R.; Grossini, E.; Surico, D. Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review. Cancers 2025, 17, 2245. https://doi.org/10.3390/cancers17132245
Aquino CI, Venkatesan S, Ligori A, Tinelli R, Grossini E, Surico D. Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review. Cancers. 2025; 17(13):2245. https://doi.org/10.3390/cancers17132245
Chicago/Turabian StyleAquino, Carmen Imma, Sakthipriyan Venkatesan, Arianna Ligori, Raffaele Tinelli, Elena Grossini, and Daniela Surico. 2025. "Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review" Cancers 17, no. 13: 2245. https://doi.org/10.3390/cancers17132245
APA StyleAquino, C. I., Venkatesan, S., Ligori, A., Tinelli, R., Grossini, E., & Surico, D. (2025). Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review. Cancers, 17(13), 2245. https://doi.org/10.3390/cancers17132245








