1. Introduction
Lung cancer stands as the leading cause of cancer-related mortality worldwide and ranks second among the most diagnosed cancers in both men and women, according to the World Health Organization (WHO) cancer statistics [
1]. Notably, data collected from the National Center for Health Statistics in the United States of America have emphasized that lung cancer had a higher mortality rate through 2021 than breast, prostate, and colorectal cancer combined [
2]. This is largely the result of diagnosis at an advanced stage, leading to an unfavorable prognosis, with a 5-year survival rate of approximately 17% [
3]. In addition, even though 16% of patients are diagnosed at an early stage, the vast majority experience disease relapse, progressing into the metastatic setting [
4]. Despite its poor prognosis, there has been a significant shift over the past few years in the management of non-small cell lung cancer (NSCLC), particularly in screening, diagnosis, and treatment. The advent of molecularly targeted therapies, immune checkpoint blockade (ICB), anti-angiogenic agents, and, more recently, bispecific antibodies and antibody–drug conjugates (ADCs), has offered new hope in this challenging disease [
5].
The development of ICB has transformed the treatment landscape across multiple cancer types. The long-term efficacy of ICB, which targets Programmed Death-1 (PD-1) and its ligand PD-L1, has been confirmed in various randomized phase III clinical trials involving patients with melanoma, NSCLC, and renal cell carcinoma (RCC), among others [
6,
7,
8]. However, objective response rates with ICB monotherapy range between 20–40% across different tumor subtypes, indicating that while these treatments have improved overall cancer survival, a significant proportion of patients do not benefit from this strategy [
9]. Furthermore, despite extensive efforts to identify predictive biomarkers of response to ICB, few have been validated, and their utility does not extend uniformly across all tumor types. The most commonly cited biomarkers are PD-L1 expression and microsatellite instability (MSI). For example, PD-L1 expression in tumor cells is associated with improved response and survival in patients with advanced NSCLC treated with pembrolizumab, but this correlation does not hold true for patients with melanoma or RCC [
10]. On the other hand, MSI-high (MSI-H)—or mismatch repair deficiency (dMMR)—has become the first tumor-agnostic biomarker approved for ICB therapy, due to its predictive role across multiple tumor types [
11]. However, the prevalence of MSI-H tumors remains low, limiting its benefit to a small subset of patients [
12,
13]. Other potential biomarkers, such as tumor mutational burden (TMB) or tumor-infiltrating lymphocytes (TILs), remain at an investigational level [
14,
15]. Despite these advances, reliable predictive biomarkers of response to immunotherapy remain an unmet need, prompting ongoing research into novel approaches to better assess and predict patient response.
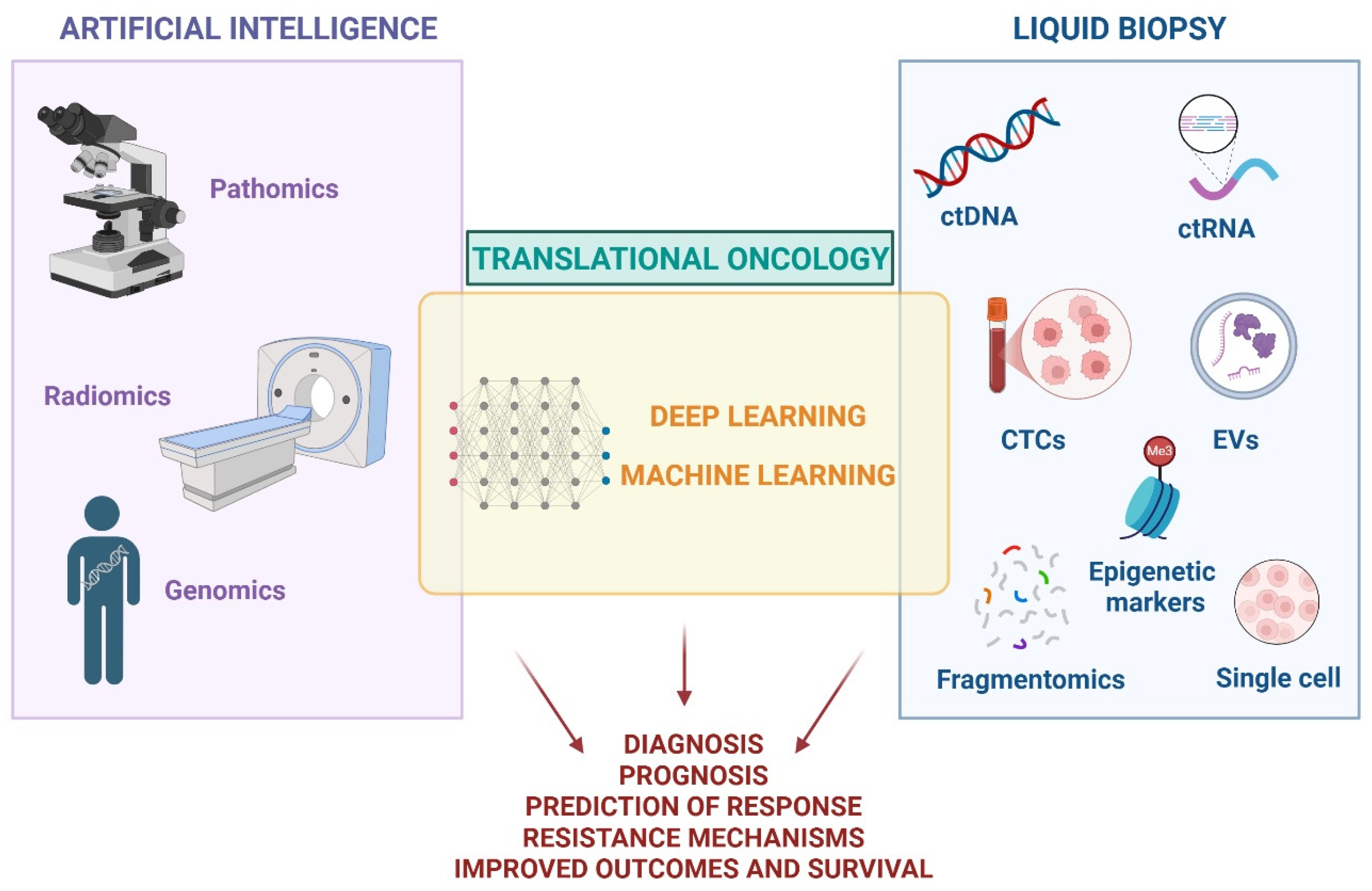
Current biomarker discovery strategies often focus on the analysis of tumor-specific molecular pathways and rarely address tumor biology as a whole, primarily due to the inherent complexity of cancer biology. Artificial intelligence (AI) and liquid biopsy are progressively gaining ground in diagnosis, prognosis, and therapeutics, offering promising new avenues. Machine learning (ML) programs—a subset of AI—can learn from data to identify patterns and generate predictive models, thus providing a comprehensive analysis of histopathological samples through imaging features to assist in diagnosis and prognosis [
16,
17]. Other emerging techniques, such as the “omics” approaches—including pathomics and radiomics—are evolving fields that might contribute to a better understanding of tumor cells, their microenvironment, and their intricate relationship with the immune system and other host factors. Liquid biopsy, particularly the analysis of circulating tumor DNA (ctDNA), is emerging as a promising diagnostic, prognostic, and predictive biomarker, offering a non-invasive method of disease evaluation [
18]. In this review, we present translational research methods and emerging techniques that contribute to a comprehensive understanding of immunotherapy within the field of oncology, with a particular focus on NSCLC.
2. The Host, Tumor Heterogeneity, and Tumor Microenvironment
The introduction of ICB into the therapeutic arsenal for NSCLC has significantly transformed the treatment landscape. However, durable clinical benefit is observed in only a subset of patients, underscoring the potential influence of host-related factors in modulating treatment efficacy.
The microbiota has been investigated as a potential biomarker of both tumorigenesis and treatment response. Current research in oncology has primarily focused on the role of intestinal microorganisms, which influence not only the efficacy of ICB treatment but also the development of treatment-related adverse effects [
19,
20]. In contrast, there is limited evidence regarding the impact of the lung or skin microbiome [
21,
22,
23,
24]. Nonetheless, studies in lung cancer patients and murine models suggest that dysregulation of the local lung microbiome may influence cancer progression by reshaping the tumor microenvironment (TME) and modulating the activity of infiltrating immune cells [
25]. Strategies aimed at modulating the microbiota, such as the use of probiotics, prebiotics, or fecal microbiota transplantation, arise as promising approaches to enhance treatment outcomes [
26].
In parallel, several host-related biomarkers have gained attention in the last decade as surrogates of prognosis in patients receiving immunotherapy. Parameters such as the neutrophil-to-lymphocyte ratio (NLR), the derived neutrophil-to-lymphocyte ratio (dNLR) or the Lung Immune Prognostic Index score (LIPI score) have demonstrated demonstrated value in capturing systemic inflammation and predicting therapeutic response and survival across multiple tumor types [
27,
28]. Moreover, understanding the molecular and genetic basis of intertumoral and intratumoral heterogeneity is crucial to elucidate the complex interplay among tumor cells, the immune system, the microbiota, and the host. While intertumoral heterogeneity reflects differences in histologically similar tumors among different patients, intratumoral heterogeneity describes molecular and phenotypic diversity within a single tumor specimen [
29,
30]. In fact, only 33% of somatic mutations are shared across all regions of the same tumor [
31]. Non-genetic heterogeneity may arise from external and internal stressors, driving the emergence of novel subclones shaped by selective pressures in the microenvironment and interactions with immune, stromal, or extracellular matrix components [
32,
33].
Importantly, the inhibition of tumor-specific T cells by stromal myeloid and lymphoid cells, as well as the activation of inflammatory pathways, underscores the need to better characterize the complex crosstalk between tumor cells and a potential immunosuppressive TME in tumor progression and treatment resistance [
34]. In this context, the analysis of single tissues and single cells has emerged as an interesting approach to dissect the cellular and molecular complexity of the TME. New working techniques, such as Single-cell RNA sequencing (scRNA-seq) and spatial RNA sequencing (spRNA-seq), together with translational techniques, including liquid biopsy or the use of AI, are increasingly used to resolve these challenges [
35]. Furthermore, transcriptomic profiling of tumor-infiltrating immune cells enables the classification of immune subpopulations, identification of mechanisms of immune-escaping and drug resistance pathways, and supports the development of more effective targeted therapies, following or combined with immunotherapy [
36]. These techniques, along with the identification of prognostic and predictive biomarkers of response to therapies, have revolutionized our understanding of cancer biology and might unravel new strategies for a more personalized approach.
3. Immune Checkpoint Blockers and Prognostic/Predictive Biomarkers
In recent years, ICB has transformed therapeutic strategies and significantly improved the prognosis of several solid tumors. In previously treated patients, ICB has demonstrated encouraging 5-year overall survival (OS) rates, reaching 34% in melanoma, 28% in RCC, and 16% in NSCLC, leading to approval of anti-PD-1, anti-PD-L1, and anti-Cytotoxic T Lymphocyte Antigen 4 inhibitor (anti-CTLA4) for its use in the metastatic setting across several malignancies [
37]. Given these favorable outcomes, the use of ICB is now being extended to earlier disease stages, with the goal of enhancing curability and long-term survival [
38]. Several biomarkers, such as TMB, PDL-1 expression, the microbiota, and the molecular and cellular characterization of the TME, have been associated with differential responses to immunotherapy, reinforcing their potential as predictive biomarkers [
39,
40,
41].
Figure 1 summarizes key prognostic and predictive biomarkers currently under investigation or in clinical use for NSCLC [
42].
3.1. PD1/PD-L1
PD-L1 was first described in the early 2000s in preclinical models, having been identified on antigen-presenting cells, endothelial cells, macrophages, and dendritic cells. It was subsequently recognized as the ligand of PD-1, a receptor primarily expressed on T cells, but also found on B cells and myeloid cells, where it exerts modulatory and inhibitory effects on immune function [
43,
44,
45]. Initially considered as an interesting predictive biomarker of response to ICB, subsequent studies revealed limitations in its reliability, as some patients with low PD-L1 expression still derived clinical benefit in specific settings [
46,
47].
In NSCLC, the phase II Checkmate 063 was the first study to demonstrate the activity of anti-PD1 therapy with nivolumab in heavily pre-treated patients [
48]. Since then, ICB has become a cornerstone in the management of metastatic NSCLC [
49,
50]. While PD-L1 expression was initially considered a strong predictor of response, subsequent evidence revealed that patients could respond regardless of PD-L1 status, challenging the biomarker’s utility as a sole determinant of treatment eligibility [
46]. Later clinical trials confirmed the predictive value of PD-L1 expression in tumor cells, with the most pronounced efficacy observed in patients with expression levels ≥50%. Nevertheless, not all high expressors respond to therapy, and some patients with low/negative PD-L1 may still achieve meaningful clinical outcomes [
51,
52].
These findings highlight the complexity of PD-L1 as a biomarker and underscore the need for further research to elucidate the mechanisms underlying response variability and to identify more robust, reliable predictors of immunotherapy benefit.
3.2. Tumor Mutational Burden
TMB, defined as the number of somatic mutations per megabase (mut/Mb) of DNA, has been associated with improved treatment responses and survival outcomes in NSCLC patients receiving ICB therapy [
53]. This association was notably supported by the CheckMate 227 study, the first phase 3 trial in lung cancer to evaluate immunotherapy efficacy in relation to TMB. In this trial, patients with high TMB (≥10 mut/Mb), who received first-line nivolumab and ipilimumab, demonstrated significantly longer progression-free survival (PFS), regardless of PD-L1 expression status [
54].
Additionally, a retrospective analysis revealed that patients with both positive PD-L1 expression (defined as ≥1%) and high TMB had significantly better objective response rates (ORR) and PFS compared to those with only one or neither biomarker [
55]. These findings suggest that TMB may provide additive predictive value to PD-L1 expression in identifying NSCLC patients most likely to benefit from immunotherapy, thereby enhancing the precision of treatment selection.
3.3. MSI-H
Among the few biomarkers that have demonstrated consistent predictive value across multiple tumor types, MSI-H stands out as the first to receive tumor-agnostic FDA approval for ICB. The underlying rationale stems from the observation that tumors with deficient DNA mismatch repair mechanisms accumulate a high mutational burden, leading to increased neoantigen production and enhanced immunogenicity—factors that improve response to PD-1/PD-L1 inhibitors. In the phase II KEYNOTE-158 basket trial, heavily pretreated patients with MSI-H/dMMR solid tumors achieved an ORR of 30.8% and a median duration of response of 47.5 months, findings that supported the tissue-agnostic approval of pembrolizumab [
56].
Although MSI-H is rare in NSCLC—occurring in fewer than 1% of cases—it defines a distinct molecular subset of patients, often associated with a heavy smoking history, markedly elevated TMB, and recurrent loss-of-function mutations, most commonly in MLH1 [
57]. Detection of MSI-H relies on immunohistochemistry (IHC) for mismatch repair proteins (MLH1, PMS2, MSH2, and MSH6), as well as Polymerase Chain Reaction (PCR)—or next-generation sequencing (NGS)-based microsatellite panels [
58]. Due to its low prevalence in NSCLC, routine MSI testing of all cases remains controversial. However, targeted evaluation is recommended when broad-panel NGS reveals an unexpectedly high TMB or when IHC demonstrates unexplained loss of MMR protein expression.
3.4. Tumor Microenvironment and Host Biomarkers
Although the aforementioned biomarkers might contribute to improved clinical outcomes, a significant proportion of patients develop resistance to therapy or even fail to respond. Consequently, the identification of reliable predictive biomarkers remains an unmet need, particularly to discern which patients are most likely to benefit from PD-1/PD-L1 blockade. Focusing on the host and tumor microenvironment, TILs, tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), blood-based inflammatory biomarkers—such as the platelet-to-lymphocyte ratio (PLR), NLR or dNLR, and LIPI score-, and the microbiota, arise as potential strategies for better selection of patients and outcomes predictions [
59,
60,
61].
Notably, the emergence of several host and TME-related biomarkers in recent years reflects a conceptual shift: markers previously regarded as purely prognostic are now being explored for their predictive potential in immunotherapy. This shift underscores the growing recognition that components of the tumor-immune interface may actively modulate therapeutic response, rather than merely reflect disease burden or biology. TILs, traditionally viewed as prognostic, are now gaining recognition for their predictive value. Their density and spatial organization within the TME may indicate a pre-existing antitumor immune response and an inflamed TME, both of which have been associated with improved outcomes following ICB in multiple tumor types, including NSCLC [
62,
63,
64]. Similarly, the presence of TAMs, TANs, and other immune and stromal components of the TME contributes to the immunological milieu and may influence immunotherapeutic efficacy. Previous studies have correlated elevated expression of TAMs, particularly the M2 phenotype, and TANs with poor prognosis [
65,
66,
67]. Beyond their prognostic value, accumulating evidence supports their role in therapeutic resistance. M2-polarized TAMs promote resistance to chemotherapy, targeted therapies, radiotherapy, and immunotherapy in NSCLC by activating immunosuppressive pathways such as JAK/STAT, PI3K/AKT, and TGF-β/Smad [
68]. Through cytokine secretion, exosomal signaling, and interactions with cancer stem cells and immune populations, TAMs may remodel the TME to favor tumor survival and immune evasion [
69]. Combination strategies targeting TAM-related mechanisms thus show promise in overcoming therapeutic resistance [
70]. In parallel, the predictive role of TINs is receiving increasing attention. Specific neutrophil phenotypes and gene expression profiles—such as CD74+high neutrophils—have been linked to enhanced antitumor immunity and may predict responsiveness to immunotherapy, either alone or in combination with chemotherapy [
71,
72]. These findings support the incorporation of TAM and TIN profiling into integrative biomarker models to refine risk stratification and guide treatment decisions in NSCLC.
The role of the microbiota as a biomarker in NSCLC is gaining increasing attention in the field of translational oncology. Emerging evidence suggests that the composition and diversity of the microbiome may influence tumor initiation, progression, and response to therapy [
73,
74]. Specific bacterial genera, such as
Haemophilus,
Prevotella, and
Streptococcus have been found in greater abundance in lung cancer patients [
75]. Such dysbiosis may contribute to carcinogenesis by promoting chronic inflammation, altering host immune responses, and activating oncogenic signaling cascades. Mechanistically, the microbiota can modulate DNA damage repair, mutagenesis, and cell cycle regulation [
76]. Metabolomic studies have further linked alterations in microbial metabolites—such as those involved in sphingolipid and autophagy pathways—to lung cancer pathophysiology [
77,
78]. Furthermore, the microbiota may impact therapeutic outcomes, particularly in patients receiving ICB, by shaping the tumor immune microenvironment [
74,
79]. Despite these promising insights, the field remains in early stages, and larger, prospective studies are needed to validate microbial biomarkers. Ultimately, integrating microbiota profiling into clinical practice could contribute to more personalized approaches in lung cancer diagnosis, prognosis, and treatment.
3.5. Other Biomarkers
In addition to molecular and immune-related biomarkers, several host- and tumor-specific factors play a pivotal role in the prognosis and therapeutic stratification of NSCLC. Established clinicopathological parameters such as tumor grade, TNM stage, standardized uptake value (SUV) from FDG-PET imaging, and proliferative index markers like Ki-67 continue to provide critical prognostic and predictive information. High Ki-67 expression and elevated SUV are generally associated with more aggressive disease and poorer outcomes, while TNM staging remains a cornerstone for treatment decisions [
80,
81]. Integrating emerging translational biomarkers with established clinical and pathological indicators may significantly improve the precision of patient stratification and facilitate a more comprehensive and personalized approach to the management of NSCLC.
Nevertheless, despite growing evidence, the intricate interplay between tumor cells, the host immune system, and the TME presents significant challenges for the routine clinical implementation of these biomarkers. In this context, AI and advanced translational research methodologies offer valuable tools for deciphering complex biological data and identifying robust, clinically applicable predictive biomarkers.
4. New Pathology Techniques in the Translational Oncology Era
AI and liquid biopsy are currently emerging as promising tools for advancing precision and personalization in cancer care (
Figure 2). However, despite their potential, AI has not yet been broadly integrated into routine clinical workflows, and liquid biopsy—though implemented in some centers—still requires broader validation, standardization, and regulatory approval. Both technologies remain active areas of research and hold considerable promise for enhancing diagnostic accuracy, optimizing treatment decisions, and ultimately improving patient outcomes and quality of life.
4.1. Artificial Intelligence
AI is defined as the capability to predict or classify objects through algorithmic analysis of existing data. This transformative technology includes disciplines such as machine learning (ML) and deep learning (DL), which are transforming the management of health care and other industries by optimizing complex data analysis [
82]. ML develops self-learning algorithms from its accumulated experience and solves complex problems without explicit programming to enhance its performance, improving in fields such as natural language processing, computer vision, and recommendation systems. DL is composed of multiple-layered ML models that achieve feature selection and model fitting simultaneously, requiring a large database input capable of dealing with more complex patterns [
83,
84]. Mimicking the structure and functionality of the human brain, DL has led to remarkable breakthroughs in complex tasks, such as image and speech recognition, language translation, and self-driving cars [
85].
In oncology, numerous studies have reported the applicability of AI in diagnostics, therapeutics, and prognostic predictions in several neoplasms [
86,
87,
88]. The so-called “omics” approach, namely pathomics, radiomics, and genomics, is increasingly evolving, representing a new pathway in translational techniques. Radiomics extracts quantitative information from radiological images, identifying complex patterns that may provide information on tumor heterogeneity and determine prognosis [
89]. Pathomics focuses on the analysis of histopathological images to extract quantitative features and characterize tissue samples in whole slide images (WSI). As a result, it allows spatial characterization of tumor and stroma, shapes and texture of nuclei, characterization of lymphocytic infiltrates and other cell types, and identifies different biomarkers by IHC [
90]. Genomics provides interpretation of gene expression patterns and dynamics, helping in the identification of prognostic biomarkers, as well as molecular mechanisms of resistance [
91]. These multi-omics approaches, when combined with ML and DL, enhance the understanding of cancer biology and refine diagnostic and prognostic capabilities.
Importantly, patient-derived xenograft (PDX) models have emerged as valuable tools for validating insights gained from multi-omics. As reported by Gu et al. (2025) [
92], PDX models retain the genetic and phenotypic complexity of the original human tumors, providing an in vivo platform for integrated molecular profiling and functional validation. When coupled with high-throughput sequencing, PDXs allow researchers to explore tumor heterogeneity at single-cell resolution and to identify rare subclones and resistance pathways that may be missed in bulk analyses. These models serve as a bridge between computational predictions and biological relevance, reinforcing the clinical utility of AI-driven omics research in oncology [
92].
Indeed, both ML and DL have the potential to impact every stage of oncology care—from diagnosis and prognosis to treatment selection and disease monitoring [
93]. However, their clinical implementation faces significant hurdles, including variability in regulatory standards, inconsistent model evaluation practices, limited interpretability, and challenges in real-world integration. Discrepancies between regulatory frameworks—such as those of the United States Food and Drug Administration (FDA) and the European CE mark system—raise concerns about premature clinical deployment and under-regulation of ML-based tools [
93]. Moreover, the lack of large, diverse, and well-annotated validation datasets limits model generalizability and may introduce bias when applied across different patient populations [
94]. Despite these challenges, as AI systems become more transparent, clinically validated, and supported by harmonized oversight, their potential to reshape precision oncology continues to grow.
In NSCLC, AI and multi-omics approaches have shown promise in improving prognostic accuracy. Several studies have demonstrated improved prediction of 1-year PFS among patients treated with immunotherapy using AI-enhanced models [
95,
96]. Furthermore, these tools have demonstrated diagnostic utility by increasing sensitivity and specificity in the detection and characterization of pulmonary nodules on conventional imaging, thereby enabling earlier and more accurate diagnosis. AI models have also shown potential in predicting
Epidermal Growth Factor Receptor (EGFR) mutation status and estimating TMB, both of which are critical for treatment planning and patient stratification [
97].
To summarize, the integration of AI technologies—including ML, DL, and multi-omics frameworks—into cancer research and clinical practice holds immense promise for improving diagnostic precision, accelerating therapeutic discovery, and advancing personalized medicine [
98].
Artificial Intelligence in Pathology Diagnosis
AI techniques applied to digital pathology involve converting conventional glass slides into WSI, enabling centralized storage, remote access, and advanced computational analysis [
99]. Slides generated by DICOM WSI can encompass more than 4 billion pixels at 40× magnification, scanning at a resolution of 0.25 μm/pixel. This extraordinary level of detail provides a detailed visualization of cellular and tissue structures [
100].
The applicability of WSI in NSCLC can effectively differentiate between histological subtypes and identify molecular alterations like
STK11, EGFR, FAT1, SETBP1, KRAS, and
TP53 mutations. A study by Coudray et al. (2018) [
101] showed an impressive accuracy in predicting molecular phenotype, ranging from 73% to 85.6% for
EGFR and
KRAS mutations, two of the most important targetable pathways in NSCLC, making a profound impact on personalized treatment strategies. Another study published by Yu et al. (2016) [
102], which gathered 2186 full-scan images from patients with lung adenocarcinoma and squamous cell carcinoma, employed ML on histopathology WSI. They successfully characterized and identified morphologic features that assisted pathologists in distinguishing malignant tumors from healthy tissue, as well as predicting survival outcomes. Features that correlated with survival in this ML model included the shape of the nuclei and cytoplasm [
102].
A recent study further advanced the field by training convolutional neural networks to classify lung adenocarcinoma subtypes and predict disease-specific survival. Using consensus images from 17 expert pathologists, the DL models demonstrated high accuracy across eight lung adenocarcinoma subtypes and were independently validated in a cohort of 133 patients [
103]. At the same time, advances in scalability and efficiency have led to the development of DL models that can be trained on entire WSIs using only slide-level labels, eliminating the need for labor-intensive, detailed annotations. In a dataset of 9662 lung cancer WSIs, this approach achieved impressive area under the curve (AUC) values of 0.9594 for adenocarcinoma and 0.9414 for squamous cell carcinoma, outperforming traditional multiple-instance learning methods and successfully localizing small lesions through class activation mapping [
104].
In a comprehensive systematic review, Davri et al. (2023) [
105] examined 96 studies applying DL to histologic and cytologic images in lung cancer, concluding that these tools significantly improve diagnostic workflows, enhance the reproducibility of subtype classification, and support predictive and prognostic decision-making based on tissue morphology.
In the context of biomarker identification, PD-L1 expression in NSCLC has also been assessed using computer-assisted scoring based on hematoxylin-eosin WSI. This model managed to accurately predict PD-L1 status by correlating PD-L1 expression with morphological features in TME [
106].
This accumulating body of evidence underscores how AI can serve as a powerful ally to pathologists, aiding in the diagnosis, phenotyping, and prognostic prediction of lung cancer by analyzing digital pathological sections. With AI assistance, diagnostic efficiency may be increased exponentially, reducing inter-observer variations and inaccuracies intrinsic to human visual interpretation. As AI continues to evolve, its transformative impact on healthcare holds the promise of providing a more accurate overview of lung cancer management. The ML and DL models applied to WSI and histopathology aim to enhance the accuracy not only of diagnostic procedures, but also of risk prediction, response detection, and treatment outcomes assessment. However, the standardization and routine clinical integration of AI techniques still require further research to validate their reliability, reproducibility, and practical applicability.
4.2. Liquid Biopsy
Liquid biopsy refers to the detection and analysis of tumor-derived components present in body fluids, offering a minimally invasive alternative to conventional tissue biopsy [
18,
107]. Its non-invasive nature allows for repeated sampling at multiple time points, enabling timely and cost-effective monitoring of treatment response, detection of resistance mechanisms, and assessment of disease relapse or progression in NSCLC patients [
108]. In addition, liquid biopsy enables the identification of new treatment targets and biomarkers, shedding light on promising avenues for the development of novel therapies [
109]. As this technology continues to evolve, its integration into routine clinical practice holds the promise of greater accuracy and improved patient outcomes, moving towards a more personalized approach.
4.2.1. Sample Types
Liquid biopsy involves the analysis of circulating tumor cells (CTCs) and tumor-derived molecules, including ctDNA, circulating-tumor RNA (ctRNA), microRNA (miRNA), or extracellular vesicles (EVs). By capturing critical genetic, epigenetic, and proteomic changes, it represents an opportunity to identify tumor-specific alterations and genetic mutations that drive cancer growth and therapy resistance. Although the blood is the most common source for liquid biopsy, other body fluids can be used, such as urine, sputum, saliva, and cerebrospinal fluid [
110].
Circulating Tumor Cells
CTCs are isolated tumor cells within the bloodstream or body fluids, though often present in very low concentrations. Various methods have emerged to improve their detectability. The Isolation by Epithelial Tumor Cell Size (ISET) approach emerged as one of the first size-based methods for CTC detection. This technique provides morphological, immunocytological, and genetic characterization of CTCs, with exceptional sensitivity and repeatability [
111]. In parallel, techniques like flow cytometry, fluorescence-activated cell sorting (FACS), and microfluidics have gained prominence, providing powerful tools to efficiently isolate CTCs from liquid biopsy samples [
112,
113]. Among these methods, the widely used epithelial cell adhesion molecule (EpCAM) immunomagnetic assay allows for the enumeration of CTCs of epithelial origin in the blood. However, it may miss a substantial cell population with “stem cell-like” properties, warranting further exploration into alternative approaches. An emerging strategy known as laser capture microdissection involves encapsulating a CTC in a hydrogel, enabling targeted extraction using a laser. This is followed by sequencing, thus providing its genetic and molecular profile [
114]. Continued research into innovative techniques for CTC detection and characterization holds the potential to enhance our understanding of cancer biology.
Focusing on the applicability of CTCs in lung cancer, their detection in patients diagnosed with NSCLC is frequently associated with poor prognosis, emphasizing the critical role they play in disease progression and treatment response [
18]. Although they are detectable in only 30% of patients, they have been associated with worse response and survival in metastatic patients treated with ICB [
115]. Several prospective studies have also assessed the prognostic role of CTCs in first-line chemotherapy-treated patients, demonstrating worse OS and PFS in the presence of a higher load of cancer cells [
116,
117]. In the early setting, detectability of CTCs pre- and post-operatively has been correlated to shorter disease-free survival [
118,
119]. Regarding their diagnostic utility, a meta-analysis of 21 studies involving 2714 patients reported that CTCs have a sensitivity of 72% and specificity of 96% for the diagnosis of NSCLC, supporting their clinical relevance [
120].
Overall, the available evidence underscores the potential of CTCs as a valuable diagnostic and prognostic tool in the management of lung cancer, offering a non-invasive approach for early detection and monitoring of the disease.
Circulating Tumor DNA
CtDNA is a component of the circulating-free DNA originating from tumor cells that have undergone apoptosis or necrosis. It has been previously described as a prognostic and predictive biomarker in various tumor types and settings, including neoadjuvant, adjuvant, and metastatic disease [
121,
122,
123]. Given the correlation between ctDNA levels and tumor burden, it represents an invaluable biomarker for disease monitoring and assessment of prognosis [
124,
125]. Classically, the quantification and analysis of ctDNA is carried out by PCR-based or NGS-based techniques [
126]. These methods represent a non-invasive and easily accessible means of capturing tumor-derived genetic material.
Current evidence highlights its use in screening, early diagnosis, detection of minimal residual (MRD) disease after radical treatment, assessment of the risk of metastasis and recurrence, and identification of resistance mechanisms in NSCLC [
127]. In a retrospective study from the Impower131 cohort including 221 patients diagnosed with metastatic NSCLC treated with chemo-immunotherapy, the ctDNA decrease from baseline was associated with improved outcomes, including better PFS and OS. Conversely, any increase in ctDNA levels during serial monitoring correlated with worse PFS and OS [
128]. Similarly, an international phase II trial of patients treated with first-line pembrolizumab demonstrated a strong concordance between ctDNA dynamics and radiological response. The study reported a sensitivity of 82% and a specificity of 75% when comparing ctDNA clearance with objective response based on RECIST criteria. Notably, patients who achieved ctDNA clearance experienced significantly longer PFS and OS compared to those with persistently detectable ctDNA [
129].
Notably, ctDNA has also demonstrated the ability to detect molecular relapse before clinical or radiological signs, allowing for earlier intervention and reduced exposure to ineffective treatments [
130]. Unlike tissue biopsy, which captures information from a single site, ctDNA reflects heterogeneity across multiple tumor clones, offering a more comprehensive overview of tumor evolution under therapeutic pressure [
110]. An example of this was described by Rolfo et al. (2021) [
131], who reported that ctDNA analysis enabled the detection of resistance mutations—such as
EGFR T790M—in NSCLC patients, facilitating timely treatment modifications without requiring repeat biopsies.
The BFAST trial further highlighted the role of ctDNA in the upfront detection of actionable mutations. In this study, ctDNA successfully identified
Anaplastic Lymphoma Kinase (ALK) rearrangements in treatment-naive NSCLC patients, enabling the initiation of targeted therapy and achieving response rates comparable to tissue-based testing [
132]. Additional studies have supported the predictive role of ctDNA in oncogene-driven NSCLC. For example, the presence of the
T790M mutation at progression in
EGFR-mutant patients treated with osimertinib, an EGFR- tyrosine kinase inhibitor (TKI) inhibitor, was associated with worse outcomes [
133]. In
ALK-rearranged NSCLC, a phase II trial demonstrated that detection of ALK translocation in plasma or tissue after TKI failure predicted better responses to lorlatinib, an ALK-TKI inhibitor, while its absence suggested off-target resistance mechanisms [
134].
In earlier-stage disease, preoperative ctDNA positivity has also been linked to poorer outcomes. A systematic review and meta-analysis by Lu et al. (2024) [
135] found that ctDNA detection in patients with stage I–II lung adenocarcinoma correlated with shorter recurrence-free survival and OS, while this association was not significant in stage III disease. Moreover, ctDNA monitoring post-surgery for MRD may offer high specificity in predicting relapse, though its sensitivity remains suboptimal [
136].
Despite promising evidence, ctDNA implementation in clinical practice is not yet fully standardized. Expert guidelines underscore its utility for MRD detection and genomic profiling, particularly when tissue biopsy is infeasible or insufficient [
137,
138,
139]. However, both ctDNA and tissue-based analyses carry non-negligible false-negative rates, reinforcing the need for complementary testing strategies to improve turnaround time and detection of targetable alterations [
139].
Overall, as the understanding of ctDNA continues to deepen, its applicability becomes increasingly evident in various clinical settings due to its prognostic and predictive value. The integration of ctDNA-based approaches into routine clinical practice holds the promise of improving patient outcomes through personalized treatment strategies. However, there is insufficient robust data yet for its widespread implementation and validation across diverse patient populations.
Circulating Tumor RNA
CtRNA, although less commonly used than ctDNA in liquid biopsy, has been described as an interesting diagnostic and prognostic biomarker. Its detection in the bloodstream, mainly in the form of long non-coding RNA (lncRNA), messenger RNA (mRNA), or miRNA, provides information on molecular changes linked to cancer development and progression, unveiling pathways that may reveal novel therapeutic strategies [
140]. CtRNA analysis can be performed by digital-droplet PCR (ddPCR), quantitative reverse transcription PCR (RT-qPCR), microarrays, and NGS methods such as RNA sequencing (RNAseq) or small RNAseq. NGS-based methods offer several advantages, including a broad dynamic range, minimal sample requirements, high reproducibility, and the ability to detect novel transcripts even in the absence of a reference genome [
141]. This has facilitated the identification of diagnostic, prognostic, and predictive biomarkers—such as lncRNAs, miRNAs, and circular RNAs (circRNAs)—as well as markers of drug resistance and neoantigen profiling [
142].
Evidence focusing on miRNAs in NSCLC supports their role as diagnostic tools in distinguishing histological subtypes. For example, increased levels of miR-181-5p and miR-361-5p have been observed in adenocarcinoma compared to squamous cell carcinoma. Additionally, some miRNAs have been associated with poor prognosis and treatment resistance. Low levels of miR-146-5p have been correlated with cisplatin resistance and worse PFS in NSCLC [
143]. A study by Liu et al. (2016) [
144], conducted on 10 patients diagnosed with NSCLC, evaluated the expression of miRNAs using a quantitative PCR array panel and found that several exosomal miRNAs, including miR-23b-3p, miR-10b-5p, and miR-21-5p, were significantly associated with poor OS. Regarding circRNA -a type of non-coding RNA- it has been previously associated with cancer development and progression. A study identified 357 dysregulated circRNAs in lung cancer patients. Overexpression of certain circRNAs was linked to treatment resistance, such as the presence of cic-CCDC66 in EGFR-inhibitor resistant patients or circ-ABCB10 in Cisplatin-resistant lung cancer [
145].
Beyond their utility as biomarkers in liquid biopsy, RNAs are also being explored as active therapeutic agents in cancer immunotherapy. Recent advances in mRNA engineering and delivery technologies have enabled the development of mRNA-based vaccines and therapeutics that can stimulate potent anti-tumor immune responses [
146]. These strategies include the delivery of tumor-specific antigens, cytokines, or immune-modulating factors via synthetic mRNA platforms. Optimized structural design and nanoparticle-based delivery systems have further improved the stability, translational efficiency, and targeting of therapeutic mRNAs [
147]. This dual role of RNA—both as a biomarker and as a therapeutic vector—illustrates its growing relevance in precision oncology and highlights the potential for ctRNA analysis not only in cancer detection but also in guiding and monitoring RNA-based immunotherapies.
Overall, ctRNA represents an interesting field of research to enhance clinical outcomes by the detection of diagnostic, prognostic, and predictive biomarkers, as well as treatment resistance factors.
4.2.2. Liquid Biopsy Techniques
While several analytical platforms are available for liquid biopsy, the most commonly employed techniques are PCR-based methods and NGS, particularly for ctDNA assessment. Due to their reliability, scalability, and increasing accessibility, both methods have become the cornerstone of ctDNA analysis in clinical and research settings [
107].
PCR-Based Techniques
PCR-based techniques are one of the most frequent techniques used in liquid biopsy, capable of detecting hot-spot mutations with driver properties and therapeutic implications. Nevertheless, it requires the presence of already-known specific mutations, restricting the ability to uncover novel genetic alterations or resistance mechanisms that may hold critical clinical significance [
153].
PCR-based approaches include digital PCR (dPCR) platforms, such as real-time PCR (RT-PCR) and BEAMing—acronym for Beams, Emulsions, Amplifications, Magnetics-, and ddPCR. RT-PCR is a semiquantitative method for analysis with an affordable price and lower sensitivity compared to ddPCR techniques, but it has high specificity for detecting known point mutations. Sensitivity varies greatly depending on the technique and type of tumor, which makes it challenging when applied to clinical practice [
154]. On the other hand, ddPCR techniques are highly sensitive and more complex methods allowing the detection of rare events and the quantification of a single molecule. However, they have a higher risk of contamination due to multiple transfer and pipetting steps, requiring the presence of specialized scientists for their proper use [
155].
Emerging techniques adapted from PCR-conventional methods based on multiplex detection include mass-spectrometry, which is ultrasensitive for detecting low-frequency mutations with as low as 0.1% minor allele frequency. UltraSEEK (high-throughput, multiplexed, ultrasensitive mutation detection) and SERS (Surface-Enhanced Raman Spectroscopy) are two mass-spectrometry techniques that require a low input of ctDNA for analysis and achieve great sensitivity and specificity targeting 1–3 mutations [
156,
157,
158].
NGS-Based Techniques
NGS-based-techniques are a powerful tool to identify multiple mutations across large genomic regions, achieving remarkable sensitivity and specificity. Indeed, techniques as Massively Parallel Shotgun Sequencing and Massively Parallel Sequencing offer the ability to quantify copy number alterations, informing on tumor genomic complexity and heterogeneity. However, its accuracy depends on the platform used, as there are various methods and profiles, including deep-sequencing, multiplex-PCR NGS, Cancer Personalized Profiling by deep-sequencing (CAPP-Seq), Tagged-Amplicon deep sequencing (TAm-seq), Safe-Sequencing System (Safe-SeqS), among others. These techniques require experienced bioinformaticians to analyze and interpret the data obtained, which is both expensive and time-consuming [
158,
159].
Two primary types of sequencing panels are commonly available for liquid biopsy analysis: targeted and untargeted panels. Targeted panels are meticulously designed to detect specific genomic sequences, offering high sensitivity in capturing low-frequency mutations, while they may miss mutations that lie beyond the pre-defined regions, thus presenting limitations in detecting new or unexpected mutations. In contrast, untargeted panels, also known as whole-genome sequencing (WGS) or whole-exome sequencing (WES), encompass a genome-wide or exome-wide exploration, offering the potential to discover previously unknown or unforeseen mutations that may offer insights into patient prognosis or new treatment strategies. However, untargeted panels may have limited sensitivity in detecting low-frequency mutations, which remains a consideration in their clinical implementation [
160,
161].
4.2.3. Liquid Biopsy Workflow
Each liquid-biopsy modality has distinct technical requirements: ctDNA workflows hinge on rapid plasma separation and rigorous nucleic-acid stabilisation; ctRNA and circRNA workflows demand stringent RNase inhibition and tight temperature control to preserve the more labile RNA species; while CTC protocols focus on maintaining cellular integrity through specialized preservative media followed by physical or immuno-enrichment [
162].
Several pre-analytical and analytical variables critically influence the accuracy and reliability of liquid biopsy results and should therefore be standardized in routine clinical practice, as outlined by the European Society for Medical Oncology (ESMO) guidelines [
163]. The timing of blood sampling must align with the clinical objective. For the assessment of MRD after surgery, blood should be collected at least 7 days postoperatively—or ideally, 14 days after major resections—to avoid contamination by cell-free DNA (cfDNA) released during wound healing. For genotyping in advanced disease, sampling should be performed outside of periods of active, radiologically confirmed treatment response, as effective therapies may suppress ctDNA shedding, increasing the likelihood of false-negative results. Regarding sample handling, EDTA tubes are appropriate when plasma can be processed within four hours. If immediate processing is not possible or if samples require transport to external laboratories, cfDNA-stabilizing tubes—permitting delays of up to seven days—are preferred. For long-term storage, plasma should be maintained at −80 °C with minimal temperature fluctuations, and repeated freeze–thaw cycles should be avoided to preserve nucleic acid integrity [
163].
Despite rigorous sampling and handling protocols, false-negative results can still occur in liquid biopsy analyses. These may arise from low ctDNA abundance, limited assay sensitivity, or the presence of intrinsically “non-shedding” tumors that release minimal detectable DNA into circulation [
164]. Conversely, false positives are frequently attributed to clonal hematopoiesis of indeterminate potential (CHIP), especially when mutations are detected in DNA repair or tumor suppressor genes such as
TP53 or
DNMT3A [
165]. To mitigate this, routine collection and parallel sequencing of the buffy coat—enriched in leukocytes—are recommended to distinguish CHIP-related variants from true tumor-derived mutations. Pathogenic germline alterations in genes such as
BRCA1,
BRCA2, or
PALB2 may also surface in plasma, necessitating reflex germline confirmation with a validated assay. Finally, clinical ctDNA workflows should incorporate computational algorithms or orthogonal purity assessments to estimate tumor-derived DNA content. This would enhance confidence in negative results and guide decisions on the necessity of complementary tissue biopsy [
163].
Meticulous control of these variables—coupled with rigorous external-quality assessment—will maximize the sensitivity and specificity of ctDNA testing, minimize interpretative pitfalls, and facilitate its seamless integration into precision-oncology workflows for NSCLC.
4.2.4. Emerging Methods for Liquid Biopsy Biomarker Discovery
Long-Read Sequencing
New sequencing methods involve third-generation technologies with longer sequencing reads, shorter preparation time, and the possibility of sequencing RNA and detect nucleic acid modifications. In addition, they allow the detection of single-nucleotide variants (SNVs) and small insertion-deletion mutations (indels) in liquid biopsies, surpassing short-read techniques. However, an error rate of 10–15% can be expected when using long-read sequencing, which can be reduced by multiple sequencing runs [
166,
167].
DNA Methylation Markers
DNA methylation is one of the hallmarks of cancer, and it is also the first and most studied epigenetic mark in cancer. Methylation signatures observed in tumor tissue are known to occur early in carcinogenesis and promote tumor progression. As a result, it has been proposed as a potential screening and diagnostic tool to improve early cancer detection [
168]. A study carried out by the Circulating Cell-free Genome Atlas, which involved 2800 participants, compared different cfDNA approaches using ML to evaluate their precision and limit of detection. Remarkably, genome-wide methylation analysis outperformed WGS and targeted sequencing approaches, emerging as a promising genomic feature for cancer signal detection. This technique managed to accurately predict cancer signal origin with greater accuracy than other techniques [
169].
As our understanding of epigenetic alterations expands, methylation technologies appear as transformative tools in precision oncology with the potential of assessing early detection and diagnosis of cancer. However, several challenges arise. Methylation occurs not only in cancer cells but also in normal cells, increasing its frequency with age. Additionally, differences among tumors and certain scenarios, such as myocardial infarction, can also promote epigenetic alterations, potentially leading to difficulties when applying these techniques [
170].
Overall, although emerging techniques based on methylation markers unravel epigenetic changes associated with tumor development and progression and can thus increase sensitivity, they still require further research for validation to enhance their applicability in clinical practice [
171].
Single-Cell Sequencing
Single-cell analysis has emerged as an interesting tool for CTCs and cancer-associated immune cells analyses. These sequencing techniques include scRNA-seq, which provides insight of the gene expression signatures of individual cells, and single-cell DNA sequencing (scDNA-seq), which helps identify single-nucleotide variants, copy number variations, or MSI. Other technologies, such as single cell proteomics, epigenomics, or metabolomics, provide information on the metabolic pathways within tumor cells, but still require further research for their widespread adoption [
172,
173].
While scDNA-seq allows the recognition of mutations throughout the genome of a single cell, it has limitations in detecting significant expression differences in heterogeneous cells within the TME. In contrast, scRNA-seq not only reveals intercellular gene expression differences but also identifies heterogeneity within phenotypically distinct immune and stromal cell populations. In NSCLC, scRNA-seq has uncovered a high degree of T cell diversity, including multiple CD8+ and CD4+ T cell subsets [
174]. A key finding from these studies is the role of B cells in establishing tertiary lymphoid structures (TLS) within the TME—facilitating lymphocyte maturation, immune activation, and clonal expansion of both T and B cells [
175]. Furthermore, scRNA-seq has shed light on the plasticity of TAMs, dendritic cell heterogeneity, and neutrophil remodeling within the tumor milieu [
176,
177].
Recent advances in single-cell analysis have significantly enhanced our understanding of the immune microenvironment in NSCLC. By allowing high-resolution mapping of intratumoral cellular components, these techniques provide a robust framework for identifying immune subpopulations that influence therapeutic response and resistance. This detailed profiling supports the design of more personalized immunotherapeutic strategies [
178].
Leader et al. (2021) [
179] contributed to this field by identifying the “lung cancer activation module” (LCAM), an immune signature composed of activated T lymphocytes, IgG plasma cells, and SPP1+ macrophages. The presence of LCAM is associated with a higher likelihood of response to immunotherapy, even among patients with high TMB, suggesting that immune cell composition provides non-redundant predictive information beyond genomic biomarkers. Moreover, multimodal analysis and spatial data integration have enabled the discovery of lymphocyte phenotypes and patterns of cellular organization that are predictive of response to ICB, thereby improving therapeutic selection in NSCLC [
180,
181].
Notably, single-cell technologies have also elucidated mechanisms of treatment resistance. In
EGFR-mutant NSCLC, single-cell analyses have identified clonal evolution and transcriptomic changes linked to resistance against tyrosine kinase inhibitors. These studies have highlighted immune remodeling and transcriptional reprogramming as central features of acquired resistance, paving the way for more precise and adaptive treatment strategies [
182,
183].
Overall, single-cell analysis techniques, and particularly scRNA-seq, enable the characterization of tumor heterogeneity, thus providing insights into potential biomarkers of clinical application. However, they require meticulous protocols and quality control measures. Library construction, after cell isolation, needs whole genome or whole transcriptome amplification (WGA/WTA) to generate sufficient genetic material for sequencing [
167]. As a result, they require further investigations to fully understand their potential in cancer research and their clinical applications.
Fragmentomics
Fragmentomics in liquid biopsy is a highly promising and innovative approach for cancer detection and monitoring. This new field focuses on the study of fragments of circulating DNA (circDNA), which are the result of genetic material released during necrosis, apoptosis, or phagocytosis [
184]. This fragmentation pattern correlates with nucleosome positioning and gene expression, thus providing valuable information on genetic and epigenetic features [
185]. Previous evidence highlighted the role of circDNA to discriminate between healthy individuals and cancer patients, and to identify specific mutations depending on the length of fragments. In fact, cancer patients have shorter median lengths and greater variability in the size of these fragments [
186]. Mouliere et al. (2018) [
187] observed that the identification of size-selected circDNA allowed the detection of actionable mutations and copy number alterations that had not been identified by other methods.
Fragmentomics has been evaluated for early diagnosis, surveillance, detection of residual disease, and identification of resistance mechanisms in NSCLC. A study of 350 healthy individuals and 432 patients with cancer merged circDNA features with five ML algorithms. This model allowed for early-stage diagnosis of lung cancer, with remarkable sensitivity and accuracy [
188]. Another study highlighted its role on patient surveillance as well as on the detection of mechanisms of resistance in
EGFR-mutated NSCLC, which was achieved by targeting shorter cfDNA fragments, which enabled the detection of the
T790M mutation [
189]. MRD after surgical resection has also been evaluated in several models combining WGS or ML with genetic and epigenetic features. These models confirmed the excellent performance of circDNA fragment assessment in predicting patient recurrence, which could guide adjuvant treatment decisions and therefore optimize patient management [
190,
191,
192].
Interestingly, fragmentomics enhances the sensitivity of ctDNA-based assays by analyzing cfDNA fragmentation patterns—such as size distribution, end motifs, and nucleosome positioning—that are altered in cancer and detectable even when mutation-based approaches fall below their detection threshold [
191,
193]. By integrating these multidimensional features, fragmentomics significantly improves early cancer detection, particularly in low tumor burden settings, with clinical studies reporting sensitivity gains from 46% to over 90% at high specificity [
194].
As fragmentomics continues to evolve, its applications in liquid biopsy research hold great promise for revolutionizing early cancer detection, monitoring, and treatment decision-making. Future research in this field might further enhance the precision and non-invasiveness of NSCLC diagnostics, improving patient outcomes and personalized care.
5. Integrating Emerging Techniques of Translational Research in Oncology
To fully harness the potential of emerging techniques of translational research in oncology, it is essential to recognize their complementary role alongside traditional diagnostic methods. AI, particularly through ML and DL, enhances rather than replaces conventional tools. In radiology, AI improves the sensitivity and specificity of imaging interpretation, aids in the early characterization of pulmonary nodules, and reduces inter-observer variability. In pathology, DL-based algorithms have shown strong performance in subtyping NSCLC, optimizing WSI analysis, and supporting immunohistochemical biomarker quantification, thereby augmenting diagnostic accuracy and reproducibility. Moreover, AI models are increasingly applied in prognostic prediction and treatment response evaluation, offering valuable clinical insights beyond static morphologic assessment.
In parallel, liquid biopsy has emerged as a minimally invasive modality capable of complementing or, in some scenarios, temporally surpassing tissue biopsy. By detecting ctDNA, ctRNA, and other tumor-derived analytes, it allows for dynamic monitoring of disease burden, early assessment of treatment response—often preceding radiologic changes—and the identification of actionable mutations and resistance mechanisms. Importantly, liquid biopsy can capture spatial and temporal tumor heterogeneity, which might be underrepresented in single-site tissue samples. Its role in MRD, assessing molecular relapse, and guiding therapeutic adaptations underscores its expanding clinical value.
Despite these advances, important challenges persist. The generalizability of AI and liquid biopsy technologies is limited by heterogeneity in study designs, variability in analytical platforms, and the absence of universally accepted technical standards. The lack of robust, prospective, multi-center clinical trials further impairs the development of consensus guidelines and regulatory endorsement. Access to these technologies remains uneven across institutions, and both financial and infrastructural barriers restrict their widespread implementation in routine care. For AI in particular, concerns regarding interpretability, model transparency, and validation across diverse populations continue to hinder clinician adoption. Similarly, liquid biopsy faces biological limitations—such as low ctDNA shedding in early-stage disease—and technical constraints in reliably detecting gene fusions or distinguishing tumor-derived alterations from clonal hematopoiesis.
Altogether, while precision medicine in oncology is evolving rapidly—with AI and liquid biopsy at the forefront—it is clear that the field is still in a formative phase. Meaningful clinical integration will require rigorous validation, regulatory harmonization, and equitable access. A multidisciplinary, evidence-driven approach that leverages the strengths of both traditional and emerging modalities will be critical to achieving the promise of truly individualized cancer care. Precision oncology is no longer a theoretical ideal, but its full realization remains a work in progress.
6. Conclusions
New advances in translational research have significantly deepened our understanding of tumor biology, particularly the dynamic interplay between cancer cells, the immune system, and the TME. These insights are beginning to translate into clinically actionable strategies that move beyond static biomarkers, toward dynamic, integrative, and biologically informed tools. While AI and liquid biopsy are among the most promising frontiers, the broader translational toolbox—including multi-omics profiling, spatial transcriptomics, and single-cell technologies—is contributing to a paradigm shift in how we approach cancer diagnostics and treatment planning.
From a clinical standpoint, the future of oncology lies in the ability to detect disease earlier, tailor therapies more precisely, and monitor patients more effectively in real time. The success of these innovations will depend not only on technological advancement but on their demonstrable impact on clinical decision-making and patient outcomes. As these techniques continue to evolve and integrate seamlessly into cancer screening and diagnostics, their transformative potential is set to redefine the landscape of healthcare, helping in the discovery of new prognostic and predictive biomarkers and paving the way to more precise and personalized treatments.
Author Contributions
Conceptualization, M.G., E.Z., A.M., V.A.-A. and J.C.B.; methodology, M.G., E.Z., A.M., V.A.-A. and J.C.B.; validation M.G., V.A.-A. and J.C.B.; investigation, M.G., E.Z., A.M. and J.C.B.; resources, M.G. and J.C.B.; data curation, M.G., E.Z., A.M., V.A.-A. and J.C.B.; writing—original draft preparation, M.G., E.Z. and A.M.; writing—review and editing, M.G., V.A.-A. and J.C.B.; visualization, M.G., V.A.-A. and J.C.B.; supervision, M.G., V.A.-A. and J.C.B.; funding acquisition, M.G. and J.C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
We confirm that no new patient data were collected for this review article, and all information was derived from previously published studies. Therefore, IRB approval or waiver was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, J.C.B.
Acknowledgments
We would like to thank our colleagues at the Virgen de la Victoria University Hospital and Gustave Roussy, and especially the patients, without whom treatments and research would not be possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Lin, Y.-T.; Lin, L.-J.; Chang, Y.-H.; Chen, H.-Y.; Wang, Y.-P.; Shih, J.-Y.; Yu, C.-J.; Yang, P.-C. Stage Shift Improves Lung Cancer Survival: Real-World Evidence. J. Thorac. Oncol. 2023, 18, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Babar, L.; Modi, P.; Anjum, F. Lung Cancer Screening; StatPearls: Petersburg, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537283/ (accessed on 4 April 2025).
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; George, S. Long-term outcomes with nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. J. Immunother. Cancer 2022, 10, e005445. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.; Kudaba, I.; Wu, Y.-L.; Lopes, G.; Kowalski, D.M.; Turna, H.Z.; Caglevic, C.; Zhang, L.; Karaszewska, B.; Laktionov, K.K.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients With Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J. Clin. Oncol. 2023, 41, 1986–1991. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Amato, M.; Franco, R.; Facchini, G.; Addeo, R.; Ciardiello, F.; Berretta, M. Microsatellite Instability: From the Implementation of the Detection to a Prognostic and Predictive Role in Cancers. Int. J. Mol. Sci. 2022, 23, 8726. [Google Scholar] [CrossRef]
- Kavun, A.; Veselovsky, E.; Lebedeva, A.; Belova, E.; Kuznetsova, O.; Yakushina, V.; Grigoreva, T.; Mileyko, V.; Fedyanin, M.; Ivanov, M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers 2023, 15, 2288. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Bonin, S.; Buriolla, S.; Bertoli, E.; Pizzichetta, M.A.; Zalaudek, I.; Puglisi, F. Find the Flame: Predictive Biomarkers for Immunotherapy in Melanoma. Cancers 2021, 13, 1819. [Google Scholar] [CrossRef]
- Ready, N.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019, 37, 992–1000. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Bülow, R.D.; Hölscher, D.L.; Costa, I.G.; Boor, P. Extending the landscape of omics technologies by pathomics. Npj Syst. Biol. Appl. 2023, 9, 38. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zang, D.; Zhang, M.; Li, X.; Liu, D.; Gao, B.; Zhou, H.; Sun, J.; Han, X.; et al. The Role of Gut Microbiota in Lung Cancer: From Carcinogenesis to Immunotherapy. Front. Oncol. 2021, 11, 720842. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z. Gut–lung axis: Role of the gut microbiota in non-small cell lung cancer immunotherapy. Front. Oncol. 2023, 13, 1257515. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef]
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016, 17, 163. [Google Scholar] [CrossRef] [PubMed]
- Raudoniute, J.; Bironaite, D.; Bagdonas, E.; Kulvinskiene, I.; Jonaityte, B.; Danila, E.; Aldonyte, R. Human airway and lung microbiome at the crossroad of health and disease (Review). Exp. Ther. Med. 2023, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Chen, E.S.; Zhao, C.; Jin, C. Host-Microbiome Interaction in Lung Cancer. Front. Immunol. 2021, 12, 679829. [Google Scholar] [CrossRef]
- Zeng, W.; Shen, J.; Bo, T.; Peng, L.; Xu, H.; Nasser, M.I.; Zhuang, Q.; Zhao, M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J. Immunol. Res. 2019, 2019, 1603758. [Google Scholar] [CrossRef]
- Benitez, J.C.; Recondo, G.; Rassy, E.; Mezquita, L. The LIPI score and inflammatory biomarkers for selection of patients with solid tumors treated with checkpoint inhibitors. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 162–174. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ramón, Y.; Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical implications of intratumor heterogeneity: Challenges and opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef]
- Fidler, I.J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978, 38, 2651–2660. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. The challenges of tumor genetic diversity. Cancer 2017, 123, 917–927. [Google Scholar] [CrossRef]
- de Sousa, V.M.L.; Carvalho, L. Heterogeneity in Lung Cancer. J. Rec. Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2018, 85, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.; Peirone, S.; Perrone, S.; Priante, F.; Varese, F.; Tirtei, E.; Fagioli, F.; Cereda, M. Artificial Intelligence in Bulk and Single-Cell RNA-Sequencing Data to Foster Precision Oncology. Int. J. Mol. Sci. 2021, 22, 4563. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Peng, M.; Tang, L.; Ouyang, J.; Xiong, F.; Guo, C.; Tang, Y.; Zhou, Y.; Liao, Q.; et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 2021, 40, 81. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Eads, J.R.; Moore, K.N.; Tatard-Leitman, V.; Wright, J.; Forde, P.M.; Ferris, R.L. Neoadjuvant immunotherapy of locoregionally advanced solid tumors. J. Immunother. Cancer 2022, 10, e005036. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef]
- Paucek, R.D.; Baltimore, D.; Li, G. The Cellular Immunotherapy Revolution: Arming the Immune System for Precision Therapy. Trends Immunol. 2019, 40, 292–309. [Google Scholar] [CrossRef]
- Pabst, L.; Lopes, S.; Bertrand, B.; Creusot, Q.; Kotovskaya, M.; Pencreach, E.; Beau-Faller, M.; Mascaux, C. Prognostic and Predictive Biomarkers in the Era of Immunotherapy for Lung Cancer. Int. J. Mol. Sci. 2023, 24, 7577. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Terawaki, S.; Ikegawa, M.; Okazaki, T.; Honjo, T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003, 198, 39–50. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Shen, X.; Zhao, B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ 2018, 362, k3529. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, B.; Chen, X.; Zhan, P.; Zhao, Y.; Zhang, T.; Liu, H.; Afzal, M.Z.; Dermime, S.; Hochwald, S.N.; et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: A meta-analysis of randomized controlled trials. Transl. Lung Cancer Res. 2019, 8, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Grossman, J.E.; Vasudevan, D.; Joyce, C.E.; Hildago, M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene 2021, 40, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Fiander, M.; Tran, D.; Korytowsky, B.; Thomas, J.-M.; Calderon, F.; Zyczynski, T.M.; Brixner, D.; Stenehjem, D.D. Tumor mutational burden in lung cancer: A systematic literature review. Oncotarget 2019, 10, 6604–6622. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852.e4. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; De Jesus Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: Updated analysis from the phase II KEYNOTE-158 study. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef]
- Yang, S.-R.; Gedvilaite, E.; Ptashkin, R.; Chang, J.; Ziegler, J.; Mata, D.A.; Villafania, L.B.; Nafa, K.; Hechtman, J.F.; Benayed, R.; et al. Microsatellite Instability and Mismatch Repair Deficiency Define a Distinct Subset of Lung Cancers Characterized by Smoking Exposure, High Tumor Mutational Burden, and Recurrent Somatic MLH1 Inactivation. J. Thorac. Oncol. 2024, 19, 409–424. [Google Scholar] [CrossRef]
- Peña-Diaz, J.; Rasmussen, L.J. Approaches to diagnose DNA mismatch repair gene defects in cancer. DNA Repair. 2016, 38, 147–154. [Google Scholar] [CrossRef]
- Sorich, M.J.; Rowland, A.; Karapetis, C.S.; Hopkins, A.M. Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated With Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J. Thorac. Oncol. 2019, 14, 1440–1446. [Google Scholar] [CrossRef]
- Yang, T.; Hao, L.; Yang, X.; Luo, C.; Wang, G.; Lin Cai, C.; Qi, S.; Li, Z. Prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in patients with non-small cell lung cancer receiving immune checkpoint inhibitors: A meta-analysis. BMJ Open 2021, 11, e049123. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef]
- Federico, L.; McGrail, D.J.; Bentebibel, S.-E.; Haymaker, C.; Ravelli, A.; Forget, M.-A.; Karpinets, T.; Jiang, P.; Reuben, A.; Negrao, M.V.; et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Ballot, E.; Ladoire, S.; Routy, B.; Truntzer, C.; Ghiringhelli, F. Tumor Infiltrating Lymphocytes Signature as a New Pan-Cancer Predictive Biomarker of Anti PD-1/PD-L1 Efficacy. Cancers 2020, 12, 2418. [Google Scholar] [CrossRef]
- Park, S.; Ock, C.-Y.; Kim, H.; Pereira, S.; Park, S.; Ma, M.; Choi, S.; Kim, S.; Shin, S.; Aum, B.J.; et al. Artificial Intelligence-Powered Spatial Analysis of Tumor-Infiltrating Lymphocytes as Complementary Biomarker for Immune Checkpoint Inhibition in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.; Chen, S.; Lu, M.; Luo, X.; Yao, S.; Liu, S.; Qin, Y.; Chen, H. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer 2011, 74, 188–196. [Google Scholar] [CrossRef]
- Rakaee, M.; Busund, L.-T.; Paulsen, E.-E.; Richardsen, E.; Al-Saad, S.; Andersen, S.; Donnem, T.; Bremnes, R.M.; Kilvaer, T.K. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 2016, 7, 72184–72196. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kadota, K.; Ikeda, T.; Yoshida, C.; Kimura, N.; Ibuki, E.; Go, T.; Yokomise, H.; Haba, R. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils in resected non-small cell lung carcinoma. Hum. Pathol. 2022, 125, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Fan, R.; Wu, J.; Gong, H.; Gao, X.; Liu, X.; Gong, Y.; Luo, B.; Zhang, Y.; Peng, X.; et al. Tumor-Associated Macrophages: Key Players in the Non-Small Cell Lung Cancer Tumor Microenvironment. Cancer Med. 2025, 14, e70670. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, T.; Zhang, R.; Chen, C.; Li, J. New insights into the role of macrophages in cancer immunotherapy. Front. Immunol. 2024, 15, 1381225. [Google Scholar] [CrossRef]
- Bae, J.; Liu, L.; Moore, C.; Hsu, E.; Zhang, A.; Ren, Z.; Sun, Z.; Wang, X.; Zhu, J.; Shen, J.; et al. IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8+ T cells to overcome immunotherapy resistance in cancer. Nat. Cell Biol. 2022, 24, 1754–1765. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, J.; Li, X.-C.; Wang, W.; Zhang, H.-Y.; Guo, Y.-Y.; Shuai, X.; Chu, Q.; Xie, C.; Lin, D.; et al. A subset of neutrophils activates anti-tumor immunity and inhibits non-small-cell lung cancer progression. Dev. Cell 2025, 60, 379–395.e8. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Q.; Chen, P.; Guo, H.; Shi, J.; Pan, Y.; Li, C.; Zhou, C. Computational recognition of LncRNA signatures in tumor-associated neutrophils could have implications for immunotherapy and prognostic outcome of non-small cell lung cancer. Front. Genet. 2022, 13, 1002699. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-N.; Ma, Q.; Ge, Y.; Yi, C.-X.; Wei, L.-Q.; Tan, J.-C.; Chu, Q.; Li, J.-Q.; Zhang, P.; Wang, H. Microbiome dysbiosis in lung cancer: From composition to therapy. Npj Precis. Oncol. 2020, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, M.; Meyer, M.-L.; Berciano-Guerrero, M.Á.; Mesas-Ruiz, A.; Cobo-Dols, M.; Perez-Ruiz, E.; Gonzalez, A.C.; Lavado-Valenzuela, R.; Barragán, I.; Oliver, J.; et al. Oncogene-addicted solid tumors and microbiome—Lung cancer as a main character: A narrative review. Transl. Lung Cancer Res. 2024, 13, 2050–2066. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Kassab, J.; Meouchy, P.; Haroun, E.; Nehme, R.; Bou Zerdan, M.; Fahed, G.; Petrosino, M.; Dutta, D.; Graziano, S. The Lung Microbiota and Lung Cancer: A Growing Relationship. Cancers 2022, 14, 4813. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Suo, L.; Zhang, W.; Cao, H.; Wang, R.; Luan, J.; Yu, X.; Dong, L.; Wang, W.; et al. Characterizing microbiota and metabolomics analysis to identify candidate biomarkers in lung cancer. Front. Oncol. 2022, 12, 1058436. [Google Scholar] [CrossRef]
- Luan, J.; Zhang, F.; Suo, L.; Zhang, W.; Li, Y.; Yu, X.; Liu, B.; Cao, H. Analyzing lung cancer risks in patients with impaired pulmonary function through characterization of gut microbiome and metabolites. BMC Pulm. Med. 2024, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, W.; Zhu, S.; Lin, T.; Mao, R.; Wu, N.; Zhang, P.; Kang, M. Gut and Intratumoral microbiota: Key to lung Cancer development and immunotherapy. Int. Immunopharmacol. 2025, 156, 114677. [Google Scholar] [CrossRef]
- Martin, B.; Paesmans, M.; Mascaux, C.; Berghmans, T.; Lothaire, P.; Meert, A.-P.; Lafitte, J.-J.; Sculier, J.-P. Ki-67 expression and patients survival in lung cancer: Systematic review of the literature with meta-analysis. Br. J. Cancer 2004, 91, 2018–2025. [Google Scholar] [CrossRef]
- Qiu, X.; Liang, H.; Zhong, W.; Zhao, J.; Chen, M.; Zhu, Z.; Xu, Y.; Wang, M. Prognostic impact of maximum standardized uptake value on 18F-FDG PET/CT imaging of the primary lung lesion on survival in advanced non-small cell lung cancer: A retrospective study. Thorac. Cancer 2021, 12, 845–853. [Google Scholar] [CrossRef]
- Bini, S.A. Artificial Intelligence, Machine Learning, Deep Learning, and Cognitive Computing: What Do These Terms Mean and How Will They Impact Health Care? J. Arthroplast. 2018, 33, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Sarker, I.H. Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, R.; Jindal, N. Machine Learning and Deep Learning Applications-A Vision. Glob. Transit. Trading 2021, 2, 24–28. [Google Scholar] [CrossRef]
- Alam, M.; Samad, M.D.; Vidyaratne, L.; Glandon, A.; Iftekharuddin, K.M. Survey on Deep Neural Networks in Speech and Vision Systems. Neurocomputing 2020, 417, 302–321. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.; Kather, J.N. A systematic analysis of deep learning in genomics and histopathology for precision oncology. BMC Med. Genom. 2024, 17, 48. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Cao, C. Cancer immunotherapy efficacy and machine learning. Expert. Rev. Anticancer. Ther. 2024, 24, 21–28. [Google Scholar] [CrossRef]
- Villa-Camacho, J.C.; Baikpour, M.; Chou, S.-H.S. Artificial Intelligence for Breast US. J. Breast Imaging 2023, 5, 11–20. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Gupta, R.; Kurc, T.; Sharma, A.; Almeida, J.S.; Saltz, J. The Emergence of Pathomics. Curr. Pathobiol. Rep. 2019, 7, 73–84. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, F.; Bronk, L.; Zhao, L. Multi-omics approaches for biomarker discovery in predicting the response of esophageal cancer to neoadjuvant therapy: A multidimensional perspective. Pharmacol. Ther. 2024, 254, 108591. [Google Scholar] [CrossRef]
- Gu, A.; Li, J.; Li, M.-Y.; Liu, Y. Patient-derived xenograft model in cancer: Establishment and applications. MedComm 2025, 6, e70059. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Wu, E.; Zhang, A.; Alizadeh, A.A.; Zou, J. From patterns to patients: Advances in clinical machine learning for cancer diagnosis, prognosis, and treatment. Cell 2023, 186, 1772–1791. [Google Scholar] [CrossRef]
- Kuperberg, S.J.; Christiani, D.C. Artificial Intelligence-Based Methods: The Path Forward in Achieving Equity in Lung Cancer Screening and Evaluation. Cancer Innov. 2025, 4, e70019. [Google Scholar] [CrossRef]
- Qian, L.; Wu, T.; Kong, S.; Lou, X.; Jiang, Y.; Tan, Z.; Wu, L.; Gao, C. Could the underlying biological basis of prognostic radiomics and deep learning signatures be explored in patients with lung cancer? A systematic review. Eur. J. Radiol. 2024, 171, 111314. [Google Scholar] [CrossRef] [PubMed]
- Shibaki, R.; Fujimoto, D.; Nozawa, T.; Sano, A.; Kitamura, Y.; Fukuoka, J.; Sato, Y.; Kijima, T.; Matsumoto, H.; Yokoyama, T.; et al. Machine learning analysis of pathological images to predict 1-year progression-free survival of immunotherapy in patients with small-cell lung cancer. J. Immunother. Cancer 2024, 12, e007987. [Google Scholar] [CrossRef]
- de Margerie-Mellon, C.; Chassagnon, G. Artificial intelligence: A critical review of applications for lung nodule and lung cancer. Diagn. Interv. Imaging 2023, 104, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, D.; Cai, S.; Guo, H.; Chen, P.; Zheng, Z.; Zhu, J.; Zhao, W.; Wang, H.; Zhao, S.; et al. The application of artificial intelligence in lung cancer: A narrative review. Transl. Cancer Res. 2021, 10, 2478–2487. [Google Scholar] [CrossRef]
- Palaskar, S.J. Technology and applications of whole slide imaging. J. Oral. Maxillofac. Pathol. 2023, 27, 614–615. [Google Scholar] [CrossRef]
- Giovagnoli, M.R.; Giansanti, D. Artificial Intelligence in Digital Pathology: What Is the Future? Part 1: From the Digital Slide Onwards. Healthcare 2021, 9, 858. [Google Scholar] [CrossRef]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Yu, K.-H.; Zhang, C.; Berry, G.J.; Altman, R.B.; Ré, C.; Rubin, D.L.; Snyder, M. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun. 2016, 7, 12474. [Google Scholar] [CrossRef] [PubMed]
- Lami, K.; Ota, N.; Yamaoka, S.; Bychkov, A.; Matsumoto, K.; Uegami, W.; Munkhdelger, J.; Seki, K.; Sukhbaatar, O.; Attanoos, R.; et al. Standardized Classification of Lung Adenocarcinoma Subtypes and Improvement of Grading Assessment Through Deep Learning. Am. J. Pathol. 2023, 193, 2066–2079. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Chen, C.-C.; Yu, W.-H.; Chen, S.-H.; Chang, Y.-C.; Hsu, T.-I.; Hsiao, M.; Yeh, C.-Y.; Chen, C.-Y. An annotation-free whole-slide training approach to pathological classification of lung cancer types using deep learning. Nat. Commun. 2021, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Davri, A.; Birbas, E.; Kanavos, T.; Ntritsos, G.; Giannakeas, N.; Tzallas, A.T.; Batistatou, A. Deep Learning for Lung Cancer Diagnosis, Prognosis and Prediction Using Histological and Cytological Images: A Systematic Review. Cancers 2023, 15, 3981. [Google Scholar] [CrossRef]
- Sha, L.; Osinski, B.L.; Ho, I.Y.; Tan, T.L.; Willis, C.; Weiss, H.; Beaubier, N.; Mahon, B.M.; Taxter, T.J.; Yip, S.S.F. Multi-Field-of-View Deep Learning Model Predicts Nonsmall Cell Lung Cancer Programmed Death-Ligand 1 Status from Whole-Slide Hematoxylin and Eosin Images. J. Pathol. Inform. 2019, 10, 24. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer: Current status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Batool, S.M.; Yekula, A.; Khanna, P.; Hsia, T.; Gamblin, A.S.; Ekanayake, E.; Escobedo, A.K.; You, D.G.; Castro, C.M.; Im, H.; et al. The Liquid Biopsy Consortium: Challenges and opportunities for early cancer detection and monitoring. Cell Rep. Med. 2023, 4, 101198. [Google Scholar] [CrossRef]
- Kilgour, E.; Rothwell, D.G.; Brady, G.; Dive, C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020, 37, 485–495. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, S.; Wang, Y.; Shi, D. Circulating tumor cell isolation for cancer diagnosis and prognosis. EBioMedicine 2022, 83, 104237. [Google Scholar] [CrossRef] [PubMed]
- Iv, C.W.S.; Reyes, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab. Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Brockley, L.J.; Souza, V.G.P.; Forder, A.; Pewarchuk, M.E.; Erkan, M.; Telkar, N.; Benard, K.; Trejo, J.; Stewart, M.D.; Stewart, G.L.; et al. Sequence-Based Platforms for Discovering Biomarkers in Liquid Biopsy of Non-Small-Cell Lung Cancer. Cancers 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.W.M.M.; Groen, H.J.M. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-J.; Li, J.; Zhu, W.-F.; Wu, Y.; Tang, X.-P.; Wang, Y.; Hu, Y.-M. Survivin mRNA-circulating tumor cells predict treatment efficacy of chemotherapy and survival for advanced non-small cell lung cancer patients. Tumor Biol. 2014, 35, 4499–4507. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Zhao, J.; Chen, M.; Xu, Y.; Zhong, W.; Xing, J.; Wang, M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016, 21, 519–525. [Google Scholar] [CrossRef]
- Bayarri-Lara, C.; Ortega, F.G.; Cueto Ladrón de Guevara, A.; Puche, J.L.; Ruiz Zafra, J.; de Miguel-Pérez, D.; Ramos, A.S.-P.; Giraldo-Ospina, C.F.; Navajas Gómez, J.A.; Delgado-Rodriguez, M.; et al. Circulating Tumor Cells Identify Early Recurrence in Patients with Non-Small Cell Lung Cancer Undergoing Radical Resection. PLoS ONE 2016, 11, e0148659. [Google Scholar] [CrossRef]
- Hofman, V.; Ilie, M.I.; Long, E.; Selva, E.; Bonnetaud, C.; Molina, T.; Vénissac, N.; Mouroux, J.; Vielh, P.; Hofman, P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch AssayTM and the isolation by size of epithelial tumor cell method. Int. J. Cancer 2011, 129, 1651–1660. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, Z.; Wang, H.; Zhang, H.; Duan, G.; Zhang, X. Role of circulating tumor cells in diagnosis of lung cancer: A systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 0300060521994926. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]
- Cavallone, L.; Aguilar-Mahecha, A.; Lafleur, J.; Brousse, S.; Aldamry, M.; Roseshter, T.; Lan, C.; Alirezaie, N.; Bareke, E.; Majewski, J.; et al. Prognostic and predictive value of circulating tumor DNA during neoadjuvant chemotherapy for triple negative breast cancer. Sci. Rep. 2020, 10, 14704. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Guo, J.; Huang, H.; Tian, L.; Xie, Y.; Wu, Q. Prognostic value of circulating tumor DNA in operable non-small cell lung cancer: A systematic review and reconstructed individual patient-data based meta-analysis. BMC Med. 2023, 21, 467. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Balar, A.; Lakhani, D.A.; Kluwe, C.; Zhao, Z.; Kopparapu, P.; Almodovar, K.; Muterspaugh, A.; Yan, Y.; York, S.; et al. Circulating Tumor DNA as a Biomarker of Radiographic Tumor Burden in SCLC. JTO Clin. Res. Rep. 2021, 2, 100110. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, L.; Murray, J.C.; Canzoniero, J.V.; Landon, B.; Jackson, J.; Scott, S.; Lam, V.; Levy, B.P.; Sausen, M.; Anagnostou, V. Liquid biopsy approaches to capture tumor evolution and clinical outcomes during cancer immunotherapy. J. Immunother. Cancer 2023, 11, e005924. [Google Scholar] [CrossRef]
- Saha, S.; Araf, Y.; Promon, S.K. Circulating tumor DNA in cancer diagnosis, monitoring, and prognosis. J. Egypt. Natl. Cancer Inst. 2022, 34, 8. [Google Scholar] [CrossRef]
- Li, C.; Shao, J.; Li, P.; Feng, J.; Li, J.; Wang, C. Circulating tumor DNA as liquid biopsy in lung cancer: Biological characteristics and clinical integration. Cancer Lett. 2023, 577, 216365. [Google Scholar] [CrossRef]
- Pellini, B.; Madison, R.W.; Childress, M.A.; Miller, S.T.; Gjoerup, O.; Cheng, J.; Huang, R.S.P.; Krainock, M.; Gupta, P.; Zou, W.; et al. Circulating Tumor DNA Monitoring on Chemo-immunotherapy for Risk Stratification in Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 4596–4605. [Google Scholar] [CrossRef]
- Anagnostou, V.; Ho, C.; Nicholas, G.; Juergens, R.A.; Sacher, A.; Fung, A.S.; Wheatley-Price, P.; Laurie, S.A.; Levy, B.; Brahmer, J.R.; et al. ctDNA response after pembrolizumab in non-small cell lung cancer: Phase 2 adaptive trial results. Nat. Med. 2023, 29, 2559–2569. [Google Scholar] [CrossRef]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Alessi, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Mok, T.; Peters, S.; Han, J.-Y.; Alatorre-Alexander, J.; Leighl, N.; Sriuranpong, V.; Pérol, M.; Junior, G.; Nadal, E.; et al. Blood First Assay Screening Trial (BFAST) in Treatment-Naive Advanced or Metastatic NSCLC: Initial Results of the Phase 2 ALK-Positive Cohort. J. Thorac. Oncol. 2021, 16, 2040–2050. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Kasahara, N.; Soda, H.; Imai, H.; Naruse, I.; Yamaguchi, H.; Itai, M.; Taguchi, K.; Uchida, M.; Sunaga, N.; et al. Predictive significance of circulating tumor DNA against patients with T790M-positive EGFR-mutant NSCLC receiving osimertinib. Sci. Rep. 2023, 13, 20848. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.-C.; Soo, R.A.; Riely, G.J.; Ou, S.-H.I.; Clancy, J.S.; Li, S.; et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 1370–1379. [Google Scholar] [CrossRef]
- Lu, J.; Feng, Y.; Guo, K.; Sun, L.; Ruan, S.; Zhang, K. Prognostic value of preoperative circulating tumor DNA in non-small cell lung cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 25. [Google Scholar] [CrossRef]
- Zhong, R.; Gao, R.; Fu, W.; Li, C.; Huo, Z.; Gao, Y.; Lu, Y.; Li, F.; Ge, F.; Tu, H.; et al. Accuracy of minimal residual disease detection by circulating tumor DNA profiling in lung cancer: A meta-analysis. BMC Med. 2023, 21, 180. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Tan, D.; Yang, J.C.-H.; Ahn, M.-J.; Batra, U.; Cho, B.-C.; Cornelio, G.; Lim, T.; Mok, T.; Prabhash, K.; et al. Expert Consensus Recommendations on Biomarker Testing in Metastatic and Nonmetastatic NSCLC in Asia. J. Thorac. Oncol. 2023, 18, 436–446. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lu, S.; Zhou, Q.; Zhang, L.; Cheng, Y.; Wang, J.; Wang, B.; Hu, C.; Lin, L.; Zhong, W.; et al. Expert consensus on treatment for stage III non-small cell lung cancer. Med. Adv. 2023, 1, 3–13. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Small Cell Lung Cancer; NCCN: Plymouth Meeting, PA, USA, 2025.
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; van ’t Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Giménez-Capitán, A.; Karachaliou, N.; Rosell, R. Comprehensive molecular screening: From the RT-PCR to the RNA-seq. Transl. Lung Cancer Res. 2013, 2, 87–91. [Google Scholar] [CrossRef]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.-T.; Huang, X.; Huang, S.; Xie, S.-J.; Xiao, Z.-D.; Zhang, H. RNA sequencing: New technologies and applications in cancer research. J. Hematol. Oncol. 2020, 13, 166. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, Z.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Xiang, Y.; Wu, N.; Wu, L.; Bai, L.; et al. Circulating exosomal microRNAs as prognostic biomarkers for non-small-cell lung cancer. Oncotarget 2016, 8, 13048–13058. [Google Scholar] [CrossRef]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Wu, J.; Wang, F. Circular RNAs Are Promising Biomarkers in Liquid Biopsy for the Diagnosis of Non-small Cell Lung Cancer. Front. Mol. Biosci. 2021, 8, 625722. [Google Scholar] [CrossRef]
- Han, Y.; Shin, S.-H.; Lim, C.G.; Heo, Y.H.; Choi, I.Y.; Kim, H.H. Synthetic RNA Therapeutics in Cancer. J. Pharmacol. Exp. Ther. 2023, 386, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, L.; Li, S.; Yang, W.; Chen, F.; Cai, Z.; Liu, X.; Xu, W.; Lehto, V.-P.; Lächelt, U.; et al. From structural design to delivery: mRNA therapeutics for cancer immunotherapy. Exploration 2024, 4, 20210146. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Andre, M.; Caobi, A.; Miles, J.S.; Vashist, A.; Ruiz, M.A.; Raymond, A.D. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer 2024, 24, 322. [Google Scholar] [CrossRef]
- Xiong, D.; Wang, C.; Yang, Z.; Han, F.; Zhan, H. Clinical Significance of Serum-Derived Exosomal LINC00917 in Patients With Non-Small Cell Lung Cancer. Front. Genet. 2021, 12, 728763. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, X.; Lou, C.; Zhou, C.; Zhao, X.; Li, N.; Tian, H.; Meng, X. PLA2G10 incorporated in exosomes could be diagnostic and prognostic biomarker for non-small cell lung cancer. Clin. Chim. Acta 2022, 530, 55–65. [Google Scholar] [CrossRef]
- Choi, E.-S.; Faruque, H.A.; Kim, J.-H.; Kim, K.J.; Choi, J.E.; Kim, B.A.; Kim, B.; Kim, Y.J.; Woo, M.H.; Park, J.Y.; et al. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics 2021, 11, 620. [Google Scholar] [CrossRef]
- Moding, E.J.; Nabet, B.Y.; Alizadeh, A.A.; Diehn, M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021, 11, 2968–2986. [Google Scholar] [CrossRef] [PubMed]
- Kemper, M.; Krekeler, C.; Menck, K.; Lenz, G.; Evers, G.; Schulze, A.B.; Bleckmann, A. Liquid Biopsies in Lung Cancer. Cancers 2023, 15, 1430. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, G.; Mantikas, K.-T.; Allsopp, R.; Yapeter, C.A.; Jahin, M.; Melnick, T.; Ali, S.; Coombes, R.C.; Toumazou, C.; Shaw, J.A.; et al. The Evolution of Affordable Technologies in Liquid Biopsy Diagnostics: The Key to Clinical Implementation. Cancers 2023, 15, 5434. [Google Scholar] [CrossRef]
- Mosko, M.J.; Nakorchevsky, A.A.; Flores, E.; Metzler, H.; Ehrich, M.; van den Boom, D.J.; Sherwood, J.L.; Nygren, A.O.H. Ultrasensitive Detection of Multiplexed Somatic Mutations Using MALDI-TOF Mass Spectrometry. J. Mol. Diagn. JMD 2016, 18, 23–31. [Google Scholar] [CrossRef]
- Vendrell, J.A.; Mau-Them, F.T.; Béganton, B.; Godreuil, S.; Coopman, P.; Solassol, J. Circulating Cell Free Tumor DNA Detection as a Routine Tool forLung Cancer Patient Management. Int. J. Mol. Sci. 2017, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Elazezy, M.; Joosse, S.A. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput. Struct. Biotechnol. J. 2018, 16, 370–378. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.-M. Liquid Biopsy, ctDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef]
- Bewicke-Copley, F.; Arjun Kumar, E.; Palladino, G.; Korfi, K.; Wang, J. Applications and analysis of targeted genomic sequencing in cancer studies. Comput. Struct. Biotechnol. J. 2019, 17, 1348–1359. [Google Scholar] [CrossRef]
- Gerber, T.; Taschner-Mandl, S.; Saloberger-Sindhöringer, L.; Popitsch, N.; Heitzer, E.; Witt, V.; Geyeregger, R.; Hutter, C.; Schwentner, R.; Ambros, I.M.; et al. Assessment of Pre-Analytical Sample Handling Conditions for Comprehensive Liquid Biopsy Analysis. J. Mol. Diagn. 2020, 22, 1070–1086. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; Mattos-Arruda, L.D.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Aredo, J.V.; Jamali, A.; Zhu, J.; Heater, N.; Wakelee, H.A.; Vaklavas, C.; Anagnostou, V.; Lu, J. Liquid Biopsy Approaches for Cancer Characterization, Residual Disease Detection, and Therapy Monitoring. Am. Soc. Clin. Oncol. Educ. Book. 2025, 45, e481114. [Google Scholar] [CrossRef] [PubMed]
- Boukovala, M.; Westphalen, C.B.; Probst, V. Liquid biopsy into the clinics: Current evidence and future perspectives. J. Liq. Biopsy 2024, 4, 100146. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, F.; Wu, J.; Schubert, J.; Li, M.M. Application of Next Generation Sequencing in Laboratory Medicine. Ann. Lab. Med. 2021, 41, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, G.A.; Vollger, M.R.; Eichler, E.E. Long-read human genome sequencing and its applications. Nat. Rev. Genet. 2020, 21, 597–614. [Google Scholar] [CrossRef]
- Lissa, D.; Robles, A.I. Methylation analyses in liquid biopsy. Transl. Lung Cancer Res. 2016, 5, 492–504. [Google Scholar] [CrossRef]
- Jamshidi, A.; Liu, M.C.; Klein, E.A.; Venn, O.; Hubbell, E.; Beausang, J.F.; Gross, S.; Melton, C.; Fields, A.P.; Liu, Q.; et al. Evaluation of cell-free DNA approaches for multi-cancer early detection. Cancer Cell 2022, 40, 1537–1549.e12. [Google Scholar] [CrossRef]
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R.-H. Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends. Mol. Med. 2021, 27, 482–500. [Google Scholar] [CrossRef]
- Board, R.E.; Knight, L.; Greystoke, A.; Blackhall, F.H.; Hughes, A.; Dive, C.; Ranson, M. DNA Methylation in Circulating Tumour DNA as a Biomarker for Cancer. Biomark. Insights 2008, 2, 307–319. [Google Scholar] [CrossRef]
- Xu, J.; Liao, K.; Yang, X.; Wu, C.; Wu, W. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol. Cancer 2021, 20, 104. [Google Scholar] [CrossRef]
- Shapiro, E.; Biezuner, T.; Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013, 14, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Zheng, L.; Zheng, C.; Song, J.; Zhang, Q.; Kang, B.; Liu, Z.; Jin, L.; Xing, R.; et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018, 24, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.-A.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.H.; Iorgulescu, J.B.; Braun, D.A.; Keskin, D.B.; Livak, K.J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 244–256. [Google Scholar] [CrossRef]
- Leader, A.M.; Grout, J.A.; Maier, B.B.; Nabet, B.Y.; Park, M.D.; Tabachnikova, A.; Chang, C.; Walker, L.; Lansky, A.; Le Berichel, J.; et al. Single-cell analysis of human non-small cell lung cancer lesions refines tumor classification and patient stratification. Cancer Cell 2021, 39, 1594–1609.e12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sadée, C.; Ozawa, M.; Howitt, B.E.; Gevaert, O. Single-cell multimodal analysis reveals tumor microenvironment predictive of treatment response in non-small cell lung cancer. Sci. Adv. 2025, 11, eadu2151. [Google Scholar] [CrossRef]
- Monkman, J.; Kim, H.; Mayer, A.; Mehdi, A.; Matigian, N.; Cumberbatch, M.; Bhagat, M.; Ladwa, R.; Mueller, S.N.; Adams, M.N.; et al. Multi-omic and spatial dissection of immunotherapy response groups in non-small cell lung cancer. Immunology 2023, 169, 487–502. [Google Scholar] [CrossRef]
- Guruprasad, P.; Lee, Y.G.; Kim, K.H.; Ruella, M. The current landscape of single-cell transcriptomics for cancer immunotherapy. J. Exp. Med. 2021, 218, e20201574. [Google Scholar] [CrossRef]
- Wlosik, J.; Fattori, S.; Rochigneux, P.; Goncalves, A.; Olive, D.; Chretien, A.S. Immune biology of NSCLC revealed by single-cell technologies: Implications for the development of biomarkers in patients treated with immunotherapy. Semin. Immunopathol. 2023, 45, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. At the dawn: Cell-free DNA fragmentomics and gene regulation. Br. J. Cancer 2022, 126, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.R. Circulating DNA fragmentomics and cancer screening. Cell Genom. 2023, 3, 100242. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef]
- Wang, S.; Meng, F.; Li, M.; Bao, H.; Chen, X.; Zhu, M.; Liu, R.; Xu, X.; Yang, S.; Wu, X.; et al. Multidimensional Cell-Free DNA Fragmentomic Assay for Detection of Early-Stage Lung Cancer. Am. J. Respir. Crit. Care Med. 2023, 207, 1203–1213. [Google Scholar] [CrossRef]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Wu, W.; Yang, H.; Jin, Y.; Wu, M.; Liu, W.; Yang, A.; Chervova, O.; Zhang, S.; et al. Multi-omics integrated circulating cell-free DNA genomic signatures enhanced the diagnostic performance of early-stage lung cancer and postoperative minimal residual disease. eBioMedicine 2023, 91, 104553. [Google Scholar] [CrossRef]
- Wang, S.; Xia, Z.; You, J.; Gu, X.; Meng, F.; Chen, P.; Tang, W.; Bao, H.; Zhang, J.; Wu, X.; et al. Enhanced Detection of Landmark Minimal Residual Disease in Lung Cancer Using Cell-free DNA Fragmentomics. Cancer Res. Commun. 2023, 3, 933–942. [Google Scholar] [CrossRef]
- Yin, R.; Wang, S.; Li, M.; Jiang, F.; Zhang, J.; Meng, F.; Tang, W.; Bao, H.; Chen, H.; Wu, X.; et al. Utilization of cell-free DNA fragmentomics in minimal residual disease detection for non-small cell lung cancer. J. Clin. Oncol. 2022, 40, 3038. [Google Scholar] [CrossRef]
- Bao, H.; Yang, S.; Chen, X.; Dong, G.; Mao, Y.; Wu, S.; Cheng, X.; Wu, X.; Tang, W.; Wu, M.; et al. Early detection of multiple cancer types using multidimensional cell-free DNA fragmentomics. Nat. Med. 2025, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Li, M.; Liu, W.; Lu, L.; Li, Y.; Chen, X.; Yang, S.; Liu, T.; Cheng, W.; et al. Ultra-short cell-free DNA fragments enhance cancer early detection in a multi-analyte blood test combining mutation, protein and fragmentomics. Clin. Chem. Lab. Med. 2024, 62, 168–177. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).