Infection Biomarkers in Children with Chemotherapy-Induced Severe Neutropenia
Simple Summary
Abstract
1. Introduction
2. Diagnostic Biomarkers of Infectious Etiology in Pediatric Febrile Neutropenia
2.1. C-Reactive Protein
2.1.1. Differentiation of Infectious vs. Non-Infectious Neutropenic Fever
2.1.2. Differentiation of Infectious Agent (GNB, GPB, Viral)
2.1.3. Risk Stratification and Antimicrobial Policy
2.2. Procalcitonin
2.2.1. Differentiation of Infectious vs. Non-Infectious Neutropenic Fever
2.2.2. Differentiation of Infectious Agent (GNB, GPB, Viral)
2.2.3. Risk Stratification and Antimicrobial Policy
2.3. Interleukin-6
2.3.1. Differentiation of Infectious vs. Non-Infectious Neutropenic Fever
2.3.2. Differentiation of Infectious Agent (GNB, GPB, Viral)
2.3.3. Risk Stratification and Antimicrobial Policy
2.4. Interleukin-8
2.4.1. Differentiation of Infectious vs. Non-Infectious Neutropenic Fever
2.4.2. Differentiation of Infectious Agent (GNB, GPB, Viral)
2.4.3. Risk Stratification and Antimicrobial Policy
2.5. Interleukin-10
2.5.1. Differentiation of Infectious vs. Non-Infectious Neutropenic Fever
2.5.2. Risk Stratification and Antimicrobial Policy
2.6. Additional Biomarkers
3. Summary
4. Discussion
- establishment of pediatric-specific reference values and cutoffs;
- standardization of assay methodologies and reporting formats;
- development of cost-effective, rapid platforms suitable for real-time decision-making;
- and validation through prospective, multicenter trials with unified protocols.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bodey, G.P.; Buckley, M.; Sathe, Y.S.; Freireich, E.J. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 1966, 64, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Dropulic, L.K.; Lederman, H.M. Overview of infections in the immunocompromised host. Microbiol. Spectr. 2016, 4, 3–50. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, O.R.; Salomão, R.; Brunialti, M.K.C.; da Silva, D.C.B.; Senerchia, A.A.; de Moraes Costa Carlesse, F.A.; Petrilli, A.S. Cytokine Kinetics in Febrile Neutropenic Children: Insights on the Usefulness as Sepsis Biomarkers, Influence of Filgrastim, and Behavior of the IL-23/IL-17 Pathway. Mediat. Inflamm. 2017, 2017, 8291316. [Google Scholar] [CrossRef] [PubMed]
- Velden, F.; Gennery, A.; Emonts, M. Biomarkers for Diagnosing Febrile Illness in Immunocompromised Children: A Systematic Review of the Literature. Front. Pediatr. 2022, 10, 828569. [Google Scholar] [CrossRef]
- Edwardson, D.W.; Parissenti, A.M.; Kovala, A.T. Chemotherapy and Inflammatory Cytokine Signalling in Cancer Cells and the Tumour Microenvironment. Adv. Exp. Med. Biol. 2019, 1152, 173–215. [Google Scholar] [CrossRef]
- Tapia, L.I.; Olivares, M.; Torres, J.P.; De la Maza, V.; Valenzuela, R.; Contardo, V.; Tordecilla, J.; Álvarez, A.M.; Varas, M.; Zubieta, M.; et al. Cytokine and chemokine profiles in episodes of persistent high-risk febrile neutropenia in children with cancer. Cytokine 2021, 148, 155619. [Google Scholar] [CrossRef]
- Hatzistilianou, M.; Rekliti, A.; Athanassiadou, F.; Catriu, D. Procalcitonin as an early marker of bacterial infection in neutropenic febrile children with acute lymphoblastic leukemia. Inflamm. Res. 2010, 59, 339–347. [Google Scholar] [CrossRef]
- Nahar, A.; Jamal, C.Y.; Refat, R.; Chowdhury, T.; Akter, S.; Karim, A.; Rahman, M.A.; Yeamin, M.B.; Saha, B.K.; Hossain, F.; et al. Procalcitonin versus C-Reactive Protein as a Biomarker for Prediction of Bacterial Infection in Children with Febrile Neutropenia in Acute Leukemia. Mymensingh Med. J. 2023, 32, 76–82. [Google Scholar]
- Martinez-Albarran, M.; Perez-Molina, J.d.J.; Gallegos-Castorena, S.; Sanchez-Zubieta, F.; Del Toro-Arreola, S.; Troyo-Sanroman, R.; Gonzalez-Ramella, O. Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatr. Hematol. Oncol. 2009, 26, 414–425. [Google Scholar] [CrossRef]
- Kharya, G.; Yadav, S.; Srivastava, L.M.; Sachdeva, A. Use of Interleukin 6, High Sensitivity CRP, TNFα and Procalcitonin as Early Markers of Sepsis in Febrile Neutropenic Children. Blood 2007, 110, 3852. [Google Scholar] [CrossRef]
- Kitanovski, L.; Jazbec, J.; Hojker, S.; Gubina, M.; Derganc, M. Diagnostic accuracy of procalcitonin and interleukin-6 values for predicting bacteremia and clinical sepsis in febrile neutropenic children with cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, J.; Påhlman, M.; Mellander, L. Interleukin 6, but not tumour necrosis factor-α, is a good predictor of severe infection in febrile neutropenic and non-neutropenic children with malignancy. Acta Pædiatrica 1997, 86, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Kumar, N.; Scott, J. Evaluation of serum procalcitonin, serum interleukin-6, and interleukin-8 as predictors of serious infection in children with febrile neutropenia and cancer. Indian J. Cancer 2021, 58, 185–189. [Google Scholar] [CrossRef]
- Diepold, M.; Noellke, P.; Duffner, U.; Kontny, U.; Berner, R. Performance of Interleukin-6 and Interleukin-8 serum levels in pediatric oncology patients with neutropenia and fever for the assessment of low-risk. BMC Infect. Dis. 2008, 8, 28. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bansal, D.; Bansal, F.; Nanda, N.; Ray, P.; Trehan, A.; Marwaha, R. Interleukin-5, interleukin-6, interleukin-8 and tumour necrosis factor-alpha levels obtained within 24 hours of admission do not predict high-risk infection in children with febrile neutropenia. Indian J. Med. Microbiol. 2013, 31, 226–229. [Google Scholar] [CrossRef]
- Doerflinger, M.; Haeusler, G.M.; Li-Wai-Suen, C.S.N.; Clark, J.E.; Slavin, M.; Babl, F.E.; Allaway, Z.; Mechinaud, F.; Smyth, G.K.; De Abreu Lourenco, R.; et al. Procalcitonin and Interleukin-10 May Assist in Early Prediction of Bacteraemia in Children with Cancer and Febrile Neutropenia. Front. Immunol. 2021, 12, 641879. [Google Scholar] [CrossRef] [PubMed]
- Urbonas, V.; Eidukaitė, A.; Tamulienė, I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine 2012, 57, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Soker, M.; Çolpan, L.; Ece, A.; Devecioğlu, C.; Haspolat, K. Serum levels of IL-1 beta, sIL-2R, IL-6, IL-8, and TNF-alpha in febrile children with cancer and neutropenia. Med. Oncol. 2001, 18, 51–57. [Google Scholar] [CrossRef]
- Riikonen, P.; Saarinen, U.; Teppo, A.; Metsärinne, K.; Fyhrquist, F.; Jalanko, H. Cytokine and acute-phase reactant levels in serum of children with cancer admitted for fever and neutropenia. J. Infect. Dis. 1992, 166, 432–436. [Google Scholar] [CrossRef]
- Miedema, K.G.E.; de Bont, E.D.; Elferink, R.O.; van Vliet, M.; Nijhuis, C.S.M.O.; Kamps, W.; Tissing, W.J.E. The diagnostic value of CRP, IL-8, PCT, and sTREM-1 in the detection of bacterial infections in pediatric oncology patients with febrile neutropenia. Support. Care Cancer 2010, 19, 1593–1600. [Google Scholar] [CrossRef]
- Bux, J.; Hofmann, C.; Welte, K. Serum G-CSF levels are not increased in patients with antibody-induced neutropenia unless they are suffering from infectious diseases. Br. J. Haematol. 1999, 105, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Urbonas, V.; Eidukaitė, A.; Tamulienė, I. The predictive value of soluble biomarkers (CD14 subtype, interleukin-2 receptor, human leucocyte antigen-G) and procalcitonin in the detection of bacteremia and sepsis in pediatric oncology patients with chemotherapy-induced febrile neutropenia. Cytokine 2013, 62, 34–37. [Google Scholar] [CrossRef]
- Santolaya, M.E.; Cofre, J.; Beresi, V. C-reactive protein: A valuable aid for the management of febrile children with cancer and neutropenia. Clin. Infect. Dis. 1994, 18, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Jaing, T.H.; Chang, C.C.; Chang, T.Y.; Chen, S.H.; Wen, Y.C.; Tsay, P.K. Diagnostic Value of C-reactive Protein and Interleukin-8 in Risk Stratification of Febrile Neutropenic Children with Allogeneic Hematopoietic Stem Cell Transplantation. Sci. Rep. 2020, 10, 2894. [Google Scholar] [CrossRef]
- Urbonas, V.; Eidukaitė, A.; Tamulienė, I. Interleukin-8 values in pediatric oncology patients with febrile neutropenia and bloodstream infections. Pediatr. Res. 2011, 70, 490. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, S.B.; Kang, J.H. Association between cytokine concentration kinetics and prolonged fever in febrile neutropenic children with bacteremia. Int. J. Immunopathol. Pharmacol. 2022, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-J.; Luo, Z.-B.; Xia, T.; Song, H.; Yang, S.-L.; Xu, W.-Q.; Ni, Y.-R.; Zhao, N.; Tang, Y.-M. Comparison of interleukin-6, interleukin-10, procalcitonin and C-reactive protein in identifying high-risk febrile illness in pediatric cancer patients: A prospective observational study. Cytokine 2019, 116, 1–6. [Google Scholar] [CrossRef]
- van der Galiën, H.T.; Loeffen, E.A.H.; Miedema, K.G.E.; Tissing, W.J.E. Predictive value of PCT and IL-6 for bacterial infection in children with cancer and febrile neutropenia. Support. Care Cancer 2018, 26, 3819–3826. [Google Scholar] [CrossRef]
- Cost, C.R.; Stegner, M.M.; Leonard, D.; Leavey, P. IL-8 predicts pediatric oncology patients with febrile neutropenia at low risk for bacteremia. J. Pediatr. Hematol. 2013, 35, 206–211. [Google Scholar] [CrossRef]
- Karakurt, D.G.; Demirsoy, U.; Corapcioglu, F.; Oncel, S.; Karadogan, M.; Arisoy, E.S. Do proinflammatory cytokine levels predict serious complication risk of infection in pediatric cancer patients? Pediatr. Hematol. Oncol. 2014, 31, 415–424. [Google Scholar] [CrossRef]
- Akçay, A.; Ağaoglu, L.; Ekmekçi, H.; Balcı Ekmekçi, O.; Saribeyoglu, E.; Atay, D.; Tuğcu, D.; Karakaş, Z.; Ünüvar, A.; Anak, S.; et al. Interleukin-8 in febrile neutropenic children with cancers: Its diagnostic value for bacteremia/sepsis is superior to that of interleukin 6, mannose binding lectin, procalcitonin and C-reactive protein. Int. J. Hematol. Oncology. UHOD 2021, 31, 230–238. [Google Scholar] [CrossRef]
- Zumrut Sahbudak Bal, Z.; Karadaş Özdemir, N.; Şen, S.; Yılmaz Karapınar, D.; Azarsız, E.; Aydemir, S.; Vardar, F. Diagnostic accuracy of interleukin-6, interleukin-8, and interleukin-10 for predicting bacteremia in children with febrile neutropenia. Turk. J. Hematol. 2017, 34, 254–257. [Google Scholar] [CrossRef]
- Gupta, M.; Kini, P.; Bhat, Y.; Aroor, S. Interleukin-6 versus C-reactive Protein as Markers for Early Detection of Bacteremia in Febrile Neutropenia in Pediatric Population. Indian J. Med. Paediatr. Oncol. 2020, 41, 702–706. [Google Scholar] [CrossRef]
- Oude Nijhuis, C.; Kamps, W.A.; Daenen, S.M.; Gietema, J.A.; van der Graaf, W.T.; Groen, H.J.; Vellenga, E.; Ten Vergert, E.M.; Vermeulen, K.M.; de Vries-Hospers, H.G.; et al. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients. J. Clin. Oncol. 2005, 23, 7437–7444. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xu, X.; Zhao, N.; Luo, Z.; Tang, Y. Comparison of the diagnostic power of cytokine patterns and procalcitonin for predicting infection among paediatric haematology/oncology patients. Clin. Microbiol. Infect. 2016, 22, 996–1001. [Google Scholar] [CrossRef]
- Hemming, V.; Jakes, A.D.; Shenton, G.; Phillips, B. Prospective cohort study of procalcitonin levels in children with cancer presenting with febrile neutropenia. BMC Pediatr. 2017, 17, 2. [Google Scholar] [CrossRef]
- Nonkulovski, D.; Tankoska, M.; Hristova, M.K.; Mandzukovska, H.; Voinovska, T.; Martinova, K. Procalcitonin-early biochemical marker for diagnosis, prognosis and treatment of sepsis in neonates and oncological patients with febrile neutropenia. J. Morphol. Sci. 2020, 3, 74–80. Available online: http://jms.mk/jms/article/download/133/86 (accessed on 25 April 2025).
- Zareifar, S.; Sanaei Dashti, A.; Masoomzade, T.; Anvarinejad, M.; Zekavat, O.R.; Bordbar, M.; Cohan, N.; Haghpanah, S. Assessment of procalcitonin as a diagnostic marker of infection in pediatrics with cancer complicated by fever and neutropenia. Iran. J. Pediatr. Hematol. Oncol. 2020, 10, 10–16. [Google Scholar] [CrossRef]
- Özdemir, Z.C.; Düzenli-Kar, Y.; Canik, A.; Küskü-Kiraz, Z.; Özen, H.; Bör, Ö. The predictive value of procalcitonin, C-reactive protein, presepsin, and soluble-triggering receptor expressed on myeloid cell levels in bloodstream infections in pediatric patients with febrile neutropenia. Turk. J. Pediatr. 2019, 61, 359–367. [Google Scholar] [CrossRef]
- Purkayastha, K.; Seth, R.; Amitabh, S.; Xess, I.; Kapil, A.; Sreenivas, V. To Determine the Role of Procalcitonin in Febrile Neutropenic Episodes ofChildren Undergoing Treatment for Childhood Cancers. J. Clin. Case Rep. 2016, 6, 805. [Google Scholar] [CrossRef]
- Baraka, A.; Zakaria, M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int. J. Hematol. 2018, 108, 184–191. [Google Scholar] [CrossRef] [PubMed]
- El-Maghraby, S.M.; Moneer, M.M.; Ismail, M.M.; Shalaby, L.M.; El-Mahallawy, H.A. The diagnostic value of C-reactive protein, interleukin-8, and monocyte chemotactic protein in risk stratification of febrile neutropenic children with hematologic malignancies. J. Pediatr. Hematol. 2007, 29, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cerasi, S.; Leardini, D.; Lisanti, N.; Belotti, T.; Pierantoni, L.; Zama, D.; Lanari, M.; Prete, A.; Masetti, R. The role of presepsin in pediatric patients with oncological and hematological diseases experiencing febrile neutropenia. Sci. Rep. 2023, 13, 6464. [Google Scholar] [CrossRef]
- Arıkan, K.; Karadag-Oncel, E.; Aytac, S.; Cetin, M.; Cengiz, A.B.; Gümrük, F.; Kara, A.; Ceyhan, M. Usage of Plasma Presepsin, C-Reactive Protein, Procalcitonin and Proadrenomedullin to Predict Bacteremia in Febril Neutropenia of Pediatric Hematological Malignancy Patients. Lab. Med. 2021, 52, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Antari, V.; Skoura, L.; Hatzipantelis, E.; Tsinopoulou, V.R.; Papakonstantinou, K.; Protonotariou, E.; Galli-Tsinopoulou, A.; Tragiannidis, A. Kinetics and role of pancreatic stone protein and midregional proadrenomedullin as predictors of sepsis and bacteremia in children with hematological malignancies. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023065. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Parisi, E.; Lucido, G.D.; Iacona, A.; Ciaccio, A.M.; Giglio, R.V.; Ziino, O.; Ciaccio, M. Presepsin and Midregional Proadrenomedullin in Pediatric Oncologic Patients with Febrile Neutropenia. Lab. Med. 2020, 51, 585–591. [Google Scholar] [CrossRef]
- Ragab, S.M.; El-Deeb, S.M.; Saeed, A.; Mahmoud, A.A. Prognostic role of mid-regional pro-adrenomedullin in predicting infection in pediatric cancer with febrile neutropenia. Clin. Exp. Pediatr. 2025, 68, 445–453. [Google Scholar] [CrossRef]
- Fawzi, M.M.; Omran, A.A.; Masood, B.A. Serum level of midregional fragment of proadrenomedullin as an early sepsis marker in severely neutropenic patients with hematologic malignancies. Egypt. J. Haematol. 2019, 44, 118–123. [Google Scholar] [CrossRef]
- Susanto, J.; Fuadi, M.R.; Andarsini, M.R. Diagnostic Value of Mid Regional Proadrenomedullin as a Sepsis Biomarker in Pediatric Patients with Cancer-Related Chemotherapy. Indian J. Forensic Med. Toxicol. 2022, 16, 658–663. [Google Scholar] [CrossRef]
- Demirkaya, M.; Tugcu, D.; Akcay, A.; Aydogan, G.; Akıcı, F.; Salcioglu, Z.; Ekmekci, H.; Sevinir, B.; Balci Ekmekci, O. Adrenomedullin—A New Marker in Febrile Neutropenia: Comparison with CRP and Procalcitonin. Pediatr. Hematol. Oncol. 2015, 32, 425–432. [Google Scholar] [CrossRef]
- Peisajovich, A.; Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev. Clin. Immunol. 2008, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Du Clos, T.W. Function of C-reactive protein. Ann. Med. 2000, 32, 274–278. [Google Scholar] [CrossRef]
- Gershov, D.; Kim, S.; Brot, N.; Elkon, K.B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: Implications for systemic autoimmunity. J. Exp. Med. 2000, 192, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Ozbay, S.; Ayan, M.; Ozsoy, O.; Akman, C.; Karcioglu, O. Diagnostic and Prognostic Roles of Procalcitonin and Other Tools in Community-Acquired Pneumonia: A Narrative Review. Diagnostics 2023, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Philipp, S.; Werner, A.; Beat, M. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: Past, present and future. BMC Med. 2011, 9, 107. [Google Scholar]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Hurst, S.; Wilkinson, T.; McLoughlin, R.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.; Topley, N.; Jones, S. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef]
- Hack, C.; de Groot, E.; Felt-Bersma, R.; Nuijens, J.; van Strack van Schijndel, R.J.; Eerenberg-Belmer, A.; Thijs, L.; Aarden, L. Increased plasma levels of interleukin-6 in sepsis. Blood 1989, 74, 1704–1710. [Google Scholar] [CrossRef]
- Vivas, M.C.; Guerrero, H.F.V.; Tascón, A.; Valderrama-Aguirre, A. Plasma interleukin-6 levels correlate with survival in patients with bacterial sepsis and septic shock. Interv. Med. Appl. Sci. 2021, 11, 224–230. [Google Scholar] [CrossRef]
- Dama, P.; Ledoux, D.; Nys, M.; Vrindts, Y.; de Groote, D.; Franchimont, P.; Lamy, M. Cytokine serum level during severe sepsis in humans: IL-6 as a marker of severity. Ann. Surg. 1992, 215, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Knall, C.; Young, S.; Nick, J.; Buhl, A.; Worthen, G.; Johnson, G. Interleukin-8 Regulation of the Ras/Raf/Mitogen-activated Protein Kinase Pathway in Human Neutrophils (*). J. Biol. Chem. 1996, 271, 2832–2838. [Google Scholar] [CrossRef]
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Cox, R.A.; Song, J.; Jeschke, M.G. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock 2015, 43, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2019, 216, 1963–1975. [Google Scholar] [CrossRef]
- Hutchins, A.P.; Takahashi, Y.; Miranda-Saavedra, D. Genomic analysis of LPS-stimulated myeloid cells identifies a common pro-inflammatory response but divergent IL-10 anti-inflammatory responses. Sci. Rep. 2015, 5, 9100. [Google Scholar] [CrossRef]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef]
- Arai, Y.; Mizugishi, K.; Nonomura, K.; Naitoh, K.; Takaori-Kondo, A.; Yamashita, K. Phagocytosis by human monocytes is required for the secretion of presepsin. J. Infect. Chemother. 2015, 21, 564–569. [Google Scholar] [CrossRef]
- Nelson, S. Role of granulocyte colony-stimulating factor in the immune response to acute bacterial infection in the nonneutropenic host: An overview. Clin. Infect. Dis. 1994, 18, 197–204. [Google Scholar] [CrossRef]
- Basu, S.; Dunn, A.; Ward, A. G-CSF: Function and modes of action (Review). Int. J. Mol. Med. 2002, 10, 3–10. [Google Scholar] [CrossRef]
- Franzke, A. The role of G-CSF in adaptive immunity. Cytokine Growth Factor Rev. 2006, 17, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kennon, C.; Overturf, G.; Bessman, S.; Sierra, E.; Smith, K.J.; Brann, B. Granulocyte colony-stimulating factor as a marker for bacterial infection in neonates. J. Pediatr. 1996, 128, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Pauksen, K.; Elfman, L.; Ulfgren, A.K.; Venge, P. Serum levels of granulocyte-colony stimulating factor (G-CSF) in bacterial and viral infections, and in atypical pneumonia. Br. J. Haematol. 1994, 88, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, P.; Nora, D.; Coelho, L.; Povoa, P. Pancreatic Stone Protein: Review of a New Biomarker in Sepsis. J. Clin. Med. 2022, 11, 1085. [Google Scholar] [CrossRef]
- Trojan, G.; Moniuszko-Malinowska, A.; Grzeszczuk, A.; Czupryna, P. Adrenomedullin as a New Prosperous Biomarker in Infections: Current and Future Perspectives. J. Clin. Med. 2024, 13, 6142. [Google Scholar] [CrossRef]
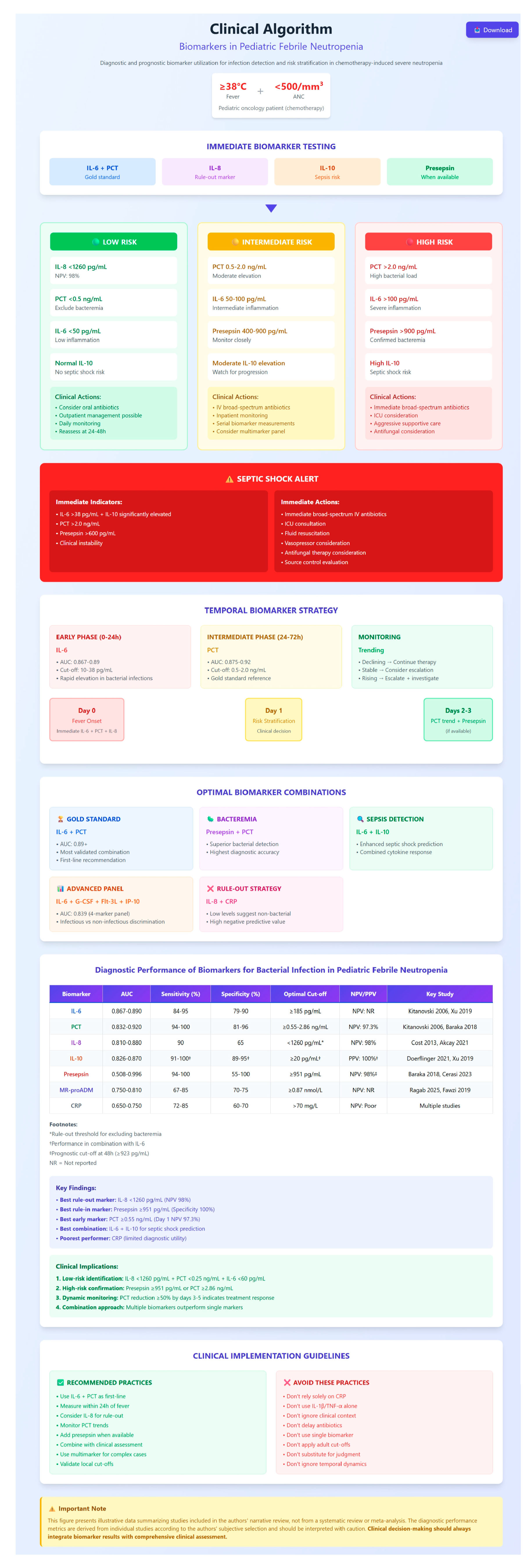
| No. | First Author (Year) [Citation No.] | Markers Studied | Population Studied | Marker(s) Results and Cutoff | Diagnostic Insights from This Study | Prognostic Value | Cytokine/Other Marker in Distinguishing Infection Etiology | Integrating Cytokine/Other Marker in Risk Stratification |
|---|---|---|---|---|---|---|---|---|
| 1 | Tapia (2021) [6] | IL-6, G-CSF, Flt-3L, IP-10 (plus 34 others) | 110 pediatric cancer patients with persistent high-risk febrile neutropenia (HRFN); multicenter study, Chile | IL-6 significantly elevated on day 4 in patients with FN-DEA vs. FN-UO: median 80.81 vs. 13.37 pg/mL; p = 0.002. IL-6: AUC = 0.701; G-CSF: AUC = 0.763; Flt-3L: AUC = 0.745. IP-10: AUC = 0.702 for viral infections. Combined G-CSF, Flt-3L, IL-6, and IP-10: AUC = 0.839, sensitivity 75%, specificity 81%. | On day 4 of persistent HRFN, cytokine/chemokine levels were significantly higher in episodes with identified infectious agent (FN-DEA), indicating a measurable immune response. | IL-6 and G-CSF were significantly elevated in FN-DEA. Two of three fatal cases occurred in FN-DEA group, suggesting potential prognostic relevance, although not statistically analyzed. | G-CSF discriminated bacterial infections (AUC = 0.665); IP-10 identified viral infections (AUC = 0.702). Flt-3L and IL-6 also showed diagnostic power. | Authors propose using combined levels of G-CSF, IL-6, Flt-3L and IP-10 on day 4 of HRFN to help differentiate infectious from non-infectious causes; AUC = 0.839. |
| 2 | Hatzistilianou (2010) [7] | PCT, CRP, TNF-α, IL-1β, IL-8, sTNFRII | 54 febrile episodes in neutropenic children with ALL (group A); comparison with non-neutropenic and non-bacterial groups, | PCT > 2 ng/mL predicted bacterial infection in neutropenic patients with sensitivity 94% and specificity 96.5%; AUC = 0.875. IL-1β AUC = 0.616; IL-8 AUC = 0.750; TNF-α AUC = 0.777; sTNFRII AUC = 0.795. | PCT levels elevated early in bacterial infection and decreased with response to therapy; best single discriminator of bacterial infection on day 1 compared to other markers | PCT decrease during treatment paralleled clinical improvement and successful antibiotic response; persistently high levels linked with prolonged or complicated infections | PCT discriminated well between bacterial and viral infections; IL-8 specificity was high in afebrile controls but limited in bacterial vs. viral discrimination. other markers (CRP, TNF-α, IL-1β, sTNFRII) were less effective | PCT proposed as valuable tool for early prediction of bacterial infections and monitoring therapy in febrile neutropenic children with ALL; no formal risk stratification model proposed |
| 3 | Nahar (2023) [8] | Procalcitonin (PCT), C-reactive protein (CRP) | 58 children with acute leukemia and febrile neutropenia, ages 1 < 18 years; single center, Bangladesh | Median PCT in bacteremia: 26.10 μg/L vs. 0.78 μg/L (no bacteremia), p = 0.002. PCT > 2 μg/L significantly associated with bacteremia. AUC: PCT = 0.797 (95% CI 0.651–0.943); CRP = 0.697 (95% CI 0.54–0.855). Median CRP: 137.4 mg/L (bacteremia) vs. 54.17 mg/L (no bacteremia), p = 0.036. | PCT and CRP measured within 24 h of FN onset; both useful in early detection of bacterial infection, PCT superior to CRP based on AUC. | not applicable | PCT > 2 μg/L significantly associated with bacteremia. PCT better discriminator than CRP based on ROC curve. | not applicable |
| 4 | Martinez-Albarran (2009) [9] | Procalcitonin (PCT), C-reactive protein (CRP) | 54 pediatric patients with febrile neutropenia and cancer, Mexico; prospective observational study | PCT (cutoff 0.67 ng/mL): sensitivity 72.2%, specificity 80.5%. CRP (cutoff 9.06 mg/dL): sensitivity 77.7%, specificity 72.2%. Median PCT: high-risk 3.2 ng/mL vs. low-risk 1.39 ng/mL (p = 0.003); CRP: high-risk 16.28 mg/dL vs. low-risk 6.78 mg/dL (p = 0.001) | PCT and CRP significantly higher in patients classified as high-risk (systemic infection or sepsis). PCT more specific; CRP more sensitive. Both useful for early identification of infection. | Mortality occurred only in high-risk group (4/18), but difference in marker levels between deceased and survivors with complications was not statistically significant | PCT and CRP both distinguished high- vs. low-risk infection. PCT had higher specificity; CRP had higher sensitivity. No data on organism-specific discrimination. | Authors recommend using PCT and/or CRP in risk group classification at FN onset. No formal algorithm provided. |
| 5 | Kharya (2007) [10] | IL-6, hsCRP, TNF-α, PCT | 62 febrile neutropenia episodes in children (5 months–17 years) with malignancy/aplastic anemia; India | IL-6 at 0 h > 137.5 pg/mL: sensitivity 67%, specificity 75%. IL-6 at 48 h > 69 pg/mL: sensitivity 75%, specificity 78%. PCT > 1.73 ng/mL at 0 h: sensitivity 73%, specificity 70%. IL-6 > 20 pg/mL: 100% sensitivity for sepsis (all 15 proven cases). PCT > 1 ng/mL: 80% sensitivity (12/15 proven cases). | IL-6 and PCT significantly elevated in sepsis vs. non-sepsis. IL-6 at admission and 48 h had AUC 0.73 and 0.77 respectively. Both useful for early identification. | All deaths (n = 6) occurred in patients with IL-6 > 20 pg/mL. Elevated IL-6 and PCT associated with proven sepsis and poor outcome. | IL-6 > 20 pg/mL and PCT > 1 ng/mL included all proven sepsis cases. Lower IL-6 values reliably excluded sepsis. | Authors suggest IL-6 and PCT could guide early discharge decisions or withholding antibiotics in low-risk patients (e.g., IL-6 < 20 pg/mL, PCT < 1 ng/mL). |
| 6 | Kitanovski (2006) [11] | IL-6, PCT, CRP | 68 febrile neutropenia episodes in 32 pediatric cancer patients (hematologic malignancies or solid tumors); Slovenia | IL-6 day 1 > 235.1 pg/mL **: AUC 0.867, sensitivity 87.5%, specificity 86.0%. PCT day 1 > 0.55 μg/L **: AUC 0.832, sensitivity 93.8%, specificity 70.6%. CRP day 1 > 60 mg/L **: AUC 0.649, sensitivity 62.5%, specificity 70.0%. | IL-6 and PCT significantly elevated in bacteremia/clinical sepsis vs. localized infection and FUO; better early markers than CRP. Sequential PCT improved diagnostic accuracy. | not applicable | No statistically significant differences in IL-6 or PCT based on pathogen type. CRP had lower initial accuracy. IL-6 and PCT better for early detection of bacteremia/sepsis. | not applicable |
| 7 | Abrahamsson (1997) [12] | IL-6, TNF-α, IFN-γ, CRP | 110 febrile episodes in 70 children with malignancy (hematologic and solid tumors), Sweden | IL-6 ≥ 50 pg/mL: sensitivity 74%, specificity 49% in detecting bacteremia. CRP ≥ 50 mg/L: sensitivity 29%, specificity 78%. | IL-6 more sensitive than CRP in detecting bacterial infection in neutropenic and non-neutropenic patients. Elevated IL-6 also seen in FUO. | not applicable | IL-6 levels higher in sepsis vs. NBF (p < 0.05). No significant difference in IL-6 between sepsis and FUO. TNF-α and IFN-γ levels less useful. | not applicable |
| 8 | Srinivasan (2021) [13] | Procalcitonin (PCT), IL-6, IL-8 | 46 febrile neutropenia episodes in 33 children with cancer; prospective study, India | PCT ≥ 2 ng/mL: sensitivity 63%, specificity 91%, NPV 88%, PPV 70%; AUC = 0.745. IL-6 (cutoff 50 pg/mL): sensitivity 54%, specificity 57%, NPV 80%, PPV 28%; AUC = 0.574. IL-8 (cutoff 130 pg/mL): sensitivity 45%, specificity 49%, NPV 73%, PPV 21%; AUC = 0.551. | PCT, IL-6, and IL-8 measured at admission. PCT showed best diagnostic performance for documented infections. IL-6 and IL-8 had lower accuracy, but favorable NPV. | not applicable | PCT better predictor of bacteremia than IL-6 and IL-8. No significant differences in IL-6/IL-8 between infected and non-infected groups. | PCT, IL-6, and IL-8 may help define low-risk patients suitable for early discharge and outpatient treatment. No formal model presented. |
| 9 | Diepold (2008) [14] | IL-6, IL-8, CRP | 123 febrile neutropenic episodes in 69 pediatric oncology patients undergoing chemotherapy; Germany | IL-6 > 42 pg/mL: sensitivity 90%, specificity 85%, PPV 94%, NPV 77%. IL-8 > 30 pg/mL: sensitivity 87%, specificity 59%, PPV 84%. CRP > 1 mg/dL: sensitivity 83%, specificity 59%, PPV 86%. | IL-6 and IL-8 measured within 24 h of fever onset. IL-6 best predictor of sepsis or prolonged fever. IL-6 > 42 pg/mL identifies high-risk patients; low levels suggest short fever duration. | not applicable | IL-6 and IL-8 higher in sepsis and prolonged fever vs short fever. IL-6 better predictor than IL-8 or CRP. No differences between Gram-positive and Gram-negative noted. | IL-6 < 42 pg/mL may help define low-risk patients for early discharge or outpatient therapy; model not formally validated. |
| 10 | Aggarwal (2013) [15] | IL-5, IL-6, IL-8, TNF-α | 52 episodes of febrile neutropenia in 48 children with hematologic malignancies; India | IL-6 > 100 pg/mL: sensitivity 36%, specificity 24%, NPV 59%. IL-8 > 300 pg/mL: sensitivity 46%, specificity 26%, NPV 59%. TNF-α > 25 pg/mL: sensitivity 71%, specificity 33%, NPV 88%. IL-5: not useful due to narrow range. | IL-5, IL-6, IL-8, TNF-α measured within 24 h of admission. None of the cytokines could predict high-risk FN, but IL-6, IL-8, TNF-α had high NPV (>80%) for ruling out serious infection. | not applicable | IL-6, IL-8 and TNF-α not significantly different between low- and high-risk groups; however, their high NPV suggests utility in excluding serious infection. | not applicable |
| 11 | Doerflinger (2021) [16] | PCT, IL-10, MIP-1β, CRP, IL-6, IL-8 (plus 28 others) | 79 febrile neutropenia episodes in 64 children with cancer; multicenter study, Australia | PCT: ≥0.425 ng/mL (sensitivity 100%, specificity 78%, AUC = 0.842). IL-10: ≥16.66 pg/mL (sensitivity 75%, specificity 83%, AUC = 0.826). Combined PCT + IL-10: ≥0.425 ng/mL + ≥4.37 pg/mL (sensitivity 100%, specificity 89%). MIP-1β: AUC = 0.780 (p = 0.010). CRP/IL-6/IL-8: Non-significant (AUC = 0.695/0.625/0.666; p > 0.05). | PCT + IL-10 best predicted bacteremia at fever onset. CRP/IL-6/IL-8 had no discriminatory value. Day 2: PCT rose 11-fold in bacteremia cases. | not applicable | PCT + IL-10 distinguished bacteremia; IL-6/IL-8 did not. | PCT + IL-10 improved AUS-rule CDR performance (84% low-risk classification vs. 73% with AUS-rule alone); supports early discharge decisions. |
| 12 | Urbonas (2012) [17] | IL-10 | 36 febrile neutropenia episodes in 24 pediatric oncology patients (19 hematologic [ALL, AML, NHL], 5 solid tumors); Lithuania | IL-10 ≥ 18 pg/mL: sensitivity 73%, specificity 92%, PPV 86%, NPV 83%; AUC = 0.87 | IL-10 significantly higher in sepsis vs. FUO group (median 39 vs. 0 pg/mL, p = 0.0006). High specificity and NPV support IL-10 as a tool to exclude infection early. | not applicable | IL-10 significantly higher in SEP vs FUO; no overlap in medians. Effective in distinguishing sepsis/bacteremia from non-infectious fever. | Rule-out tool for sepsis/bacteremia (high NPV/specificity). Not a standalone predictor (no formal CDR model) |
| 13 | Soker (2001) [18] | IL-1β, sIL-2R, IL-6, IL-8, TNF-α | 48 febrile neutropenia episodes in 23 pediatric cancer patients (ALL, AML, NHL); Turkey | IL-6, sIL-2R, and IL-8 significantly higher in culture-positive vs. culture-negative and control groups (p < 0.001). IL-6: 57 pg/mL vs. 8.6 pg/mL vs. 4.0 pg/mL; IL-8: 305 pg/mL vs. 23 pg/mL vs. 5 pg/mL. IL-1β and TNF-α not significantly different. | IL-6, IL-8, sIL-2R elevated early in febrile neutropenia, especially in culture-positive episodes. May guide empirical antibiotic initiation. | not applicable | IL-6, IL-8, sIL-2R significantly higher in Gram-negative vs. Gram-positive bacteremia (p = 0.042, 0.023, 0.006). IL-1β and TNF-α not useful in identifying infection type. | not applicable |
| 14 | Riikonen (1992) [19] | TNF-α, IL-1β, IL-6, CRP, SAA | 81 febrile episodes in 46 neutropenic children with cancer; Finland | TNF-α > 40 ng/L in most patients; range up to 520 ng/L. IL-1β > 20 ng/L in all groups. IL-6 detectable in 68% of episodes, with elevated concentrations in 15%. SAA > 5 mg/L in all bacteremia cases (mean normal = 2.1 ± 3.2 mg/L). CRP < 20 mg/L in 32% of bacteremia episodes. CRP and SAA correlated (r = 0.63, p < 0.001). | All markers elevated in fever, but none differentiated bacteremia from other causes. IL-6 correlated with CRP on days 1 and 2. SAA more sensitive than CRP for early detection of bacteremia. | not applicable | No cytokine or acute-phase protein clearly differentiated between bacteremia, FUO, focal or non-bacterial infection. | not applicable |
| 15 | Miedema (2010) [20] | IL-8, PCT, CRP, sTREM-1 | 43 febrile neutropenic episodes in 29 children with cancer; Netherlands; prospective study | IL-8 (cutoff 60 ng/L): AUC = 0.81 (95% CI: 0.656–0.965), sensitivity 92%, specificity 54% PCT (cutoff 0.25 ng/mL): AUC = 0.84 (95% CI: 0.710–0.970), sensitivity 79%, specificity 77%, significant predictor at 24–48 h (p = 0.047) CRP (cutoff 40 mg/L): AUC = 0.60 (95% CI: 0.388–0.803), sensitivity 69%, specificity 62% (p = 0.183) IL-8 + PCT combination: 100% sensitivity, 52% specificity for MDI | IL-8 and PCT measured at admission. Both markers performed well in early detection of microbiologically documented infection (MDI). | not applicable | IL-8 and PCT significantly higher in MDI vs. no infection. IL-8 median: 318.5 vs. 55 ng/L (p = 0.002); PCT: 0.81 vs. 0.10 ng/mL (p = 0.001). | IL-8 + PCT combination showed potential for risk stratification; 100% sensitivity in identifying MDI. |
| 16 | Bux (1999) [21] | G-CSF | 63 patients with antibody-induced neutropenia (including neonatal, autoimmune, and drug-induced forms) | G-CSF elevated (60–1006 pg/mL) only in patients with concurrent infections; normal < 39 pg/mL G-CSF levels decreased to normal after successful antibiotic treatment” | Elevated G-CSF levels observed only in presence of infection, not due to neutropenia itself | not applicable | not applicable | not applicable |
| 17 | Urbonas (2013) [22] | PCT, sIL-2R, sHLA-G, presepsin | 62 febrile neutropenia episodes in 37 pediatric oncology patients (ALL, AML, NHL, solid tumors); Lithuania | PCT: AUC = 0.79, optimal cutoff 0.38 ng/mL; diagnostic cutoff 0.65 ng/mL: specificity 92%, PPV 82%. sIL-2R: AUC = 0.73; screening cutoff 1558 ng/L: sensitivity 85%, specificity 56%. Presepsin: median 401 vs. 356 ng/L (p = 0.82); sHLA-G: p = 0.07 | PCT and sIL-2R significantly higher in BS vs FUO group. sHLA-G showed a non-significant trend (p = 0.07); presepsin not useful diagnostically. | not applicable | PCT and sIL-2R helped distinguish bacteremia/sepsis from FUO; sHLA-G and presepsin did not. | Combined PCT + sIL-2R AUC = 0.82, but no improvement over PCT alone (AUC = 0.79); PCT recommended as primary marker. |
| 18 | Santolaya (1994) [23] | CRP | 85 febrile neutropenia episodes in 75 pediatric cancer patients (ALL, lymphoma, solid tumors); Chile. Group I = confirmed bacterial infection (culture-positive); Group II = probable bacterial infection (severe clinical/radiologic findings, culture-negative); Group III = viral or no infection. | CRP > 40 mg/L: sensitivity 100%, specificity 76.6% for demonstrated bacterial infection (day 1); mean CRP: group I = 194 mg/L, group II = 143 mg/L, group III = 29 mg/L; p < 0.001 | CRP > 40 mg/L on day 1 significantly associated with bacterial infection; CRP useful for early diagnosis and monitoring | Persistently high CRP associated with poor outcomes; patients with unfavorable course had non-decreasing CRP | CRP > 40 mg/L on day 1 discriminated bacterial from viral/no infection; p < 0.001 | Authors propose using day 1–2 CRP levels (>40 mg/L) to guide antibiotic initiation; CRP decline supports treatment efficacy |
| 19 | Jaing (2020) [24] | IL-8, CRP | 30 febrile neutropenic pediatric patients after allogeneic HSCT; Taiwan. Group I = unexplained fever; Group II = clinically/radiologically documented infection. | IL-8 ≥ 60 ng/L and CRP ≥ 40 mg/L associated with documented infection; IL-8 decreased earlier than CRP; IL-8 and CRP positively correlated (r = 0.289, p = 0.039) | IL-8 elevated earlier than CRP; both markers higher in infection group; CRP significantly higher in group II (p < 0.05); IL-8 not significantly different | not applicable | IL-8 and CRP useful in distinguishing unexplained fever from documented infectious episodes | IL-8 < 60 ng/L (2 consecutive values) used to identify low-risk patients eligible for antibiotic de-escalation |
| 20 | Urbonas (2011) [25] | IL-8 | 16 pediatric oncology patients with febrile neutropenia and bloodstream infections (11 Gram-negative, 5 Gram-positive); Lithuania | IL-8 measured on day 1 and day 2 of FN. Median IL-8 values in Gram-negative infection group were 3.9–4.3 times higher than in Gram-positive infection group. Specific values or cutoff not provided. Mann–Whitney test used for comparison. | Distinct cytokine response to Gram-negative vs. Gram-positive bacteria; IL-8 elevated significantly more in Gram-negative infections on day 1–2 | not applicable | IL-8 levels significantly higher in Gram-negative bloodstream infections (3.9–4.3×, p < 0.05). | Proposed as adjunct diagnostic tool for early bacteremia typing (days 1–2), but requires validation in larger cohorts. Not yet validated for risk stratification. |
| 21 | Kim (2022) [26] | IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, IL-17A, IL-17F, IL-21, IL-22, TNF-α, IFN-γ | 10 pediatric patients (3–18 yrs) with febrile neutropenia and bacteremia during chemotherapy or HCT for hematologic malignancies; Korea | IL-6 and IL-10: highest on Day 1 (median IL-6: 429.68 pg/mL (73.07–8030.00)), significantly decreased by Day 4 (p < 0.001 and p = 0.001, respectively); IL-2 higher in GPB vs. GNB on Day 1 (6.05 vs. 3.00 pg/mL, p = 0.038); IL-22 higher in GNB vs. GPB on Day 8 (76.46 vs. 47.32 pg/mL, p = 0.010); no consistent cutoff values reported | IL-6 and IL-10 concentrations were significantly elevated at onset of FN and declined within 4 days regardless of fever duration; IL-2 and IL-22 showed statistical differences depending on bacterial type (GPB vs. GNB) | No severe complications (shock, ARDS, death); cytokine kinetics not associated with prolonged fever ≥3 days; all patients survived except two who died due to leukemia progression | IL-2 (Day 1) higher in GPB; IL-22 (Day 8) higher in GNB; other cytokines not discriminatory; potential for IL-2/IL-22 in early etiology differentiation | Cytokine kinetics (esp. IL-6/IL-10) peaked early and normalized by Day 4; no link to fever duration; authors conclude immune-modulating therapy not supported for prolonged fever |
| 22 | Xu (2019) [27] | CRP, PCT, IL-6, IL-10, TNF-α, IFN-γ | 3118 febrile illness episodes in 1115 pediatric cancer patients (mainly ALL, AML, lymphoma); China | For GNB: IL-10 AUC = 0.81 (cutoff 18.5 pg/mL), IL-6 AUC = 0.77 (cutoff 185 pg/mL), PCT AUC = 0.68, CRP AUC = 0.56. For septic shock: IL-6 AUC = 0.89 (cutoff 185 pg/mL, Se 84.4%, Sp 79.3%), IL-10 AUC = 0.87 (cutoff 20 pg/mL), PCT AUC = 0.78, CRP AUC = 0.65 | IL-6 and IL-10 were significantly elevated in GNB and septic shock; better predictors than CRP or PCT; CRP not independently predictive | IL-6 ≥ 185 pg/mL (OR 8.21) and IL-10 ≥ 20 pg/mL (OR 4.28) independently predicted septic shock; 12 deaths recorded (8 with shock) | IL-6 and IL-10 identified GNB and septic shock more accurately than CRP/PCT; IL-10 ≥ 18.5 pg/mL and IL-6 ≥ 185 pg/mL discriminated severe infection | IL-6 < 185 pg/mL and IL-10 < 20 pg/mL combination identified low-risk patients (shock rate 0.7%); proposed for early risk stratification |
| 23 | van der Galiën (2018) [28] | IL-6, PCT | 77 febrile neutropenia episodes in 55 children with cancer (hematologic, solid, brain tumors); Netherlands | IL-6 cutoff = 60 ng/L: AUC T0 = 0.88, Se 100%, Sp 34.7%, NPV 100%; IL-6 T1: AUC = 0.86, Se 100%, Sp 54.2%; PCT cutoff = 0.25 ng/mL: AUC T0 = 0.70, Se 93.3%, Sp 42.8%; PCT T1: AUC = 0.77, Se 90%, Sp 37.1% | IL-6 and PCT significantly higher in bacterial vs non-bacterial episodes; IL-6 more sensitive than PCT; combining both improved accuracy | not applicable | IL-6 and PCT (individually and combined) were significantly elevated in bacterial infection; IL-6 alone identified all bacterial cases | IL-6 > 60 and/or PCT > 0.25 ng/mL at admission: sensitivity 100%, NPV 100%; Combined rule (IL-6 ≥ 60 ng/L or PCT ≥ 0.25 ng/mL) achieved 100% sensitivity/NPV; the inverse rule (both biomarkers below cutoffs) identified 41% as low-risk |
| 24 | Cost (2013) [29] | IL-8 (plus IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α, IFN-γ, GM-CSF), CRP | 195 febrile neutropenia episodes in 116 pediatric oncology patients (chemotherapy or radiotherapy; no HSCT); single center, USA | IL-8 < 1260 pg/mL predicted low risk for bacteremia: sensitivity 90%, specificity 65%, NPV 98%, PPV 25%; AUC = 0.81; IL-8 levels significantly higher in bacteremia vs. non-bacteremia (median 4479 vs. 553 pg/mL, p < 0.001) | IL-8 most predictive marker in multivariate model; outperformed clinical predictors and other cytokines; proposed as single-variable risk stratification tool | 2 deaths due to bacteremia (Pseudomonas + Enterococcus, and S. pneumoniae); | IL-8 significantly elevated in bacteremia vs non-bacteremia; not influenced by concurrent respiratory viral infection | IL-8 < 1260 pg/mL identified low-risk patients with 98% NPV; authors propose IL-8-based triage to reduce unnecessary hospitalizations |
| 25 | Karakurt (2014) [30] | IL-6, IL-8, sTNFRII, sIL-2R, CRP, PCT | 50 febrile neutropenia episodes in 31 pediatric cancer patients (non-leukemic); Turkey | IL-6: median 94.7 pg/mL (6.7–1729.9), IL-8: 125.9 pg/mL (18.2–3362.4), sTNFRII: 628.7 pg/mL, sIL-2R: 12.1 ng/mL, CRP: 1.8 mg/dL. IL-6, IL-8, sTNFRII significantly higher in fever >3 days (p = 0.02, 0.01, 0.04). IL-8 higher in treatment modification group (p = 0.048). PCT ≥ 1 ng/mL associated with prolonged fever (p = 0.014). No cutoff values defined. | IL-6, IL-8, sTNFRII correlated with prolonged fever; IL-8 also associated with treatment modification; no single marker predictive of severe infectious complications | not applicable | IL-6, IL-8, sTNFRII levels higher in microbiologically proven infections; CRP paradoxically lower in infection vs. FUO; differences not statistically significant | IL-6, IL-8, sTNFRII suggested as supportive markers in early identification of prolonged febrile course; authors recommend cautious use in clinical decisions |
| 26 | Akcay (2021) [31] | IL-6, IL-8, MBL, CRP, PCT | 54 febrile neutropenic episodes in 30 pediatric cancer patients (ALL, AML, NHL, neuroblastoma); Turkey | IL-8 ≥ 200 pg/mL: sensitivity 80%, specificity 65%, NPV 92%; IL-6 ≥ 100 pg/mL: sensitivity 80%, specificity 40%, NPV 88%; PCT ≥ 10 ng/mL: specificity 100%, PPV 100%, NPV 84%; CRP not discriminatory; MBL ≥ 400 ng/mL: sensitivity 80%, specificity 59%, NPV 93% | IL-8 significantly higher in B/S vs. CMDI (p = 0.038) and FUO (p = 0.012); IL-6, PCT, MBL highest in B/S but without statistical significance; CRP not useful diagnostically | not applicable | IL-8 was best marker for distinguishing B/S from CMDI/FUO; other markers showed no significant differences between infection types | IL-8 ≥ 200 pg/mL had the highest discriminatory power and 92% NPV for B/S, but its standalone use is limited by 65% specificity. The study advocates combining it with PCT (specificity 100%) for risk stratification.; proposed as part of early low-risk identification strategy |
| 27 | Şahbudak Bal (2017) [32] | IL-6, IL-8, IL-10 | 59 febrile neutropenia episodes in 38 pediatric patients with ALL or AML; prospective study, Turkey | IL-8 ≥ 61.3 pg/mL: Sens. 64.1%, Spec. 75.6%, PPV 45%, NPV 87.2%; IL-10 ≥ 5.04 pg/mL: Sens. 92.9%, Spec. 44.4%, PPV 34.2%, NPV 95.2%; IL-6 ≥ 98.8 pg/mL: Sens. 50%, Spec. 71.1%, PPV 35%, NPV 81.1% | IL-6, IL-8, and IL-10 levels were significantly higher during infection. IL-10 was the most sensitive and IL-8 the most specific for predicting culture-confirmed infection. | not applicable | IL-8 showed the highest specificity and NPV for Gram-negative bacteremia, with AUC = 0.772 (p = 0.008); IL-10 had the highest sensitivity for overall culture-confirmed infections, AUC = 0.725 (p = 0.003). | IL-8 may be useful in ruling out Gram-negative infections; IL-10 may be used to rule out bacterial infection in general due to high NPV. |
| 28 | Araujo (2017) [3] | IL-1β, IL-6, IL-8, IL-10, IL-12/23p40, IL-17, IL-21, TNF-α, G-CSF, GM-CSF, PCT | 35 febrile neutropenic pediatric oncology patients, Brazil | IL-6 > 170 pg/mL: Sens. 69%, Spec. 95%, PPV 90%, NPV 84%; IL-8 > 240 pg/mL: Sens. 69%, Spec. 100%, PPV 100%, NPV 85%; IL-10 > 6 pg/mL: Sens. 69%, Spec. 86%, PPV 75%, NPV 83%; PCT > 180 pg/mL: Sens. 80%, Spec. 68%, PPV 50%, NPV 89% | IL-6, IL-8, IL-10, and PCT were significantly elevated on day 1 in patients who developed sepsis. IL-8 and IL-10 decreased after day 2. IL-12/23p40 and IL-17 were higher in non-septic patients. Filgrastim significantly increased IL-6, IL-8, IL-10 levels, but had minimal effect on diagnostic performance. | Deficiency in IL-23/IL-17 axis and IL-21 expression in septic patients may reflect immunosuppression; not quantitatively analyzed as prognostic markers. | IL-12/23p40 and IL-17 were significantly higher in non-septic patients, suggesting their role in mucosal immunity and bacterial translocation prevention. | Combination of markers (e.g., IL-8 > 240 + high risk, or IL-6 > 50 + PCT > 100) improved stratification performance. |
| 29 | Gupta (2020) [33] | IL-6, CRP | 32 episodes of febrile neutropenia in 25 pediatric oncology patients in South India; prospective observational study | IL-6 median values: Gram-negative 169, Gram-positive 17.5, sterile 52 pg/mL; CRP median: Gram-negative 60.7, Gram-positive 85.5, sterile 44.2 mg/L. IL-6 levels ranged from 8 to 5000 pg/mL; CRP: 0.66–288 mg/L. | IL-6 was elevated in all episodes and significantly higher in Gram-negative bacteremia (p = 0.017); CRP did not show such discriminatory power (p = 0.796). | All 4 deaths had IL-6 > 100 pg/mL; MDI had the highest IL-6 and worst outcomes (3/7 deaths, prolonged neutropenia in 85.8%). | IL-6 was a better predictor of Gram-negative sepsis than CRP. | IL-6 may help identify high-risk Gram-negative infections; CRP remains useful when IL-6 is unavailable. |
| 30 | Nijhuis (2005) [34] | IL-8 | 196 febrile neutropenic episodes in 128 cancer patients (children and adults), Netherlands; prospective, interventional single-center study | IL-8 cutoff: 60 ng/L. Low-risk: IL-8 ≤ 60 ng/L and no abnormal vital signs. Median IL-8 levels: low-risk 23 (7–45), medium-risk 120 (29–4927), high-risk 92 (13–9384) ng/L. | IL-8-based model (with vitals) correctly identified low-risk patients. Sensitivity: 100%, specificity: 21%, NPV: 100%, PPV: 13%. No bacteremia or failures in low-risk group. | not applicable | IL-8 levels at admission helped stratify risk of bacterial infection; no bacteremia occurred in patients with IL-8 ≤ 60 ng/L and no signs of sepsis. | IL-8 combined with clinical parameters allowed identification of patients safe for early discharge and without need for antibiotics, reducing costs and hospitalization. |
| 31 | Xia (2016) [35] | IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, PCT, CRP | 2819 febrile episodes in 828 pediatric hematology/oncology patients, China; retrospective study | IL-6 ≥ 500 pg/mL: Sens. 54.4%, Spec. 92.5%; IL-10 ≥ 100 pg/mL: Sens. 47.1%, Spec. 94.9%; PCT ≥ 2 ng/mL: Sens. 29.4%, Spec. 95% (severe infection). TNF-α ≥ 50 pg/mL: Sens. 18.1%, IFN-γ ≥ 25 pg/mL: Sens. 25.6%, IL-6/IL-10 positivity in Gram-negative bacteremia: 96.8%/90.3% vs. TNF-α/IFN-γ: 18.1%/25.6%. | IL-6 and IL-10 were superior to TNF-α/IFN-γ in detecting bacteremia/severe infection. TNF-α/IFN-γ had low sensitivity (<30%) but correlated with inflammation severity. | IL-6/IL-10 levels correlated with infection severity and septic shock risk; TNF-α/IFN-γ had limited prognostic utility. | IL-6/IL-10 had highest accuracy for severe infection (AUC 0.875/0.839), but moderate accuracy for Gram-negative bacteremia (AUC 0.686/0.747) | IL-6/IL-10 thresholds (≥500/≥100 pg/mL) identify high-risk patients; TNF-α/IFN-γ are not recommended for risk stratification. |
| 32 | Hemming (2017) [36] | PCT | 48 episodes of febrile neutropenia in 27 pediatric cancer patients, UK; prospective cohort study | PCT > 2 ng/dL: LR = 26 [95% CI 3.5–190]; PCT < 0.5: LR = 0.32; intermediate (0.5–2): LR = 0.39. No correlation with neutrophil count (r = −0.08). | High PCT on admission significantly associated with severe infection; clinical decision rules alone frequently overstated risk. | PCT > 2 ng/dL correlated with severe outcomes, but data insufficient to assess multiple-day predictive value. | not applicable | No definitive benefit in using PCT in pediatric FN |
| 33 | Nonkulovski (2020) [37] | PCT | 20 pediatric hemato-oncology patients with febrile neutropenia and sepsis; North Macedonia; retrospective-prospective study | All 20 patients had PCT ≥ 2 ng/mL within 24 h of admission. In septic shock: PCT > 10 ng/mL. PCT decreased after 3–5 days of antibiotic therapy; further drop by 6–14 days. | Elevated PCT confirmed sepsis early; levels decreased with treatment. Used to guide initiation and discontinuation of antibiotics. | PCT > 10 ng/mL associated with septic shock and fatal outcomes; dynamic PCT decline reflected response to treatment. | not applicable | PCT used to tailor duration and intensity of antibiotic therapy; supports rational antibiotic use and resistance prevention. |
| 34 | Zareifar (2020) [38] | PCT, CRP, ESR | 107 pediatric cancer patients with febrile neutropenia; cross-sectional study, Iran | PCT ≥ 0.70 ng/mL: Sens. 76%, Spec. 74.4%, PPV 47.5%, NPV 91%; AUC = 0.74 (95% CI: 0.61–0.87); p < 0.001 | PCT levels significantly higher in patients with positive blood cultures (median 2.17 vs. 0.32 ng/mL, p < 0.001); superior to ESR and comparable to CRP. | not applicable | PCT was significantly higher in culture-positive cases; suggests value in detecting infection, especially bloodstream infection. | High NPV suggests PCT may help rule out serious infection in febrile neutropenic patients; useful to guide clinical management. |
| 35 | Özdemir (2019) [39] | PCT, CRP, Presepsin, sTREM-1 | 47 episodes of febrile neutropenia in 30 pediatric oncology patients (vs. 27 controls); Turkey | PCT (cutoffs): Day 1 = 0.5 ng/mL (Sens. 61.5%, Spec. 89.4%, AUC =0.722), Day 2 = 0.25 ng/mL (Sens. 84.6%, Spec. 55.9%, AUC = 691), Day 7 = 0.5 ng/mL (Sens. 53.8%, Spec. 85.3%). CRP (cutoffs): Day 1 = 2.5 mg/dL, Day 2 = 4 mg/dL, Day 7 = 7 mg/dL. Presepsin and sTREM-1 had no significant diagnostic performance. | PCT and CRP were significantly higher in culture-positive vs. culture-negative episodes. PRE-SEP and sTREM-1 were elevated but lacked discriminatory power. | PCT and CRP levels on days 2 and 7 were higher in patients with clinical signs of sepsis; sTREM-1 levels were numerically higher in sepsis but not statistically significant. | Presepsin and sTREM-1 did not distinguish culture-positive from negative; PCT and CRP had moderate diagnostic accuracy. | PCT and CRP within 24 h useful for guiding therapy; Presepsin and sTREM-1 not recommended for risk stratification in this context. |
| 36 | Purkayastha (2016) [40] | PCT, CRP | 82 febrile neutropenia episodes in pediatric oncology patients; India; prospective observational study | PCT ≥ 0.25 ng/mL: Sens. 73.3%, Spec. 29.4%, PPV 18.6%, NPV 83.3%; CRP ≥ 110 mg/L: Sens. 13.3%, Spec. 77.2%, PPV 13.3%, NPV 77.2% | PCT was more sensitive than CRP, particularly in pulmonary infections. However, specificity was low, and PCT values showed wide variability. CRP had higher specificity but poor sensitivity. | not applicable | PCT levels were higher in pulmonary than extrapulmonary infections; CRP was less useful in distinguishing etiologies. | Authors emphasize that PCT should be used routinely. Its low specificity and high variability limit its standalone value. Further validation is needed. |
| 37 | Baraka (2018) [41] | Presepsin, PCT, CRP | 60 pediatric FN patients with hematological malignancies (ALL, AML, NHL, HD); Egypt; case-control study | Presepsin ≥ 1014 pg/mL (bacteremia): Sens. 100%, Spec. 85.7%, AUC = 0.95; ≥ 951 pg/mL (bacteremia + CPI): Sens. 93.8%, Spec. 100%, AUC = 0.996. PCT ≥ 2.86 ng/mL: Sens. 100%, Spec. 81%, AUC = 0.92. CRP ≥ 105 mg/L: Sens. 77.8%, Spec. 66.7%, AUC = 0.75. | Presepsin showed excellent accuracy (AUC 0.996) for bacteremia + CPI, outperforming CRP but comparable to PCT (AUC 0.92). PCT retained 100% sensitivity for bacteremia. Presepsin levels were higher in bacteremia vs. CPI and FUO; positively correlated with PCT and CRP, negatively with ANC. | not applicable | Presepsin levels did not significantly differ between Gram-positive and Gram-negative infections. | Authors recommend combining presepsin with CRP to improve diagnostic sensitivity. PCT remains valuable due to its high sensitivity. Study limited by small sample size and lack of external validation. |
| 38 | El-Maghraby (2007) [42] | CRP, IL-8, MCP-1-a | 85 febrile neutropenia episodes in 76 pediatric patients with hematologic malignancies (ALL, ANLL, NHL); Egypt; prospective study | CRP ≥ 90 mg/L: Sens. 69.5%, Spec. 73.1%, PPV 85.4%, NPV 51.4%. IL-8 ≥ 62 pg/mL: Sens. 71.2%, Spec. 76.5%, PPV 87.5%, NPV 54.1%. MCP-1-a ≥ 350 pg/mL: Sens. 64.4%, Spec. 92.3%, PPV 95%, NPV 53.3%. | CRP, IL-8 and MCP-1-a were significantly elevated in infected patients (p < 0.001). CRP ≥ 90 mg/L had highest sensitivity (100%) for bacteremia; MCP-1-a had the highest specificity. IL-8 and MCP-1-a had limited sensitivity for bacteremia. | not applicable | CRP levels were higher in bacteremia (especially Gram-negative and S. aureus); MCP-1-a and IL-8 levels did not differ significantly between Gram(+) and Gram(−) infections. | CRP showed superior sensitivity (100%) for bacteremia at ≥90 mg/L, while MCP-1-a had the highest specificity (92.3%). Combining markers improved accuracy: 78% of infected patients had ≥2 elevated markers vs. 16% in non-infectious cases. Authors recommend CRP as the primary marker and highlight study limitations. |
| 39 | Cerasi (2023) [43] | Presepsin, CRP, PCT, IL-6 | 41 pediatric patients with hematological/oncological diseases experiencing 50 febrile neutropenia episodes; 100 healthy controls; Italy | Presepsin T0 (at fever onset, before antibiotics): cutoff 410 pg/mL (AUC = 0.508; Se = 0.53, Sp = 0.55, PPV = 0.84, NPV = 0.22); T1 (48 h later): cutoff 213 pg/mL (AUC = 0.423; Se = 0.90, Sp = 0.50, PPV = 0.90, NPV = 0.50). IL-6 T0: AUC = 0.748; PCT T0: AUC = 0.793 | Presepsin was not useful for detecting bacteremia (low sensitivity/specificity, AUC < 0.6). PCT and IL-6 showed better accuracy. | Presepsin T1 ≥ 923 pg/mL predicted unfavorable outcome: Se = 0.75, Sp = 0.89, PPV = 0.37, NPV = 0.98, AUC = 0.792. IL-6 and CRP at T1 were also significant predictors. | Presepsin was ineffective in differentiating Gram+ from Gram− bacteremia; IL-6 was more useful at T0 for distinguishing bacterial infections. | Authors suggest presepsin may help rule out poor outcome due to its high NPV at T1; recommend combining biomarkers (IL-6, PCT, presepsin) for early risk stratification. |
| 40 | Arıkan (2021) [44] | Presepsin, proADM, CRP, PCT | 47 febrile neutropenia episodes in 39 pediatric patients with hematologic malignancies; 40 healthy controls; Turkey | Presepsin day 7 cutoff 750 pg/mL (AUC = 0.74; Se = 85%, Sp = 64%); PCT day 1 cutoff 0.36 ng/mL (AUC = 0.74; Se = 82%, Sp = 62%); proADM day 1 cutoff 57.95 pg/mL (AUC = 0.78; Se = 98%, Sp = 75%) | Presepsin and PCT effective in detecting bacteremia in FN; proADM most valuable early marker with highest sensitivity (98%); notably, presepsin levels were higher in healthy controls than FN patients due to neutropenia effect | Study focused on diagnostic accuracy for bacteremia detection, not prognostic outcomes; proADM showed highest diagnostic performance for early bacteremia detection | PCT significantly higher in Gram− vs. Gram+ bacteremia (p = 0.01 for day 1, p = 0.008 for day 2); CRP higher in Gram− vs. Gram+ only on day 2 (p = 0.02, NS on day 1); presepsin not significantly different between bacterial subtypes | Multivariate analysis showed that combining presepsin day 1 with PCT1/CRP1 was superior to individual markers for early bacteremia prediction in FN episodes |
| 41 | Antari (2023) [45] | PSP; MR-proADM, CRP | 70 children (<18 years) with hematological malignancies (ALL, AML, NHL); 70 episodes of febrile neutropenia; Greece | PSP on day 1: median 179 ng/mL (sepsis) vs. 80 ng/mL (no sepsis), p < 0.00001. MR-proADM: 0.559 vs. 0.196 nmol/L, p = 0.02. CRP: 7.08 vs. 3.04 mg/dL, p = 0.06. Diagnostic performance: PSP–Se = 84%, Sp = 82%; MR-proADM–Se = 74%, Sp = 70%; CRP–Se = 88%, Sp = 57% | PSP and MR-proADM were effective in early sepsis detection; PSP showed the best overall diagnostic accuracy | Prognostic evaluation not detailed; outcomes included 4% 28-day mortality and 10% ICU admission | PSP and MR-proADM levels were significantly higher in bloodstream infections (p = 0.03 and p = 0.04, respectively) | Not explicitly discussed, but PSP considered promising for early identification of high-risk cases (e.g., ICU need) |
| 42 | Agnello (2020) [46] | Presepsin, MR-ProADM | 37 febrile neutropenia episodes in 26 pediatric oncology patients; single-center study; Italy | Presepsin and mr-proADM were elevated at admission (T0) and decreased by day 5 (T2). Presepsin differed significantly between bacteremia and FUO groups at T1 only (median 416 vs. 271 pg/mL, p = 0.021). Diagnostic accuracy was low: Presepsin AUC = 0.58; mr-proADM AUC = 0.62 | Both biomarkers showed poor utility in identifying blood culture positivity; low diagnostic value in distinguishing infection etiology | Presepsin and mr-proADM at T0 were significant predictors of hospital length of stay (PSP: p = 0.00007; mr-proADM: p = 0.0038), but not of fever duration | Only PSP showed significant differences between bacteremia and FUO at T1; mr-proADM did not differ significantly at any timepoint | Elevated Presepsin and mr-proADM were associated with longer hospitalization, suggesting limited prognostic value; no specific risk stratification model proposed |
| 43 | Ragab (2025) [47] | CRP, PCT, MR-ProADM | 137 pediatric patients with chemotherapy-induced febrile neutropenia; Egypt | MR-ProADM cutoff: 489 pg/mL (AUC = 0.964, Se = 90.5%, Sp = 82.6%, PPV = 87.9%, NPV = 88.2%). CRP and MR-ProADM levels significantly higher in bacterial infection (CRP: 114.2 ± 12.16 vs. 68.9 ± 10.56 mg/dL; MR-ProADM: 703.65 ± 100.3 vs. 492.04 ± 124.9 pg/mL; p < 0.001) | MR-ProADM, CRP, and PCT were all elevated in bacterial infections; MR-ProADM showed highest diagnostic accuracy for infection prediction | Higher levels of MR-ProADM, CRP, and PCT correlated with longer FN duration, hospital stay, and mortality. MR-ProADM showed strongest correlation with hospitalization length (r = 0.838) | MR-ProADM and CRP significantly higher in bacteremia cases than non-bacteremia | MR-ProADM suggested as a reliable diagnostic and prognostic biomarker in FN; no formal algorithm proposed but strong potential indicated |
| 44 | Fawzi (2019) [48] | CRP, MR-ProADM | 100 pediatric patients (1–15 years) with hematologic malignancies and severe neutropenia; Egypt | MR-proADM day 1 cutoff: 2.4 nmol/L (AUC = 0.939, Se = 91.6%, Sp = 85.1%, PPV = 83.3%, NPV = 92.4); CRP day 1 cutoff: 94 mg/L (AUC = 0.509, Se = 52.6%, Sp = 70.9%). MR-proADM levels significantly higher in BS vs PUO on both days 1 and 2 | MR-proADM significantly outperformed CRP in early detection of bacteremia/sepsis versus PUO; CRP was a poor diagnostic marker (AUC ~0.51) | Prognostic value not assessed due to short follow-up; no data on outcomes beyond diagnostic performance | MR-proADM effectively differentiated BS from PUO from day 1; CRP levels showed no significant differences | MR-proADM considered a reliable early biomarker to aid in sepsis diagnosis in febrile neutropenic children with hematologic malignancies |
| 45 | Susanto (2022) [49] | MR-ProADM | 60 pediatric patients (1–18 years) with cancer-related chemotherapy (30 with sepsis, 30 without sepsis); Indonesia | MR-ProADM cutoff: 2.88 nmol/L; AUC = 0.707; Se = 60.0%, Sp = 56.67%, PPV = 58.06%, NPV = 58.62%, LR+ = 1.38, LR− = 0.71, diagnostic accuracy = 59.33%. Unexpectedly, median MR-ProADM levels were higher in the non-sepsis group (2.51 vs. 0.194 nmol/L), p = 0.006 | MR-ProADM showed moderate overall diagnostic accuracy (AUC = 0.707) for sepsis in pediatric cancer patients, but limited sensitivity and specificity | Not evaluated; the study focused only on diagnostic performance | MR-ProADM levels were paradoxically higher in non-sepsis group than in sepsis group, suggesting inverse association with clinical diagnosis | Authors concluded that MR-ProADM alone has limited diagnostic value; may be more effective when used alongside clinical criteria or in combination with other biomarkers |
| 46 | Demirkaya (2015) [50] | MR-ProADM CRP, PCT | 50 febrile neutropenia episodes in 37 pediatric cancer patients (leukemia, lymphoma, solid tumors); Turkey | ADM levels on day 3 were significantly higher in MDI group vs CDI and FUO [MDI: 191.7 (32.1–695.4), CDI: 42.8 (21.2–110.1), FUO: 46.0 (23.3–91.3), sepsis: 58.7 (19.7–100.1) pg/mL]; no cutoff provided | ADM could identify microbiologically documented infections on day 3 but was not helpful in differentiating sepsis; diagnostic utility limited compared to PCT | ADM levels were not associated with mortality or infection severity. In contrast, PCT was significantly elevated in deceased patients and in severe infections | ADM levels significantly higher in MDI vs FUO/CDI but not in sepsis; CRP showed no significant differentiation across groups; PCT most useful for identifying infection severity | Due to ADM’s rapid clearance, authors suggest limited standalone utility and recommend future studies using proADM for improved stability and stratification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bal, W.; Piasecka, Z.; Szuler, K.; Chaber, R. Infection Biomarkers in Children with Chemotherapy-Induced Severe Neutropenia. Cancers 2025, 17, 2227. https://doi.org/10.3390/cancers17132227
Bal W, Piasecka Z, Szuler K, Chaber R. Infection Biomarkers in Children with Chemotherapy-Induced Severe Neutropenia. Cancers. 2025; 17(13):2227. https://doi.org/10.3390/cancers17132227
Chicago/Turabian StyleBal, Wioletta, Zuzanna Piasecka, Klaudia Szuler, and Radosław Chaber. 2025. "Infection Biomarkers in Children with Chemotherapy-Induced Severe Neutropenia" Cancers 17, no. 13: 2227. https://doi.org/10.3390/cancers17132227
APA StyleBal, W., Piasecka, Z., Szuler, K., & Chaber, R. (2025). Infection Biomarkers in Children with Chemotherapy-Induced Severe Neutropenia. Cancers, 17(13), 2227. https://doi.org/10.3390/cancers17132227






