FAP-Directed Imaging and Therapy in Head and Neck Cancer of Unknown Primary
Simple Summary
Abstract
1. Introduction
2. Conventional Imaging
3. FAPI-PET
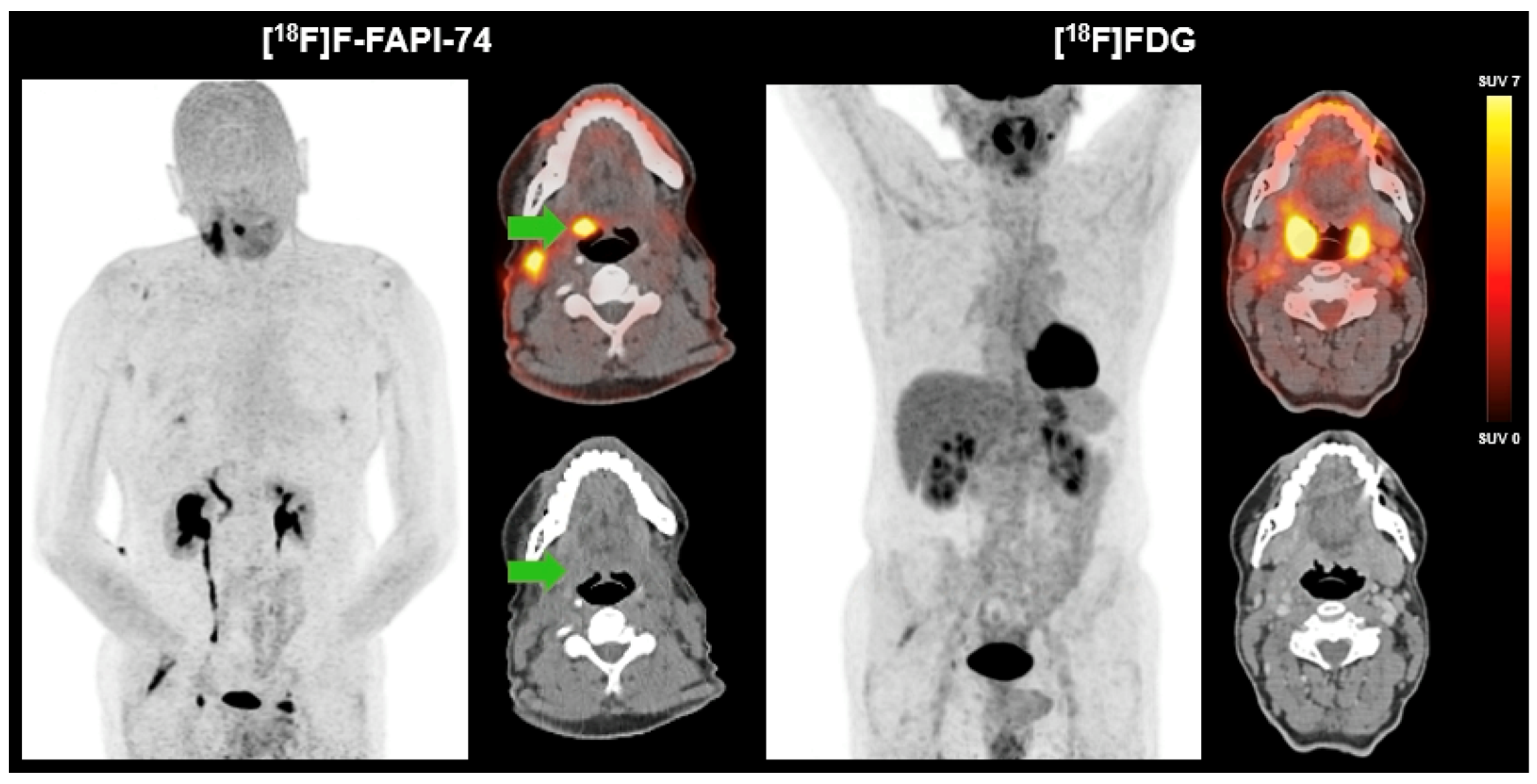
3.1. Literature Review
3.2. Case Study
4. FAP-Directed Theranostics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| CUP | Cancer of unknown primary |
| FAP | Fibroblast activation protein |
| FAPI | Fibroblast activation protein inhibitor |
| FDG | 18F-Fluorodeoxyglucose |
| HN | Head and neck cancer |
| HNCUP | Head and neck cancer of unknown primary |
| MRI | Magnetic resonance imaging |
| PET | Positron emission tomography |
| SSC | Squamous cell carcinoma |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Grau, C.; Johansen, L.V.; Jakobsen, J.; Geertsen, P.; Andersen, E.; Jensen, B.B. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2000, 55, 121–129. [Google Scholar] [CrossRef]
- Arosio, A.D.; Pignataro, L.; Gaini, R.M.; Garavello, W. Neck lymph node metastases from unknown primary. Cancer Treat. Rev. 2017, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Ferlito, A.; Medina, J.E.; Woolgar, J.A.; Rinaldo, A.; Robbins, K.T.; Fagan, J.J.; Mendenhall, W.M.; Paleri, V.; Silver, C.E.; et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013, 35, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.M.; Galloway, T.J.; Holdbrook, T.; Ruth, K.; Yang, D.; Dubyk, C.; Flieder, D.; Lango, M.N.; Mehra, R.; Burtness, B.; et al. p16 status, pathologic and clinical characteristics, biomolecular signature, and long-term outcomes in head and neck squamous cell carcinomas of unknown primary. Head Neck 2014, 36, 1677–1684. [Google Scholar] [CrossRef]
- Conway, A.M.; Mitchell, C.; Kilgour, E.; Brady, G.; Dive, C.; Cook, N. Molecular characterisation and liquid biomarkers in Carcinoma of Unknown Primary (CUP): Taking the ‘U’ out of ‘CUP’. Br. J. Cancer 2019, 120, 141–153. [Google Scholar] [CrossRef]
- Greco, F.A.; Erlander, M.G. Molecular Classification of Cancers of Unknown Primary Site. Mol. Diagn. Ther. 2009, 13, 367–373. [Google Scholar] [CrossRef]
- Pavlidis, N.; Pentheroudakis, G. Cancer of unknown primary site. Lancet 2012, 379, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Koivunen, P.; Laranne, J.; Virtaniemi, J.; Bäck, L.; Mäkitie, A.; Pulkkinen, J.; Grenman, R. Cervical Metastasis of Unknown Origin: A Series of 72 Patients. Acta Oto-Laryngol. 2002, 122, 569–574. [Google Scholar] [CrossRef]
- Nieder, C.; Gregoire, V.; Ang, K.K. Cervical lymph node metastases from occult squamous cell carcinoma: Cut down a tree to get an apple? Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 727–733. [Google Scholar] [CrossRef]
- Haas, I.; Hoffmann, T.K.; Engers, R.; Ganzer, U. Diagnostic strategies in cervical carcinoma of an unknown primary (CUP). Eur. Arch. Oto-Rhino-Laryngol. 2002, 259, 325–333. [Google Scholar] [CrossRef]
- Lee, J.R.; Kim, J.S.; Roh, J.-L.; Lee, J.H.; Baek, J.H.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Detection of Occult Primary Tumors in Patients with Cervical Metastases of Unknown Primary Tumors: Comparison of18F FDG PET/CT with Contrast-enhanced CT or CT/MR Imaging—Prospective Study. Radiology 2015, 274, 764–771. [Google Scholar] [CrossRef]
- Singnurkar, A.; Poon, R.; Metser, U. Comparison of 18F-FDG-PET/CT and 18F-FDG-PET/MR imaging in oncology: A systematic review. Ann. Nucl. Med. 2017, 31, 366–378. [Google Scholar] [CrossRef]
- Rusthoven, K.E.; Koshy, M.; Paulino, A.C. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 2004, 101, 2641–2649. [Google Scholar] [CrossRef]
- Noij, D.P.; Martens, R.M.; Zwezerijnen, B.; Koopman, T.; de Bree, R.; Hoekstra, O.S.; de Graaf, P.; Castelijns, J.A. Diagnostic value of diffusion-weighted imaging and 18F-FDG-PET/CT for the detection of unknown primary head and neck cancer in patients presenting with cervical metastasis. Eur. J. Radiol. 2018, 107, 20–25. [Google Scholar] [CrossRef]
- Martens, R.M.; van der Stappen, R.; Koopman, T.; Noij, D.P.; Comans, E.F.; Zwezerijnen, G.J.; Vergeer, M.R.; Leemans, C.R.; de Bree, R.; Boellaard, R.; et al. The Additional Value of Ultrafast DCE-MRI to DWI-MRI and 18F-FDG-PET to Detect Occult Primary Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2826. [Google Scholar] [CrossRef]
- Fukui, M.B.; Blodgett, T.M.; Snyderman, C.H.; Johnson, J.J.; Myers, E.N.; Townsend, D.W.; Meltzer, C.C. Combined PET-CT in the head and neck: Part 2. Diagnostic uses and pitfalls of oncologic imaging. Radiographics 2005, 25, 913–930. [Google Scholar] [CrossRef]
- Karam, M.B.; Doroudinia, A.; Naini, A.S.; Kaghazchi, F.; Koma, A.Y.; Mehrian, P.; Hossein, F.A. Role of FDG PET/CT Scan in Head and Neck Cancer Patients. Arch. Iran Med. 2017, 20, 452–458. [Google Scholar]
- Newbold, K.; Powell, C. PET/CT in Radiotherapy Planning for Head and Neck Cancer. Front. Oncol. 2012, 2, 39080. [Google Scholar] [CrossRef]
- Ciernik, I.; Dizendorf, E.; Baumert, B.G.; Reiner, B.; Burger, C.; Davis, J.; Lütolf, U.M.; Steinert, H.C.; Von Schulthess, G.K. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): A feasibility study. Int. J. Radiat. Oncol. 2003, 57, 853–863. [Google Scholar] [CrossRef]
- Talaat, O.; Maher, S.; Hassan, M.; Farouk, S. Impact of 18F-FDG-PET/CT in detection of the primary site and change management in patients with metastases of unknown primary. Egypt. J. Nucl. Med. 2019, 19, 36–49. [Google Scholar] [CrossRef]
- Galloway, T.J.; Ridge, J.A. Management of Squamous Cancer Metastatic to Cervical Nodes With an Unknown Primary Site. J. Clin. Oncol. 2015, 33, 3328–3337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, N. 18F-fluorodeoxyglucose positron emission tomography-computed tomography as a diagnostic tool in patients with cervical nodal metastases of unknown primary site: A meta-analysis. Surg. Oncol. 2013, 22, 190–194. [Google Scholar] [CrossRef]
- Szyszko, T.; Cook, G. PET/CT and PET/MRI in head and neck malignancy. Clin. Radiol. 2018, 73, 60–69. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Koczorowska, M.; Tholen, S.; Bucher, F.; Lutz, L.; Kizhakkedathu, J.; De Wever, O.; Wellner, U.; Biniossek, M.; Stahl, A.; Lassmann, S.; et al. Fibroblast activation protein-α, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol. Oncol. 2015, 10, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Rettig, W.J.; Garin-Chesa, P.; Beresford, H.R.; Oettgen, H.F.; Melamed, M.R.; Old, L.J. Cell-surface glycoproteins of human sarcomas: Differential expression in normal and malignant tissues and cultured cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3110–3114. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Raj, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and Preliminary Dosimetry Estimate of 2 DOTA-Containing FAP-Targeting Agents in Patients with Various Cancers. J. Nucl. Med. 2018, 60, 386–392. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A Tumor-Imaging Method Targeting Cancer-Associated Fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Wegen, S.; van Heek, L.; Linde, P.; Claus, K.; Akuamoa-Boateng, D.; Baues, C.; Sharma, S.J.; Schomäcker, K.; Fischer, T.; Roth, K.S.; et al. Head-to-Head Comparison of [68 Ga]Ga-FAPI-46-PET/CT and [18F]F-FDG-PET/CT for Radiotherapy Planning in Head and Neck Cancer. Mol. Imaging Biol. 2022, 24, 986–994. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. 2021, 48, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. 2020, 47, 2836–2845. [Google Scholar] [CrossRef]
- Promteangtrong, C.; Siripongsatian, D.; Jantarato, A.; Kunawudhi, A.; Kiatkittikul, P.; Yaset, S.; Boonkawin, N.; Chotipanich, C. Head-to-Head Comparison of 68Ga-FAPI-46 and 18F-FDG PET/CT for Evaluation of Head and Neck Squamous Cell Carcinoma: A Single-Center Exploratory Study. J. Nucl. Med. 2021, 63, 1155–1161. [Google Scholar] [CrossRef]
- Gu, B.; Xu, X.; Zhang, J.; Ou, X.; Xia, Z.; Guan, Q.; Hu, S.; Yang, Z.; Song, S. The Added Value of (68)Ga-FAPI PET/CT in Patients with Head and Neck Cancer of Unknown Primary with (18)F-FDG-Negative Findings. J. Nucl. Med. 2022, 63, 875–881. [Google Scholar] [CrossRef]
- Gu, B.; Yang, Z.; Du, X.; Xu, X.; Ou, X.; Xia, Z.; Guan, Q.; Hu, S.; Yang, Z.; Song, S. Imaging of Tumor Stroma Using68Ga-FAPI PET/CT to Improve Diagnostic Accuracy of Primary Tumors in Head and Neck Cancer of Unknown Primary: A Comparative Imaging Trial. J. Nucl. Med. 2024, 65, 365–371. [Google Scholar] [CrossRef]
- Eisenmenger, L.B. Non-FDG Radiopharmaceuticals in Head and Neck PET Imaging: Current Techniques and Future Directions. Semin. Ultrasound CT MRI 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Maghami, E.; Ismaila, N.; Alvarez, A.; Chernock, R.; Duvvuri, U.; Geiger, J.; Gross, N.; Haughey, B.; Paul, D.; Rodriguez, C.; et al. Diagnosis and Management of Squamous Cell Carcinoma of Unknown Primary in the Head and Neck: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2570–2596. [Google Scholar] [CrossRef]
- Kothari, P.; Randhawa, P.S.; Farrell, R. Role of tonsillectomy in the search for a squamous cell carcinoma from an unknown primary in the head and neck. Br. J. Oral Maxillofac. Surg. 2008, 46, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, M.; Mancuso, A.A.; Amdur, R.J.; Werning, J.W.; Kirwan, J.; Morris, C.G.; Mendenhall, W.M. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope 2009, 119, 2348–2354. [Google Scholar] [CrossRef]
- Waltonen, J.D.; Ozer, E.; Schuller, D.E.; Agrawal, A. Tonsillectomy vs. deep tonsil biopsies in detecting occult tonsil tumors. Laryngoscope 2008, 119, 102–106. [Google Scholar] [CrossRef]
- Serfling, S.; Zhi, Y.; Schirbel, A.; Lindner, T.; Meyer, T.; Gerhard-Hartmann, E.; Lapa, C.; Hagen, R.; Hackenberg, S.; Buck, A.K.; et al. Improved cancer detection in Waldeyer’s tonsillar ring by 68Ga-FAPI PET/CT imaging. Eur. J. Nucl. Med. 2020, 48, 1178–1187. [Google Scholar] [CrossRef]
- Leeman, J.E.; Li, J.-G.; Pei, X.; Venigalla, P.; Zumsteg, Z.S.; Katsoulakis, E.; Lupovitch, E.; McBride, S.M.; Tsai, C.J.; Boyle, J.O.; et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol. 2017, 3, 1487–1494. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Zou, G.; Zheng, S.; Zheng, K.; Zhang, J.; Huang, C.; Yao, S.; Miao, W. Accurate preoperative staging with [68Ga]Ga-FAPI PET/CT for patients with oral squamous cell carcinoma: A comparison to 2-[18F]FDG PET/CT. Eur. Radiol. 2022, 32, 6070–6079. [Google Scholar] [CrossRef]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either (18)F-AlF or Cold-Kit (68)Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2021, 62, 201–207. [Google Scholar] [CrossRef]
- Quinn, B.; Dauer, Z.; Pandit-Taskar, N.; Schoder, H.; Dauer, L.T. Radiation dosimetry of 18F-FDG PET/CT: Incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Hildingsson, S.; Gebre-Medhin, M.; Zschaeck, S.; Adrian, G. Hypoxia in relationship to tumor volume using hypoxia PET-imaging in head & neck cancer—A scoping review. Clin. Transl. Radiat. Oncol. 2022, 36, 40–46. [Google Scholar] [CrossRef]
- Marcus, C.; Sheikhbahaei, S.; Shivamurthy, V.K.N.; Avey, G.; Subramaniam, R.M. PET Imaging for Head and Neck Cancers. Radiol. Clin. N. Am. 2021, 59, 773–788. [Google Scholar] [CrossRef]
- Privé, B.M.; Boussihmad, M.A.; Timmermans, B.; van Gemert, W.A.; Peters, S.M.B.; Derks, Y.H.W.; van Lith, S.A.M.; Mehra, N.; Nagarajah, J.; Heskamp, S.; et al. Fibroblast activation protein-targeted radionuclide therapy: Background, opportunities, and challenges of first (pre)clinical studies. Eur. J. Nucl. Med. 2023, 50, 1906–1918. [Google Scholar] [CrossRef]
- Fu, K.; Pang, Y.; Zhao, L.; Lin, L.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. FAP-targeted radionuclide therapy with [177Lu]Lu-FAPI-46 in metastatic nasopharyngeal carcinoma. Eur. J. Nucl. Med. 2021, 49, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandus, J.; Fendler, W.P.; Farolfi, A.; Washington, S.; Mohammad, O.; Pampaloni, M.H.; Scott, P.J.; Rodnick, M.; Viglianti, B.L.; Eiber, M.; et al. PSMA PET Validates Higher Rates of Metastatic Disease for European Association of Urology Biochemical Recurrence Risk Groups: An International Multicenter Study. J. Nucl. Med. 2021, 63, 76–80. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Kovan, B.M.; Sanli, Y.; Buyukkaya, F.; Simsek, D.H.; Özkan, Z.G.; Isik, E.G.; Ekenel, M.; Turkmen, C. Safety of Fibroblast Activation Protein–Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021, 46, 641–646. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, L.; Fang, J.; Chen, J.; Meng, L.; Sun, L.; Wu, H.; Guo, Z.; Lin, Q.; Chen, H. Development of FAPI Tetramers to Improve Tumor Uptake and Efficacy of FAPI Radioligand Therapy. J. Nucl. Med. 2023, 64, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. 2021, 49, 871–880. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jäger, D.; Lindner, T.; Haberkorn, U. [153Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. 2021, 48, 3011–3013. [Google Scholar] [CrossRef]
| Higher tumor-to-background ratio (FAPI vs. FDG) | [33,34] |
| Positive FAPI scan in patients with negative FDG scan is possible (higher sensitivity and accuracy) | [36] |
| Fewer diagnostic tonsillectomies due to improved primary detection with FAPI-PET/CT | [44] |
| FAPI-PET/CT is more accurate than FDG at assessing the N0 neck status (100% vs. 29%) | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunte, S.C.; Sheikh, G.T.; Giesel, F.L.; Canis, M.; Werner, R.A. FAP-Directed Imaging and Therapy in Head and Neck Cancer of Unknown Primary. Cancers 2025, 17, 2205. https://doi.org/10.3390/cancers17132205
Kunte SC, Sheikh GT, Giesel FL, Canis M, Werner RA. FAP-Directed Imaging and Therapy in Head and Neck Cancer of Unknown Primary. Cancers. 2025; 17(13):2205. https://doi.org/10.3390/cancers17132205
Chicago/Turabian StyleKunte, Sophie C., Gabriel T. Sheikh, Frederik L. Giesel, Martin Canis, and Rudolf A. Werner. 2025. "FAP-Directed Imaging and Therapy in Head and Neck Cancer of Unknown Primary" Cancers 17, no. 13: 2205. https://doi.org/10.3390/cancers17132205
APA StyleKunte, S. C., Sheikh, G. T., Giesel, F. L., Canis, M., & Werner, R. A. (2025). FAP-Directed Imaging and Therapy in Head and Neck Cancer of Unknown Primary. Cancers, 17(13), 2205. https://doi.org/10.3390/cancers17132205







